Abstract
There is often a pressing need for reconstruction after cancer surgery. Regenerative therapy holds the promise of more natural and esthetic functional tissue. In the case of breast reconstruction postmastectomy, volume retention problems associated with autologous fat transfer could be ameliorated by augmentation with cells capable mediating rapid vascularization of the graft. Intentional placement of regenerating tissue at the site of tumor resection raises questions concerning the possibility of promoting cancer recurrence. Here we review coculture and animal models of tumor/mesenchymal stem cell interactions under regenerating conditions. Available evidence from case reports, cell lines, and clinical isolates favors the interpretation that regenerating tissue promotes the growth of active, high-grade tumor. In contrast, dormant cancer cells do not appear to be activated by the complex signals accompanying wound healing and tissue regeneration, suggesting that engineered tissue reconstruction should be deferred until cancer remission has been firmly established.
Introduction
Cancer surgery can be disfiguring
Restoring acceptable human appearance after cancer extirpation is an important part of the treatment process. In particular surgical excision of head and neck cancer or breast cancer, can lead to disfiguring aesthetic deformities and reconstruction is highly desirable. The field of regenerative medicine promises new alternatives to surgical reconstruction. Through the use of scaffolds and multipotent adult tissue stem cells, the restoration of stable, functional, and natural appearing tissue is envisioned. A major concern in the application of regenerative therapies after cancer, especially cell based therapies, is whether these new treatments will increase the risk of tumor recurrence. Unfortunately, the factors that accompany tissue regeneration and revascularization are also critical to cancer growth and metastasis. This article reviews what is known concerning interactions between multipotent mesenchymal stem cells (MSC) and cancer, with a view to assess the potential risks of regenerative therapy after cancer surgery.
Autologous fat transfer
Autologous fat transfer (AFT) for soft tissue reconstruction was initially described more than a century ago in a German-language article entitled Fettransplantation (Fat Transplantation). The initial indication for fat transplantation was for correction of facial defects1 and was soon after introduced for breast reconstruction postmastectomy.2 However, fat injection into the breast became controversial among plastic surgeons because of potential complications such as local calcifications and interference with mammographic breast cancer surveillance.3 AFT remains an attractive reconstructive technique with low complication rates.4 The first successful application of soft tissue regenerative therapy after cancer was performed more than a century ago by Czerny,2 who restored breast symmetry postmastectomy by transplanting a benign autologous lipoma. Although transplantation of autologous fat yields satisfactory short-term cosmetic results, volume retention has been a recurring problem.5,6 Recent fat transfer and lipoinjection protocols have focused on the addition of autologous adipose-derived stem/stromal cells (ASC) or freshly isolated adipose stromal vascular cells to promote graft volume retention.7–9 Enthusiasm for the use of stem cell-augmented adipose transplantation for breast reconstruction has been tempered by the fear that the transplantation of self-vascularizing, self-renewing adipose tissue may promote tumor recurrence by supporting reactivation of occult breast cancer cells.
Potential advantages of cellular therapy
The variability in long-term graft survival6,10 has been attributed to differences in local angiogenesis.11,12 Recently, the addition of ASC to whole fat grafts was proposed to support the formation of a new vasculature8,13 and promote graft retention.14 Fat tissue is a rich source of both endothelial progenitors15,16 which can mediate angiogenesis and multipotent MSC.17 Both populations are present in the freshly isolated adipose stromal vascular fraction (SVF).18 The SVF, when expanded in short term culture,17,19,20 has been termed ASC21 and resembles MSC in many important respects, which will be discussed below. The rationale for combining whole fat or lipoaspirate with SVF cells or ASC is that the organized fat tissue may serve as a scaffold upon which more concentrated stem/progenitor cells can organize and differentiate. Another attractive approach for guiding the three-dimensional organization of engineered tissue reconstruction is through the use of scaffolding,22–24 which may be mineral, synthetic polymers, or biological and may incorporate growth factors naturally25 or by design.26–28
Models of tumor cell/MSC interactions
Therapy for epithelial cancers is rarely curative and late recurrence after apparently successful therapy provides prima facia evidence for the persistence of dormant cancer cells. Precisely how regenerating engineered tissues may interact with active and dormant cancer cells in vivo is currently unknown. However, interactions of proliferating ASC and MSC with tumor cells have been addressed in coculture and human/murine xenotransplantation models. The major contribution of these studies is that they have provided insight into mechanisms of tumor invasion, demonstrating that secretion of the chemokine CCL5 by bone marrow-derived MSC (BM-MSC)29 or ASC increase the motility of breast cancer cell lines in in vitro models of tumor invasion. These findings were reproduced by the addition of exogenous CCL5 to breast cancer cell line cultures.30 Further, BM-MSC have been shown to promote in vitro epithelial to mesenchymal transition of breast cancer cells and reduce expression of proliferation-associated genes.31
Because these studies utilized immortalized cancer cell lines that grow rapidly in culture and give rise to large tumors in a very short time, they fail to model the crucial aspects of tumor heterogeneity, tumor dormancy, and reactivation of occult tumor cells. For example, coculture of MDA-MB-231, a hormone-independent breast cancer cell line, with MSC resulted in greater cell expansion after 4 days in culture (1.7-fold more cells), but tumor cells alone had a doubling time of ∼24 h.32 This extreme lack of clinical realism is also apparent in tumor transplantation models. For example, Yu et al. coinjected 1 × 106 each of H460 cells (a human lung cancer cell line) and human ASC and reported measurable tumors in 5 days that grew to 20 mm3 in 10 days.33 Muehlberg et al. injected 5000 4T1 cells (a murine breast cancer cell line) and observed 125 mm3 tumors in 21 days. Addition of murine ASC increased the size of 21 day tumors to 400 cubic mm.34 In the model reported by Karnoub et al., tumors ranging in size from 50 to 500 mm3 were detectable by day 30.29 Clearly if these models bear any relevance to human disease, it would be in the context of rapidly growing high-grade therapy unresponsive tumors, where reconstructive surgery would not be a consideration. Our own approach has relied on a model system utilizing unpassaged sort-purified clinical isolates injected in limiting numbers (100 cells).35,36 This model requires 3–6 months for the generation of vascularized epithelial tumors in the size range of 5–10 mm3.
Apart from the ability to promote the growth of existing tumors, there is also a report in which implantation of ceramic scaffold charged with syngeneic murine MSC gave rise to sarcomas in a proportion of treated mice.37
There is also a degree of contradiction in the literature, in which ASC or MSC have been shown to inhibit the growth of tumor cells. For example, ASC have been reported to inhibit tumor growth of MDA-MB-231 cells,38 and induce cell death of pancreatic adenocarcinoma cells lines, hepatocarcinoma, colon cancer, and prostate cancer.39 MSC reportedly inhibit tumorigenesis from Kaposi's sarcoma cells40 and hepatoma cells,41 bone metastasis of prostate cancer,42 and in vitro growth of hepatoma, lymphoma, and insulinoma cell lines.43 Similarly, MSC derived from human umbilical cord blood are cytotoxic for human malignant glioma cells.44 Multiple mechanisms have been invoked to relate these findings to the cytokines, chemokines, and prostaglandins secreted by MSC,39 but little experimental evidence has been provided to define the mechanisms of tumor inhibition. Khakoo et al.40 proposed contact-dependant inhibition of Akt protein kinase, whereas Qiao et al. cited down regulation of Wnt signaling, c-Myc, and Bcl-2.41
Phenotypic and functional characteristics of ASC and MSC
ASC phenotypically resemble BM-MSC19 and share their multipotentiality and many surface markers17,45 (Table 1), but are orders of magnitude more prevalent in fat than BM-MSC are in BM aspirates. In disaggregated freshly isolated adipose tissue, phenotypically defined pericytes comprise 1.7%, whereas CD34+ supra-adventitial stromal cells represent 27% of nucleated cells.18 In contrast MSC represent only 0.001%–0.004% of BM aspirate cells.20,46 Although ASC and BM-MSC are both promising candidates for reconstructive cellular therapy after tumor resection, the potential risk of promoting tumor reactivation is controversial. This is especially germane in breast cancer considering that up to 20% of patients will suffer from cancer recurrence during the first decade after adjuvant therapy.47
Table 1.
Functional and Phenotypic Characteristics of Mesenchymal and Adipose-Derived Stem Cells
| MSC | ASC | |
|---|---|---|
| Developmental origin | Mesoderm, neural crest | Mesoderm, neural crest |
| Tissue of origin | BM | White adipose tissue |
| Selection | Plastic adherence | Plastic adherence |
| Principal antigenic markers (in vitro) | CD10, CD13, CD29, CD44, CD49a-f, CD63, CD73, CD90, CD105, CD106, CD140b, CD146, CD166, CD271, STRO-145,75–86 | CD10, CD13, CD29, CD34, CD44, CD49a, CD63, CD73, CD90, CD105, CD106, CD140b, CD146, CD166, CD271, STRO-119,83–90 |
| Differentiation potential | Adipose tissue91,a | Adipose tissue87,a |
| Bone91,a | Bone87,a | |
| Cartilage91,a | Cartilage87,a | |
| Smooth muscle92,a | Smooth muscle93,94 | |
| Skeletal muscle95 | Skeletal muscle87,a | |
| Cardiac muscle96 | Cardiac muscle97 | |
| Endothelium98 | Endothelium15,16 | |
| Neurons99 | Neurons100 | |
| Hepatocytes101 | Hepatocytes102 | |
| Epithelium103 | Epithelium104 | |
| Telomerase | Absent105–107 | Absent or low108,109 |
| Expansion in vitro | 20–50 population doublings110 | 44–80 population doublings17,111 |
| Precursors in vivo | Pericytes (subendothelial reticular cells, CD146+)76,112 | CD34+ Supra-adventitial, pericytes18 |
| Frequency estimate in vivo (percent of nucleated cells) | 0.001%–0.004%20,46 | 2%–27%18 |
Multipotentiality demonstrated at the clonal level.
ASC, adipose-derived stem cell; BM, bone marrow; MSC, mesenchymal stem cell.
These data indicate strong phenotypic and functional similarities between ASC and BM-MSC. Like BM-MSC, ASC are able to give rise to differentiated progeny with characteristics of bone, cartilage, fat, and vessels and, like MSC, were predominantly CD105+/CD73+/CD90+/CD44+ (Table 1). However, low passage ASC also contain minor populations expressing the adipose pericyte-associated marker CD14618 and CD34, a marker not associated with BM-MSC. The in vitro secretomes of ASC and MSC are also similar (Table 2). However, ASC secrete significantly higher quantities of leptin (140-fold) and adipsin (20-fold), and lower quantities of the angiogenic factors vascular endothelial growth factor and soluble vascular cell adhesion molecule (sVCAM). Production of leptin and adipsin is characteristic of mature adipocytes, but has also been described in ASC cultures.35 The hormone leptin has been reported to increase angiogenic and proliferative signaling in ER+ and ER− breast cancer cell lines.48 Adipsin, a trypsin-like serine protease increases the concentration of acylation-stimulating protein, another adipose-derived hormone that regulates triglyceride synthesis.49 It has no known role in cancer.
Table 2.
Cytokines, Chemokines, and Growth Factors Produced by Mesenchymal Stem Cell and Adipose-Derived Stem Cell
| Cytokines/chemokines/growth factors | BM-MSC mean (ng/mL) | ASC mean (ng/mL) |
|---|---|---|
| Adiponectin | <0.34 | <0.34 |
| Adipsin (CFD) | 3.8 | 74.0 |
| CCL2 (MCP1) | 2.3 | 1.6 |
| CCL5 (RANTES) | <0.02 | <0.02 |
| CRP | <0.03 | <0.03 |
| IL-1b | <0.0001 | <0.0001 |
| IL-2 | <0.0001 | <0.0001 |
| IL-4 | <0.0001 | <0.0001 |
| IL-5 | <0.0001 | <0.0001 |
| IL-6 | 2.2 | 1.1 |
| IL-10 | <0.0001 | <0.0001 |
| IL-12 | <0.0001 | <0.0001 |
| IL-13 | <0.0001 | <0.0001 |
| Leptin | 0.05 | 7.0 |
| PAI-1 (serpine2) | >50 | >50 |
| Resistin | <0.04 | <0.04 |
| TGF-β1 | 1.2 | 1.2 |
| TNF-α | <0.0001 | <0.0001 |
| sVCAM (CD106) | 30 | 0.5 |
| VEGF | 3.4 | 0.2 |
BM-MSC data are unpublished internal control data of the authors using a single MSC isolate at passage 3. Analytes were measured by Luminex assay of supernatants harvested 3 days after plating when cultures were ∼80% confluent. All supernatants (MSC and ASC) were processed simultaneously. The methods, media, standards, and blanks were identical to those published for ASC35. Bolded rows show major differences.
CRP, C-reactive protein; IL, interleukin; PAI, plasminogen activator inhibitor; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
In vitro model of ASC interaction using clinical isolates
In vitro model systems can detect effects of secreted growth factors and cell adhesion-mediated effects, but lack the complexity of xenograft models. Through an in vitro coculture model utilizing heterogeneous cells isolated from clinical isolates (malignant pleural effusions), we confirmed the enhancing effect of ASC on the proliferation of breast cancer cells35 which appeared as nest of epithelioid cells among a monolayer of carboxyfluorescein succinimidyl ester (CFSE)-labeled stromal cells. Our results are in agreement with those studies showing that the presence of mesenchymal cells promotes the growth of highly proliferative tumor cells.32,50
Tumor cell heterogeneity
The epithelial component of breast cancer clinical isolates is heterogeneous. Initially, a tumor cell subset identified as CD44+/CD24-/CD326 (ESA, EpCAM)+ was shown to be enriched for tumorigenic cells as detected in a xenotransplantation model.51 In an accompanying editorial it was hypothesized that these cells represent breast cancer stem cells.52 Gradually, evidence has accrued substantiating the fact that clonogenic tumor cells share, constitutively or conditionally, many characteristics with adult tissue stem cells. Most prominently these include self-renewal53,54 and therapy resistance.36,55 As a cause or consequence of these functional similarities, adult tissue stem cells and clonogenic epithelial tumor subsets share expression of several markers. We previously demonstrated the tumorigenicity of CD90+ breast cancer cells separated on the basis of light scatter into resting (low light scatter) and active (high light scatter) populations. Small resting CD90+ cells gave rise to tumors with high efficiency (50–100 cells/injection),36,56 whereas large, active CD90+ cells were tumorigenic at high (600–13,000 cells),36 but not low56 dose. We further explored the phenotypic differences between resting and active CD90+ tumor cells, where low light scatter resting CD90+ nonheme cells were mostly quiescent in contrast to their high light scatter counterpart, which had a higher proportion of cycling/aneuploid cells.56 We have demonstrated that ASC failed to augment the tumorigenicity of small resting CD90+ tumor cells, whereas they markedly enhanced tumorigenesis mediated by active CD90+ tumor cells.35 Dormant and proliferating breast cancer cells display distinct genome-wide expression signatures, including differences for a high number of angiogenesis-related genes.57 This is consistent with the hypothesis that ASC may support survival and proliferation of tumor cells in vivo by promoting angiogenesis, to which active cells are preferentially receptive.
Discussion
Escape from tumor dormancy: A working hypothesis
Throughout this article we have drawn a distinction between two potentially tumorigenic populations, resting (dormant) and active (proliferating) tumor cells. This distinction has parallels with adult tissue stem cells versus transit amplifying populations. The notion of cancer dormancy is prevalent in the cancer literature, but ill defined. Dormant cancer is subclinical cancer, and is known because of tumor recurrence after a symptom-free interval. It is not known whether dormant tumor cells are out of cell cycle (i.e., G0), or persisting in a dynamic state of balanced proliferation and death. The same can be said for normal tissue stem cells.58 Regardless of the mechanism by which subclinical tumor persists, it is useful to hypothesize that dormancy is an intrinsic characteristic of the resting tumor cell, perhaps imposed by epigenetic programming.59 As such, transition between dormant and active states requires genetic reprogramming and not merely the presence of signals such as those provided by hormones and hormone receptors, or even the presence of mutations that bypass the need for such signaling. Whatever the stimuli that drive dormant stem-like cancer cells into an active tumorigenic state, we hypothesize that they are distinct from those that favor the survival and proliferation of active progenitor-like tumor cells (Fig. 1). Therefore, the introduction of pro-angiogenic autologous mesenchymal stem cells to the site of a tumor bed would be non-contributory to local recurrence. Indeed, if increased local vascularization at the site of a tumor bed was an independent risk factor for recurrence, this fact would have become clear after observing patients reconstructed with local tissue flaps. In this commonly accepted method of reconstruction, a well vascularized tissue flap is in direct contact with the tumor bed and extensive local vascular remodeling occurs. Immediate autologous tissue flap reconstruction has not been correlated with increased recurrence rates.113
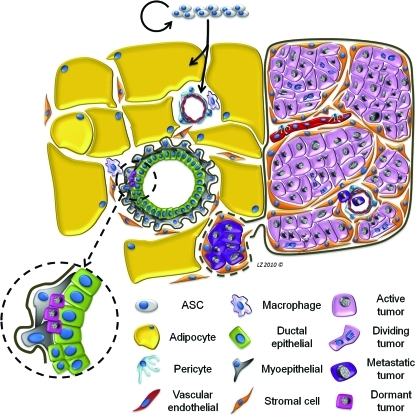
FIG. 1.
Working hypothesis of the interaction between regenerating tissue and an epithelial cancer. The effect of adipose-derived stem cell administration on active breast cancer is depicted here. Introduction of ASC, which self-replicate and give rise to adipose, vessels, and stroma, promotes the growth of active tumor (purple) and induces motility and upregulation of adhesion molecules, promoting invasion and metastasis (deep purple). Dormant cancer cells, shown here nested in a normal breast duct (hot pink, inset), are unaffected by the wound healing signals resulting from regenerative therapy. ASC, adipose-derived stem cells. Color images available online at www.liebertonline.com/ten.
Risks assessment
In the absence of substantial clinical experience with tissue-engineered reconstructive surgery after cancer, one can also look to biological parallels to estimate the risks imposed by intentionally placing regenerating tissue at the site of tumor resection. The first and most obvious parallel is the wound healing that normally accompanies cancer therapy of any kind, whether surgical, chemotherapeutic, or radio-ablative. Even before antineoplastic therapy has been initiated, the tumor microenvironment has much in common with a wound.60 The similarities have been well described61 and include the presence of inflammation, growth factors, cross-linked fibrin, fibroblast activation, angiogenesis, and the deposition of a network of extracellular matrix. Thus, it is difficult in most cases to determine the extent to which wound healing associated with treatment contributes to relapse at the primary tumor site. Cancer recurrence in mastectomy scars has been reported, but is rare.62 In colon cancer, recurrence at scar sites is also infrequent and associated with aggressive systemic disease.63 There are even case reports of high-grade tumors recurring along the tracks of laparoscopy.64–66 In stage I/II invasive breast cancer patients treated with radiation, in whom the incidence of early local recurrence is very low, high mitotic activity and high tumor grade were the major predictors of local recurrence.67 Thus, the common feature shared by cancers that are recruited into treatment-associated wounds appears to be the presence of aggressive active disease.
A second, less obvious parallel comes from experience with allogeneic and autologous hematopoietic stem cell transplantation for hematologic malignancies, where there are decades of experience in regenerative therapy for cancer.68,69 When disease is active at the time of transplantation (i.e., the patient has had multiple remissions or has active disease at the time of myeloablative therapy), relapse often occurs early in the peritransplant period when BM regeneration (and associated reestablishment of the components of the BM microenvironment) is at its peak.70 When this occurs, the recurrent disease is usually very aggressive. In contrast, patients transplanted in first remission often have extended disease-free survival. The proportion of patients who eventually relapse often do so years after transplant, indicating that (1) residual disease survived myeloablative therapy; (2) these cells were dormant; and (3) dormant residual malignant cells were not reactivated as a result of massive injury to the BM and explosive hematopoietic regeneration ediated by the graft. An analogous argument can be made for cytokine mobilization in the context of autologous hematopoietic stem cell transplantation, where patients in remission are treated with consolidation chemotherapy and the hematopoietic growth factor granulocyte colony stimulating factor (G-CSF).71 The patients experience BM suppression followed by explosive regeneration without triggering early relapse.
In the 1990s autologous hematopoietic transplantation was widely used to rescue the BM of breast cancer patients undergoing dose-intensive chemotherapy. This practice was discontinued when randomized trials failed to show superiority over conventional therapy.72 However, the massive damage to all proliferative tissues by high-dose cytotoxic therapy and the ensuing tissue regeneration (including marrow reconstitution, revascularization, and reepithelialization) did not promote breast cancer relapse.
Currently, there is relatively little clinical experience pertaining specifically to regenerative therapy in the context of epithelial cancers. Delay et al. published a series of 880 breast reconstructions with autologous fat alone performed by injection of lipoaspirate. Some of the participants included patients undergoing nipple-areola reconstruction after mastectomy. In this cohort, which had a maximal follow-up of 10 years, there was no detectable increase in the risk of local recurrence or new cancer development.4 A similar series was reported by Illouz and Sterodimas, who performed AFT on 820 patients, 381 of whom were treated for asymmetry after mastectomy and breast reconstruction.73 Although 230 patients were followed with yearly mammography and ultrasonography for an average of 11.3 years, the author made no mention of the incidence of locoregional recurrence or metastasis among the breast cancer patients. Yoshimura et al. reported on the use of SVF-augmented AFT for cosmetic breast augmentation in 40 women, none of whom experienced serious complications during a follow-up interval ranging from 6 to 42 months.8 Among the adverse effects reported after SVF-augmented breast reconstruction were calcifications and cyst formation in 4 of 40 patients. An additional cohort of 15 patients had successful breast reconstruction after experiencing complications of breast implant surgery.74 In a cohort study (mean 7.2 year follow-up) reported by Rigotti, 137 patients treated with AFT breast reconstruction did not show increased risk of local recurrence after this treatment. While this study lacked a formal control group (the recurrence rates after treatment were referenced to both historical data and pre-reconstruction recurrence rates for the cohort), the data suggest that the addition of autologous adipose cells to a tumor bed does not impact any nascent cancer cells.114 Yoshimura et al. also performed cell-augmented AFT on eight patients undergoing breast reconstruction after mastectomy,8 but the clinical status of these patients was not reported.
Taken together with our published results in a xenograft model,35 which indicate that ASC augment the growth of active but not resting breast cancer cells, the available data suggest that the critical factor determining whether regeneration augments tumor growth is the state of residual tumor: active disease is promoted, whereas dormant tumor is insensitive. This suggests that reconstructive therapy utilizing ASC-augmented whole fat should be deferred until cancer remission has been firmly established.
Acknowledgments
This work was supported by grants BC032981 and BC044784 from the Department of Defense, grants R01CA114246 (JPR) and NO1 HB 37165 from the NIH, the Hillman Foundation, the Glimmer of Hope Foundation. and the Commonwealth of Pennsylvania, through the McGowan Institute of Regenerative Medicine. Vera S. Donnenberg is a CDMRP Era of Hope Scholar.
Authors Contributions
Vera S. Donnenberg and Albert D. Donnenberg and Ludovic Zimmerlin, graduate student in the Donnenberg laboratory, contributed equally to this article. J. Peter Rubin provided clinical perspective and expertise in AFT.
Disclosure Statement
None of the authors have competing interests that would have influenced the preparation of this article.
References
- 1.Neuber G.A. Fettransplantation. Chir Kongr Verhandl Deutsche Gesellschaft für Chirurgie. 1893;22:66. [Google Scholar]
- 2.Czerny V. Plastischer Ersatz der Brustdruse durch ein Lipom. Chir Kong Verhandl. 1895;2:216. [Google Scholar]
- 3.ASPRS. Report on autologous fat transplantation. ASPRS Ad-Hoc Committee on New Procedures. Plast Surg Nurs. 1987 1987 Sep 30;7:140. [PubMed] [Google Scholar]
- 4.Delay E. Garson S. Tousson G. Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29:360. doi: 10.1016/j.asj.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Niechajev I. Sevcuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg. 1994;94:496. doi: 10.1097/00006534-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Peer L.A. Loss of weight and volume in human fat grafts: with postulation of a “Cell Survival Theory”. Plast Reconstr Surg. 1950;5:217. [Google Scholar]
- 7.Yoshimura K. Sato K. Aoi N. Kurita M. Inoue K. Suga H., et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura K. Sato K. Aoi N. Kurita M. Hirohi T. Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthet Plast Surg. 2008;32:6–7. doi: 10.1007/s00266-007-9019-4. 48; discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto D. Sato K. Gonda K. Takaki Y. Shigeura T. Sato T., et al. Cell- assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 10.Calabria R. Hills B. Fat grafting: fact or fiction? Aesthet Surg J. 2005;25:55. doi: 10.1016/j.asj.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Billings E., Jr. May J.W., Jr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83:368. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen A. Pasyk K.A. Bouvier T.N. Hassett C.A. Argenta L.C. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:87–89. 378; discussion. [PubMed] [Google Scholar]
- 13.Moseley T.A. Zhu M. Hedrick M.H. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118:121S. doi: 10.1097/01.prs.0000234609.74811.2e. [DOI] [PubMed] [Google Scholar]
- 14.Zhu M. Zhou Z. Chen Y. Schreiber R. Ransom J.T. Fraser J.K., et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 15.Miranville A. Heeschen C. Sengenes C. Curat C.A. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 16.Planat-Benard V. Silvestre J.S. Cousin B. Andre M. Nibbelink M. Tamarat R., et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 17.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerlin L. Donnenberg V.S. Pfeifer M.E. Meyer E.M. Peault B. Rubin J.P., et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77A:22. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronthos S. Franklin D.M. Leddy H.A. Robey P.G. Storms R.W. Gimble J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 20.Strem B.M. Hicok K.C. Zhu M. Wulur I. Alfonso Z. Schreiber R.E., et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 21.Daher S.R. Johnstone B.H. Phinney D.G. March K.L. Adipose stromal/stem cells: basic and translational advances: the IFATS collection. Stem Cells. 2008;26:2664. doi: 10.1634/stemcells.2008-0927. [DOI] [PubMed] [Google Scholar]
- 22.Rubin J.P. Bennett J.M. Doctor J.S. Tebbets B.M. Marra K.G. Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plast Reconstr Surg. 2007;120:414. doi: 10.1097/01.prs.0000267699.99369.a8. [DOI] [PubMed] [Google Scholar]
- 23.Stacey D.H. Hanson S.E. Lahvis G. Gutowski K.A. Masters K.S. In vitro adipogenic differentiation of preadipocytes varies with differentiation stimulus, culture dimensionality, and scaffold composition. Tissue Eng Part A. 2009;15:3389. doi: 10.1089/ten.TEA.2008.0293. [DOI] [PubMed] [Google Scholar]
- 24.Leong D.T. Nah W.K. Gupta A. Hutmacher D.W. Woodruff M.A. The osteogenic differentiation of adipose tissue-derived precursor cells in a 3D scaffold/matrix environment. Curr Drug Discov Technol. 2008;5:319. doi: 10.2174/157016308786733537. [DOI] [PubMed] [Google Scholar]
- 25.Hodde J.P. Record R.D. Liang H.A. Badylak S.F. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y. Liu T. Ye H. Song K. Ma X. Cui Z. Enhancement of adipose-derived stem cell differentiation in scaffolds with IGF-I gene impregnation under dynamic microenvironment. Stem Cells Dev. 2010. Sep 6, [Epub ahead of print]. [DOI] [PubMed]
- 27.Ahmed T.A. Dare E.V. Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev. 2008. May 1, [Epub ahead of print]. [DOI] [PubMed]
- 28.Ota T. Gilbert T.W. Schwartzman D. McTiernan C.F. Kitajima T. Ito Y., et al. A fusion protein of hepatocyte growth factor enhances reconstruction of myocardium in a cardiac patch derived from porcine urinary bladder matrix. J Thorac Cardiovasc Surg. 2008;136:1309. doi: 10.1016/j.jtcvs.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnoub A.E. Dash A.B. Vo A.P. Sullivan A. Brooks M.W. Bell G.W., et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y. Yao F. Yao X. Yi C. Tan C. Wei L., et al. Role of CCL5 in invasion, proliferation and proportion of CD44+/CD24- phenotype of MCF-7 cells and correlation of CCL5 and CCR5 expression with breast cancer progression. Oncol Rep. 2009;21:1113. [PubMed] [Google Scholar]
- 31.Martin F.T. Dwyer R.M. Kelly J. Khan S. Murphy J.M. Curran C., et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT) Breast Cancer Res Treat. 2010. Jan 20, [Epub ahead of print]. [DOI] [PubMed]
- 32.Pinilla S. Alt E. Abdul Khalek F.J. Jotzu C. Muehlberg F. Beckmann C., et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284:80. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Yu J.M. Jun E.S. Bae Y.C. Jung J.S. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 34.Muehlberg F.L. Song Y.H. Krohn A. Pinilla S.P. Droll L.H. Leng X., et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerlin L. Donnenberg A.D. Rubin J.P. Basse P. Landreneau R.J. Donnenberg V.S. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng. 2010. Jul 30, [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 36.Donnenberg V.S. Luketich J.D. Landreneau R.J. DeLoia J.A. Basse P. Donnenberg A.D. Tumorigenic epithelial stem cells and their normal counterparts. Ernst Schering Found Symp Proc. 2006;5:245. doi: 10.1007/2789_2007_054. [DOI] [PubMed] [Google Scholar]
- 37.Tasso R. Augello A. Carida M. Postiglione F. Tibiletti M.G. Bernasconi B., et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30:150. doi: 10.1093/carcin/bgn234. [DOI] [PubMed] [Google Scholar]
- 38.Sun B. Roh K.H. Park J.R. Lee S.R. Park S.B. Jung J.W., et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289. doi: 10.1080/14653240902807026. 1 p following 298. [DOI] [PubMed] [Google Scholar]
- 39.Cousin B. Ravet E. Poglio S. De Toni F. Bertuzzi M. Lulka H., et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS ONE. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khakoo A.Y. Pati S. Anderson S.A. Reid W. Elshal M.F. Rovira I.I., et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiao L. Xu Z. Zhao T. Zhao Z. Shi M. Zhao R.C., et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 42.Chanda D. Isayeva T. Kumar S. Hensel J.A. Sawant A. Ramaswamy G., et al. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in prostate cancer bone metastasis. Clin Cancer Res. 2009;15:7175. doi: 10.1158/1078-0432.CCR-09-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu Y.R. Yuan Y. Wang X.J. Wei L.L. Chen Y.N. Cong C., et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245. doi: 10.4161/cbt.7.2.5296. [DOI] [PubMed] [Google Scholar]
- 44.Kang S.G. Jeun S.S. Lim J.Y. Kim S.M. Yang Y.S. Oh W.I., et al. Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Childs Nerv Syst. 2008;24:293. doi: 10.1007/s00381-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 45.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 46.Wexler S.A. Donaldson C. Denning-Kendall P. Rice C. Bradley B. Hows J.M. Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 47.Brewster A.M. Hortobagyi G.N. Broglio K.R. Kau S.W. Santa-Maria C.A. Arun B., et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rene Gonzalez R. Watters A. Xu Y. Singh U. Mann D. Rueda B., et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldo A. Sniderman A.D. St. Luce S. Avramoglu R.K. Maslowska M. Hoang B., et al. The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J Clin Invest. 1993;92:1543. doi: 10.1172/JCI116733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kucerova L. Matuskova M. Hlubinova K. Altanerova V. Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9:129. doi: 10.1186/1476-4598-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Hajj M. Wicha M.S. Benito-Hernandez A. Morrison S.J. Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dick J.E. Breast cancer stem cells revealed. Proc Natl Acad Sci U S A. 2003;100:3547. doi: 10.1073/pnas.0830967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abraham B.K. Fritz P. McClellan M. Hauptvogel P. Athelogou M. Brauch H. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154. [PubMed] [Google Scholar]
- 54.Sheridan C. Kishimoto H. Fuchs R.K. Mehrotra S. Bhat-Nakshatri P. Turner C.H., et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kakarala M. Wicha M.S. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnenberg V.S. Donnenberg A.D. Zimmerlin L. Landreneau R.J. Bhargava R. Wetzel R.A., et al. Localization of CD44, CD90 positive cells to the invasive front of breast tumors. Cytometry B Clin Cytom. 2010. Apr 30, [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 57.Almog N. Ma L. Raychowdhury R. Schwager C. Erber R. Short S., et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 58.Quesenberry P.J. Dooner M.S. Aliotta J.M. Stem cell plasticity revisited: the continuum marrow model and phenotypic changes mediated by microvesicles. Exp Hematol. 2010;38:581. doi: 10.1016/j.exphem.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones P.A. Laird P.W. Cancer-epigenetics comes of age. Nat Genet. 1999;21:163. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 60.Dvorak H.F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 61.Hofer S.O. Molema G. Hermens R.A. Wanebo H.J. Reichner J.S. Hoekstra H.J. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999;25:231. doi: 10.1053/ejso.1998.0634. [DOI] [PubMed] [Google Scholar]
- 62.Warner R.M. Wallace D.L. Ferran N.A. Erel E. Park A.J. Prinsloo D.J., et al. Mastectomy scars following breast reconstruction: should routine histologic analysis be performed? Plast Reconstr Surg. 2009;123:1141. doi: 10.1097/PRS.0b013e31819f25d5. [DOI] [PubMed] [Google Scholar]
- 63.Hughes E. McDermott F. Polglase A. Johnson W. Tumor recurrence in the abdominal wall scar tissue after large-bowel cancer surgery. Dis Colon Rectum. 1983;26:571. doi: 10.1007/BF02552962. [DOI] [PubMed] [Google Scholar]
- 64.Winston C.B. Chen J.W. Fong Y. Schwartz L.H. Panicek D.M. Recurrent gallbladder carcinoma along laparoscopic cholecystectomy port tracks: CT demonstration. Radiology. 1999;212:439. doi: 10.1148/radiology.212.2.r99au17439. [DOI] [PubMed] [Google Scholar]
- 65.Faught W. Fung Kee Fung M. Port site recurrences following laparoscopically managed early stage endometrial cancer. Int J Gynecol Cancer. 1999;9:256. doi: 10.1046/j.1525-1438.1999.09912.x. [DOI] [PubMed] [Google Scholar]
- 66.Agostini A. Cohen D. Cravello L. Bretelle F. Roger V. Blanc B. Port-site recurrence following laparoscopic para-aortic lymphadenectomy for squamous carcinoma of the cervix. Eur J Obstet Gynecol Reprod Biol. 2001;98:258. doi: 10.1016/s0301-2115(01)00335-9. [DOI] [PubMed] [Google Scholar]
- 67.Schnitt S.J. Connolly J.L. Harris J.R. Hellman S. Cohen R.B. Pathologic predictors of early local recurrence in stage I and II breast cancer treated by primary radiation therapy. Cancer. 1984;53:1049. doi: 10.1002/1097-0142(19840301)53:5<1049::aid-cncr2820530506>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 68.Thomas E.D. Lochte H.L., Jr. Cannon J.H. Sahler O.D. Ferrebee J.W. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709. doi: 10.1172/JCI103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santos G.W. Sensenbrenner L.L. Burke P.J. Colvin M. Owens A.H., Jr. Bias W.B., et al. Marrow transplantation in man following cyclophosphamide. Transplant Proc. 1971;3:400. [PubMed] [Google Scholar]
- 70.Pasquini M.C. Wang Z. Current use and outcome of hematopoietic stem cell transplantation. CIBMTR Summary Slides. 2010. www.cibmtr.org www.cibmtr.org
- 71.Heimfeld S. Fogarty B. McGuire K. Williams S. Berenson R.J. Peripheral blood stem cell mobilization after stem cell factor or G-CSF treatment: rapid enrichment for stem and progenitor cells using the CEPRATE immunoaffinity separation system. Transplant Proc. 1992;24:2818. [PubMed] [Google Scholar]
- 72.Stadtmauer E.A. O'Neill A. Goldstein L.J. Crilley P.A. Mangan K.F. Ingle J.N., et al. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. N Engl J Med. 2000;342:1069. doi: 10.1056/NEJM200004133421501. [DOI] [PubMed] [Google Scholar]
- 73.Illouz Y.G. Sterodimas A. Autologous fat transplantation to the breast: a personal technique with 25 years of experience. Aesthet Plast Surg. 2009;33:706. doi: 10.1007/s00266-009-9377-1. [DOI] [PubMed] [Google Scholar]
- 74.Yoshimura K. Asano Y. Aoi N. Kurita M. Oshima Y. Sato K., et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169. doi: 10.1111/j.1524-4741.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 75.Simmons P.J. Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55. [PubMed] [Google Scholar]
- 76.Sacchetti B. Funari A. Michienzi S. Di Cesare S. Piersanti S. Saggio I., et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 77.Benayahu D. Akavia U.D. Shur I. Differentiation of bone marrow stroma-derived mesenchymal cells. Curr Med Chem. 2007;14:173. doi: 10.2174/092986707779313363. [DOI] [PubMed] [Google Scholar]
- 78.Park P.C. Selvarajah S. Bayani J. Zielenska M. Squire J.A. Stem cell enrichment approaches. Semin Cancer Biol. 2007;17:257. doi: 10.1016/j.semcancer.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Quirici N. Soligo D. Bossolasco P. Servida F. Lumini C. Deliliers G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 80.Delorme B. Ringe J. Gallay N. Le Vern Y. Kerboeuf D. Jorgensen C., et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 81.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 82.Vogel W. Grunebach F. Messam C.A. Kanz L. Brugger W. Buhring H.J. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88:126. [PubMed] [Google Scholar]
- 83.Wagner W. Wein F. Seckinger A. Frankhauser M. Wirkner U. Krause U., et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Lee R.H. Kim B. Choi I. Kim H. Choi H. Suh K., et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 85.Roche S. Delorme B. Oostendorp R.A. Barbet R. Caton D. Noel D., et al. Comparative proteomic analysis of human mesenchymal and embryonic stem cells: towards the definition of a mesenchymal stem cell proteomic signature. Proteomics. 2009;9:223. doi: 10.1002/pmic.200800035. [DOI] [PubMed] [Google Scholar]
- 86.Noel D. Caton D. Roche S. Bony C. Lehmann S. Casteilla L., et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell J.B. McIntosh K. Zvonic S. Garrett S. Floyd Z.E. Kloster A., et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 89.Traktuev D.O. Merfeld-Clauss S. Li J. Kolonin M. Arap W. Pasqualini R., et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 90.Ishimura D. Yamamoto N. Tajima K. Ohno A. Yamamoto Y. Washimi O., et al. Differentiation of adipose-derived stromal vascular fraction culture cells into chondrocytes using the method of cell sorting with a mesenchymal stem cell marker. Tohoku J Exp Med. 2008;216:149. doi: 10.1620/tjem.216.149. [DOI] [PubMed] [Google Scholar]
- 91.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 92.Delorme B. Ringe J. Pontikoglou C. Gaillard J. Langonne A. Sensebe L., et al. Specific lineage-priming of bone marrow mesenchymal stem cells provides the molecular framework for their plasticity. Stem Cells. 2009;27:1142. doi: 10.1002/stem.34. [DOI] [PubMed] [Google Scholar]
- 93.Harris L.J. Abdollahi H. Zhang P. McIlhenny S. Tulenko T.N. Dimuzio P.J. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res. 2009. Sep 4, [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 94.Kim Y.M. Jeon E.S. Kim M.R. Jho S.K. Ryu S.W. Kim J.H. Angiotensin II-induced differentiation of adipose tissue-derived mesenchymal stem cells to smooth muscle-like cells. Int J Biochem Cell Biol. 2008;40:2482. doi: 10.1016/j.biocel.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 95.Dezawa M. Ishikawa H. Itokazu Y. Yoshihara T. Hoshino M. Takeda S., et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 96.Toma C. Pittenger M.F. Cahill K.S. Byrne B.J. Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 97.Planat-Benard V. Menard C. Andre M. Puceat M. Perez A. Garcia-Verdugo J.M., et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 98.Oswald J. Boxberger S. Jorgensen B. Feldmann S. Ehninger G. Bornhauser M., et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 99.Woodbury D. Schwarz E.J. Prockop D.J. Black I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 100.Safford K.M. Hicok K.C. Safford S.D. Halvorsen Y.D. Wilkison W.O. Gimble J.M., et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 101.Lee K.D. Kuo T.K. Whang-Peng J. Chung Y.F. Lin C.T. Chou S.H., et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 102.Seo M.J. Suh S.Y. Bae Y.C. Jung J.S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 103.Spees J.L. Olson S.D. Ylostalo J. Lynch P.J. Smith J. Perry A., et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brzoska M. Geiger H. Gauer S. Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330:142. doi: 10.1016/j.bbrc.2005.02.141. [DOI] [PubMed] [Google Scholar]
- 105.Banfi A. Bianchi G. Notaro R. Luzzatto L. Cancedda R. Quarto R. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng. 2002;8:901. doi: 10.1089/107632702320934001. [DOI] [PubMed] [Google Scholar]
- 106.Simonsen J.L. Rosada C. Serakinci N. Justesen J. Stenderup K. Rattan S.I., et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 107.Zimmermann S. Voss M. Kaiser S. Kapp U. Waller C.F. Martens U.M. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003;17:1146. doi: 10.1038/sj.leu.2402962. [DOI] [PubMed] [Google Scholar]
- 108.Katz A.J. Tholpady A. Tholpady S.S. Shang H. Ogle R.C. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 109.Lin G. Garcia M. Ning H. Banie L. Guo Y.L. Lue T.F., et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009;12:105. doi: 10.1089/rej.2009.0830. [DOI] [PubMed] [Google Scholar]
- 111.Noer A. Boquest A.C. Collas P. Dynamics of adipogenic promoter DNA methylation during clonal culture of human adipose stem cells to senescence. BMC Cell Biol. 2007;8:18. doi: 10.1186/1471-2121-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S., et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 113.Min S.Y. Kim H.Y. Jung S.Y. Kwon Y. Shin K.H. Lee S. Kim S.W. Kang H.S. Yun Y.H. Lee E.S. Oncological safety and quality of life associated with mastectomy and immediate breast reconstruction with a latissimus dorsi myocutaneous flap. Breast J. 2010;16:356. doi: 10.1111/j.1524-4741.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- 114.Rigotti G. Marchi A. Stringhini P. Baroni G. Galiè M. Molino A.M. Mercanti A. Micciolo R. Sbarbati A. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg. 2010;34:475. doi: 10.1007/s00266-010-9481-2. [DOI] [PubMed] [Google Scholar]