Abstract
Advanced melanoma has proven difficult to treat for many years, and no previous agent has shown improved survival in a phase 3 trial. The deepening understanding of tumor immunobiology and the complexity of the interactions between host T cells and cancer have led to novel treatment approaches. Among these, ipilimumab is a first-in-class T-cell potentiator that works by blocking cytotoxic T-lymphocyte antigen-4, a critical negative regulator of the antitumor T-cell response. From phase 1 studies, ipilimumab has shown encouraging activity in melanoma and other cancers, with unusual response patterns and mechanism-related, predictable toxicities that are medically manageable and mostly reversible but can sometimes be life threatening unless recognized and treated early. Early indications of a survival benefit in phase 2 studies have been confirmed recently in the first randomized phase 3 trial; the primary endpoint of the trial, overall survival (OS), was met with ipilimumab significantly prolonging median OS both as a single agent (10.1 months; p = 0.003) and combined with gp100 vaccine (10.0 months; p < 0.001) compared with vaccine control (6.4 months). Even more noteworthy was the improvement in long-term survival at 24 months from 13.7% (gp100 alone) to 21.6% and 23.5% for the combination and single ipilimumab, respectively. The addition of gp100 vaccine did not appear to impact OS since data for ipilimumab alone were similar to those for the combination with vaccine. Re-induction with ipilimumab in selected patients who progressed gave further clinical benefits. Ipilimumab has also shown promising activity in melanoma patients with brain metastases, and patients with non–small cell lung cancer, renal cell cancer, and castrate-resistant prostate cancer. Ipilimumab not only has a novel mechanism of action but demonstrates unique immune-related toxicities that require particular care in their recognition and treatment.
Key words: antitumor, CTLA-4, immune system, ipilimumab, malignant melanoma
Introduction
Despite advances in treatment, advanced stage melanoma has resolutely defied improvements in survival. Of stage IV patients found to have distant spread, only about 11%–15% will be alive at 5 years,1,2 and the preferred management for those who are not amenable to surgery is enrollment in a clinical trial.3 After 30 years and despite having no impact on survival, dacarbazine (DTIC) remains the reference single agent.3 Although some responses have been seen with the advent of immunotherapies such as high-dose interleukin-2 (IL-2), median survival for stage IV disease is between 6 and 9 months.4,5 Among those few patients who achieve a complete remission with high-dose IL-2, responses can often last over a decade, demonstrating the ability of immunotherapy to control and possibly even cure advanced melanoma.6,7
Activated T cells and antibodies targeting tumor-associated antigens (TAAs) have been detected frequently in blood from patients with various types of tumor,8 supporting an active role for a host immune response against tumor. In melanoma, T-cell infiltrates in primary melanoma have prognostic significance,9 and T-cell infiltrates within regional nodal metastases predict benefit in patients treated with neoadjuvant interferon-α-2b therapy.10–12 It is now well-accepted that tumors are able to evade detection and destruction by the immune system, even though many tumor types, especially melanoma, are capable of eliciting a strong immune response.13 Tumor immune evasion can be considered in two categories: induction of immune tolerance and resistance to killing by activated immune effector cells.14 Tumors manipulate their microenvironment by creating complex local and regional immunosuppressive networks comprising various tumor-derived cytokines and other soluble factors.15
One of the most promising strategies to support and enhance the patient's natural antitumor response consists of blocking the immunoregulatory mechanisms that brake host responses to TAAs thereby—therapeutically—releasing the brakes. One such critical inhibitory checkpoint is cytotoxic T lymphocyte antigen-4 (CTLA-4), a molecule that prevents unwanted autoimmunity and establishes tolerance to self-antigens by downregulating T-cell activation via a homeostatic feedback loop.16 Chronic T-cell stimulation by TAAs results in persistently high CTLA-4 expression and immune cells that are primed but no longer able to respond.
Ipilimumab is a first-in-class monoclonal antibody against CTLA-4. This novel drug has now been studied in 2901 patients enrolled in 25 clinical trials and has shown durable responses and prolonged survival in advanced stage IV melanoma.17–19 Ipilimumab is currently under investigation in several registrational clinical trials under special protocol assessment agreements with the U.S. Food and Drug Administration for the treatment of advanced melanoma—as a first-line treatment in combination with DTIC and as a second-line treatment—and its approval is expected in 2011.20 Its mode of action, patterns of responses, and safety profile are very different from conventional chemotherapy or even other forms of immunotherapy.
Mechanism of Action
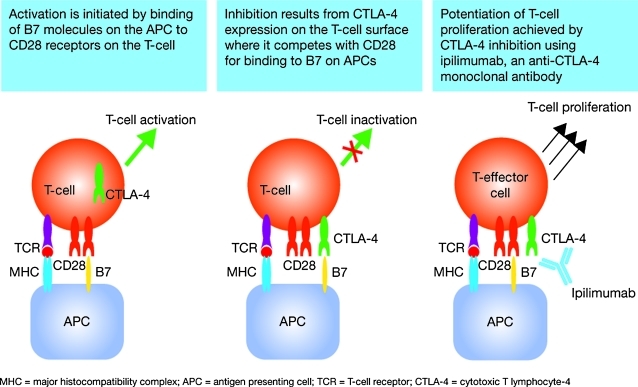
Full T-cell activation requires two signals.16,21 The first is initiated by T-cell receptor binding to TAAs presented by antigen presenting cells (APCs) via major histocompatibility complexes I and II. The second signal is generated when the principal costimulatory receptor on the T cell, CD28, binds to B7 ligand subtypes CD80 and CD86 on the APC. The resulting dual signaling induces changes including T-cell proliferation and cytokine release, triggering and then amplifying the immune response. In response to T-cell activation, CTLA-4 is upregulated and competes with CD28 for CD80 and CD86 binding on APCs but with significantly higher affinity, therefore downregulating—or deactivating—the T cell (Fig. 1).16 CTLA-4, therefore, downregulates T-cell responses and APC function, resulting in a decreased immune response to TAAs and immune tolerance.16,21
FIG. 1.
T-cell activation and mechanism of action of ipilimumab (adapted with permission from Weber51). APC, antigen presenting cell; CTLA-4, cytotoxic T lymphocyte antigen-4; TCR, T-cell receptor; MHC, major histocompatibility complex.
CTLA-4 signaling contributes to the immunosuppressive function of regulatory T cells (Tregs)22; binding of Treg-associated CTLA-4 to APCs decreases APC function and effector T-cell proliferation.21 These factors all contribute to a natural homeostatic mechanism designed to prevent unwanted autoimmunity against self-antigens by inducing peripheral immune tolerance. CTLA-4 is the main negative regulator of T-cell-mediated antitumor immune responses and therefore represents a critical immunity checkpoint, controlling both the duration and the intensity of an immune response.16,21,23
Anti-CTLA-4 monoclonal antibodies like ipilimumab inhibit CD80, and CD86 on APCs forms binding to CTLA-4 on T cells (Fig. 1). The resulting blockade of CTLA-4 signaling prolongs T-cell activation, restores T-cell proliferation, and thus amplifies T-cell-mediated immunity, which theoretically enhances the patient's capacity to mount an antitumor immune response.16,21 Cancer patients with reduced CTLA-4 expression have shown a more pronounced response to blockade of the CTLA-4 pathway, and less likelihood of subsequent relapse.24
Pharmacokinetics and Dosing
Ipilimumab is a fully human monoclonal IgG1κ antibody. Limited pharmacokinetic data have been presented to date, but a study of single-dose ipilimumab in patients with castrate-resistant prostate cancer showed that the pharmacokinetics of ipilimumab were consistent with other clinically used monoclonal antibodies tested in humans. The relatively long terminal half-life (12.5 days) means that a single dose of 3 mg/kg results in a serum concentration that will permit dosing at 3–4 week intervals.25 Although the second-line, phase 3 trial used a dose of 3 mg/kg every 3 weeks for four doses without any maintenance, most trials initiated after 2006 use a dose of 10 mg/kg every 3 weeks for four doses and then one dose every 12 weeks for up to at least 3 years. The 10 mg/kg dose was selected based on superior clinical and pharmacokinetic data and a comparable safety profile.
Clinical Trials
During the early development of ipilimumab, various mouse models of cancer showed that CTLA-4 blockade could not only enhance endogenous immune responses to immunogenic tumors, but that it could also synergize with multiple other interventions such as chemotherapy or vaccination to support and enhance host antitumor immune responses in less strongly immunogenic cancers.16 Findings from phase 1 trials extended the preclinical data, showing antitumor activity in patients with various advanced solid tumors, particularly in melanoma, and that ipilimumab was safe and well tolerated alone and in combination (Table 1).25–35 As a result, ipilimumab clinical development progressed and is now in phase 3 (Table 2).17–19,36–39
Table 1.
Summary of Phase 1 Clinical Trials with Ipilimumab
| Patient population | Design/schedule | Activity | Conclusions | Citation |
|---|---|---|---|---|
| Untreated advanced melanoma, n = 36 | Ipilimumab 0.1–3 mg/kg plus IL-2 720,000 IU/kg | Responses in 8/36 patients (22%); durations of 11–19 months | No synergistic effect in combination; antitumor effects of ipilimumab result from T-cell activation | Maker et al.26,27 |
| Prostate cancer, colon cancer, and NHL, n = 11 (after no response to tumor-specific vaccination) | Ipilimumab 3 mg/kg induction then 1.5 mg/kg maintenance monthly for four cycles | Tumor regression in 2/4 patients with lymphoma | Evidence of activity and well tolerated; reduction in Tregs noted | O'Mahony et al.28 |
| Advanced melanoma and ovarian cancer, n = 9 | Single dose—3 mg/kg (patients previously vaccinated—GVAX) | Activity and extensive tumor necrosis with lymphocyte and granulocyte infiltration in 3 melanoma patients; reduced/stabilized CA-125 in 2 ovarian cancer patients | No serious toxicities and evidence of increased tumor immunity | Hodi et al.29 |
| Advanced melanoma, n = 11; ovarian cancer, n = 9 | Single dose—3 mg/kg (retreatment permitted after 2/3 months)—following GVAX | Antitumor effect in 8/11 melanoma patients (including 3 PRs); 4/9 ovarian cancer patients had SD | Ipilimumab following GVAX gives clinically meaningful antitumor immunity without grade 3 or 4 toxicity in most melanoma patients | Hodi et al.30 |
| Advanced melanoma, n = 14 | Ipilimumab 3 mg/kg Q3W plus peptide vaccine | Two CRs and 1 PR | Immune-related AEs and antitumor activity suggests a role for CTLA-4 in breaking tolerance to cancer antigens | Phan et al.31 |
| CRPC, n = 14 (including pretreated patients) | Single dose—3 mg/kg | PSA decrease of ≥50% in 2/14 patients | Single 3 mg/kg dose is safe; no significant autoimmunity | Small et al.25 |
| Untreated CRPC, n = 24 | Dose escalation: ipilimumab 0.5–3 mg/kg plus GM-CSF 250 μg/m2/day | 3/6 patients on highest dose had PSA declines >50% | Evidence of activity and 3 mg/kg associated with more frequent expansion of circulating activated cytotoxic T cells than lower doses | Fong et al.32 |
| Metastatic CRPC n = 36 | GM-CSF 250 μg/m2/day (days 1–14 of 28-day cycle) + ipilimumab at escalating doses (0.5–10 mg/kg) on day 1 of each cycle × 6 | 3/6 patients treated with ipilimumab 3 mg/kg had confirmed PSA decline ≥50% and TTP 22, 26, and 103 weeks; 1 patient had PR in hepatic metastases; 1 patient on 10 mg/kg ipilimumab had PSA decline ≥50% and TTP 39 weeks | Ipilimumab combined with GM-CSF induced clinical responses in CRPC with the highest response proportion at the 3 mg/kg dose level | Harzstark et al.33 |
| Metastatic CRPC n = 30 | PSA vaccine days 1, 15, and 29, then monthly + ipilimumab 1, 3, 5, or 10 mg/kg in SEQ dose levels monthly; after 6 months patients could receive maintenance ipilimumab Q3M | Median OS (all patients) 31.8 months, with 74% survival probability at 24 months; median OS for chemotherapy naïve subset 30.9 months OS was dose independent | Based on historical data for the same vaccine (OS ∼26 months), addition of ipilimumab may augment the clinical benefit of vaccines in CRPC | Madan et al.34 |
| Relapsed/refractory B-cell NHL n = 18 | Ipilimumab 3 mg/kg, then monthly at 1 mg/kg × 3 (dose level 1), then escalation to 3 mg/kg monthly × 4 | 1 patient with diffuse large B-cell lymphoma had CR (>31 months), 1 patient with follicular lymphoma had PR (>19 months) | Ipilimumab has antitumor activity in patients with B-cell lymphoma | Ansell et al.35 |
IL-2, interleukin-2; GVAX, irradiated, autologous tumor cells engineered to secrete granulocyte macrophage colony-stimulating factor; PR, partial response; SD, stable disease; Q3W, every 3 weeks; CR, complete response; AEs, adverse events; CTLA-4, cytotoxic T lymphocyte antigen-4; CRPC, castrate-resistant prostate cancer; PSA, prostate-specific antigen; GM-CSF, granulocyte macrophage colony-stimulating factor; TTP, time to progression; OS, overall survival; SEQ, sequential; Q3M, every 3 months; NHL, non-Hodgkin's lymphoma.
Table 2.
Summary of Phase 2 and 3 Clinical Trials with Ipilimumab
| Phase/patient population | Design/schedule | Activity | Conclusions | Citation |
|---|---|---|---|---|
| Phase 2 (MDX10-08)/untreated advanced melanoma, n = 72 | Ipilimumab (3 mg/kg/month for 4 months) with or without DTIC (250 mg/m2 for 5 days monthly for 4 months) | Ipilimumab: DCR = 21.6%, durable disease control (≥24 weeks) in 2 patients, and OS 11.4 months | Ipilimumab resulted in clinically meaningful responses in advanced melanoma in combo. With DTIC. Combination was well tolerated; AEs were medically manageable | Hersh et al.17 |
| Ipilimumab/DTIC: DCR = 37.1%, durable disease control in 4 patients, and OS 14.3 months | ||||
| Phase 1/2 (CA184-022)/pretreated advanced melanoma, n = 217 | 0.3, 3, or 10 mg/kg Q3W for four doses then maintenance (Q12W) | 0.3 mg/kg: DCR = 13.7%, durable disease control 0%, and OS 8.6 months 3 mg/kg: DCR = 26.4%, durable disease control 3%, and OS 8.7 months 10 mg/kg: DCR = 29.2%, durable disease control 7%, and OS 11.4 months |
Ipilimumab had a dose-dependent effect on efficacy and safety, supporting further studies at a dose of 10 mg/kg | Wolchok et al.19 |
| Phase 2 (CA184-008)/pretreated advanced melanoma, n = 155 | 10 mg/kg ipilimumab Q3W for four doses then maintenance (Q12W) | BORR = 5.8%; DCR = 27.1%; 1-year survival rate = 47.2%; 2-year survival rate = 32.8%, median OS of 10.2 months | Ipilimumab has clinical activity with encouraging long-term survival | O'Day et al.18 |
| Phase 2 (CA184-007)/pretreated and treatment-naïve advanced melanoma, n = 115 | 10 mg/kg Q3W for four doses and randomized to receive prophylactic budesonide (arm A; n = 58) or placebo (arm B; n = 57) | Arm A: BORR = 12.1%, OS = 17.7 months Arm B: BORR = 15.8%, OS = 19.3 months |
Ipilimumab has activity with encouraging survival and manageable AEs | Weber et al.36 |
| Phase 2 (CA184-042)/melanoma with brain metastases, steroid-free (n = 51; arm A) or requiring steroids (n = 21; arm B) | 10 mg/kg Q3W for four doses; responding or stable patients could receive maintenance 10 mg/kg Q12W | Budesonide use did not affect the rate of grade 2 or higher diarrhea Arm A data at week 12: Global lesions (brain + non-CNS) = 4 PR, 5 SD Brain lesions only = 5 PR, 6 SD Response duration = 3–12 + months, SD duration = 1–7 months, median global PFS = 1.9 months |
Ipilimumab had similar activity in brain and non-CNS lesions | Lawrence et al.37 |
| Phase 3 (CA184-020)/patients with previously treated advanced melanoma, n = 676 | Randomized, double-blind study of 3 mg/kg Q3W × 4 ± gp100 vaccine, or vaccine only | Ipilimumab + gp100 (n = 403): CR = 1 (0.2%), PR = 22 (5.5%), SD = 58 (14.4%), BORR = 5.7%, median OS = 10.0 months, 1-year survival rate = 43.6%, 2-year survival rate = 21.6% | Ipilimumab with or without gp100 vaccine improved OS compared with vaccine alone; re-induction with ipilimumab at the time of disease progression can produce further clinical benefit | Hodi et al.38 |
| Ipilimumab only (n = 137): CR = 2 (1.5%), PR = 13 (9.5%), SD = 24 (17.5%), BORR = 10.9%, median OS = 10.1 months, 1-year survival rate = 45.6%, 2-year survival rate = 23.5% | ||||
| gp100 only (n = 136): CR = 0, PR = 2 (1.5%), SD = 13 (9.6%), BORR = 1.5%, median OS = 6.4 months, 1-year survival rate = 25.3%, 2-year survival rate = 13.7% | ||||
| Phase 2 (CA184-041)/chemo-naïve recurrent/metastatic NSCLC | Randomized, double-blind study of first-line ipilimumab (10 mg/kg Q3W) + CON P/C (175 mg/m2 AUC = 6 Q3W, (n = 70); or SEQ P/C (n = 68), or PBO, (n = 66); after P/C, ipilimumab maintenance therapy Q12W until toxicity or PD | SEQ regimen: Median PFS = 5.68 months versus 4.63 (PBO), p = 0.026 Median irPFS = 5.13 versus 4.21 (PBO), p = 0.024 No statistically significant differences in PFS for CON regimen or in OS for CON/SEQ (vs. PBO) |
Ipilimumab addition to P/C in a SEQ regimen extended PFS and irPFS in NSCLC patients compared with P/C alone | Lynch et al.39 |
DTIC, dacarbazine; DCR, disease control rate (CR + PR + SD/n); Q12W, every 12 weeks; BORR, best objective response rate; CNS, central nervous system; PFS, progression-free survival; NSCLC, nonsmall cell lung cancer; CON, consecutive; PBO, paclitaxel/carboplatin only; irPFS, immune-related progression-free survival; P/C, paclitaxel/carboplatin; PD, progressive disease.
Phase 2 trials
In a phase 2 trial (MDX010-08) of 72 patients with previously untreated advanced melanoma who were randomized to receive ipilimumab 3 mg/kg with or without DTIC, ipilimumab produced durable objective clinical responses and encouraging overall survival (OS) both alone and in combination. One (1) patient in the combination group had progressive disease (PD) according to Response Evaluation Criteria In Solid Tumors (RECIST), but showed loss of positron emission tomography reactivity and had subsequent tumor shrinkage, whereas another had stable disease (SD) at day 85 and subsequently achieved a partial response (PR).17
Long-term survival data for patients treated with ipilimumab in both MDX010-08 and MDX010-15, a phase 1/2 dose-ranging study in which 23 patients were treated with 10 mg/kg ipilimumab every 3 weeks, demonstrated a trend toward better survival with the higher dose (e.g., a 2-year survival rate of 36% vs. 22% for 10 vs. 3 mg/kg).40
In a randomized, double-blind, multicenter, phase 1/2 study (CA184-022), 217 patients with previously treated advanced melanoma received induction therapy with one of three doses of ipilimumab followed by maintenance therapy, beginning at week 24. Disease control rate (DCR) was 13.7%, 26.4%, and 29.2% in the 0.3, 3, and 10 mg/kg groups, respectively. The best overall response rates were 11.1% (95% confidence interval [CI] 4.9–20.7) at 10 mg/kg, 4.2% (95% CI 0.9–11.7) at 3 mg/kg, and 0% (95% CI 0–4.9) at 0.3 mg/kg. The safety profile was comparable for the 3 and 10 mg/kg cohorts with an overall immune-related adverse event (irAE) rate of 70% at 10 mg/kg and 65% at 3 mg/kg.19 Since these data favored the 10 mg/kg dose, in 2007 10 mg/kg every 3 weeks for four doses, then every 3 months as maintenance, was adopted for subsequent trials.
In a multicenter, single-arm, phase 2 study (CA184-008) in 155 patients with previously treated, advanced melanoma, the best objective response rate was 5.8% and the DCR was 27.1%. Survival rates at 1 and 2 years were 47.2% and 32.8%, respectively, with a median OS of 10.2 months.18 It was hoped that with no U.S. Food and Drug Administration–approved therapy available for pretreated patients with advanced melanoma, a high response rate in this study would speed approval; however, the 5.8% response rate was lower than expected. This may have been because of the patient population or a reflection of the inability of conventional RECIST to capture delayed responses after initial tumor progression, something addressed by the development of the novel immune-related response criteria (irRC) that are discussed later.
In an attempt to prevent ipilimumab-induced colitis, in study CA184-007 115 previously treated and treatment-naïve patients with advanced melanoma were randomized to receive open-label ipilimumab with prophylactic budesonide (group A) or placebo (group B). Overall response rate and survival were similar between the groups and there was no difference in the incidence of diarrhea or colitis in the groups or in response between first-line and previously treated patients.36
Analysis of the long-term survival of patients who received ipilimumab 10 mg/kg during the three key phase 2 trials (CA184-007, CA184-008, and CA184-022) showed OS ranging from 10.2 months in previously treated patients to 22.5 months in treatment-naïve patients after median follow-ups of between 10.1 and 16.3 months.41 The 12-month survival rates across these three studies ranged from 47.2% to 71.4% for previously treated patients, corresponding 18-month survival rates ranged from 34.5% to 39.4%.42 Long-term survivors included patients with PD by World Health Organization (WHO) criteria. Despite lactate dehydrogenase (LDH) levels being a known prognostic factor for poor long-term survival,43 patients with high LDH levels were equally likely to respond to ipilimumab and there were no negative associations between LDH and DCR or OS (data pooled from studies CA184-008 and CA184-022 only).44
There are case reports of patients with brain metastases from melanoma having prolonged responses to ipilimumab, even in the absence of local therapy to the brain.45 A retrospective analysis of data from one of the previously mentioned phase 2 studies of ipilimumab (CA184-007) showed that in the 12 patients with brain metastases at baseline, 1 patient had PR and 3 had SD; further, 1 patient with an index brain lesion showed a decrease in its size to below measureable levels by week 12. Treatment was associated with survival benefits in 8 of these patients.46 Subsequently, a prospective phase 2 study of ipilimumab in patients with melanoma and brain metastases was recently completed. In a preliminary analysis of data from 51 steroid-free patients, for brain lesions only, 5 patients had a PR and 6 had SD at week 12, with additional unconfirmed responses. These outcomes were similar to those considering global lesions (brain and noncentral nervous system). Response duration ranged from 3 to 12 + months, and SD duration from 1 to 7 months; there were no unexpected toxicities.37 Thus, responses and some cases of prolonged survival have been seen in melanoma patients traditionally felt to have little chance of benefit from therapy, that is, those with elevated LDH, previous treatment, or brain metastases.
Finally, patients who achieved disease control in trials CA184-004, CA184-007, CA184-008, and CA184-022 but subsequently progressed were eligible to receive ipilimumab re-induction therapy (10 mg/kg kg Q3W × 4, then maintenance Q12W beginning at week 24) in trial CA184-025. Re-induction was allowed within 28 days of documented progression, provided the following criteria were met: response to the initial cycle of therapy was SD lasting ≥6 months from baseline, complete response (CR), or PR; no grade 3 irAE that precluded further ipilimumab dosing (such as colitis or iritis), and no grade 4 toxicity of any type.38 An interim analysis of data from CA184-025 suggests that of the 28 patients to date who have received re-induction therapy following progression, 50% responded (by modified WHO criteria).47 Although early investigations of the combination of ipilimumab with high-dose IL-2 in advanced melanoma were disappointing because of an increased incidence of colitis, recent follow-up analysis of these phase 1 data has revealed an objective tumor regression rate of 25% and a durable CR rate of 17% in patients receiving this regimen.48 This is much higher than that for other ipilimumab-based regimens, notably those containing vaccines, suggesting the possibility of a synergistic effect and prompting further study.
In addition to its proven activity in melanoma, ipilimumab is undergoing an active program of evaluation in several other cancers. Early data from phase 1 studies have been encouraging for ovarian and prostate cancer and for non-Hodgkin's lymphoma, and ipilimumab has also shown promising efficacy in later development for some tumors. In a phase 2 trial, ipilimumab-induced cancer regression in some of 61 patients with metastatic clear cell renal cancer, even in those previously unresponsive to IL-2.49 A randomized, double-blind, placebo-controlled, multicenter, phase 2 trial compared the efficacy and safety of ipilimumab (10 mg/kg Q3W) in combination with paclitaxel/carboplatin against the chemotherapy doublet alone in patients with untreated lung cancer. Preliminary analysis showed that the combination of ipilimumab and chemotherapy was well tolerated with some evidence of improved progression-free survival (PFS) and OS.39
Phase 3 trials
Because of earlier experimental data suggesting synergy between ipilimumab and vaccines in melanoma, a phase 3 randomized trial of 676 pretreated patients comparing a combination of ipilimumab with a peptide gp100 vaccine to both vaccine and ipilimumab monotherapies was started in 2006. The vaccine arms meant this second-line trial was restricted to HLA-A2 patients, and ipilimumab induction therapy was given at 3 mg/kg every 3 weeks for four doses without maintenance, with responding patients eligible for re-induction with ipilimumab if they relapsed. The trial's original primary endpoint was response rate, but when it became apparent from ipilimumab studies that survival was more impressive than response rate by traditional WHO criteria, the primary endpoint was amended to OS with subsequent data showing a significant benefit in favor of ipilimumab (Table 2).
Primary analysis of data from this study showed that median OS increased from 6.4 to 10.0 months with the addition of ipilimumab to gp100 vaccine (HR 0.68, p < 0.0001). DCR improved from 11.0% to 20.1% (p = 0.02). Both median OS and long-term survival rates improved, with 21.6% of patients receiving the ipilimumab and vaccine combination still alive at 24 months compared with only 13.7% of patients on vaccine alone; ∼20% in the group treated with ipilimumab alone were alive at 4 years.50 In addition, some patients who progressed after an initial response to ipilimumab induction therapy had objective responses or SD upon re-induction with ipilimumab with or without gp100. Overall, 31 patients who received such re-induction had an objective response rate of 19%, and a further 48% had SD.50 Addition of gp100 vaccine did not lead to improvement over that achieved by ipilimumab alone; in fact, PFS was worse in patients treated with ipilimumab and vaccine compared with PFS in patients on ipilimumab alone (p = 0.04). Going forward, a phase 3 trial of ipilimumab (10 mg/kg) with DTIC versus DTIC alone in treatment-naïve patients with advanced melanoma completed accrual in 2008 and survival data are awaited (Table 3).
Table 3.
Key Ongoing Phase 2 and 3 Clinical Trials with Ipilimumab
| Indication | Phase/identifier | Design and interventions | Primary endpoint | Status |
|---|---|---|---|---|
| Treatment-naïve, advanced melanoma | Phase 3; CA184-024 | Randomized; ipilimumab (10 mg/kg Q3W × 4, then Q12W)/DTIC versus DTIC | Improvement in OS | Active, not recruiting |
| High risk, stage III melanoma | Phase 3; CA184-029, EORTC 18071 | Randomized; ipilimumab (10 mg/kg Q3W × 4) versus placebo | Improvement in recurrence-free survival | Recruiting |
| Pretreated CRPC | Phase 3; CA184-043 | Randomized, ipilimumab (10 mg/kg Q3W × 4) versus placebo following radiotherapy | Improvement in OS | Recruiting |
| Treatment-naïve CRPC (minimally symptomatic) | Phase 3; CA184-095 | Randomized; ipilimumab (10 mg/kg Q3W × 4, then Q12W) versus placebo | Improvement in OS | Recruiting |
| Treatment-naïve, advanced melanoma with brain metastases | Phase 2; NA | Non randomized; ipilimumab (10 mg/kg Q3W × 4, then Q12W) plus temozolomide | 6-month PFS | Recruiting |
Immune-Related Adverse Events
Ipilimumab produces a novel, mechanism-related spectrum of autoimmune or irAEs different from those typically encountered with chemotherapy and even other forms of immunotherapy. These irAEs are dose dependent and can occur quite rapidly. They include severe rash (50% of cases); grade 3–4 enterocolitis (up to 16% of cases); hypophysitis (∼5% of cases); hepatitis (<5% of cases); and, more rarely, uveitis, pancreatitis, neuropathy, severe leucopenia, and red cell aplasia.51 These irAEs seen consistently across studies17–19,25,32,35,49,52 are generally manageable and reversible without long-term consequences if recognized early and treated promptly with corticosteroids. Permanent pituitary dysfunction in patients with hypophysitis may occur, however, requiring long-term hormone replacement therapy.51 Further, corticosteroid use did not appear to preclude an antitumor response to ipilimumab.36,51,52 An earlier investigation on the prophylactic use of budesonide for immune-related diarrhea did not reduce the incidence of this irAE and as such should not be used in this manner.36
Grade 1 and 2 AEs are the most common and usually resolve spontaneously or after symptomatic treatment with over-the-counter medications (such as hydroxyzine or diphenhydramine for rash). More severe or persistent toxicities require early intervention with agents, usually corticosteroids, not typically used to manage chemotherapy-related toxicity.21,53,54 Even the higher-grade toxicities can be managed with prompt identification and initiation of immunosuppressive therapy. Ipilimumab therapy must be held if the patient develops a grade 2 diarrhea or iritis or any grade 3 or 4 ipilimumab-related toxicity.54 Prompt recognition of irAE symptoms by physicians and patients is key to successful intervention and outcomes. Diarrhea resulting from immune-related enterocolitis is the most common serious toxicity, which if untreated may lead to a fatal bowel perforation.55 Ipilimumab colitis histologically and clinically resembles ulcerative colitis, and may occur more frequently in patients with a family history of colitis. Colonoscopy is not essential for diagnosis, but infectious etiologies should be excluded first. Any patient with severe, watery diarrhea (grade 2) on ipilimumab presents a potentially serious problem that should be treated aggressively and monitored closely. Safety guidelines recommend systemic steroids as first-line intervention in severe ipilimumab-related diarrhea with most patients responding to this treatment.52,56 When the antitumor necrosis factor antibody infliximab is used together with mesalamine and hydrocortisone, enemas has shown promise in pre-empting worsening of and improving grade 2 ipilimumab-related diarrhea without the need for systemic steroids.55 Patients with grade 1 diarrhea usually respond well to dietary modifications and loperamide.
Of all the adverse effects associated with ipilimumab, hypophysitis is the most unusual and possibly the hardest to recognize. The protean symptoms are nonspecific and may include weakness, malaise, arthralgias, headaches, hyponatremia, hyperkalemia, hypotension, or shock. Plasma cortisol level, adrenocorticotropic hormone level, and a brain magnetic resonance imaging looking for sellar enlargement should be obtained in patients with these symptoms, with prompt corticosteroid replacement begun while awaiting the results.54 A short course of high-dose dexamethasone followed by physiologic hormone replacement has produced a partial recovery of pituitary function in some patients, but pituitary dysfunction may be permanent.57 Hepatitis with elevation of transaminase to over 500 U/L may have few clinical symptoms, so liver function test results should be monitored before each dose of ipilimumab. Guidelines state that grade 3 or 4 hepatitis should be treated with steroids in the first instance, followed sequentially by mycophenolate mofetil, tacrolimus and eventually, infliximab if the patient does not respond.56 Uveitis can be resolved with topical corticosteroids.21 Some pancreatitis symptoms can be confused with enterocolitis and laboratory investigations of enzyme levels are needed to distinguish it; thereafter, patients should be hospitalized and treated with parenteral or oral steroids as indicated.58 Overall, toxicities associated with CTLA-4 blockade are usually less severe but more delayed than those seen with high-dose IL-2.
Patients experiencing an irAE have a higher chance of having an antitumor response to ipilimumab31,36,49,52,57,59,60 and higher irAE incidences have been associated with longer survival.51 This connection between irAEs and efficacy has not been absolute51,61 as disease control and survival benefits have been seen also in patients without irAEs.62 Therefore, patients without an irAE should continue to receive ipilimumab, and ipilimumab should not be dosed to induce an irAE.
Immune-Related Response Criteria
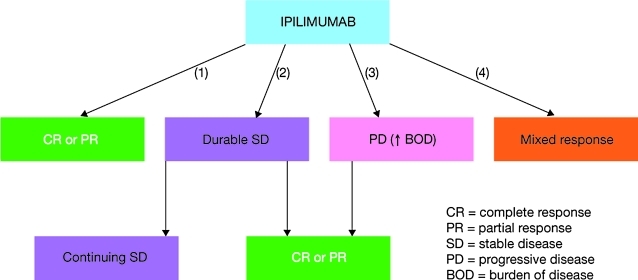
Responses to ipilimumab are typically heterogeneous and have been seen during, at the end of, and beyond the 12-week induction period.63 This, perhaps, is not surprising given that the drug's mechanism of action relies on amplification of the patient's immune response to their cancer and is unlikely to elicit an immediate response (in contrast to conventional anticancer agents).63 Similar response patterns have been seen with vaccines and other immunotherapeutic agents, prompting the proposal and development of more appropriate assessment criteria.64 Four distinct response patterns to ipilimumab have been described: (1) shrinkage of baseline index lesions without new lesions appearing; (2) durable SD followed (in some cases) by slow, steady decline in tumor burden; (3) response after an increase in tumor burden, and (4) response in the presence of new lesions (Fig. 2).65 Because of the difference between the mechanisms and patterns of response to ipilimumab/other immunotherapies and conventional chemotherapy, RECIST or WHO criteria may be underestimating efficacy and leading to premature treatment withdrawal.51,65 Novel irRC, which allow for maturation of an antitumor immune response, were therefore developed (Table 4). In common with RECIST and WHO, these are based on total disease burden at baseline, but differ in the timing of first disease assessment, which is typically later, and define PD only after a 25% increase in total disease burden has been breached twice and at least 4 weeks apart (irrespective of individual lesion sizes or appearance of new lesions).51,65 A major advantage of the irRC is that patients with new lesions, but a response in one or more index lesions, will not automatically be considered to have PD, as would be the conclusion by RECIST or WHO. These patients could therefore continue to receive ipilimumab, and potentially benefit from treatment.
FIG. 2.
Four response patterns to ipilimumab have been seen: (1) response from the outset; (2) durable SD with or without subsequent response; (3) response after PD, and (4) response in some lesions accompanied by the appearance of new ones.65
Table 4.
Key Differences Between World Health Organization and Immune-Related Response Criteria
| WHO criteria | irRC | |
|---|---|---|
| New lesions | Always mean progressive disease | Incorporated into measurement of total tumor burden that determines response |
| Progressive disease | ≥25% increase in sum of products of diameters at any time | ≥25% increase in sum of products of diameters, confirmed by repeat scans at least 4 weeks later |
WHO, World Health Organization; irRC, immune-related response criteria.
These irRC were used to evaluate response in two of the previously described ipilimumab phase 2 trials demonstrating, in particular, that patients with PD by WHO criteria can go on to achieve clinically meaningful SD or PR. In trial CA184-022, 2 patients allocated 10 mg/kg ipilimumab achieved a PR (1 confirmed) at week 15 after initial PD (by WHO criteria) at week 12. One (1) patient in the 3 mg/kg group achieved a reduction in total tumor burden after PD. SD followed by a slow, steady decline in total tumor burden was noted in all three dose groups.19 In CA184-008, 87 patients had PD by WHO criteria and 43 of these were evaluated by irRC; 12 patients had evidence of clinical activity resulting in a total DCR of 35%. Of the 12 patients with evidence of activity, 7 patients had irSD with a slow, steady decline in total tumor burden, 1 patient had irPR after irPD, 3 had irPR after the appearance of new lesions, and 1 patient experienced a late PR/irPR. OS was similar between patients with activity per WHO criteria and patients with WHO-defined PD but activity according to irRC.18
A combined analysis of data from 227 patients treated with ipilimumab (10 mg/kg) enrolled in two phase 2 trials (CA184-008 and CA184-022) showed a best overall response rate using irRC of 7.5% (17/227), and SD in 20.3% (46/227). By WHO criteria, 123 patients had PD at weeks 12 and 57 of them continued to be followed; 5 of them went on to have a PR (defined using irRC) and 17 had SD (also by irRC).65
Discussion
Patterns of response to ipilimumab
Although responses to ipilimumab can mirror those found with standard chemotherapy, they can also (1) be preceded by an apparent disease progression, and (2) produce varying reactions throughout different lesions; these responses can take weeks, and even months, to develop.63,65 There is some evidence that the apparent on-treatment disease progression seen in some patients receiving ipilimumab may be a function of this agent's mode of action, whereby therapy-induced immune and inflammatory cell infiltration transiently bulks up individual lesions and produces tumor enlargement.66 The on-treatment progression may also be due to tumor growth occurring before a sufficient immune response has developed.65 It is recommended that PD at week 12 be confirmed (e.g., by computed tomography scan) at least 4 weeks later as true PD before changing therapy. In contrast, if a response to chemotherapy is going to occur it is generally evident within the first few weeks of therapy with tumor growth or the development of new disease indicating therapeutic failure.
Biomarkers and predictors of response
As with many targeted oncologic agents, identifying a predicative biomarker of response would assist in the development and clinical optimization of ipilimumab.67 At present, it remains unclear why some patients respond to therapy and others do not. Studies are in progress to identify which immunologic, tumor, or other parameters affect outcome and could be used as biomarker, but success has been limited thus far.
In a pooled analysis of studies CA184-007, CA184-008, and CA184-022 (and confirmed prospectively in study CA184-004), higher peripheral blood absolute lymphocyte counts (ALC) were significantly associated with clinical activity.68,69 Similarly, in a smaller single-institution analysis of 51 patients who received ipilimumab, higher ALC also correlated with clinical benefit. Patients with an ALC ≥1000/μL after two ipilimumab doses (week 7) had a significantly improved clinical benefit rate and median OS than those with ALC <1000/μL (51% vs. 0%; 11.9 vs. 1.4 months).70 This increase in blood lymphocytes is mostly due to an increase in CD8 rather than CD4 T cells. Changes in peripheral blood CD4+ CD25+ (suppressor T cells) do not correlate with response.71 Perhaps ALC may represent an easily accessible way to show that an immune reaction is occurring in response to ipilimumab. As well as an association with an increase in ALC, freedom from relapse after ipilimumab treatment has also been associated with an increase in IL-17-secreting helper T cells.36
Similar to IL-2 associated vitiligo, data have emerged recently showing that hair depigmentation develops alongside durable responses to ipilimumab in patients with advanced melanoma.72 In two trials involving a total of 43 patients, 6 patients who developed hair depigmentation after a median of 10 months' treatment all had SD or CR ranging from 24 to 36 months. No nonresponsive patient had hair depigmentation, although 5 patients without hair depigmentation had durable SD ranging from 24 to 48 months. Hair depigmentation suggests an association between induced autoimmunity and clinical benefit and could be a potential surrogate for response in some patients. Finally, recent data found an association between low baseline C-reactive protein and survival benefit from tremelimumab, another anti-CTLA-4 antibody, in patients with advanced melanoma.73,74 It is likely that a similar association will be found for ipilimumab.
Future questions and strategies
Although the same dosage schedule has been used in all the ipilimumab trials in the last 3 years, questions remain about the importance of maintenance therapy and the best use of re-induction courses. In addition, some patients have no side-effects and do not develop any lymphocytosis with the current induction therapy schedule, raising the question of whether these patients require a higher dosage of ipilimumab for sufficient immune activation.
Targeted immunotherapeutic agents like ipilimumab have opened the gateway to novel therapies in cancer care while at the same time presenting new challenges in the management of unique drug toxicities. Since their mechanism of action relies on indirectly promoting host immunity, it is unlikely that any single immunotherapeutic agent will be active across a high percentage of patients. Several future strategies, including combinations with conventional cytotoxic agents and cytokine therapies, have already been proposed. The pairing of conventional cytotoxic agents that maximize tumor antigen presentation along with immunotherapies that boost host immunity could be one promising route.75 The combination of tremelimumab with high-dose interferon-α-2b has also shown significant results in phase 2 testing,74 which along with updated data from the combination of ipilimumab and high-dose IL-2 will likely lead to further testing of cytokine combinations in the near future. Results from the ongoing trials of ipilimumab as adjuvant therapy for melanoma might show CTLA-4 inhibition to be more effective when disease burden is lower and immune tolerance is less advanced, enabling immunologic memory to protect against tumor recurrence. An ongoing neo-adjuvant study with ipilimumab for patients with bulky operable stage III melanoma provides a promising therapeutic strategy for this population. In addition, through access to tumor tissue at baseline and following induction therapy, as well as serial serum and peripheral blood mononuclear cell collection, it allows for novel biomarker studies that may have significant prognostic and therapeutic predictive value. Finally, when we consider the possibility of patient-tailored therapy the future becomes even more exciting if more complex.
Conclusions
Ipilimumab is a first-in-class T-cell potentiator that has significant activity against stage IV melanoma with durable remissions in multiple phase 2 trials and now has demonstrated a significant survival benefit in a phase 3 randomized trial. In melanoma it has shown marked benefit in patient populations previously refractory to treatment—patients with elevated LDH, patients with brain metastases, and patients who have progressed on prior systemic treatments. Ipilimumab also has shown activity in melanoma as re-induction therapy in patients who progress when either off therapy or when receiving maintenance doses. Its unique toxicities demand diligent education of the physicians who use this agent, as some toxicities, like hypophysitis, may be difficult to recognize, and some, like colitis, may be difficult to manage. The unique pattern of delayed responses necessitates a revision in our tumor response criteria in an effort to optimize our therapeutic strategies with this class of drugs and maximize survival benefits.
Although melanoma is known for its responses to immunotherapy, the mode of action of ipilimumab means it may have important therapeutic activity against other neoplasms. We eagerly await the conclusion of current ongoing trials in lung cancer and prostate cancer, and hope to see new trials in lymphoma, lung cancer, kidney cancer, prostate cancer, ovarian cancer, and other tumors where there was some evidence of benefit in phase 1/2 trials.
The phase 3, second-line trial used a dose of 3 mg/kg; most of the trials in the last 3 years, including the pivotal first-line phase 3 trial, used the higher dose of 10 mg/kg based on superior efficacy and pharmacokinetic data, and comparable safety profile. Although some patients have durable remissions after a single course of therapy, most patients appear to benefit from maintenance therapy with ipilimumab. However, the best schedule and duration for maintenance therapy is uncertain. The U.S. Intergroup E1609 adjuvant trial has sought to limit therapy to 60 weeks and this, when compared with the EORTC 18871 adjuvant trial of 3 years' planned treatment, may be most useful in answering this question. Additional work is needed to define the optimal therapy for ipilimumab-induced colitis, and whether acute therapy with steroids can prevent long-term pituitary damage in cases of hypophysitis. In trials so far, patients who developed grade 3 or 4 irAEs could not be retreated even if they had a CR and then relapsed; we need to learn if safe retreatment of these patients is possible with dose or schedule modification.
Undoubtedly, combination therapy with ipilimumab will be the subject of intense investigation in the next decade. There is no a priori reason not to combine ipilimumab with other forms of immunotherapy, chemotherapy, targeted therapies, or antiangiogenic therapy. A trial of ipilimumab and bevacizumab in melanoma is already underway, and the trial in lung cancer combined ipilimumab and chemotherapy. Combining ipilimumab with IL-2 requires careful monitoring because of the increased risk of severe toxicities, but data showing a higher CR rate after this regimen have produced renewed interest in evaluating this combination. Phase 3 data for the combination of ipilimumab with gp100 vaccine were disappointing, and it seemed that the addition of vaccine was not beneficial. We now have a new weapon in the immunotherapy of cancer, and we can hope that a new era for the immunotherapy of cancer has begun.
Acknowledgments
The authors take full responsibility for the content of this publication, and confirm that it reflects their viewpoint and medical expertise. They also wish to acknowledge StemScientific, funded by Bristol-Myers Squibb, for providing writing and editing support. Bristol-Myers Squibb did not influence the content of the article, nor did the authors receive financial compensation for authoring the article.
Disclosure Statement
Drs. Tarhini and Minor have received research support from Bristol-Myers Squibb, and Dr. Minor has acted as an advisor to the company.
References
- 1.Balch CM. Buzaid AC. Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review, 1975–2007. In: Altekruse SF, editor; Kosary CL, editor; Krapcho M, et al., editors. Based on November 2009 SEER data submission, posted to the SEER web site, 2010. Bethesda, MD: National Cancer Institute; [Jul 1;2010 ]. [Google Scholar]
- 3.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. www.nccn.org. [Jul 1;2010 ]. www.nccn.org Melanoma, Version 1.2010.
- 4.Manola J. Atkins M. Ibrahim J, et al. Prognostic factors in metastatic melanoma: A pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 5.Bedikian AY. Millward M. Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB. Kunkel L. Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: Long-term survival update. Cancer J Sci Am. 2000;6(suppl1):S11. [PubMed] [Google Scholar]
- 7.Atkins MB. Lotze MT. Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 8.Nagorsen D. Scheibenbogen C. Marincola FM, et al. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296. [PubMed] [Google Scholar]
- 9.Clemente CG. Mihm MC., Jr. Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Håkansson A. Gustafsson B. Krysander L, et al. Tumour-infiltrating lymphocytes in metastatic malignant melanoma and response to interferon alpha treatment. Br J Cancer. 1996;74:670. doi: 10.1038/bjc.1996.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihm MC., Jr. Clemente CG. Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: A histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43. [PubMed] [Google Scholar]
- 12.Moschos SJ. Edington HD. Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 13.Swann JB. Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake CG. Jaffee E. Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim R. Emi M. Tanabe K, et al. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 16.Peggs KS. Quezada SA. Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer. Curr Opin Immunol. 2006;16:206. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Hersh EM. O'Day SJ. Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2010. Jan 16 [Epub ahead of print]. [DOI] [PubMed]
- 18.O'Day SJ. Maio M. Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann Oncol. 2010;21:1712. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 19.Wolchok JD. Neyns B. Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 20.Movva S. Verschraegen C. The monoclonal antibody to cytotoxic T lymphocyte antigen 4, ipilimumab (MDX-010), a novel treatment strategy in cancer management. Expert Opin Biol Ther. 2009;9:231. doi: 10.1517/14712590802643347. [DOI] [PubMed] [Google Scholar]
- 21.Robert C. Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist. 2009;14:848. doi: 10.1634/theoncologist.2009-0028. [DOI] [PubMed] [Google Scholar]
- 22.Read S. Malmstrom V. Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenfield EA. Nguyen KA. Kuchroo VK. CD28/B7 costimulation: A review. Crit Rev Immunol. 1998;18:389. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 24.Sanderson K. Scotland R. Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 25.Small EJ. Tchekmedyian NS. Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 26.Maker AV. Attia P. Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maker AV. Phan GQ. Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: A phase I/II study. Ann Surg Oncol. 2005;12:1005. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Mahony D. Morris JC. Quinn C, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 29.Hodi FS. Mihm MC. Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodi FS. Butler M. Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phan GQ. Yang JC. Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong L. Kwek SS. O'Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harzstark AL. Fong L. Weinberg VK, et al. Final results of a phase I study of CTLA-4 blockade in combination with GM-CSF for metastatic castration resistant prostate cancer (mCRPC) [abstr] J Clin Oncol. 2010;28(suppl):7s.4689. [Google Scholar]
- 34.Madan RA. Mohebtash M. Arlen PM, et al. Overall survival (OS) analysis of a phase l trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab (Ipi) in the treatment of metastatic castration-resistant prostate cancer (mCRPC) [abstr] J Clin Oncol. 2010;28(suppl):7s.2550. [Google Scholar]
- 35.Ansell SM. Hurvitz SA. Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber J. Thompson JA. Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable Stage III or IV melanoma. Clin Cancer Res. 2009;15:5591. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence DP. Hamid O. McDermott DF, et al. Phase II trial of ipilimumab monotherapy in melanoma patients with brain metastases [abstr] J Clin Oncol. 2010;28(suppl):7s.8523. [Google Scholar]
- 38.Hodi FS. O'Day S. McDermott DF, et al. Re-induction with ipilimumab, gp100 peptide vaccine, or a combination of both from a phase III, randomized, double-blind, multicenter study of previously treated patients with unresectable stage III or IV melanoma [abstr] J Clin Oncol. 2010;28(suppl):15s.8509. [Google Scholar]
- 39.Lynch TJ. Bondarenko IN. Luft A, et al. Phase II trial of ipilimumab (IPI) and paclitaxel/carboplatin (P/C) in first-line stage IIIb/IV non-small cell lung cancer (NSCLC) [abstr] J Clin Oncol. 2010;28(suppl):7s.7531. [Google Scholar]
- 40.Hersh EM. Weber J. Powderly J, et al. Long-term survival of patients (pts) with advanced melanoma treated with ipilimumab with or without dacarbazine [abstr] J Clin Oncol. 2009;27(suppl):15s.9038. [Google Scholar]
- 41.Maio M. Lebbé C. Sileni VC, et al. Long-term survival in advanced melanoma patients treated with ipilimumab at 10 mg/kg: Ongoing analyses from completed phase II trials [abstr] Eur J Cancer. 2009;7(suppl):578.P-9307. [Google Scholar]
- 42.O'Day S. Weber J. Lebbe C, et al. Effect of ipilimumab treatment on 18-month survival: Update of patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials [abstr] J Clin Oncol. 2009;27(suppl):15s.9033. [Google Scholar]
- 43.Bedikian AY. Johnson MM. Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26:624. doi: 10.1080/07357900802027073. [DOI] [PubMed] [Google Scholar]
- 44.Schadendorf D. Wolchok J. Neyns B, et al. Activity of ipilimumab at 10 mg/kg in patients with advanced melanoma is independent of baseline prognostic factors [abstr] Eur J Cancer. 2009;7(suppl):578.P-9308. [Google Scholar]
- 45.Hodi FS. Oble DA. Drappatz J, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5:557. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 46.Weber JS. Berman D. Siegel J, et al. Clinical activity of ipilimumab in patients with advanced melanoma and brain metastases [abstr] Ann Oncol. 2008;19(suppl8):viii244.786P. [Google Scholar]
- 47.Harmankaya K. Minor D. Linette G, et al. Ipilimumab re-induction after progression in patients with advanced melanoma enrolled in phase II clinical trials [abstr] Eur J Cancer. 2009;7(suppl):582.P-9317. [Google Scholar]
- 48.Prieto PA. Yang JC. Sherry RM, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade with ipilimumab: Long-term follow-up of 179 patients with metastatic melanoma [abstr] J Clin Oncol. 2010;28(suppl):7s.8544. [Google Scholar]
- 49.Yang JC. Hughes M. Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodi FS. O'Day SJ. McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber J. Ipilimumab: Controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beck KE. Blansfield JA. Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ridolfi L. Ridolfi R. Anti-CTLA-4 therapy in melanoma: Role of ipilimumab (MDX-010) Expert Rev Dermatol. 2009;4:199. [Google Scholar]
- 54.Chin K. Ibrahim R. Berman D, et al. Treatment guidelines for the management of immune-related adverse events in patients treated with ipilimumab, an anti-CTLA4 therapy [abstr] Ann Oncol. 2008;19(suppl8):viii244.787P. [Google Scholar]
- 55.Minor D. Chin K. Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24:321. doi: 10.1089/cbr.2008.0607. [DOI] [PubMed] [Google Scholar]
- 56.O'Day SJ. Hamid O. Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): A novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 57.Blansfield JA. Beck KE. Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Giacomo AM. Danielli R. Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58:1297. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Attia P. Phan GQ. Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Downey SG. Klapper JA. Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen blockade. Clin Cancer Res. 2007;13:6681. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maker AV. Yang JC. Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29:455. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lutzky J. Wolchok J. Hamid O, et al. Association between immune-related adverse events (irAEs) and disease control or overall survival in patients (pts) with advanced melanoma treated with 10 mg/kg ipilimumab in three phase II clinical trials [abstr] J Clin Oncol. 2009;27(suppl):15s.9034. [Google Scholar]
- 63.Saenger YM. Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: Patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 64.Hoos A. Parmiani G. Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 65.Wolchok JD. Hoos A. O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 66.Ribas A. Chmielowski B. Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15:7116. doi: 10.1158/1078-0432.CCR-09-2376. [DOI] [PubMed] [Google Scholar]
- 67.Kirkwood JM. Tarhini AA. Biomarkers of therapeutic response in melanoma and renal cell carcinoma: Potential inroads to improved immunotherapy. J Clin Oncol. 2009;27:2583. doi: 10.1200/JCO.2008.21.1540. [DOI] [PubMed] [Google Scholar]
- 68.Berman DM. Wolchok J. Weber J, et al. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients (pts) with advanced melanoma treated with ipilimumab [abstr] J Clin Oncol. 2009;27(suppl):15s.3020. [Google Scholar]
- 69.Hamid O. Chasalow SD. Tsuchihashi Z, et al. Association of baseline and on-study tumor biopsy markers with clinical activity in patients (pts) with advanced melanoma treated with ipilimumab [abstr] J Clin Oncol. 2009;27(suppl):15s.9008. [Google Scholar]
- 70.Ku GY. Yuan J. Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: Lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang A. Kendle RF. Ginsberg BA, et al. CTLA-4 blockade with ipilimumab increases peripheral CD8+ T cells: Correlation with clinical outcomes [abstr] J Clin Oncol. 2010;28(suppl):15s.2555. [Google Scholar]
- 72.Pavlick AC. Ott PA. Kannan R, et al. Hair depigmentation as an indicator of durable response to CTLA-4 therapy [abstr] J Clin Oncol. 2010;28(suppl):7s.8571. [Google Scholar]
- 73.Marshall MA. Ribas A. Huang B. Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma [abstr] J Clin Oncol. 2010;28(suppl):7s.2609. [Google Scholar]
- 74.Tarhini AA. Moschos SJ. Tawbi H, et al. Phase II evaluation of tremelimumab (Treme) combined with high-dose interferon alpha-2b (HDI) for metastatic melanoma [abstr] J Clin Oncol. 2010;28(suppl):15s.8524. [Google Scholar]
- 75.Peggs KS. Segal NH. Allison JP. Targeting immunosupportive cancer therapies: Accentuate the positive, eliminate the negative. Cancer Cell. 2007;12:192. doi: 10.1016/j.ccr.2007.08.023. [DOI] [PubMed] [Google Scholar]