Abstract
Several new antiretroviral (ARV) agents for treatment experienced HIV-infected patients have been approved since June 2006, including darunavir (DRV) and raltegravir (RAL). While efficacious in clinical trials, the effectiveness, durability, and tolerability of these new ARVs remains understudied in the context of routine clinical care. The Darunavir Outcomes Study is a prospective cohort study of three-class ARV-experienced patients changing regimens at the 1917 Clinic after 1/7/2006. All treatment decisions were at the discretion of primary providers. Multivariate (MV) logistic regression for 48 week VL <400c/ml and Cox models for regimen durability were completed. Propensity score methods controlled for sociodemographics. Among 108 patients, mean age of 46, 48% were white, 80% male, with prior exposure to a mean 10.5 ARVs. Overall, 64% of patients achieved 48-week VL <400 c/ml. In MV modeling DRV/rll (OR = 5.77;95%CI = 1.62–20.58) and RAL (OR = 3.84;95%CI = 1.23–11.95) use increased odds of 48-week suppression. Use of these agents exhibited a trend towards prolonged regimen durability in Cox models. Among those highly ARV-experienced, regimens containing DRV/r and/or RAL were more likely to achieve 48-week VL <400 c/ml and exhibited a trend towards prolonged durability. New agents have transformed the treatment landscape for ARV-experienced patients, with effectiveness in routine clinical care mirroring efficacy in clinical trials.
Introduction
The approval of the first agents in new therapeutic drug classes [raltegravir (RAL), maraviroc (MVC)] and second generation agents in existing drug classes [etravirine (ETR), darunavir (DRV)] since 2006 has greatly expanded the availability of antiretroviral therapy (ART) options for treatment-experienced patients. With various clinical trials highlighting the safety and superior efficacy of these newer agents, complete virologic suppression has become the therapeutic goal for all HIV-infected patients, including those who are treatment-experienced.1–12
Despite the accumulating body of evidence from clinical trials, a paucity of published data addressing the effectiveness of these newer agents in routine clinical care settings exists. Evaluating both the efficacy and effectiveness of newer agents is important to ensure that the results obtained from clinical trials are generalizable to populations treated through routine care.13 The Darunavir Outcome Study is a prospective cohort study of three-class antiretroviral experienced patients initiating a new ART regimen since 2006, which marked US Food and Drug Administration approval of the first novel aforementioned antiretroviral agent for treatment-experienced patients, darunavir. We previously reported an increased likelihood of 24-week virologic suppression among three-class experienced patients treated with DRV/r (Darunavir/ritonavir), RAL or both of these agents in routine clinical care.1 In the present analysis, we evaluated the comparative effectiveness of 48-week virologic suppression, comparative regimen durability, and the safety of these agents comparing baseline to 48-week laboratory values.
Methods
The Darunavir Outcomes Study (DOS) is a prospective cohort study of three-class antiretroviral experienced patients initiating new ART since 2006. The DOS study is nested within the University of Alabama at Birmingham (UAB) 1917 Clinic Cohort and was sponsored by Tibotec Therapeutics. The UAB 1917 Clinic Cohort (www.uab1917cliniccohort.org; last accessed February 1, 2010) is an ongoing, institutional review board (IRB) approved, prospective clinical cohort study that collects psychosocial, socio-demographic, and clinical information from patients receiving outpatient care at the UAB 1917 HIV/AIDS Clinic (www.1917clinic.org; last accessed February 1, 2010). The cohort was established in 1992 and has been described in detail previously.14,15 Electronic data capture began shortly after the cohort was established. In 2004, the UAB 1917 Clinic Cohort transitioned from an interval to a clinical cohort with electronic data capture at the point-of-care by providers through a locally programmed electronic medical record. This application imports all laboratory values directly from the central UAB laboratory, includes electronic prescription for all medications, and contains detailed encounter notes. The electronic medical record and databases are 100% quality assured, whereby all provider notes, diagnostic data, and medication orders are reviewed within 72 hours of entry into the system to ensure accurate data capture. Providers select treatment regimens independently as no clinic-based therapeutic recommendations or treatment algorithms exist.
The present study was approved by the UAB IRB, and all participants provided written informed consent. According to the study protocol, participants completed a questionnaire at each visit and had extra blood drawn at each clinic visit. The scheduling of appointments, medication treatment, and other decisions pertaining to individual patient care were made by the primary care providers, independent of research personnel. The study was observational in design and did not provide antiretroviral medications, laboratory tests, or remuneration to participants. Representatives from Tibotec Therapeutics participated in the study design, data interpretation, and manuscript preparation but did not contribute to data collection or analysis. Academic authors made final determination of manuscript content.
Study sample and procedures
Inclusion criteria
(1) Three-class antiretroviral-experienced patients (previously prescribed an agent from three of the following drug classes: nucleoside reverse transcriptase inhibitor [NRTI], non-nucleoside reverse transcriptase inhibitor [NNRTI], protease inhibitor-boosted or unboosted and fusion inhibitor) changing regimens between 1 July 2006 and 30 April 2008; and (2) plasma HIV viral load above 1,000 copies/ml (c/ml). As an effectiveness trial of patients in routine clinical care, there were no CD4 count or medical co-morbidity based exclusion criteria in an effort to capture a broad and representative patient population. Patients were only excluded from study if they were enrolled in a clinical trial evaluating antiretroviral medications as this would have influenced regimen selection. Providers had access to all available patient-level clinical data, ordered resistance testing at their discretion, and choose subsequent antiretroviral regimens without the involvement of study personnel.
Independent variables
At study enrollment, the following socio-demographic and clinical data were obtained: age, sex, race, health insurance, baseline plasma HIV viral load (c/ml), and baseline CD4+ T-lymphocyte count (cells/mm3). Antiretroviral treatment history was determined by electronic database search and confirmed by medical record abstraction. If an HIV resistance assay was obtained prior to regimen change, genotypic and phenotypic data were recorded by study personnel in an electronic database. The number of active drugs in each regimen was determined by analysis of genotypic mutations with the Stanford Genotype Database with scores below 30 denoting an active agent. RAL was considered an active agent for all study participants as none of the patients had previously been exposed to an integrase inhibitor. Antiretroviral regimen composition or treatment strategy was characterized as: (1) protease inhibitor sparing (PI-Sparing); (2) darunavir-containing regimen (DRV/r); or (3) other protease inhibitor-containing regimen (Other PI). Models were adjusted for concomitant RAL use. As MVC and ETR were prescribed in fewer than 20 participants, these agents were not evaluated in adjusted analyses.
Outcome variables
The primary outcome variables were 48 week virologic suppression (in a ± 120 day window); and regimen durability, defined as time to regimen change or discontinuation. Viral load (VL) was measured with two different tests during the study period, initially the Roche COBAS HIV-1 Ampliprep Amplicor Monitor Test, v.1.5 (Amplicor) was used and subsequently the COBAS Ampliprep/COBAS TaqMan HIV-1, v.1.0 assay (TaqMan) was utilized starting 9 June 2008. Regimen durability or persistency measures the time from initiation to regimen change or discontinuation of therapy.14–17 Regimen change or discontinuation reasons were retrieved electronically, then subsequently confirmed through chart abstraction. Changes in treatment strategy (PI-Sparing, DRV/r, or Other PI) motivated by concerns over toxicity or effectiveness were considered a regimen change while changes due to convenience or regimen simplification were not (i.e.,exchange of injected enfuvirtide [ENF] for an alternative oral agent, or change of single NRTIs to agents in fixed-dosed combination). Changes in NRTI backbones were also allowed and not considered regimen discontinuations as the treatment strategy, which was the primary study focus, remained intact.
Statistical analysis
Descriptive statistics were employed to evaluate overall patient and regimen level characteristics and to ensure distributional assumptions for statistical tests were met. The distribution of socio-demographic and regimen characteristics per treatment strategy (PI-sparing, DRV/r, or Other PI) was characterized and subsequently compared using analysis of variance (ANOVA).
Due to the modest sample size and emphasis on clinical and regimen characteristics, propensity score methods were employed to determine a composite (propensity) score for DRV/r receipt on the basis of age, sex, race, and insurance provider. The use of the propensity score for DRV/r receipt allowed for adjustment for multiple variables without over-fitting the multivariable comparative effectiveness models for 48-week virologic suppression and regimen durability.
Univariate and multivariable logistic regression modeling (adjusted for propensity score) were used to evaluate the comparative effectiveness of 48-week virologic suppression among study participants according to treatment strategy. In vitro evidence and reports from our cohort and other sites have questioned the clinical implications of employing the TaqMan VL assay at the lower end of the dynamic range due to increased reports of detectable low-level viremia and “blips” in previously well-controlled individuals.18–21 Because of concerns over the use of the TaqMan assay v.1.0 during the study period, VL <400 c/ml was utilized for the analysis of comparative effectiveness of 48-week virologic suppression. Primary viral load effectiveness analyses employed a missing is equal to missing approach, excluding patients who did not have a viral load measure within the 48-week assessment window. Sensitivity analyses employed a missing is equal to failure approach to assess the impact of missing 48-week viral load data on study findings.
We analyzed regimen durability according to treatment strategy by the Kaplan–Meier method and modeled time to regimen discontinuation with univariate and propensity score adjusted multivariable Cox proportional hazards models. Finally, we evaluated grade 3–4 laboratory-based adverse events (includes: hemoglobin, low density lipoprotein, total bilirubin, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, creatinine, and glucose) according to the AIDS Clinical Trials Group severity ranges (grades) for laboratory based adverse events in adults. Week 48 laboratory values were evaluated for changes from baseline values. All p values were two-sided, with values less than 0.05 considered statistically significant. All statistical analyses were performed using SAS software version 9.1.3 (SAS Institute, Cary, NC).
Results
A total of 108 three-class experienced patients were enrolled. DRV/r (n = 51; 47%) was the most commonly used treatment strategy, followed by Other PI (n = 32; 30%) and PI-Sparing (n = 25; 23%). Only sex was significantly different among treatment strategies [males: other PI = 72%; n = 23, DRV/r = 94%; n = 48, PI-sparing = 80%; n = 20, p = 0.02)] while all other socio-demographic characteristics including age, race, and insurance status were well balanced. No statistically significant differences in mean baseline CD4 count, mean prior antiretroviral drug exposure, and number of active drugs in each regimen were observed across treatment strategy. Mean baseline HIV VL was highest in the DRV/r group (4.54 ± 0.88 log10 c/ml; p = 0.01) (Table 1).
Table 1.
Baseline Characteristics of 108 Three-Class Antiretroviral-Experienced Patients Changing Regimens in the Darunavir Outcomes Study, July 2006-May 2008
| Other-PI1 N = 32 | Darunavir/r N = 51 | PI-Sparing N = 25 | P value | |
|---|---|---|---|---|
| Socio-demographic characteristics | ||||
| Age, year | ||||
| Mean ± SD | 46.1 ± 8.0 | 46.7 ± 9.1 | 46.4 ± 8.8 | 0.94 |
| Gender, n (%) | ||||
| Male | 23 (71.9%) | 48 (94.1%) | 20 (80.0%) | |
| Female | 9 (28.1%) | 3 (5.9%) | 5 (20.0%) | 0.02 |
| Race, n (%) | ||||
| White | 15 (46.9%) | 29 (56.9%) | 11 (44.0%) | |
| Black/Other | 17 (53.1%) | 22 (43.1%) | 14 (56.0%) | 0.49 |
| Insurance, n (%) | ||||
| Private | 16 (50.0%) | 21 (41.2%) | 6 (24.0%) | |
| Public | 12 (37.5%) | 22 (43.1%) | 15 (60.0%) | |
| Uninsured | 4 (12.5%) | 8 (15.7%) | 4 (16.0%) | 0.37 |
| Clinical and regimen characteristics | ||||
| Baseline HIV VL, log10 copies/ml | ||||
| Mean ± SD | 3.81 ± 1.27 | 4.54 ± 0.88 | 4.19 ± 1.07 | 0.01 |
| Baseline CD4 count, cells/mm3 | ||||
| Mean ± SD | 233 ± 214 | 213 ± 173 | 226 ± 232 | 0.90 |
| ≥ 200 | 17 (53.1%) | 27 (52.9%) | 12 (48.0%) | |
| 200–350 | 8 (25.0%) | 14 (27.5%) | 9 (36.0%) | |
| ≥ 350 | 7 (21.9%) | 10 (19.6%) | 4 (16.0%) | 0.91 |
| Previous ARVs2 | ||||
| Mean ± SD | 9.4 ± 3.5 | 11.3 ± 4.2 | 10.3 ± 4.8 | 0.12 |
| Active drugs (based on GSS2) | ||||
| 1 or 2 | 11 (34.4%) | 22 (43.1%) | 10 (40.0%) | |
| 3 or 4 | 14 (43.7%) | 23 (45.1%) | 7 (28.0%) | |
| Unknown | 7 (21.9%) | 6 (11.8%) | 8 (32.0%) | 0.25 |
| Other ARVs2 | ||||
| Raltegravir No | 21 (65.6%) | 16 (31.4%) | 6 (24.0%) | |
| Yes | 11 (34.4%) | 35 (68.6%) | 19 (76.0%) | <0.001 |
| Enfuvirtide No | 30 (93.8%) | 37 (72.6%) | 25 (100.0%) | |
| Yes | 2 (6.2%) | 14 (27.4%) | 0 (0.0%) | 0.002 |
| Etravirine No | 32 (100.0%) | 43 (84.3%) | 15 (60.0%) | |
| Yes | 0 (0.0%) | 8 (15.7%) | 10 (40.0%) | <0.001 |
Included atazanavir (n = 17; 12 ritonavir boosted, 5 no ritonavir), lopinavir/ritonavir (n = 11), fosamprenavir/ritonavir (n = 2), nelfinavir (n = 1), and one regimen with both atazanavir and lopinavir/ritonavir.
ARVs: antiretrovirals; GSS: genotypic susceptibility score.
Statistically significant differences were observed regarding the use of RAL, ETR and ENF among treatment strategies (MVC was not included as prescribed in one subject only). DRV/r was most commonly paired with RAL (n = 35, 69%) and ENF (n = 14; 27%), and less frequently with ETR (n = 8; 16%). Regimens in the Other PI group included: atazanavir (n = 17; 12 ritonavir boosted, 5 unboosted), lopinavir/ritonavir (n = 11), fosamprenavir/ritonavir (n = 2), nelfinavir (n = 1), and one regimen with both atazanavir and lopinavir/ritonavir. PIs other than DRV/r were most frequently combined with RAL (n = 11; 34%). The PI-Sparing strategy most commonly consisted of RAL (n = 19; 76%) followed by ETR (n = 10; 40%) (Table 1). A total of 95 patients (88%) had virologic data available for 48-week analyses. In all, 61 of 108 individuals (64%) had a viral load <400 c/ml at week 48. Of these 61 subjects, 53% (n = 32) were treated with the DRV/r strategy, 31% (n = 19) with the Other PI strategy, and 16% (n = 10) with the PI-Sparing strategy (data not shown).
A propensity score adjusted logistic regression model for comparative effectiveness of 48-week virologic suppression (VL <400 c/ml) was fit. Compared to the PI-Sparing regimen strategy, the use of DRV/r (Odds ratio [OR] = 5.77; 95% confidence interval [CI] = 1.62–20.58) was associated with an increased likelihood of suppression while the use of the Other PI (OR = 2.85; 95% CI = 0.73–11.21) strategy was not. RAL-containing regimens (OR = 3.84; 95% CI = 1.23–11.95) were found to increase the odds of 48-week virologic suppression (Table 2). Sensitivity analysis using a missing is equal to failure approach yielded findings consistent with primary analysis with parameter estimates of comparable magnitude (data not shown).
Table 2.
Factors Associated with Plasma HIV Viral Load <400 Copies/ml at Week 48 Among 108 Patients in Unadjusted and Multivariate Logistic Regression Analyses
| VL <400 c/ml (n = 61) | VL >400 c/ml (n = 34) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Propensity score (age, gender, race, insurance; OR for 10% increment) | ||||
| Mean ± SD | 0.47 ± 0.14 | 0.46 ± 0.14 | 1.07 (0.80–1.44) | 0.82 (0.55–1.23) |
| HIV VL, log10 copies/ml 0 | ||||
| Mean ± SD | 4.05 ± 1.17 | 4.50 ± 1.00 | 0.68 (0.45–1.04) | 0.63 (0.37–1.07) |
| CD4+, cells/mm3 (O.R. per 50 cells) | ||||
| Mean ± SD | 246 ± 193 | 163 ± 191 | 1.14 (1.00–1.30) | 1.14 (0.99–1.32) |
| Active drugs | ||||
| 1 or 2 | 22 (36.1%) | 15 (44.1%) | 1.0 | 1.0 |
| 3 or 4 | 28 (45.9%) | 12 (35.3%) | 1.59 (0.62–4.08) | 0.91 (0.31–2.73) |
| Unknown | 11 (18.0%) | 7 (20.6%) | 1.07 (0.34–3.39) | 0.63 (0.15–2.76) |
| Strategy | ||||
| PI-sparing | 10 (16.4%) | 12 (35.4%) | 1.0 | 1.0 |
| Other-PI1 | 19 (31.1%) | 11 (32.3%) | 2.07 (0.68–6.36) | 2.85 (0.73–11.21) |
| Darunavir/r | 32 (52.5%) | 11 (32.3%) | 3.49 (1.18–10.31) | 5.77 (1.62–20.58) |
| Raltegravir | ||||
| No | 19 (31.2%) | 18 (52.9%) | 1.0 | 1.0 |
| Yes | 42 (68.8%) | 16 (47.1%) | 2.49 (1.05–5.90) | 3.84 (1.23–11.95) |
Included atazanavir (n = 17; 12 ritonavir boosted, 5 no ritonavir), lopinavir/ritonavir (n = 11), fosamprenavir/ritonavir (n = 2), nelfinavir (n = 1), and one regimen with both atazanavir and lopinavir/ritonavir.
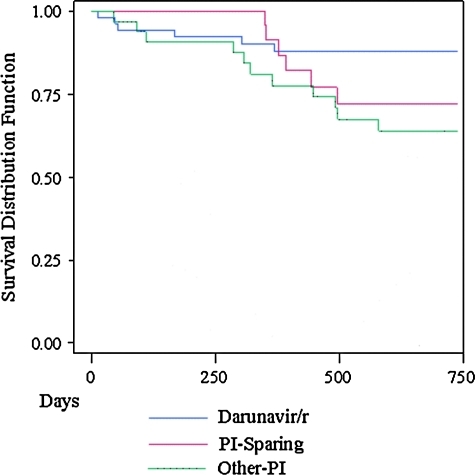
Kaplan–Meier analysis of regimen durability by treatment strategy was completed (Fig. 1). Overall, 26 patients (24%) changed or discontinued their regimens by Week 48: 6 DRV/r (6 of 51, 12%), 14 Other PI (14 of 32, 44%), and 6 PI-Sparing (6 of 25, 24%). Eighteen of the 26 patients (69%) discontinued due to nonadherence or virologic failure: 4 for DRV/r, 11 for Other PI, and 3 for the PI-Sparing treatment strategy. Four (15%) of 26 patients discontinued due to toxicity: 2 for DRV/r (hyperglycemia, rash), and 2 for the Other PI treatment strategy (nausea, diarrhea). The remaining 4 patients discontinued for other reasons: 2 for medication interactions (both Other PI strategy: proton pump inhibitor use with atazanavir); 1 for enrollment in a clinical trial, and 1 for a need to change to liquid medication (data not shown). A Cox proportional hazard model including propensity score adjustment for demographic factors was fit. Higher baseline CD4 values at the time of regimen initiation led to a decreased hazard of early regimen discontinuation (hazard ratio [HR] = 0.77 per 50 cells/mm3 increase; 95%CI = 0.65–0.92). A trend towards an increased hazard of early regimen discontinuation was seen with the use of non-DRV/r regimen strategies (Other PI vs. DRV/r HR = 3.27, 95%CI = 0.97–10.96; PI-Sparing vs. DRV/r HR = 3.43, 95% CI = 0.95–12.32). Additionally, a trend towards prolonged regimen durability was observed with RAL use (HR = 0.35; 95% CI = 0.12–1.03) (Table 3).
FIG. 1.
Kaplan–Meier plot of comparative regimen durability for Darunavir/r (D), Other-PI (PI), and PI-Sparing (N) regimens. (Color image can be found at www.liebertonline.com/aid).
Table 3.
Cox Proportional Hazard Model of Comparative Regimen Durability Among 108 Study Patients in the DOS Study
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Propensity score (age, gender, race, insurance; OR for 10% increment) | 0.90 (0.69–1.17) | 1.18 (0.85–1.64) |
| HIV VL, log10 copies/ml | 0.94 (0.65–1.35) | 0.89 (0.60–1.32) |
| CD4+, cells/mm3 (H.R. per 50 cells) | 0.82 (0.70–0.96) | 0.77 (0.65–0.92) |
| Active drugs | ||
| 1 or 2 | 1.0 | 1.0 |
| 3 or 4 | 1.14 (0.48–2.70) | 1.77 (0.66–4.75) |
| Unknown | 0.76 (0.24–2.44) | 1.18 (0.33–4.24) |
| Strategy | ||
| Darunavir/r | 1.0 | 1.0 |
| Other-PI1 | 3.26 (1.22–8.71) | 3.27 (0.97–10.96) |
| PI-Sparing | 2.21 (0.71–6.88) | 3.43 (0.95–12.32) |
| Raltegravir | ||
| No | 1.0 | 1.0 |
| Yes | 0.44 (0.20–1.00) | 0.35 (0.12–1.03) |
Included atazanavir (n = 17; 12 ritonavir boosted, 5 unboosted), lopinavir/ritonavir (n = 11), fosamprenavir/ritonavir (n = 2), nelfinavir (n = 1), and one regimen with both atazanavir and lopinavir/ritonavir.
Overall, incident grade 3–4 lab abnormalities were infrequent across treatment strategies. The most common incident grade 3–4 adverse events per treatment strategy at 48 weeks involved creatinine (n = 2; 6%) and low density lipoprotein (n = 2; 6%) for Other PI; alanine aminotransferase (n = 1; 2%) for DRV/r, and elevated glucose (n = 2, 8%) for PI-Sparing (Table 4).
Table 4.
Laboratory Grade 3–4 Adverse Events at Baseline and 48 Weeks in DOS Study Patients with Available Values Collected as Part of Routine Care
| |
Other-PI1 |
Darunavir/r |
PI-Sparing |
|||
|---|---|---|---|---|---|---|
| Laboratory value | Baseline (n = 32) | Week 48 (n = 32) | Baseline (n = 48) | Week 48 (n = 50) | Baseline (n = 25) | Week 48 (n = 25) |
| Hemoglobin | 0 | 0 | 0 | 0 | 0 | 0 |
| LDL2 cholesterol | 0 | 2 (6.3%) | 0 | 0 | 1 (4.0%) | 0 |
| Glucose | 0 | 1 (3.1%) | 0 | 0 | 0 | 2 (8.0%) |
| Creatinine | 3 (9.4%) | 5 (15.6%) | 0 | 0 | 0 | 0 |
| Total bilirubin | 0 | 1 (3.1%) | 0 | 0 | 1 (4.0%) | 0 |
| Alkaline phosphatase | 0 | 0 | 0 | 0 | 0 | 0 |
| AST3 | 0 | 1 (3.1%) | 0 | 0 | 0 | 0 |
| ALT4 | 1 (3.1%) | 0 | 0 | 1 (2.0%) | 0 | 0 |
Included atazanavir (n = 17; 12 ritonavir boosted, 5 no ritonavir), lopinavir/ritonavir (n = 11), fosamprenavir/ritonavir (n = 2), nelfinavir (n = 1), and one regimen with both atazanavir and lopinavir/ritonavir.
LDL, low density lipoprotein; 3AST, aspartate aminotransferase; 4ALT, alanine aminotransferase.
Discussion
Overall, 64% of three-class experienced individuals starting new ART regimens after 1 July 2006 achieved 48-week virologic suppression (VL <400 c/ml). This rate of effectiveness in routine clinical care compares favorably with 48-week efficacy of virologic suppression rates in clinical trials using newer ART agents, although caution must be exercised when comparing results across study designs. In the TITAN study that compared an optimized background regimen (OBR) plus DRV/r vs. lopinavir/ritonavir, 48-week virologic suppression (<400 c/ml) was achieved in 77% and 68% of patients, respectively, though these patients were less treatment experienced than those in the present study.10 Pooled analysis of the DUET 1 and 2 trials (OBR including DRV/r in all patients, plus ETR vs. Placebo) showed 48-week virologic suppression in (VL <50 c/ml) 61% (ETR) and 40% (Placebo) of individuals.8 In the BENCHMRK trial, the 48-week virologic efficacy (VL <400 c/ml) of OBR plus RAL was 62% vs. 33% for OBR plus placebo.12 The accumulating body of evidence regarding both the efficacy and effectiveness of newer agents underscores the arrival of a new era in the therapy of treatment experienced patients post-2006.1 Achieving viral load suppression among experienced patients as endorsed by the most recent treatment guidelines has become a more achievable objective than in previous years.
The inclusion of DRV/r and/or RAL in the ART regimen of heavily treatment experienced patients was associated with an increased likelihood of regimen effectiveness as measured by 48-week VL suppression (<400 c/ml) in both primary and sensitivity analyses. Whereas data on the efficacy and safety of combinations of these newer agents await further clinical trial confirmation and are just recently starting to emerge,22 providers in contemporary practice settings have been combining newer agents since their release. In our sample, 35 patients were prescribed DRV/r and RAL simultaneously, with 80% achieving 48-week virologic suppression (<400 c/ml). Our findings of greater 48-week virologic effectiveness among patients treated with newer antiretroviral agents serves to complement clinical trial findings and provide early insight into the result of combinations of these newer agents in routine care settings.
Regimen durability or persistency is defined as the time from initiation to change or discontinuation of therapy.16 Durability or persistency is a distinct concept from treatment adherence (extent to which a patient takes medication in agreement with dosing and frequency recommendations) and more prolonged persistency has been associated with decreased adverse outcomes in other chronic conditions (diabetes, hyperlipidemia, and hypertension).23 Though we have previously reported on the durability/persistency of initial ART in the 1917 Clinic Cohort and a cohort in the developing world,14,15,17 the persistency of therapy in heavily treatment experienced individuals remains understudied. We saw a trend towards an increased risk of regimen discontinuation for Other PI and PI-Sparing strategies when compared to the DRV/r treatment strategy, as well as a trend toward prolonged regimen durability in regimens containing RAL. Though these findings were affected by the modest sample size, these trends may point to prolonged regimen durability/persistency of these newer antiretroviral agents. Higher baseline CD4 values were associated with prolonged regimen durability/persistency, underscoring the importance of prompt changes before more marked CD4 declines occur. The impact of durability/persistency on long term treatment outcomes of HIV/AIDS awaits further study.
The ART regimens selected by clinicians in the context of routine care in the present study were generally well tolerated. In the DRV/r treatment strategy group, no patients initiated new ART with baseline grade 3–4 laboratory adverse events, while 4 patients and 2 patients started ART in the Other PI and PI-Sparing groups, respectively, with such laboratory abnormalities. Per treatment strategy at 48 weeks, grade 3–4 laboratory adverse events were found most commonly in the Other PI group and less commonly with other strategies (Other PI = 10, PI-Sparing = 2, DRV/r = 1) (Table 4). The development of grade 3–4 laboratory adverse events led to treatment discontinuation in one study patient (DRV/r). The observation of lower rates of adverse events in the DRV/r and PI-Sparing treatment strategies echo the favorable safety profiles for post-2006 treatment options reported in recent clinical trials.2–4, 6–12
The results of this study should be interpreted within the context of its limitations. As a single academic HIV clinic in the Southeastern USA, results may not be generalizable to other locations. Unmeasured confounders related to antiretroviral regimen selection and measured outcomes may have influenced study findings. As with all observational studies, the results identify associations, but cannot attribute causality. Data were collected through routine practice patterns and 21 patients lacked resistance testing prior to starting a new regimen. However, post-hoc chart review indicated these patients had not been on therapy at the time of regimen change due to nonadherence, side effects that led to the discontinuation of their previous regimen, or return to care after long absences. Though a limitation of the study and often avoided in clinical efficacy trials, such situations are common in the routine care of HIV-infected individuals. As such, our findings are reflective of the realities of contemporary ambulatory HIV care and contribute effectiveness data on newer treatment options in a routine care setting.
In summary, the results of this observational trial found superior 48-week effectiveness of HIV virologic suppression in three-class antiretroviral experienced patients receiving DRV/r and RAL individually or together as part of a combination ART regimen, corroborating the clinical trial efficacy of these agents.2,3,6–12 A trend towards enhanced regimen durability/persistency with these agents was also observed. Though future study into optimal combinations of novel antiretroviral agents released post-2006 is needed, the increased efficacy, effectiveness, and enhanced durability/persistency of these agents are indicative of important advancements in the care of heavily treatment experienced patients.
Acknowledgments
The UAB 1917 HIV/AIDS Clinic Cohort Observational Database project receives financial support from the following: UAB Center for AIDS Research (Grant P30-AI27767), CFAR-Network of Integrated Clinical Systems, CNICS (Grant 1 R24 AI067039-1), and the Mary Fisher CARE Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of any agency providing support for this study. Funding is also from the Tibotec Therapeutics.
Author Disclosure Statement
J. H. Willig: Consultant and/or has received investigational funding from the following companies: Tibotec Therapeutics, Bristol-Myers Squibb, Pfizer, Definicare, and Gilead. I. Aban, C.R. Nevin, Y. Ye, and J.L. Raper report no conflict. J. A. McKinnel has received investigational funding from Bristol-Myers Squibb. L. L. DeLaitsch, J. M. Mrus, and G. R. De La Rosa were employees of Tibotec Therapeutics during the time of this analysis. M. J. Mugavero is a consultant and/or has received investigational funding from the following companies: Tibotec Therapeutics, Bristol-Myers Squibb, Pfizer, and Gilead. M.S. Saag is a consultant and/or receives research grants from the following companies: Adrea Pharmaceuticals, Avexa, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Merck, Monogram Biosciences, Panacos, Pfizer, Progenics, Roche, Serono, Tanox, Tibotec, Trimeris, and Vertex.
References
- 1.McKinnell JA. Lin HY. Nevin CN, et al. Early virologic suppression with three-class experienced patients: 24-week effectiveness in the darunavir outcomes study. AIDS. 2009;23:1539–1546. doi: 10.1097/QAD.0b013e32832c7b5c. [DOI] [PubMed] [Google Scholar]
- 2.Arasteh K. Yeni P. Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antivir Ther. 2009;14:859–864. doi: 10.3851/IMP1301. [DOI] [PubMed] [Google Scholar]
- 3.Clotet B. Bellos N. Molina JM, et al. Efficacy, safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1, 2: A pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369:1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 4.Gulick RM. Lalezari J. Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer SM. Eron JJ., Jr. Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 6.Imaz A. Del Saz SV. Ribas MA, et al. Raltegravir, etravirine, ritonavir-boosted darunavir: A safe and successful rescue regimen for multidrug-resistant HIV-1 infection. J Acquir Immune Defic Syndr. 2009;52:382–386. doi: 10.1097/QAI.0b013e3181b17f53. [DOI] [PubMed] [Google Scholar]
- 7.Katlama C. Esposito R. Gatell JM, et al. Efficacy, safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. AIDS. 2007;21:395–402. doi: 10.1097/QAD.0b013e328013d9d7. [DOI] [PubMed] [Google Scholar]
- 8.Katlama C. Haubrich R. Lalezari J, et al. Efficacy, safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS. 2009;23:2289–2300. doi: 10.1097/QAD.0b013e3283316a5e. [DOI] [PubMed] [Google Scholar]
- 9.Lazzarin A. Campbell T. Clotet B, et al. Efficacy, safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]
- 10.Madruga JV. Berger D. McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: A randomised controlled phase III trial. Lancet. 2007;370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 11.Madruga JV. Cahn P. Grinsztejn B, et al. Efficacy, safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]
- 12.Steigbigel RT. Cooper DA. Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 13.Routman JS. Willig JH. Westfall AO, et al. Comparative efficacy versus effectiveness of initial antiretroviral therapy in clinical trials versus routine care. Clin Infect Dis. 2010;50:574–584. doi: 10.1086/650004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen RY. Westfall AO. Mugavero MJ, et al. Duration of highly active antiretroviral therapy regimens. Clin Infect Dis. 2003;37:714–722. doi: 10.1086/377271. [DOI] [PubMed] [Google Scholar]
- 15.Willig JH. Echevarria J. Westfall AO, et al. Durability of initial antiretroviral therapy in a resource constrained setting and the potential need for zidovudine weight-based dosing. J Acquir Immune Defic Syndr. 2010;53:215–221. doi: 10.1097/QAI.0b013e3181bc0f10. [DOI] [PubMed] [Google Scholar]
- 16.Cramer JA. Roy A. Burrell A, et al. Medication compliance, persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 17.Willig JH. Abroms S. Westfall AO, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22:1951–1960. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatanaga H. Tsukada K. Honda H, et al. Detection of HIV type 1 load by the Roche Cobas TaqMan assay in patients with viral loads previously undetectable by the Roche Cobas Amplicor Monitor. Clin Infect Dis. 2009;48:260–262. doi: 10.1086/595707. [DOI] [PubMed] [Google Scholar]
- 19.Lima V. Harrigan R. Montaner JS. Increased reporting of detectable plasma HIV-1 RNA levels at the critical threshold of 50 copies per milliliter with the Taqman assay in comparison to the Amplicor assay. J Acquir Immune Defic Syndr. 2009;5:3–6. doi: 10.1097/QAI.0b013e31819e721b. [DOI] [PubMed] [Google Scholar]
- 20.Smit E. Bhattacharya S. Osman H. Taylor S. Increased frequency of HIV-1 viral load blip rate observed after switching from Roche Cobas Amplicor to Cobas Taqman assay. J Acquir Immune Defic Syndr. 2009;51:364–365. doi: 10.1097/QAI.0b013e3181aa13b3. [DOI] [PubMed] [Google Scholar]
- 21.Willig JH. Nevin CR. Willig AL, et al. Cost ramifications of increased reporting of detectable plasma HIV-1 RNA levels by the Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 version 1.0 viral load test. J Acquir Immune Defic Syndr. 2010;54:442–444. doi: 10.1097/QAI.0b013e3181d01d1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazdanpanah Y. Fagard C. Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: Results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49:1441–1449. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 23.Cramer JA. Benedict A. Muszbek N. Keskinaslan A. Khan ZM. The significance of compliance, persistence in the treatment of diabetes, hypertension, dyslipidaemia: A review. Int J Clin Pract. 2008;62:76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]