Abstract
Medroxyprogesterone acetate (MPA) is widely known for its use in combination hormone therapy for postmenopausal women. However, MPA is also commonly used in young women for contraception and treatment of a number of gynecological conditions. Despite its widespread use, the cardiovascular effects of MPA in young women are unclear. Therefore, the purpose of this study was to determine the acute effects of MPA when used in combination with estradiol on markers of cardiovascular risk in young women. We suppressed endogenous estrogens and progesterone in 10 premenopausal women using a gonadotropin-releasing hormone antagonist (GnRHa) for 10 days. On day 4 of GnRHa subjects received 0.1 mg of estradiol (GnRHa+E2), and on day 7 5 mg of MPA was added (GnRHa+E2+MPA). Endothelium-dependent vasodilation and endothelium-independent vasodilation of the brachial artery, lipids, homocysteine, high-sensitivity C-reactive protein, and endothelin-1 were assessed during treatment with GnRHa, GnRHa+E2, and GnRHa+E2+MPA. Four additional subjects were tested to validate the efficacy of the GnRHa model and confirm the findings. Endothelium-dependent vasodilation was greater during GnRHa+E2 than during GnRHa or GnRHa+E2+MPA (P = 0.006). Endothelin-1 was lower during GnRHa+E2 than GnRHa alone (P = 0.039). Endothelin-1 increased with the addition of MPA and was not significantly different from GnRHa alone. There were no differences in the other markers of cardiovascular risk between hormone treatment days. These data suggest that acute MPA administration negates the beneficial effects of estradiol on endothelium-dependent vasodilation in young women. In addition, these data suggest that estradiol decreases endothelin-1 concentrations and the addition of MPA may counteract the effect of estradiol on endothelin-1.
Keywords: endothelium, hormones, flow-mediated vasodilation, endothelin-1, progestins
There is substantial evidence to suggest that estrogens have a cardioprotective influence in women by improving endothelial function (11, 14, 19, 24) and low-density lipoprotein (LDL) concentrations (45) while decreasing concentrations of endothelin-1 (ET-1) (4, 26, 31, 32, 56) and homocysteine (9). In contrast, medroxyprogesterone acetate (MPA) has been shown to counteract the beneficial effects of estrogens in animals (1, 52) and in some studies in postmenopausal women (18, 44, 49). Recently, the estrogen plus MPA arm of the Women’s Health Initiative clinical trial was terminated because of a trend toward negative cardiovascular outcomes (37). These findings raise questions about the use of progestins, and specifically MPA, in hormone treatments.
In addition to postmenopausal women, premenopausal women are also commonly prescribed MPA. MPA is used in the injectable progestin-only contraceptive Depo-Provera, which is a popular contraceptive choice, particularly for younger premenopausal women because of the ease of use and high compliance. Oral MPA hormone treatments are also used to treat a number of gynecological conditions in young women, such as endometriosis, polycystic ovarian syndrome, and irregular uterine bleeding (7). Despite numerous reports that MPA may impair the benefits of estradiol in postmenopausal women (18, 49), MPA continues to be used regularly in conjunction with estrogens to treat young women for many of the common disorders listed above. However, the effect of clinically relevant doses of MPA on cardiovascular indexes in young women has not been evaluated. Thus it remains unknown whether MPA antagonizes the beneficial cardiovascular effects of estrogen in reproductive-age women.
Therefore, the aim of our study was to investigate whether MPA antagonizes the favorable effects of exogenous estradiol on vascular function and biomarkers of cardiovascular risk by measuring endothelium-dependent vasodilation of the brachial artery, serum lipid concentrations, homocysteine, high-sensitivity C-reactive protein (hs-CRP), and ET-1 in young women after acute estradiol and combination estradiol and MPA treatment. We hypothesized that short-term estradiol treatment would increase endothelium-dependent vasodilation, improve lipid panel variables, and decrease homocysteine, hs-CRP, and ET-1 concentrations. We further hypothesized that the addition of MPA to estradiol treatment would negate the improvements in these markers of cardiovascular risk in young women.
METHODS
Fourteen female subjects between the ages of 19 and 27 yr participated in this study. All subjects were healthy nonsmokers and were not taking any medications, with the exception of oral or vaginal combined contraceptives, which were discontinued for at least 72 h before beginning study medications. Subjects were screened to ensure that they did not have any of the following health conditions: cardiovascular disease, hypertension, hypercholesterolemia, metabolic disorders, menstrual disorders, and a personal or family history of blood clots. Subjects underwent medical screening by a physician specializing in obstetrics and gynecology to ensure that they had no contra-indications to using a gonadotropin-releasing hormone antagonist (GnRHa) and/or hormone treatments (described below). Approval of this protocol was granted by the Institutional Review Board of the University of Oregon. Each subject provided oral and written consent before participation. Subjects were required to take a pregnancy test and show negative results immediately before the start of each study day. Subjects abstained from exercise, vitamins, alcohol, and over-the-counter medications for 24 h and abstained from food and caffeine for 12 h before participating. All studies were conducted at the same time of day for each subject.
Gonadotropin-releasing hormone antagonist
Endogenous female sex hormones were suppressed for the duration of the study in each subject via a daily 250 μg/0.5 ml gonadotropin-releasing hormone antagonist (GnRHa) subcutaneous injection (Antagon, Organon International, Roseland, NJ) for 10 days beginning with the onset of menses. GnRHas are synthetic decapeptides that compete with natural gonadotropin-releasing hormone by competitively binding to its receptor, thereby suppressing the production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). This action subsequently prevents production of estrogen and progesterone, and within 36–48 h of the onset of treatment endogenous estrogen and progesterone are fully suppressed (29). Thus use of the gonadotropin-releasing hormone suppression and hormone add-back model allows for the study of exogenous hormones in premenopausal women without the competing influence of endogenous sex hormones. All 14 subjects completed the 10-day GnRHa treatment. During this treatment, the subjects were randomly assigned to either the main treatment group or one of the two follow-up groups (described below).
Primary experimental design
Ten subjects participated in group 1, which was our primary experimental group. Subjects in group 1 were studied in the morning on day 4 of GnRHa treatment. After completion of the first study, subjects in group 1 began estradiol supplementation via a 0.1-mg-release 17β-estradiol (E2) transdermal patch and returned to the laboratory early in the morning on day 7 of GnRHa administration and estradiol (GnRHa+E2). After completion of the second study, subjects in group 1 added 5 mg/day of oral MPA to the estradiol supplementation and returned to the laboratory early in the morning on day 10 of GnRHa administration, estradiol, and MPA (GnRHa+E2+MPA).
This design allowed subjects to be studied during three distinct hormone profiles: once in a low-hormone state, after 72 h of GnRHa treatment only (GnRHa); once in an estradiol-only state, after 72 h of exogenous estradiol add-back (GnRHa+E2); and once in an estradiol and MPA hormone state, after 72 h of combination estradiol and MPA add-back (GnRHa+E2+MPA). This design permitted within-subject comparisons of estradiol and MPA effects on acute changes in biomarkers of cardiovascular health in young women.
Follow-up experimental design
To verify that the results from our primary protocol were attributable to the specific sex hormone treatments and not the GnRHa suppression model, we completed studies in a limited number of subjects assigned to one of two follow-up groups. Four young women completed the 10-day GnRHa treatment (used in the primary experimental design) paired with estradiol add-back only (group 2; n = 2) or no hormone add-back (group 3; n = 2).
Subjects in group 2 were studied in the morning on day 4 of GnRHa treatment. After completion of the first study, subjects in group 2 were studied on two separate study days after beginning estradiol supplementation via a 0.1-mg-release E2 transdermal patch in addition to GnRHa treatment. Group 2 participants returned to the laboratory once during the early morning on day 7 of GnRHa and transdermal estradiol administration [GnRHa+E2(I)] and again on day 10 of GnRHa and transdermal estradiol administration [GnRHa+E2(II)].
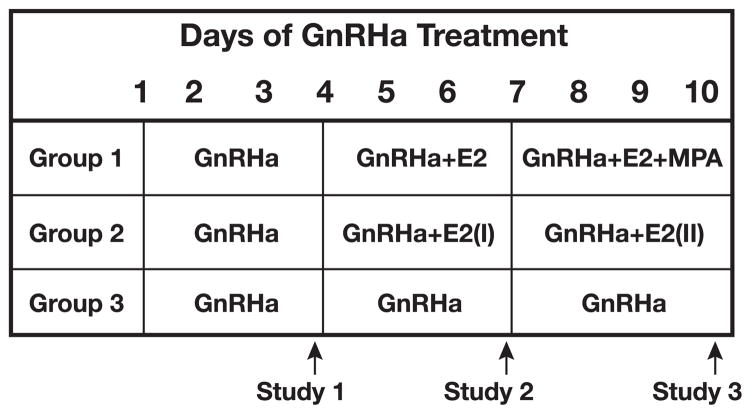
Group 3 received no hormone add-back during the 10-day GnRHa treatment and were studied on day 4 (GnRHa-D4), day 7 (GnRHa-D7), and day 10 (GnRHa-D10) of GnRHa treatment in order to rule out a time effect and to serve as a control for our hormone add-back groups 1 and 2. Figure 1 provides a schematic diagram of the experimental design used in the groups.
Fig. 1.
Schematic diagram of the experimental design used in the primary protocol with group 1 (n = 10) and in the follow-up protocols with group 2 (n = 2) and group 3 (n = 2). GnRHa, gonadotropin-releasing hormone antagonist; E2, 17β-estradiol; MPA, medroxyprogesterone acetate.
Protocol
Subjects were instrumented with electrocardiogram (ECG) and three cuffs: one blood pressure cuff on the ring finger of the left hand, one blood pressure cuff on the left upper arm, and one occlusion cuff on the right forearm just below the antecubital fossa. Subjects were positioned supine with their right arm supported at heart level at an 80–90° angle from their torso. Subjects rested for 10–15 min in this position before the collection of venous blood samples. Subjects continued to rest in the supine position for 45–60 min before endothelial function testing was completed with techniques previously described by Celemajer et al. (5) and summarized below.
Endothelium-dependent vasodilation
After a clear image of the brachial artery was obtained with the ultrasound transducer held in place above the subject’s upper arm with a stereotactic clamp, 2 min of baseline data was recorded before rapid inflation (E20 Rapid Cuff Inflater, D. E. Hokanson, Bellevue, WA) of the occlusion cuff (Zimmer, Dover, OH) on the subject’s forearm to 300 mmHg and holding this pressure constant for 5 min. On release of the occlusion cuff, arterial blood flow through the brachial artery increases and imposes a shear stress on the vascular endothelium, resulting in vasodilation. This vasodilation has been termed flow-mediated vasodilation (FMD) and is primarily dependent on the release of the potent vasodilator nitric oxide from endothelial cells (17). Data collection continued for 10 min after cuff release. Subjects then rested in the supine position for 20 min before the second trial of flow-mediated, endothelium-dependent vasodilation.
Endothelium-independent vasodilation
After completing the endothelium-dependent, flow-mediated vasodilation testing, subjects completed a second 20-min supine rest period. Subsequently, 2 min of baseline data was collected for the endothelium-independent vasodilation test, followed by the administration of 0.04 mg of sublingual nitroglycerin and 10 min of data recording.
Measurement techniques
Venous blood samples were collected on each study day from an antecubital vein for analysis of estrogen, progesterone, LH, FSH, homocysteine, ET-1, hs-CRP, and a complete lipid panel. The lipid panel analysis consisted of the following variables: LDL, high-density lipoproteins (HDL), total cholesterol (TC), triglycerides (TRG), and the total cholesterol-to-HDL ratio (TC/HDL). Blood samples were collected into the appropriate collection tubes (BD Vacutainer, Franklin, NJ) indicated for each test. The samples were centrifuged at 1,300 g relative centrifugal force for 15 min at 4°C, separated, and stored frozen at −70°C within 30 min until being transported for analysis to Oregon Medical Laboratories (Eugene, OR) or the University of Minnesota Central Laboratory (for ET-1; Minneapolis, MN).
Heart rate and blood pressure were measured continuously throughout the protocol. Heart rate was measured with a five-lead ECG dually interfaced with our data acquisition computer and a Doppler ultrasound system. Blood pressure was measured with a finger blood pressure cuff (Portapres model-2, Amsterdam, The Netherlands) and was corrected against arm blood pressure measured noninvasively from the left arm via automated brachial auscultation (CardioCap, Datex-Ohmeda, Louisville, CO).
Brachial artery diameter and blood velocity were assessed by imaging the brachial artery with a Doppler ultrasound machine (Acuson 128XP) with a 7.0-MHz linear array transducer. The transducer was placed ~3–10 cm proximal to the antecubital fossa. Within this range, we selected the area with the greatest clarity of the near and far intimal-medial borders of the arterial wall and fixed the transducer in place over the subject’s right arm with a stereotactic clamp. Ultrasound parameters were set to optimize longitudinal, B-mode images of the lumen-arterial wall interface while insonating the lumen of the artery at an angle of 60° to determine blood velocity.
Data analysis
Heart rate and blood pressure signals were digitized and stored on a computer at 20 Hz and saved for later analysis with signal processing software (WinDaq, DataQ Instruments, Akron, OH).
Brachial artery diameter and blood velocity were recorded to a computer interfaced with custom-designed edge detection and wall-tracking analysis software (DICOM; Perth, Australia). The custom analysis software allows real-time video images of the brachial artery to be captured from the ultrasound machine, encoded, and stored at 30 frames/s for later analysis of vessel diameter in synchrony with end diastole (53). Endothelium-dependent, flow-mediated vasodilation (FMD) was calculated as the percent change in brachial artery diameter from baseline to after cuff release {endothelium-dependent FMD − [peak diameter (mm) − baseline diameter (mm)]/baseline diameter (mm) × 100}. Likewise, endothelium-independent, nitroglycerin-mediated vasodilation was calculated as the percent change in brachial artery diameter from baseline to after nitroglycerin administration {endothelium-independent, nitroglycerin-mediated vasodilation − [peak diameter (mm) × baseline diameter (mm)]/baseline diameter (mm) × 100}. This software allows for more accurate and reproducible analysis measurements by decreasing observer error and eliminating observer bias. Through these means, this software can detect a 1.5–2.0% change in endothelium-dependent vasodilation in 6–8 subjects with a power of 80% and in 7–11 subjects with a power of 90%, substantially decreasing the number of subjects needed to detect significant differences compared with other methods of analysis (53).
To assess the intensity of the FMD stimulus, shear rate was calculated by dividing blood velocity (cm/s) by diameter (mm) (33, 35). Because peak vasodilation may not occur until ≥60 s after occlusion cuff release (3, 28), we calculated shear rate for 90 s after cuff release during the endothelium-dependent, flow-mediated vasodilation test. From these data, we plotted shear rate vs. time and determined the 90-s shear rate area under the curve (90-s SR AUC) (33, 35). We also determined the time to peak (TTP) diameter during the endothelium-dependent vasodilation test, plotted shear rate vs. time, and determined the TTP shear rate area under the curve (TTP SR AUC) (34). Finally, we normalized the data to shear rate (%FMD/90-s SR AUC and %FMD/TTP SR AUC) (34). We observed no statistical differences when evaluating the 90-s SR AUC and TTP SR AUC or when evaluating absolute and normalized endothelium-dependent vasodilation in this study. However, we have reported the data in multiple forms in Table 3 for comparison.
Table 3A.
Endothelial function values in group 1
| GnRHa | GnRHa+E2 | GnRHa+E2+MPA | P Value | |
|---|---|---|---|---|
| Baseline FMD diameter, mm | 3.15±0.12 | 3.10±0.10 | 3.14±0.10 | 0.410 |
| Shear rate 90-s AUC, velocity/diameter | 9,897±715 | 10,043±1,003 | 10,216±1,110 | 0.937 |
| Shear rate TTP AUC, velocity/diameter | 6,806±671 | 6,975±651 | 6,874±740 | 0.847 |
| Normalized response, % FMD/shear rate 90-s AUC | 0.0006±0.0001 | 0.0010±0.0001* | 0.0006±0.0001 | 0.001 |
| Normalized response, % FMD/shear rate TTP AUC | 0.0009±0.0001 | 0.0014±0.0002* | 0.0009±0.0001 | 0.001 |
| Baseline NTG diameter, mm | 3.15±0.12 | 3.11±0.11 | 3.15±0.10 | 0.598 |
| NTG, % change | 15.34±1.40 | 15.62±1.55 | 15.68±0.88 | 0.926 |
Values are means ± SE; n = 10 subjects. FMD, flow-mediated, endothelium-dependent vasodilation; NTG, nitroglycerin-mediated, endothelium-independent vasodilation; AUC, area under the curve; TTP, time to peak.
Significant difference, GnRHa+E2 vs. GnRHa and GnRHa+E2+MPA.
Reproducibility
The intraobserver variability of brachial artery diameter measurements was assessed by comparing baseline diameter measurements from endothelium-dependent and endothelium-independent dilation for every subject on all study days. The coefficient of variation (SD/mean × 100) was 1.2% for this study. We waited 20 min after completion of the first flow-mediated, endothelium-dependent vasodilation before repeating the test to determine reproducibility. The average SE between the first and second flow-mediated, endothelium-dependent vasodilation tests was ±0.2342.
Statistical analysis
Subject characteristics were compared between groups with one-way ANOVAs. Within-group comparisons between hormone treatments were made with one-way repeated-measures ANOVAs. When significance was achieved with ANOVAs, the Holm-Sidak post hoc test was used to locate the differences. Statistical significance was defined as P < 0.05. All data are expressed as means ± SE.
RESULTS
Subject characteristics and baseline hemodynamics
Subjects were all young, healthy women and were randomly assigned to groups; thus there were no differences in age (22 ± 1 yr) height (165 ± 3 cm), weight (60 ± 6 kg), or body mass index (22 ± 3). Tables 1A, 1B, and 1C display baseline measurements across hormone treatments in groups 1, 2, and 3, respectively. In group 1, estradiol was significantly higher during treatment with GnRHa+E2 and GnRHa+E2+MPA than during GnRHa treatment alone (P < 0.001) and FSH was greater during GnRHa treatment alone than during GnRHa+E2 or GnRHa+E2+MPA treatment (P < 0.001), consistent with estrogen receptor-mediated downregulation of FSH. Tables 1B and 1C also provide baseline measures for groups 2 and 3, respectively, but statistical comparisons were not made. Within group 2, estradiol was low during treatment with GnRHa alone (<20.0 ± 0 pg/ml) and increased to higher values as predicted during GnRHa+E2(I) (198.0 ± 122 pg/ml) and GnRHa+E2(II) (276.0 ± 88 pg/ml). There were no differences in baseline progesterone, LH, systolic blood pressure, diastolic blood pressure, or mean arterial pressure across hormone treatments in group 1, group 2, or group 3.
Table 1A.
Baseline characteristics in group 1
| GnRHa | GnRHa+E2 | GnRHa+E2+MPA | P Value | |
|---|---|---|---|---|
| Estradiol, pg/ml | 22.1±1.4* | 157.9±27.0 | 175.2±25.7 | <0.001 |
| Progesterone, ng/ml | 0.85±0.11 | 0.77±0.08 | 0.73±0.07 | 0.159 |
| FSH, mIU/ml | 4.91±1.06* | 1.99±0.43 | 1.71±0.48 | <0.001 |
| LH, mIU/ml | 0.53±0.14 | 0.36±0.09 | 0.35±0.07 | 0.198 |
| Blood pressure, mmHg | ||||
| Systolic | 114±2 | 114±2 | 112±2 | 0.115 |
| Diastolic | 74±2 | 74±2 | 72±2 | 0.368 |
| Mean arterial | 87±2 | 87±2 | 85±2 | 0.160 |
| Heart rate, beats/min | 64±3 | 62±3 | 63±3 | 0.453 |
Values are means ± SE; n = 10 subjects. GnRHa, gonadotropin-releasing hormone antagonist; E2, 17β-estradiol; MPA, medroxyprogesterone acetate; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Significant difference, GnRHa vs. GnRHa+E2 and GnRHa+E2+MPA.
Table 1B.
Baseline characteristics in group 2
| GnRHa | GnRHa+E2(I) | GnRHa+E2(II) | |
|---|---|---|---|
| Estradiol, pg/ml | <20.0 | 198.0±122 | 276±88 |
| Progesterone, ng/ml | 0.86±0.43 | 0.66±0.14 | 0.74±0.12 |
| FSH, mIU/ml | 5.00±0.60 | 2.00±0.60 | 1.25±0.05 |
| LH, mIU/ml | 0.50±0.20 | 0.25±0.05 | 0.50±0.20 |
| Blood pressure, mmHg | |||
| Systolic | 122±2 | 125±1 | 121±1 |
| Diastolic | 80±6 | 78±1 | 75±1 |
| Mean arterial | 94±3 | 94±1 | 90±1 |
| Heart rate, beats/min | 67±3 | 65±4 | 68±1 |
Values are means ± SE; n = 2 subjects.
Table 1C.
Baseline characteristics in group 3
| GnRHa-D4 | GnRHa-D7 | GnRHa-D10 | |
|---|---|---|---|
| Estrogen, pg/ml | <20 | <20 | <20 |
| Progesterone, ng/ml | 0.96±0.12 | 0.92±0.18 | 0.80±0.10 |
| FSH, mIU/ml | 2.70±2.40 | 5.00±3.00 | 5.40±3.60 |
| LH, mIU/ml | 0.35±0.15 | 0.50±0.30 | 0.30±0.10 |
| Blood pressure, mmHg | |||
| Systolic | 110±6 | 110±7 | 112±4 |
| Diastolic | 74±9 | 71.0±4 | 76±11 |
| Mean arterial | 86±7 | 84±5 | 88±9 |
| Heart Rate, beats/min | 63±3 | 63±1 | 62±1 |
Values are means ± SE; n = 2 subjects. D4, D7, D10, days 4, 7, and 10.
Biomarkers of cardiovascular risk
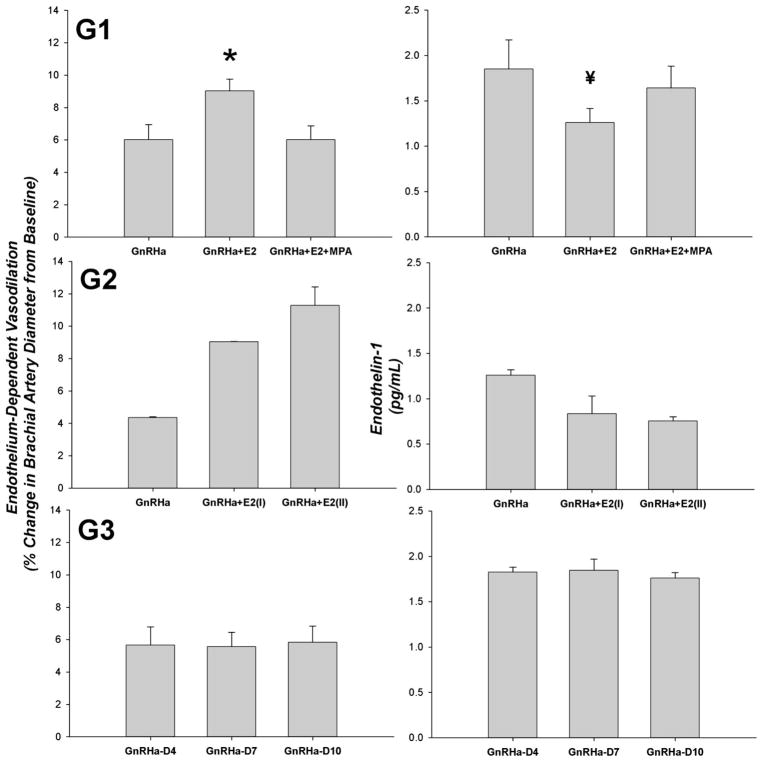
Table 2 displays the biomarkers of cardiovascular risk across hormone treatments in group 1. ET-1 was significantly lower during GnRHa+E2 treatment than during GnRHa treatment alone (P = 0.039). ET-1 increased with the addition of MPA treatment and was no longer significantly different from GnRHa treatment alone. Likewise, we also observed a lower ET-1 with the addition of E2 administration in the subjects in group 2, because ET-1 decreased from GnRHa only (1.3 ± 0.06 pg/ml) to GnRHa+E2(I) (0.84 ± 0.20 pg/ml) and GnRHa+E2(II) (0.76 ± 0.05 pg/ml). ET-1 remained consistent across study days in group 3. There were no differences in HDL, LDL, TC, TRG, TC/HDL, hs-CRP, or homocysteine across hormone treatments in group 1, group 2, or group 3.
Table 2.
Biomarkers of cardiovascular risk in group 1
| GnRHa | GnRHa+E2 | GnRHa+E2+MPA | P Value | |
|---|---|---|---|---|
| HDL, mg/dl | 62±5 | 58±6 | 57±6 | 0.231 |
| LDL, mg/dl | 123±11 | 119±11 | 114±12 | 0.169 |
| TC, mg/dl | 201±13 | 198±15 | 188±16 | 0.087 |
| TRG, mg/dl | 78±9 | 104±13 | 80±6 | 0.063 |
| TC-to-HDL ratio | 3.3±0.3 | 3.5±0.3 | 3.4±0.3 | 0.242 |
| hs-CRP, mg/l | 1.50±0.37 | 2.24±0.80 | 2.00±0.73 | 0.404 |
| Coronary risk index | 1.7±0.3 | 2.2±0.5 | 2.0±0.6 | 0.142 |
| Endothelin-1, pg/ml | 1.85±0.32 | 1.26±0.16* | 1.64±0.24 | 0.039 |
| Homocysteine, μmol/l | 5.91±0.31 | 5.84±0.47 | 6.02±0.45 | 0.892 |
Values are means ± SE; n = 10 subjects. HDL, high-density lipoproteins; LDL, low-density lipoprotein; TC, total cholesterol; TRG, triglycerides; hs-CRP, high-sensitivity C-reactive protein.
Significant difference, GnRHa+E2 vs. GnRHa.
Endothelium-dependent and endothelium-independent vasodilation
Tables 3A, 3B, and 3C display the vascular responsiveness of the brachial artery across hormone treatments in groups 1, 2, and 3, respectively. In group 1, endothelium-dependent vasodilation was significantly higher during treatment with GnRHa+E2 than during GnRHa and GnRHa+E2+MPA treatment (P = 0.006). In group 2 treatment with E2 increased endothelium-dependent vasodilation from that observed during GnRHa-only treatment in both subjects, and endothelium-dependent vasodilation was consistent over the course of GnRHa treatment in group 3. A visual representation of the endothelium-dependent vasodilation responses discussed above is presented in Fig. 2.
Table 3B.
Endothelial function values in group 2
| GnRHa | GnRHa+E2(I) | GnRHa+E2(II) | |
|---|---|---|---|
| Baseline FMD diameter, mm | 2.88±0.02 | 2.90±0.05 | 2.87±0.01 |
| Shear rate 90-s AUC, velocity/diameter | 11,817±1,805 | 12,039±2,179 | 12,751±1,893 |
| Shear rate TTP AUC, velocity/diameter | 6,806±317 | 6,653±227 | 6,989±13 |
| Normalized response, % FMD/shear rate 90-s AUC | 0.0004±0.0001 | 0.0008±0.0001 | 0.0009±0.0001 |
| Normalized response, % FMD/shear rate TTP AUC | 0.0006±0.0001 | 0.0013±0.0002 | 0.0016±0.0001 |
| Baseline NTG diameter, mm | 2.89±0.04 | 2.90±0.05 | 2.90±0.05 |
| NTG, % change | 14.55±1.05 | 15.06±1.74 | 15.22±2.35 |
Values are means ± SE; n = 2 subjects.
Table 3C.
Endothelial function values in group 3
| GnRHa-D4 | GnRHa-D7 | GnRHa-D10 | |
|---|---|---|---|
| Baseline FMD diameter, mm | 3.05±0.20 | 3.16±0.19 | 3.16±0.32 |
| Shear rate 90-s AUC, velocity/diameter | 7,419±2,217 | 7,648±2,896 | 8,106±2,488 |
| Shear rate TTP AUC, velocity/diameter | 6,023±2,196 | 5,853±2,688 | 6,037±1,449 |
| Normalized response, % FMD/shear rate 90-s AUC | 0.0009±0.0004 | 0.0009±0.0005 | 0.0008±0.0004 |
| Normalized response, % FMD/shear rate TTP AUC | 0.0012±0.0006 | 0.00012±0.0007 | 0.0011±0.0004 |
| Baseline NTG diameter, mm | 3.03±0.30 | 3.13±0.28 | 3.21±0.39 |
| NTG, % change | 19.77±0.45 | 20.16±0.02 | 20.17±0.43 |
Values are means ± SE; n = 2 subjects.
Fig. 2.
Endothelium-dependent vasodilation of the brachial artery and endothelin-1 concentrations across hormone treatments in group 1 (G1; n = 10), group 2 (G2; n = 2), and group 3 (G3; n = 2). D4, D7, D10, days 4, 7, 10. Values are means ± SE. *P = 0.006 vs. GnRHa and GnRHa+E2+MPA; ¥P = 0.039 vs. GnRHa.
There were no significant differences in baseline brachial artery diameters or 90-s SR AUC and TTP SR AUC between hormone phases (Tables 3A–3C). This finding verifies that the stimulus for flow-mediated, endothelium-dependent vasodilation was consistent across all hormone phases. Additionally, there were no differences in endothelium-independent vasodilation between hormone treatments (Tables 3A–3C), which suggests that the observed changes in endothelium-dependent vasodilation are not due to changes in smooth muscle cell responsiveness but rather due to hormonal influences on the vascular endothelium.
DISCUSSION
In support of our hypothesis, this study demonstrated that although acute estradiol administration increased endothelium-dependent vasodilation and decreased resting plasma ET-1 in healthy young women, the addition of MPA treatment antagonized these beneficial effects. In contrast to our hypothesis, we observed no changes in lipids, homocysteine, or hs-CRP with acute estradiol or combination estradiol + MPA treatment in young women.
Endothelial function
Estrogen therapy increases endothelium-dependent vasodilation in postmenopausal women (18, 24, 38, 49). Similarly, young amenorrheic women treated with a combination oral contraceptive containing ethinyl estradiol show significant improvement in endothelium-dependent vasodilation (36). Additionally, endothelium-dependent vasodilation fluctuates across the menstrual cycle, tracking the fluctuations in estrogen (14, 19). The present study supports these previous observations, because endothelium-dependent vasodilation was significantly higher after acute estradiol administration than during treatment with GnRHa alone.
The present study further demonstrates that the addition of MPA to E2 treatment reduced endothelium-dependent vasodilation of the brachial artery. Although the specific mechanism remains unknown, these data show that MPA reduces endothelium-dependent vasodilation in young women even in the presence of variable but elevated serum concentrations of E2. On the basis of these data alone, we cannot definitively conclude whether MPA is antagonizing estradiol, having an independent effect, or some combination of these options. Yet, despite a concrete understanding of the mechanism, these data suggest that 5 mg/day of oral MPA negates the beneficial effects of 0.1 mg/day transdermal E2 on endothelium-dependent vasodilation in young women. Endothelium-dependent vasodilation has been shown to be reduced in young women using MPA in Depo-Provera (42); however, chronic Depo-Provera use results in a hypoendogenous estrogen status, and thus it could not be determined whether the reduction in endothelial function is purely attributable to the use of MPA or to lowered estrogen. We anticipate that further mechanistically driven research will help to elucidate the specific mechanism by which MPA affects the vasculature of young healthy women.
MPA is a highly androgenic progestin. Its androgenic nature has been suggested to be one mechanism through which MPA may antagonize estrogen. This is supported by data showing endothelium-dependent vasodilation increases in men during androgen suppression (15) and decreases in men using anabolic androgenic steroids (10). Additionally, elevated androgen profiles in young women with polycystic ovarian syndrome are associated with decreased endothelial function (30). In contrast to our findings with MPA, Gerhard and colleagues (12) demonstrated that micronized progesterone did not attenuate the effects of estrogen on endothelium-dependent vasodilation. The androgenic nature of MPA compared with progesterone provides additional support to the notion that different progestogens may have divergent effects on vascular function (43). This is one reason that the most recent clinical trial in post-menopausal women, the KEEPS clinical trial, is utilizing progesterone rather than MPA as the progestogenic steroid (27). However, the idea of utilizing progestogens with little or no androgenic properties, such as progesterone, has not been translated to younger women, specifically those women taking MPA.
In postmenopausal women, studies investigating the influence of MPA on improvements in endothelium-dependent vasodilation with estrogen administration have resulted in conflicting conclusions. For example, endothelium-dependent vasodilation was reported to be improved after 2 mo of treatment with 0.625 mg of conjugated equine estrogen plus 10 mg of MPA (21) and after 6 wk of treatment with 0.625 mg of conjugated equine estrogen plus 2.5 mg of MPA (16) compared with pretreatment. These studies have been viewed as evidence that MPA does not have adverse effects on the vasculature (22). However, neither of these studies had an estradiol-only treatment group with which to compare their results and thus cannot rule out that endothelium-dependent vasodilation would not have been highest with estrogen treatment alone. In support of this concept, Kawano et al. (18) observed a significant increase in endothelium-dependent vasodilation from before treatment to after treatment with 2 mg of E2 every 2 days plus 2.5 mg of MPA per day but saw a more substantial increase in endothelium-dependent vasodilation from before treatment to after treatment when E2 was given unopposed by MPA. Furthermore, endothelium-dependent vasodilation was reported to be increased with 3 mo of 0.625-mg conjugated equine estrogen treatment alone but did not increase with the combination of 0.625 mg of conjugated equine estrogen plus 2.5 mg of MPA or 0.0625 mg of conjugated equine estrogen plus 5.0 mg of MPA (50). Together, these data suggest that MPA may antagonize, but not completely negate, the beneficial effects of estrogen on endothelium-dependent vasodilation in postmenopausal women. However, route of administration and dose of both estrogen and MPA may have influenced these findings and are areas in need of further investigation.
Role of endothelin-1
Vascular smooth muscle tone is controlled locally by the production/release of vasoactive substances. ET-1 is produced and released by the vascular endothelium and is the most potent vasoconstrictor of vascular smooth muscle known to date. Evidence suggests that nitric oxide may indirectly inhibit the production and/or release of ET-1 (42). There is also evidence to suggest that estrogen can alter ET-1 production and/or release in women. For example, ET-1 concentrations decrease in postmenopausal women during estrogen replacement therapy (4, 56). Additionally, ET-1 fluctuates across the course of the menstrual cycle, decreasing when estrogen is high (26, 32). Consistent with previous findings, we observed a significant decrease in ET-1 with estradiol treatment. In addition, our study expands on these findings by showing that the estradiol-induced decrease in ET-1 was reversed by the addition of MPA. This finding is strengthened by the results of experiments in our follow-up subjects: ET-1 decreased when estradiol was added to GnRHa treatment (group 2) and yet remained consistent and elevated over the three study days with GnRHa only (group 3). Because MPA is a highly androgenic progestin, the increase in ET-1 observed in male-to-female transsexuals receiving androgen treatments (48) is consistent with our finding that MPA has the ability to increase ET-1 in young women.
Estrogen increases the bioavailability of nitric oxide, and nitric oxide has been shown to impair ET-1 production and/or action (23). Because of the direct and indirect effects of ET-1 on the development or promotion of atherosclerosis, this may be one of the mechanisms through which estrogen exerts cardioprotection in women. The finding that MPA may antagonize this process is consistent with its antagonistic action on endothelium-dependent vasodilation observed in this study and may provide another mechanism through which MPA antagonizes the beneficial effects of estrogen on endothelial function. However, we are not able to conclude a causative effect of ET-1 on endothelial function in this study.
Despite previous studies that suggest that female sex hormones have the ability to effect hs-CRP, homocysteine, and lipid concentrations (9, 26, 45, 55), no differences were observed in these variables in the present study. Treatment of postmenopausal women with oral conjugated equine estrogen has been shown to increase hs-CRP, but no change in hs-CRP has been observed with the use of transdermal estrogen (20), suggesting that the lack of change in hs-CRP across hormone treatments in this study may be due to the transdermal route of estrogen delivery. Additionally, MPA has been shown to inhibit the increase in hs-CRP often seen with independent estrogen treatment (50, 55).
There is substantial evidence to suggest that oral estrogens decrease plasma homocysteine concentrations in postmenopausal women (9, 47). In addition, most (13, 51, 55), but not all (2, 41) studies in postmenopausal women show that combination hormone therapy with MPA also decreases homocysteine. On the basis of these data, we can only speculate that the lack of change in homocysteine concentrations in the present study may be due to the route of estrogen delivery or the acute nature of the estrogen and MPA treatments. Finally, there is a large base of literature suggesting that female sex hormones have the ability to alter lipid concentrations (25, 39, 40, 44 – 46). The majority of the literature suggests that oral estrogen appears to decrease LDL and increase HDL in postmenopausal women (45, 46). Similar changes in LDL and HDL have been observed with combination estrogen plus progestin hormone therapy (46), although some suggest that progestins with high androgenic properties may antagonize the beneficial changes in LDL and HDL induced by estrogen (39, 40, 46). The previous studies used oral estrogens, which could have a greater influence on lipid concentrations due to first-pass metabolism via the liver than transdermal estrogens. Therefore, we speculate that the lack of observed changes in hs-CRP, homocysteine, and lipid concentrations across hormone treatments may be due to the route of estradiol administration or the acute nature of the hormone treatments used in our experimental protocol.
Study limitations
A relatively small sample size is often a limitation in human studies. However, we do not believe the results of our primary group (group 1) would have been different by increasing the sample size in the present study. The differences we observed were highly significant, and our sample size of 10 subjects is greater than the estimated sample size required to detect significant changes in endothelium-dependent vasodilation in a repeated-measures study design using our custom edge detection software (53). Therefore, we feel confident that the differences in endothelium-dependent vasodilation observed in this group are valid. Four subjects completed follow-up protocols (groups 2 and 3) to help us validate the observations from our primary group. The findings were not surprising based on evidence in the literature, and data were included only to strengthen the findings from group 1 in support of the GnRH suppression model. Because of the low number of subjects in these groups, the data were more variable.
A second limitation of this study was that our experimental design did not lend itself to the study of MPA treatment alone. This would allow us to better understand whether MPA is specifically acting as an antagonist to the effects of estrogen, or whether MPA has direct effects on biomarkers of cardiovascular risk. Further study of this question is warranted.
Perspectives
From a clinical standpoint it has been clearly shown that depomedroxyprogesterone acetate (DMPA), a commonly used injectable, progestin-only contraceptive, decreases endogenous estrogen levels in women, which can lead to significant reductions in endothelial function (42) and bone mineral density (6, 54). In 2001, the Food and Drug Administration issued a “black box” warning for the use of DMPA to inform young women of its deleterious effects on bone mineral density. Since this time, some clinicians have suggested supplementing DMPA users with estradiol add-back treatment to counteract the reduction in bone mineral density (8). In addition to arresting the decline in bone mineral density, some clinicians have speculated that estradiol supplementation may also provide cardiovascular benefits to young women using DMPA. The results of the present study, which suggest that MPA antagonizes the beneficial effects of estradiol in young women, lead one to question whether or not estradiol add-back therapy would provide cardiovascular benefits when administered to long-term DMPA users and highlights the importance of conducting further research to evaluate the cardiovascular effects of estrogen add-back in this clinical population.
Additionally, because this study provides strong evidence that MPA antagonizes the beneficial effects of estrogens in young women (similar to the action observed in postmenopausal women), it may be advantageous for clinicians to consider prescribing progestins with low androgenic or antiandrogenic properties in young women, which will achieve the same clinical goals but with less detrimental cardiovascular effects.
Conclusions
In conclusion, this study demonstrated that although acute estradiol supplementation increased endothelium-dependent vasodilation and decreased ET-1 concentrations, the addition of MPA negated these changes in young women. These data suggest that MPA antagonizes the beneficial effects of clinical doses of short-term estradiol treatment on endothelium-dependent vasodilation and ET-1 in young women.
Acknowledgments
We extend our appreciation to the research subjects. Additionally, we gratefully acknowledge Sarah Williams and Sarah Luther for help with data collection.
GRANTS
This study was supported by Medical Research Foundation of Oregon no. 446121, National Heart, Lung, and Blood Institute R01-HL-081671 (to C. T. Minson), and the Eugene and Clarissa Evonuk Graduate Fellowship (to J. R. Meendering).
References
- 1.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Barnes JF, Farish E, Rankin M, Hart DM. Effects of two continuous hormone therapy regimens on C-reactive protein and homocysteine. Menopause. 2005;12:92–98. doi: 10.1097/00042192-200512010-00016. [DOI] [PubMed] [Google Scholar]
- 3.Berry KL, Skyrme-Jones RA, Meredith IT. Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery after different circulatory occlusion conditions. Clin Sci (Lond) 2000;99:261–267. [PubMed] [Google Scholar]
- 4.Best PJ, Berger PB, Miller VM, Lerman A. The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-1 levels in postmenopausal women. Ann Intern Med. 1998;128:285–288. doi: 10.7326/0003-4819-128-4-199802150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 6.Clark MK, Sowers M, Levy B, Nichols S. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86:1466–1474. doi: 10.1016/j.fertnstert.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Cullins VE. Noncontraceptive benefits and therapeutic uses of depot medroxyprogesterone acetate. J Reprod Med. 1996;41:428–433. [PubMed] [Google Scholar]
- 8.Cundy T, Ames R, Horne A, Clearwater J, Roberts H, Gamble G, Reid IR. A randomized controlled trial of estrogen replacement therapy in long-term users of depot medroxyprogesterone acetate. J Clin Endocrinol Metab. 2003;88:78–81. doi: 10.1210/jc.2002-020874. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrova KR, DeGroot K, Myers AK, Kim YD. Estrogen and homocysteine. Cardiovasc Res. 2002;53:577–588. doi: 10.1016/s0008-6363(01)00462-x. [DOI] [PubMed] [Google Scholar]
- 10.Ebenbichler CF, Sturm W, Gèanzer H, Bodner J, Mangweth B, Ritsch A, Sandhofer A, Lechleitner M, Fèoger B, Patsch JR. Flow-mediated, endothelium-dependent vasodilatation is impaired in male body builders taking anabolic-androgenic steroids. Atherosclerosis. 2001;158:483–490. doi: 10.1016/s0021-9150(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 11.English JL, Jacobs LO, Green G, Andrews TC. Effect of the menstrual cycle on endothelium-dependent vasodilation of the brachial artery in normal young women. Am J Cardiol. 1998;82:256–258. doi: 10.1016/s0002-9149(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 12.Gerhard M, Walsh BW, Tawakol A, Haley EA, Creager SJ, Seely EW, Ganz P, Creager MA. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation. 1998;98:1158–1163. doi: 10.1161/01.cir.98.12.1158. [DOI] [PubMed] [Google Scholar]
- 13.Gol M, Akan P, Dogan E, Karas C, Saygili U, Posaci C. Effects of estrogen, raloxifene, and hormone replacement therapy on serum C-reactive protein and homocysteine levels. Maturitas. 2006;53:252–259. doi: 10.1016/j.maturitas.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 15.Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arterioscler Thromb Vasc Biol. 1997;17:2004–2009. doi: 10.1161/01.atv.17.10.2004. [DOI] [PubMed] [Google Scholar]
- 16.Herrington DM, Werbel BL, Riley WA, Pusser BE, Morgan TM. Individual and combined effects of estrogen/progestin therapy and lovastatin on lipids and flow-mediated vasodilation in postmenopausal women with coronary artery disease. J Am Coll Cardiol. 1999;33:2030–2037. doi: 10.1016/s0735-1097(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 17.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 18.Kawano H, Motoyama T, Hirai N, Yoshimura T, Kugiyama K, Ogawa H, Okamura H, Yasue H. Effect of medroxyprogesterone acetate plus estradiol on endothelium-dependent vasodilation in postmenopausal women. Am J Cardiol. 2001;87:238–240. A9. doi: 10.1016/s0002-9149(00)01329-1. [DOI] [PubMed] [Google Scholar]
- 19.Kawano H, Motoyama T, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Okumura K, Yasue H. Menstrual cyclic variation of endothelium-dependent vasodilation of the brachial artery: possible role of estrogen and nitric oxide. Proc Assoc Am Physicians. 1996;108:473–480. [PubMed] [Google Scholar]
- 20.Kawano H, Yasue H, Hirai N, Yoshida T, Fukushima H, Miyamoto S, Kojima S, Hokamaki J, Nakamura H, Yodoi J, Ogawa H. Effects of transdermal and oral estrogen supplementation on endothelial function, inflammation and cellular redox state. Int J Clin Pharmacol Ther. 2003;41:346–353. doi: 10.5414/cpp41346. [DOI] [PubMed] [Google Scholar]
- 21.Koh KK, Jin DK, Yang SH, Lee SK, Hwang HY, Kang MH, Kim W, Kim DS, Choi IS, Shin EK. Vascular effects of synthetic or natural progestagen combined with conjugated equine estrogen in healthy post-menopausal women. Circulation. 2001;103:1961–1966. doi: 10.1161/01.cir.103.15.1961. [DOI] [PubMed] [Google Scholar]
- 22.Koh KK, Sakuma I. Should progestins be blamed for the failure of hormone replacement therapy to reduce cardiovascular events in randomized controlled trials? Arterioscler Thromb Vasc Biol. 2004;24:1171–1179. doi: 10.1161/01.ATV.0000131262.98040.65. [DOI] [PubMed] [Google Scholar]
- 23.Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993;92:99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, Yeung AC, Creager MA. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann Intern Med. 1994;121:936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Lobo RA. Clinical review 27: effects of hormonal replacement on lipids and lipoproteins in postmenopausal women. J Clin Endocrinol Metab. 1991;73:925–930. doi: 10.1210/jcem-73-5-925. [DOI] [PubMed] [Google Scholar]
- 26.Merki-Feld GS, Imthurn B, Keller PJ. The effect of the menstrual cycle and of ethinylestradiol on nitric oxide, endothelin-1 and homocysteine plasma levels. Horm Metab Res. 2000;32:288–293. doi: 10.1055/s-2007-978638. [DOI] [PubMed] [Google Scholar]
- 27.Miller VM, Clarkson TB, Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Naftolin F, Santoro N. Women, hormones, and clinical trials: a beginning, not an end. J Appl Physiol. 2005;99:381–383. doi: 10.1152/japplphysiol.00248.2005. [DOI] [PubMed] [Google Scholar]
- 28.Naylor LH, Weisbrod CJ, O’Driscoll G, Green DJ. Measuring peripheral resistance and conduit arterial structure in humans using Doppler ultrasound. J Appl Physiol. 2005;98:2311–2315. doi: 10.1152/japplphysiol.01047.2004. [DOI] [PubMed] [Google Scholar]
- 29.Oberyé JJ, Mannaerts BM, Huisman JA, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999;72:1006–1012. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- 30.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, Baron AD. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–1415. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 31.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–432. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 32.Polderman KH, Stehouwer CD, van Kamp GJ, Schalkwijk CG, Gooren LJ. Modulation of plasma endothelin levels by the menstrual cycle. Metabolism. 2000;49:648–650. doi: 10.1016/s0026-0495(00)80042-6. [DOI] [PubMed] [Google Scholar]
- 33.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 34.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 35.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL. Oral contraceptives improve endothelial function in amenorrheic athletes. J Clin Endocrinol Metab. 2005;90:3162–3167. doi: 10.1210/jc.2004-1964. [DOI] [PubMed] [Google Scholar]
- 37.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 38.Sitges M, Heras M, Roig E, Durán M, Masotti M, Zurbano MJ, Roqué M, Sanz G. Acute and mid-term combined hormone replacement therapy improves endothelial function in post-menopausal women with angina and angiographically normal coronary arteries. Eur Heart J. 2001;22:2116–2124. doi: 10.1053/euhj.2001.2631. [DOI] [PubMed] [Google Scholar]
- 39.Sitruk-Ware R. New progestogens: a review of their effects in perimenopausal and postmenopausal women. Drugs Aging. 2004;21:865–883. doi: 10.2165/00002512-200421130-00004. [DOI] [PubMed] [Google Scholar]
- 40.Sitruk-Ware R, Husmann F, Thijssen JH, Skouby SO, Fruzzetti F, Hanker J, Huber J, Druckmann R. Role of progestins with partial antiandrogenic effects. Climacteric. 2004;7:238–254. doi: 10.1080/13697130400001307. [DOI] [PubMed] [Google Scholar]
- 41.Smolders RG, Vogelvang TE, Mijatovic V, van Baal WM, Neele SJ, Netelenbos JC, Kenemans P, van der Mooren MJ. A 2-year, randomized, comparative, placebo-controlled study on the effects of raloxifene on lipoprotein(a) and homocysteine. Maturitas. 2002;41:105–114. doi: 10.1016/s0378-5122(01)00280-8. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen MB, Collins P, Ong PJ, Webb CM, Hayward CS, Asbury EA, Gatehouse PD, Elkington AG, Yang GZ, Kubba A, Pennell DJ. Long-term use of contraceptive depot medroxyprogesterone acetate in young women impairs arterial endothelial function assessed by cardiovascular magnetic resonance. Circulation. 2002;106:1646–1651. doi: 10.1161/01.cir.0000030940.73167.4e. [DOI] [PubMed] [Google Scholar]
- 43.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 44.The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 45.Tikkanen MJ. Estrogens, progestins and lipid metabolism. Maturitas. 1996;23(Suppl):S51–S55. doi: 10.1016/0378-5122(96)01012-2. [DOI] [PubMed] [Google Scholar]
- 46.Tikkanen MJ. The menopause and hormone replacement therapy: lipids, lipoproteins, coagulation and fibrinolytic factors. Maturitas. 1996;23:209–216. doi: 10.1016/0378-5122(95)00950-7. [DOI] [PubMed] [Google Scholar]
- 47.van Baal WM, Smolders RG, van der Mooren MJ, Teerlink T, Kenemans P. Hormone replacement therapy and plasma homocysteine levels. Obstet Gynecol. 1999;94:485–491. doi: 10.1016/s0029-7844(99)00412-3. [DOI] [PubMed] [Google Scholar]
- 48.van Kesteren PJ, Kooistra T, Lansink M, van Kamp GJ, Asscheman H, Gooren LJ, Emeis JJ, Vischer UM, Stehouwer CD. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost. 1998;79:1029–1033. [PubMed] [Google Scholar]
- 49.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation. 2001;104:1773–1778. doi: 10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- 50.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on vascular inflammatory markers in postmenopausal women receiving estrogen. Circulation. 2002;105:1436–1439. doi: 10.1161/hc1202.105945. [DOI] [PubMed] [Google Scholar]
- 51.Walsh BW, Paul S, Wild RA, Dean RA, Tracy RP, Cox DA, Anderson PW. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2000;85:214–218. doi: 10.1210/jcem.85.1.6326. [DOI] [PubMed] [Google Scholar]
- 52.Williams JK, Honoré EK, Washburn SA, Clarkson TB. Effects of hormone replacement therapy on reactivity of atherosclerotic coronary arteries in cynomolgus monkeys. J Am Coll Cardiol. 1994;24:1757–1761. doi: 10.1016/0735-1097(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 53.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol. 2001;91:929–937. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- 54.Wooltorton E. Medroxyprogesterone acetate (Depo-Provera) and bone mineral density loss. CMAJ. 2005;172:746. doi: 10.1503/cmaj.050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yildirir A, Aybar F, Tokgozoglu L, Yarali H, Kabakci G, Bukulmez O, Sinici I, Oto A. Effects of hormone replacement therapy on plasma homocysteine and C-reactive protein levels. Gynecol Obstet Invest. 2002;53:54–58. doi: 10.1159/000049412. [DOI] [PubMed] [Google Scholar]
- 56.Ylikorkala O, Orpana A, Puolakka J, Pyèorèalèa T, Viinikka L. Postmenopausal hormonal replacement decreases plasma levels of endothelin-1. J Clin Endocrinol Metab. 1995;80:3384–3387. doi: 10.1210/jcem.80.11.7593457. [DOI] [PubMed] [Google Scholar]