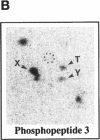
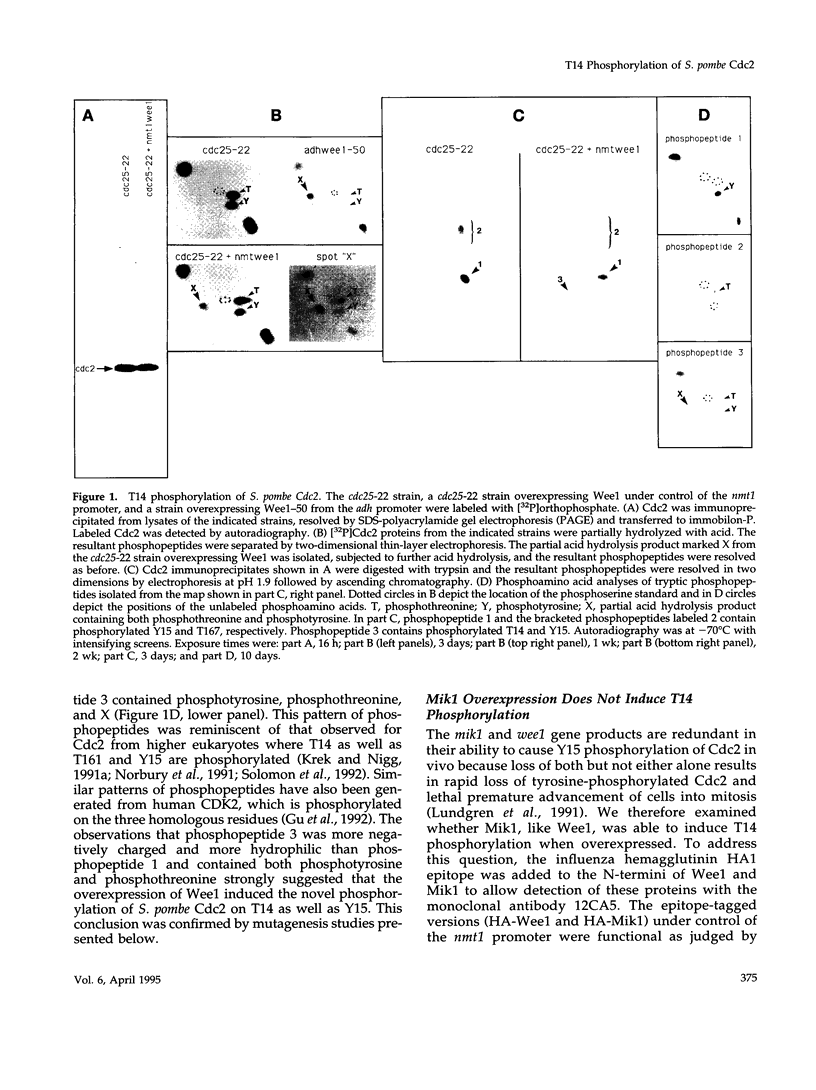
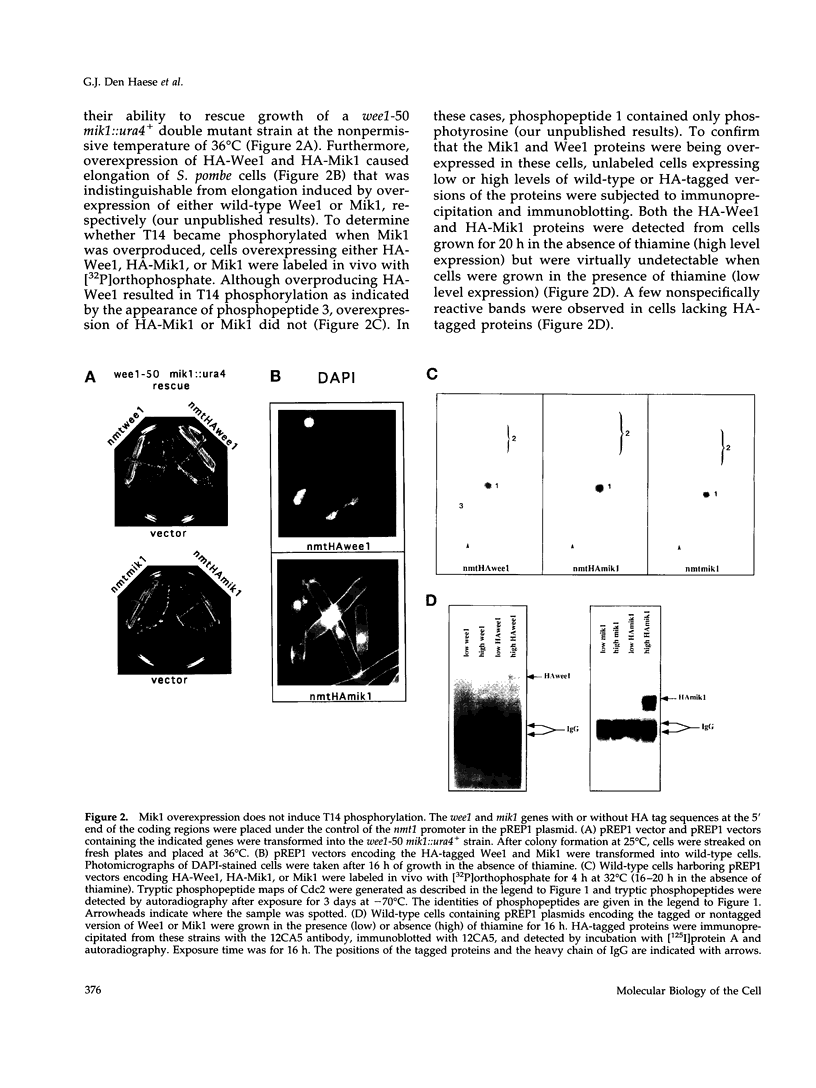
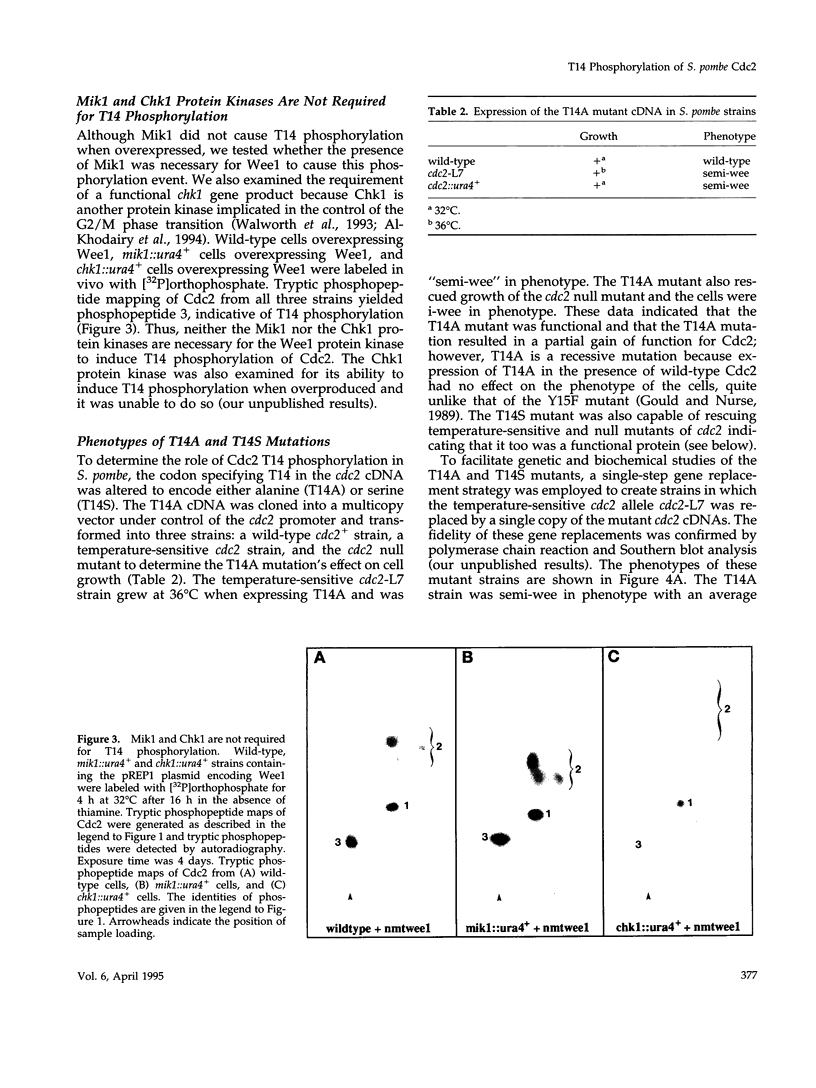
Abstract
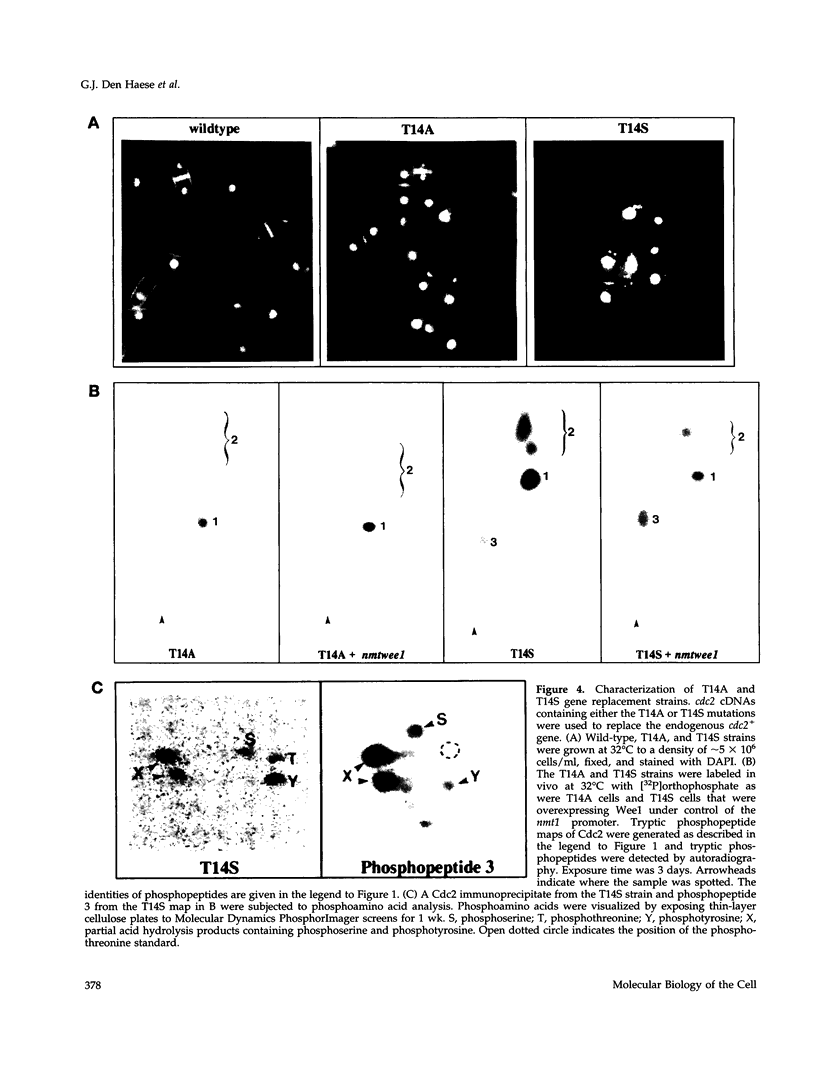
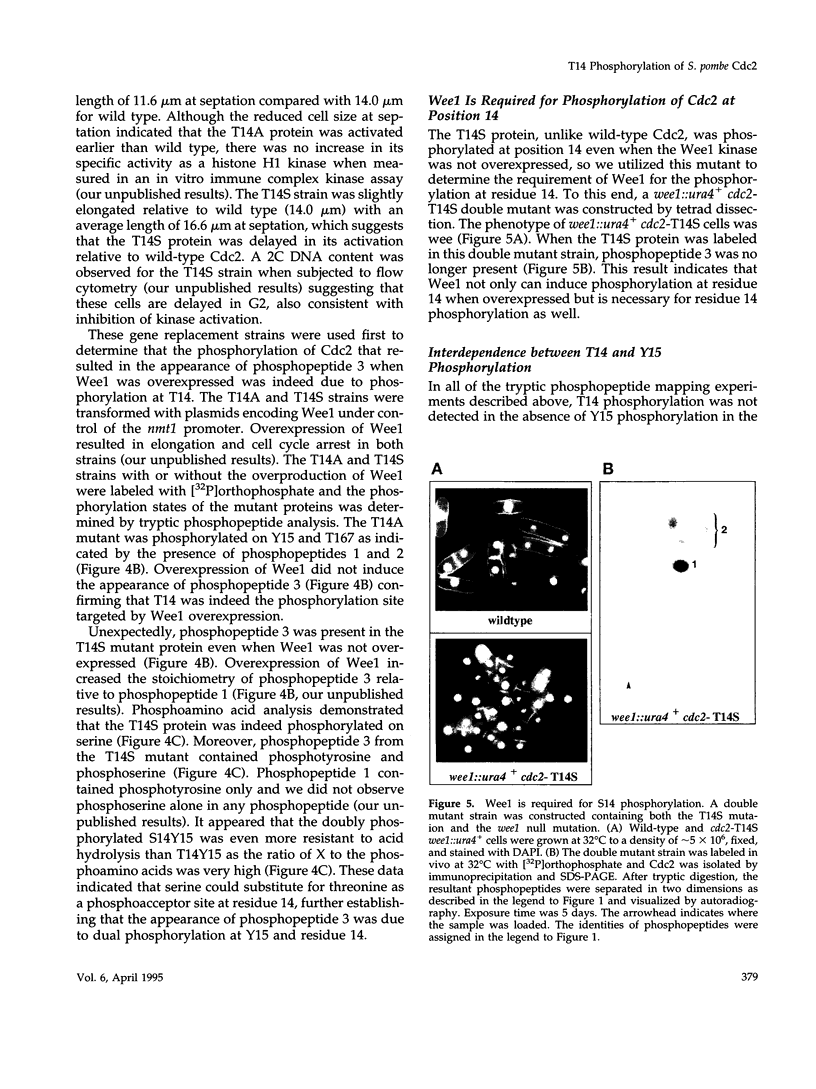
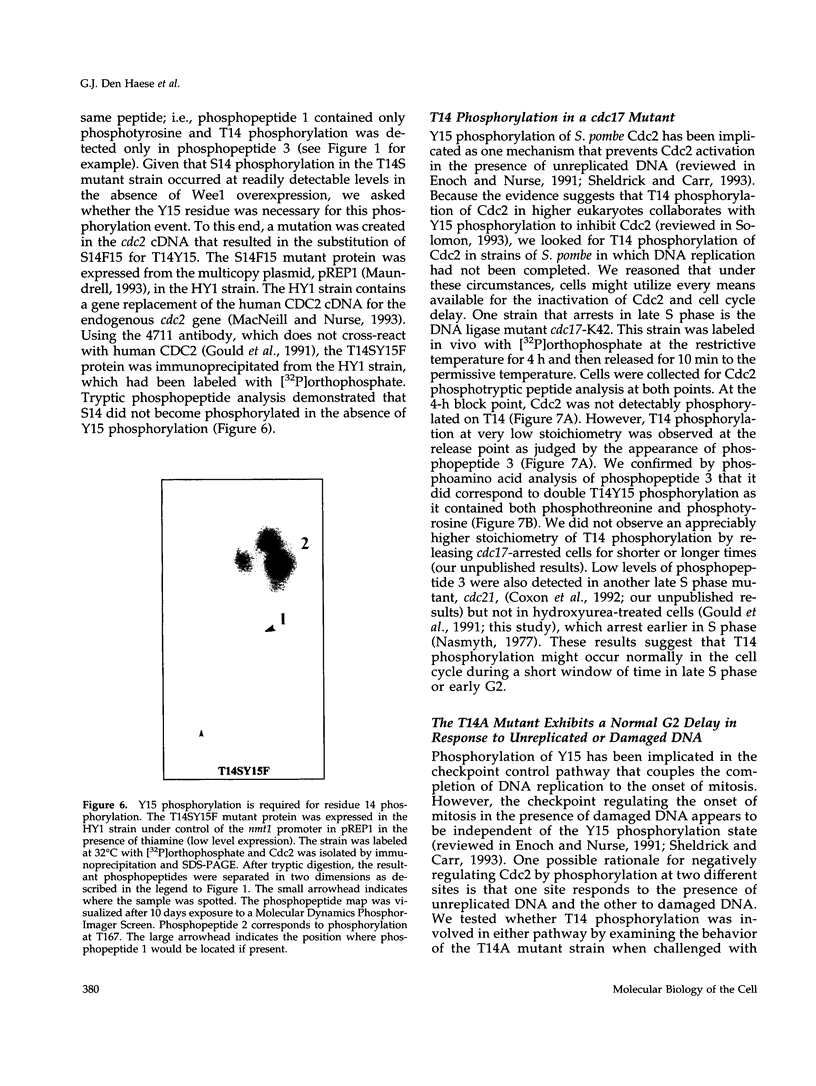
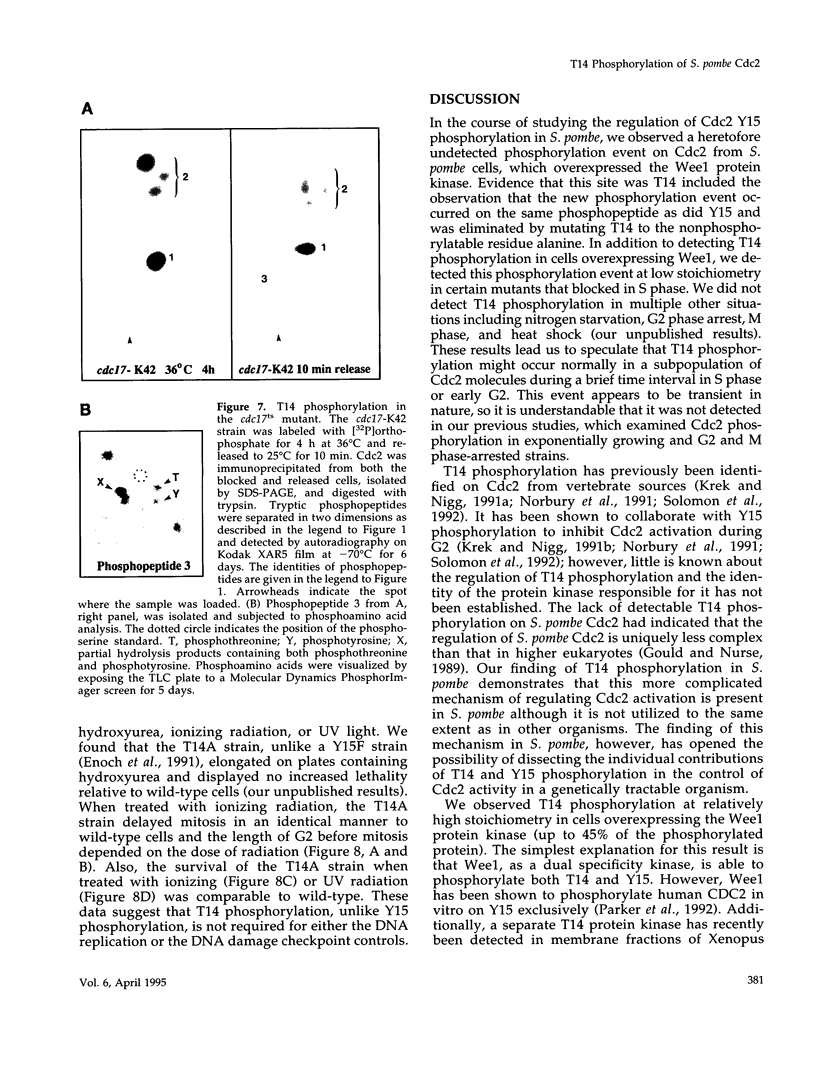
The Cdc2 protein kinase is a key regulator of the G1-S and G2-M cell cycle transitions in the fission yeast Schizosaccharomyces pombe. The activation of Cdc2 at the G2-M transition is triggered by dephosphorylation at a conserved tyrosine residue Y15. The level of Y15 phosphorylation is controlled by the Wee1 and Mik1 protein kinases acting in opposition to the Cdc25 protein phosphatase. Here, we demonstrate that Wee1 overexpression leads to a high stoichiometry of phosphorylation at a previously undetected site in S. pombe Cdc2, T14. T14 phosphorylation was also detected in certain cell cycle mutants blocked in progression through S phase, indicating that T14 phosphorylation might normally occur at low stoichiometry during DNA replication or early G2. Strains in which the chromosomal copy of cdc2 was replaced with either a T14A or a T14S mutant allele were generated and the phenotypes of these strains are consistent with T14 phosphorylation playing an inhibitory role in the activation of Cdc2 as it does in higher eukaryotes. We have also obtained evidence that Wee1 but not Mik1 or Chk1 is required for phosphorylation at this site, that the Mik1 and Chk1 protein kinases are unable to drive T14 phosphorylation in vivo, that residue 14 phosphorylation requires previous phosphorylation at Y15, and that the T14A mutant, unlike Y15F, is recessive to wild-type Cdc2 activity. Finally, the normal duration of G2 delay after irradiation or hydroxyurea treatment in a T14A mutant strain indicates that T14 phosphorylation is not required for the DNA damage or replication checkpoint controls.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton-Fessler S., Liu F., Gabrielli B., Lee M. S., Peng C. Y., Piwnica-Worms H. Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell. 1994 Sep;5(9):989–1001. doi: 10.1091/mbc.5.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet N. C., Carr A. M. Fission yeast wee1 protein kinase is not required for DNA damage-dependent mitotic arrest. Nature. 1993 Aug 26;364(6440):824–827. doi: 10.1038/364824a0. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Coxon A., Maundrell K., Kearsey S. E. Fission yeast cdc21+ belongs to a family of proteins involved in an early step of chromosome replication. Nucleic Acids Res. 1992 Nov 11;20(21):5571–5577. doi: 10.1093/nar/20.21.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G. The decision to enter mitosis. Trends Cell Biol. 1994 Jun;4(6):202–207. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Richman R., Hall F. L., Williams R. T., Lodgson N., Harper J. W. CDK2 encodes a 33-kDa cyclin A-associated protein kinase and is expressed before CDC2 in the cell cycle. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2907–2911. doi: 10.1073/pnas.89.7.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Carr A. M., Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 1992 Nov;6(11):2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Enoch T., Gould K. L., Nurse P. Mitotic checkpoint control in fission yeast. Cold Spring Harb Symp Quant Biol. 1991;56:409–416. doi: 10.1101/sqb.1991.056.01.048. [DOI] [PubMed] [Google Scholar]
- Enoch T., Nurse P. Coupling M phase and S phase: controls maintaining the dependence of mitosis on chromosome replication. Cell. 1991 Jun 14;65(6):921–923. doi: 10.1016/0092-8674(91)90542-7. [DOI] [PubMed] [Google Scholar]
- Enoch T., Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990 Feb 23;60(4):665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- Featherstone C., Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991 Feb 28;349(6312):808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Fesquet D., Labbé J. C., Derancourt J., Capony J. P., Galas S., Girard F., Lorca T., Shuttleworth J., Dorée M., Cavadore J. C. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993 Aug;12(8):3111–3121. doi: 10.1002/j.1460-2075.1993.tb05980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993 Jun 25;21(12):2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Gu Y., Rosenblatt J., Morgan D. O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992 Nov;11(11):3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley J., Phear G. A. Sequence of the cell division gene CDC2 from Schizosaccharomyces pombe; patterns of splicing and homology to protein kinases. Gene. 1984 Nov;31(1-3):129–134. doi: 10.1016/0378-1119(84)90203-8. [DOI] [PubMed] [Google Scholar]
- Hunt T. Cell biology. Cell cycle gets more cyclins. Nature. 1991 Apr 11;350(6318):462–463. doi: 10.1038/350462a0. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Sebastian B., Hunter T., Newport J. Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol Biol Cell. 1994 Mar;5(3):273–282. doi: 10.1091/mbc.5.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Differential phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J. 1991 Feb;10(2):305–316. doi: 10.1002/j.1460-2075.1991.tb07951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Mutations of p34cdc2 phosphorylation sites induce premature mitotic events in HeLa cells: evidence for a double block to p34cdc2 kinase activation in vertebrates. EMBO J. 1991 Nov;10(11):3331–3341. doi: 10.1002/j.1460-2075.1991.tb04897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Enoch T., Piwnica-Worms H. mik1+ encodes a tyrosine kinase that phosphorylates p34cdc2 on tyrosine 15. J Biol Chem. 1994 Dec 2;269(48):30530–30537. [PubMed] [Google Scholar]
- Lundgren K., Walworth N., Booher R., Dembski M., Kirschner M., Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991 Mar 22;64(6):1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- MacNeill S. A., Nurse P. Genetic analysis of human p34CDC2 function in fission yeast. Mol Gen Genet. 1993 Sep;240(3):315–322. doi: 10.1007/BF00280381. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993 Jan 15;123(1):127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990 Jul 5;265(19):10857–10864. [PubMed] [Google Scholar]
- Millar J. B., Russell P. The cdc25 M-phase inducer: an unconventional protein phosphatase. Cell. 1992 Feb 7;68(3):407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A. Temperature-sensitive lethal mutants in the structural gene for DNA ligase in the yeast Schizosaccharomyces pombe. Cell. 1977 Dec;12(4):1109–1120. doi: 10.1016/0092-8674(77)90173-8. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Control of the yeast cell cycle by the Cdc28 protein kinase. Curr Opin Cell Biol. 1993 Apr;5(2):166–179. doi: 10.1016/0955-0674(93)90099-c. [DOI] [PubMed] [Google Scholar]
- Norbury C., Blow J., Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991 Nov;10(11):3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics. 1980 Nov;96(3):627–637. doi: 10.1093/genetics/96.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Parker L. L., Atherton-Fessler S., Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993 Jun;18(6):195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- Poon R. Y., Yamashita K., Adamczewski J. P., Hunt T., Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993 Aug;12(8):3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice H. L. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1992 Feb 11;20(3):621–621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley R., Subramani S., Young P. G. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 1992 Apr;11(4):1335–1342. doi: 10.1002/j.1460-2075.1992.tb05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987 May 22;49(4):559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sheldrick K. S., Carr A. M. Feedback controls and G2 checkpoints: fission yeast as a model system. Bioessays. 1993 Dec;15(12):775–782. doi: 10.1002/bies.950151202. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Solomon M. J. Activation of the various cyclin/cdc2 protein kinases. Curr Opin Cell Biol. 1993 Apr;5(2):180–186. doi: 10.1016/0955-0674(93)90100-5. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Harper J. W., Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993 Aug;12(8):3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992 Jan;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth N., Davey S., Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993 May 27;363(6427):368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- al-Khodairy F., Carr A. M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992 Apr;11(4):1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Khodairy F., Fotou E., Sheldrick K. S., Griffiths D. J., Lehmann A. R., Carr A. M. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994 Feb;5(2):147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]