Abstract
Gliomas represent the most common primary brain tumor and among the most aggressive of cancers. Patients with glioma typically relapse within a year of initial diagnosis. Recurrent glioma is associated with acquired therapeutic resistance. Although neurosurgical resection, radiation and chemotherapy provide clear benefit, survival remains disappointing. It is, therefore, critical that we identify effective medical therapies and appropriate tumor biomarkers in patients at initial presentation, to promote durable responses in glioma. Pathways linking receptor tyrosine kinases, PI3 kinase, Akt, and mTOR feature prominently in this disease and represent therapeutic targets. Small molecules that inhibit one or more of these kinases are now being introduced into the clinic and may have some activity. Disappointingly, however, preclinical studies demonstrate these agents to be primarily cytostatic rather than cytotoxic to glioma cells. Here, we detail activation of the EGFR-PI3K-Akt-mTOR signaling network in glioma, review class I PI3K inhibitors, discuss roles for Akt, PKC and mTOR, and the importance of biomarkers. We further delineate attempts to target both single and multiple components within the EGFR-PI3K-Akt-mTOR axes. Lastly, we discuss the need to combine targeted therapies with cytotoxic chemotherapy, radiation and with inhibitors of survival signaling to improve outcomes in glioma.
1 Introduction
Gliomas represent the most common primary brain tumor and are among the most lethal of all cancers. Prognosis for glioma differs from most other cancer types in that grade (mitotic features, microvascular proliferation, and necrotic tissue surrounded by anaplastic cells, so-called pseudopalisading necrosis) is much more important than stage (extent of disease). Astrocytomas are the most frequently occurring type of glioma. The vast majority of patients (~90%) present at diagnosis with high-grade glioblastoma multiforme tumors (GBM). Both GBM (grade IV) and grade III astrocytomas (high-grade without pseudopalisading necrosis) comprise “malignant gliomas”. Standard-of-care therapy for GBM includes surgery and radiation therapy, resulting in a median survival of approximately 1 year from the time of diagnosis (reviewed in Persson et al. 2007). Over the past decade, addition of the alkylating agent temozolomide, administered both during and after radiotherapy, has been justifiably viewed as a major advance in the care of these patients, improving survival by approximately 3 m overall (Stupp et al. 2005).
Genetic alterations in GBM typically deregulate pathways involving tumor suppressors p53 (87%), RB (78%), and receptor-tyrosine kinase (RTK)/RAS/PI3K (88%) (Cancer Genome Atlas Research Network 2008). Among these, the RTK/RAS/PI3K pathway is distinguished in requiring a number of key kinase intermediates, and currently represents the pathway most amenable to pharmacologic intervention. Mutations such as amplification of EGFR (45%), gain of function in PIK3CA (15%), or loss of PTEN (36%) all activate the lipid kinase PI3K and its downstream target, the plekstrin-homology-domain serine threonine kinase Akt. Akt has over 40 downstream targets (Manning and Cantley 2007). Prominent among these are GSK-3, PRAS40, FOXO, BAD, mTOR, and the TSC1/2 proteins (Fig. 1). Although EGFR and downstream signaling components all represent attractive targets for therapy, initial clinical studies focused on inhibiting EGFR have been disappointing in glioma (Prados et al. 2006; Rich et al. 2004). In addition, preclinical studies inhibiting EGFR and other RTKs, as well as PI3K and mTOR have shown only modest efficacy in GBM. Can an understanding of the molecular and genetic abnormalities in GBM lead to improved therapies using single agents or combination protocols, enabling these pathways to be targeted effectively in patients?
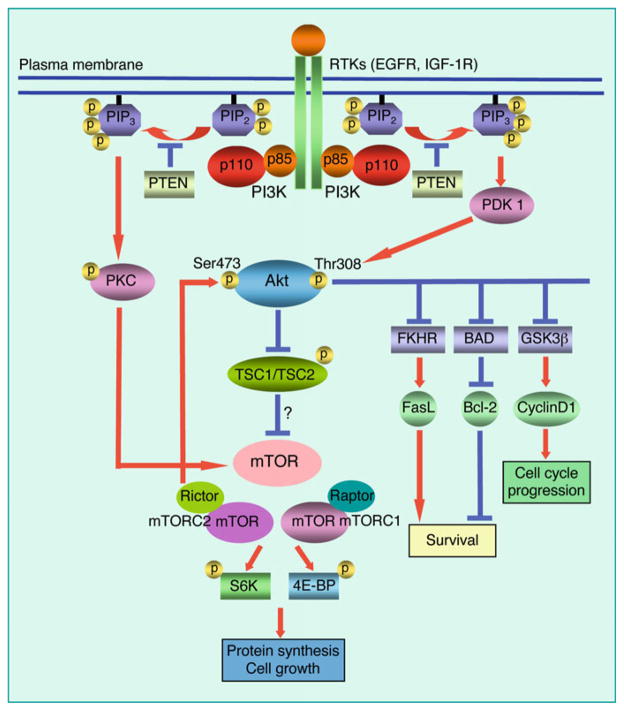
Fig. 1.
PI3 kinase signaling pathway in glioma. Class I PI3 kinases are activated by upstream signals from receptor tyrosine kinases (RTKs) including EGFR and other RTKs. PI3 kinase catalyzes production of the second messenger PIP3, which actives both Akt and PKC. Akt and PKC phosphorylate multiple downstream substrates. We found Akt was dispensable for mitogenic signaling between EGFR and mTOR in glioma cells, whereas PKC was critical (33). PIP3 is negatively regulated by the tumor suppressor PTEN, a phosphatase driving dephosphorylation of PIP3
2 The Epidermal Growth Factor Receptor Pathway
EGFR is commonly mutated in GBM, leading to overexpression and activation of downstream signaling pathways. The EGFR gene is amplified in 40–50% tumors, and overexpressed in a majority of GBM. Approximately 40% of tumors with EGFR amplification also have gene rearrangements, most commonly deleting the ligand binding domain, resulting in a constitutively active EGFRvIII allele (Cancer Genome Atlas Research Network 2008; Jones et al. 2008). EGFR signals through a complex network of intermediates including PI3K, AKT, MAPK and PLCγ. Overactivity of the EGFR pathway results in proliferation, invasiveness, motility, angiogenesis and inhibition of apoptosis, and is associated with resistance to radiation and chemotherapy (reviewed in Brandes et al. 2008).
Since EGFR is a driving oncogene in malignant glioma, it was anticipated that inhibition of EGFR signaling would represent an effective therapeutic strategy. Two small-molecule tyrosine kinase inhibitors of the EGFR (erlotinib and gefitinib) were evaluated in malignant gliomas. Initial results with EGFR inhibitors in GBM have been disappointing however, with most patients not responding. Only patients with high expression of wild-type EGFR and low levels of phosphorylated Akt in one study (Haas-Kogan et al. 2005), and coexpression of EGFRvIII and wild-type PTEN in another study (Mellinghoff et al. 2005) showed a radiographic response to EGFR kinase inhibitors. It was not clear that these changes were durable, and such patients represented a minority population (~10%).
Gefitinib (ZD1839, Iressa) is a small molecule inhibitor of the EGFR tyrosine kinase that has been tested in a phase II study in recurrent GBM. Median event-free survival was 8.1 weeks. No radiographic responses were observed and the 6-month median progression-free survival (PFS) was 17% (Rich et al. 2004). Another phase II trial also reported the ineffectiveness of gefitinib in patients with high-grade glioma (Franceschi et al. 2007). Gefitinib is rarely used currently in the treatment of GBM.
Erlotinib (OSI-774, Tarceva) inhibits the tyrosine kinase activity of EGFR and EGFRvIII. Partial response rates of 6% were reported in a phase II study, in which progression free survival for patients was 12 weeks. All patients progressed by 24 weeks (de Groot et al. 2008). It is unclear whether erlotinib is more effective than gefitinib for radiographic response rate in high-grade glioma. A recently published Phase II study demonstrated that erlotinib in combination with temozolomide chemotherapy resulted in improved survival, again correlating with PTEN immunopositivity (Prados et al. 2009).
Amplification of EGFR is prominent in glioma. It was, therefore, quite disappointing although perhaps not surprising that blockade of this kinase had such a modest effect in patients. At least two observations can help to explain this apparent paradox. First, EGFR is one among many kinases activated in glioma. The abundance of RTKs expressed in GBM suggests a redundancy that may preclude observing clinical improvement in response to targeting any single RTK in this disease (Stommel et al. 2007). This observation is somewhat at odds with one of many lessons learned from CML patients treated with imatinib however; kinases activated by mutation are generally better targets than kinases activated in the absence of genetic mutation (Sawyers 2004). With so many RTKs apparently over-expressed in glioma, why does mutational activation of EGFR occur so much more commonly than mutational activation of other RTKs?
A second contributor to the failure of EGFR inhibitors in glioma relates to EGFR-independent mutational activation in coupled signaling pathways, leading to sustained activation of downstream signaling even in the setting of effective upstream blockade. To fully understand this issue requires a brief review of lipid kinase signaling downstream of EGFR.
3 The PI3K/Akt/mTOR Axis in Glioma
PI3Ks are lipid kinases activated by a wide range of RTKs to generate the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 couples PI3K to downstream effectors such as Akt, a serine-threonine kinase that suppresses apoptosis, promotes growth and drives proliferation. PIP3 also indirectly activates the protein kinase mTOR, which is critical for cell growth and contains a PI3K homology domain (making mTOR a PIK related Kinase – PIKK), although mTOR itself has no lipid kinase activity. The lipid phosphatase Pten acts on PIP3 to antagonize PI3K signaling and shows frequent inactivation, deletion, or epigenetic silencing in GBM. Inactivation of PTEN and activating mutations in PI3K itself collectively occur in a substantial fraction of GBM tumors, effectively uncoupling PI3K from upstream control by EGFR.
It is perhaps not surprising then that inhibition of EGFR in PTEN mutant glioma has shown little therapeutic effect, an observation made preclinically by us and others (Fan et al. 2003, 2007; Wang et al. 2006) and subsequently borne out clinically in studies described above. That responses to clinical inhibitors of EGFR were seen only in patients with tumors that were wild-type for PTEN, or that showed low levels of phosphorylated Akt (a histological surrogate for PTEN proficiency), suggests that responses were limited to tumors in which inhibition of EGFR led to blockade of downstream PI3K signaling. The role played by PTEN mutation in uncoupling EGFR and PI3K also suggests that the efficacy of EGFR inhibition in PTEN mutant glioma should be augmented by addition of a PI3K inhibitor. In preclinical studies, we therefore transduced PTEN mutant human glioma cells with the constitutively active, tumor-derived EGFRvIII allele, established flank xenografts, and treated these with the EGFR inhibitor gefitinib or the pan-PI3K inhibitor LY294002. While low doses of either monotherapy had no effect on tumor burden, combination low-dose therapy efficiently blocked further growth of established tumor xenografts. These and other preclinical studies (Fan et al. 2003; Wang et al. 2006) support the use of such a combination approach in EGFR-driven, PTEN mutant glioma. However, the availability of clinical PI3K inhibitors, now in clinical trials, has to date precluded clinical trials that test this combination.
4 Isoform Specific Inhibitors of Class I PI3K Inhibitors
The PI3K-Akt-mTOR signaling pathway is currently one of the most attractive therapeutic targets in GBM. The eight mammalian PI3Ks are divided into three classes according to their structure, regulation, and substrate specificity. Most PI3K enzymes consist of a p110 catalytic subunit that heterodimerizes with a separate regulatory subunit. Of particular interest are the four Class I PI3K isoforms (α, β, δ, and γ), which are activated by receptor tyrosine kinases or by heterotrimeric G-proteins. Although gain-of function mutations in the p110α gene (PIK3CA) are found uniquely in human cancers, other p110 isoforms also have oncogenic potential when overexpressed. PI3Kβ is associated with development of thrombotic diseases through activation of platelets (Jackson et al. 2005). Both PI3Kδ and PI3Kγ play major roles in the immune system. Knockout of either the δ or γ isoforms of PI3K led to impaired immune responses (Okkenhaug et al. 2002; Sasaki et al. 2000).
Two recent studies show that the PI3Kβ also has a role in glucose metabolism that was not required for Akt activation. In conditional knock out mice, ablation of PI3Kβ in the livers led to impaired insulin sensitivity and glucose homeostasis with little change in phosphorylation of Akt (Jia et al. 2008). Interestingly, deletion of PI3Kβ (but not PI3Ka) blocked prostate tumor formation in genetically engineered mice deleted for PTEN, and was associated with decreased levels of p-Akt in this setting. Mice expressing a catalytically inactive PI3Kβ (K805R) developed mild insulin resistance detectable from 6 months of age, and were protected from breast tumor formation driven by a Her2 transgene (Ciraolo et al. 2008). Comparison of wild-type MEFs and MEFs homozygous for the deleted or inactive PI3Kβ kinase revealed that the catalytic function of PI3Kβ was not required for Akt activation shortly after growth factor stimulation, but was required for signaling via GPCRs.
Much of our understanding of the biology of PI3Ks has resulted from experiments using panselective PI3K inhibitors such as LY294002 and wortmannin, which inhibit a broad range of p110 enzymes. However, this same diversity of functions within the PI3K family has limited the utility of panselective inhibitors of PI3Ks to validate particular PI3K isoforms as potential therapeutic targets. In addition, use of LY294002 and wortmannin has been restricted to preclinical studies because of lack of selectivity, toxic effects, and poor pharmaceutical properties. Despite these inadequacies, both compounds have proven invaluable for the early study of PI3K inhibition and serve as pathfinders in the development of PI3K inhibitors.
To assess the impact of inhibiting individual PI3Ks, efforts in both academia and industry have recently developed several isoform-selective inhibitors of class I PI3Ks that show significant selectivity. All are ATP-competitive inhibitors. Because ATP-binding pockets of different kinases are structurally similar, these inhibitors generally show activities against a number of PI3 kinases. Several isoform-selective inhibitors of the PI3Ks are therefore being evaluated in malignant gliomas.
To test the efficacy of these agents, we screened a genetically heterogeneous panel of glioma cell lines using a panel of small molecule PI3K inhibitors, including chemotypes of compounds likely to be further developed as clinical inhibitors (Fan et al. 2006; Knight et al. 2006). We observed biochemical activity (against phospho-Akt) using a number of agents that blocked PI3K alpha and PI3K beta, but not in response to a more limited number of agents that blocked PI3K gamma or PI3K delta. Although agents blocking PI3K beta were equivalent to agents that blocked PI3K alpha in terms of their activity against Akt, only PI3K alpha inhibitors were effective as antiproliferative agents. These observations suggest that PI3K alpha is the principal PI3K driving proliferation in glioma. That inhibitors of PI3K beta showed activity against p-Akt but were ineffective in blocking proliferation suggests that Akt is a poor biomarker for the antiproliferative activity of PI3K inhibitors in glioma (discussed in further detail below).
Interestingly, the Piramed inhibitor PI-103 was unique among inhibitors tested in showing the most potent antiproliferative activity. We traced the antiproliferative activity of PI-103 to its independent inhibitory activities against both PI3Kα and mTOR. This combined inhibition of p110α and mTOR eliminated the increased Akt signaling often observed using allosteric inhibitors of mTOR as monotherapy (Fig. 2) and likely antagonizing the potential efficacy of mTOR inhibitors (Fan et al. 2006). Interestingly, the ability of this and other compounds to inhibit mTOR was largely overlooked during initial development of these drugs, likely because mTOR inhibition was presumed to be a consequence of PI3K blockade. We subsequently demonstrated that inhibitors of PI3K do not block mTOR in glioma (Fan et al. 2009), suggesting that dual inhibitors of PI3K and mTOR in this disease affect two parallel pathways, rather than a single linear one (see below).
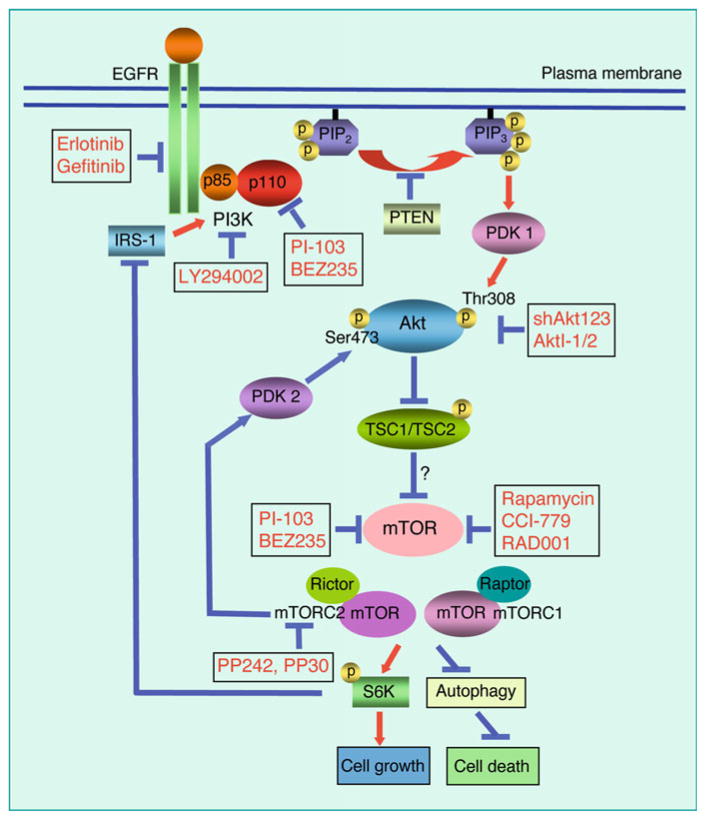
Fig. 2.
Sites of action for inhibitors and shRNAs in the EGFR-PI3K-Akt-mTOR pathway. Agents that inhibit the EGFR-PI3K-Akt-mTOR pathway at multiple sites may contribute to anticancer effects in malignant glioma. Agents that inhibit only one target within the EGFR-PI3K-Akt-mTOR axis generally show only modest efficacy and fail to induce appreciable apoptosis. This disappointing efficacy stems in-part from multiple nodes of activation in the EGFR-PI3K-Akt-mTOR axis. For example, loss of PTEN activates PI3K signaling. A feedback loop between S6K and IRS-1 also lead to PI3K activation. Inhibition of the PI3K-Akt-mTOR pathway may also induce autophagy, enabling cancer cells to survive some small molecule inhibitors of this pathway
5 Targeting mTOR Signaling
The mTOR kinase (also known as FRAP, RAFT or RAPT) is intimately linked to PI3K/Akt signaling and to the regulation of protein synthesis, cell growth and survival. Activation of mTOR in response to growth factor signals is thought to be regulated through the PI3K/Akt pathway. Stimulation of PI3K leads to activation of Akt, with subsequent phosphorylation of mTOR on ser-2448 by phospho-Akt. In addition, phospho-Akt is able to phosphorylate the Tsc1/2 (hamartin-tuberin) complex on the Thr-1462 of tuberin that is inhibitory to mTOR.
Rapamycin and analogs temsirolimus (CCI-779), everolimus (RAD001), and deforolimus (AP23573) represent allosteric mTOR inhibitors that posses antiproliferative and antitumor activity as single agents both in vitro and in vivo, and have been evaluated in a limited clinical setting in malignant glioma. Two recently published phase II studies of temsirolimus monotherapy in recurrent GBM demonstrated a range of radiographic improvement without survival benefit (Chang et al. 2005; Galanis et al. 2005), suggesting that the efficacy of rapamycin analogs might be augmented through combination therapy approaches. A recent Phase I trial tested geftinib and CCI-779 in combination and showed very few responses, all of which were limited to patients with amplification of EGFR and high levels of p-Akt (Reardon et al. 2006)
The mTOR kinase exists as a component of two distinct protein complexes. mTORC1 is activated by growth factors and nutrients, phosphorylates substrates including S6K at Thr389 and EIF4E at Ser209, and is sensitive to rapamycin. In contrast, the role played by growth factors and/or nutrients in regulating mTORC2 is less certain. The mTORC2 complex itself phosphorylates Akt at S473. Although S473 is generally resistant to rapamycin and rapamycin-like inhibitors, some cancer cell lines do show inhibition in response to rapamycin (Copp et al. 2009). Rapamycin and its analogs inhibit mTOR by altering the conformation of this kinase, inactivating mTORC1 (with limited effect on mTORC2) and until recently defining the activity of the mTORC1 kinase complex. In contrast, dual inhibitors of PI3K and mTOR compete with ATP in binding the active site of these kinases, and inactivate PI3K, mTORC1 and mTORC2. Two recent reports have characterized ATP-competitive inhibitors of mTOR kinase that do not concomitantly inhibit PI3K (Feldman et al. 2009; Thoreen et al. 2009). In contrast to rapamycin, ATP competitive inhibitors of mTOR blocked the phosphorylation of Akt at S473 and prevented its activation (an mTORC2 function). Preclinically, these compounds were more effective antiproliferative agents than rapamycin. Studying MEFs deficient in components of mTORC2 enabled a direct comparison of allosteric inhibition of mTORC1 versus ATP-competitive inhibition of this same complex. Surprisingly, the improved antiproliferative proficiency of ATP-competitive inhibitors was traced to inhibition of mTORC1 and to the improved activities of ATP competitive inhibitors in blocking cap-dependent translation and in inducing autophagy (Feldman et al. 2009; Thoreen et al. 2009). Thus, ATP-competitive inhibitors of mTOR, alone or as a component of dual inhibitors of PI3K and mTOR provide a new class of agents and therapeutics for glioma and other cancers.
6 Targeting the EGFR-PI3K-Akt-mTOR Axis: The Importance of Akt
The AKT family of serine threonine kinases consists of three members: Akt 1 (PKBα), Akt 2 (PKBβ), and Akt 3 (PKBγ), all of which have been implicated in cancer. Akt is a central node in the complex cascade of PI3K signaling, with crosstalk and feedback loops influencing regulation of this kinase. Akt is also well known for its antiapoptotic activity when overexpressed. Surprisingly, however, inhibiting components of the PI3K-Akt pathway in glioma often does not typically induce apoptosis (reviewed in Cheng et al. 2009). Several small molecule Akt inhibitors with varying potencies and specificities for the different Akt isoforms have now been developed.
Because inhibitors of PI3K should signal through Akt to mTOR, it is perhaps surprising that dual inhibitors of PI3K and mTOR show enhanced efficacy when compared with mono-specific inhibitors of PI3K. We presumed initially that mTOR had additional upstream inputs, so that inhibition of PI3K would only partially impair mTOR function. To test this hypothesis, we took advantage of the observation that EGFR-driven glioma cell lines wild-type for PTEN showed proliferative arrest in response to inhibitors of EGFR, and that comparable cell lines mutant for PTEN did not. In PTEN mutant lines, levels of phospho-Akt decreased significantly in response to EGFR inhibition. These data were aligned with our earlier demonstration that inhibitors of PI3K beta showed pronounced activity against p-Akt in the absence of affecting proliferation, and collectively suggest that p-Akt represents a poor biomarker for the antiproliferative activity of EGFR and PI3K inhibitors (Fan et al. 2006). In contrast, levels of p-mTOR and downstream kinases, including p-rpS6 kinase were robust biomarkers for the antiproliferative activity of both EGFR and PI3K inhibitors (Fan et al. 2007, 2009).
We went on to show that neither blockade nor knockdown of Akt1, 2 or 3 (alone or in combination) impacted proliferation or levels of p-mTOR and downstream targets, even in a setting where the canonical Akt targets Tsc2 and Gsk3 were potently inhibited (Fan et al. 2009). In addition, a constitutively activated allele of Akt affected neither proliferation nor response to the EGFR inhibitor erlotinib in glioma cells. Our observations suggest that Akt is not a central regulator of proliferation in glioma. We subsequently identified protein kinase C as a key intermediate linking EGFR and mTOR in glioma, and showed that PKC alpha contributed prominently to this signaling. Since PKC signals downstream of EGFR and PTEN, and upstream of mTOR, we hypothesized that inhibitors of PKC might show efficacy even in PTEN mutant cells. Using the tool compound bis-indolylmaleic acid, a pan-PKC inhibitor, we showed that this agent blocked viability in glioma irrespective of PTEN status. These data raise questions regarding whether and how to incorporate inhibitors of Akt into a combination treatment regimen for GBM, an important area of experimental therapeutic investigation more fully detailed below.
7 Combination Strategies Within the EGFR-PI3K-mTOR Axis to Improve Therapeutic Efficacy
Current targeted therapies that inhibit EGFR and downstream PI3K signaling pathways in malignant gliomas have shown modest benefits. At least one component contributing to the relatively disappointing anti-cancer efficacy of these agents stems from multiple nodes of activation in the EGFR-PI3K axis, through which EGFR inhibitors fail to block PI3K signaling as a function of PTEN or PIK3CA mutational status. Given the complexity of cellular signal transduction and the ability of cancer cells to compensate for acute changes in signaling (in-part through feedback loops activated in response to treatment with kinase inhibitors) it is not surprising that agents that target only one or a few kinases in the EGFR-PI3K-mTOR signaling pathway may be disappointing as they enter clinical trials (Fig. 2). Thus, effective therapy in GBM and other RTK-PI3K-driven cancers likely requires targeting multiple components in EGFR-PI3K-mTOR axis, with the caveat that the toxicities associated with a complex cocktail of agents remain acceptable to patients and clinicians.
Preclinical examples of such approaches include combination therapies using EGFR inhibitors with dual PI3K/mTOR inhibitors, which were superior to either monotherapy or therapy combining an EGFR inhibitor with either a PI3K inhibitor or an mTOR inhibitor in blocking proliferation (but not in inducing apoptosis) in glioma cell lines (Fan et al. 2007). The concept of combining inhibitors of EGFR and mTOR is also translating into clinical trials using EGFR inhibitors with allosteric inhibitors of mTOR in patients (Doherty et al. 2006).
What will the future hold? ATP-competitive inhibitors of EGFR and allosteric inhibitors of mTOR are now in clinical use. Irreversible inhibitors of EGFR, ATP-competitive inhibitors of PI3K and dual inhibitors of PI3K and mTOR are now in clinical trials. ATP-competitive inhibitors of mTOR are likely to be tested clinically in the near future. With such an array of compounds now available or soon to be available, is there a logical approach to testing these alone and in combination in GBM? In this section, we provide a rationale for preclinical therapeutic studies over the next few years, ultimately with the goal of guide clinicians in prioritizing agents for clinical studies.
8 A Role for EGFR Inhibitors in Combination Therapy
Inhibitors of EGFR present a paradox. Multiple RTKs are typically activated in glioma (Stommel et al. 2007). EGFR is the most prominent of these, showing mutational in addition to epigenetic activation. A simple solution to the problem of multiple RTK activation would be to target downstream nodes into which these signals coalesce; however aside from inhibitors of PI3K, such therapeutic reagents are only slowly entering clinical use. At present therefore, there is a clear rationale for continued use of EGFR inhibitors in order to block MAP kinase and other pathways not directly impacted by PI3K blockade, and for which specific targeted therapies are not yet available. It is likely that irreversible inhibitors of EGFR will be more active than the reversible agents currently available, and that these irreversible inhibitors will show improved activity in GBM. Since many GBM tumors are associated with RTK-independent activation of PI3K, it is likely that inhibitors of EGFR should be combined with inhibitors of PI3K in GBM (Fig. 2).
9 Inhibitors of mTOR, PI3K and Dual PI3K/mTOR Inhibitors
Allosteric inhibitors of mTOR, inhibitors of PI3K and inhibitors of PI3K/mTOR are now all in clinical trials. As mentioned above, inhibitors of PI3K fail to impact mTOR in preclinical studies of GBM, providing a clear rationale for the clinical use of dual inhibitors of PI3K and mTOR. Is there a setting where mono-specific inhibitors of PI3K or allosteric inhibitors of mTOR should be used instead of dual PI3K/mTOR inhibitors? Toxicity issues aside (which are as yet uncertain for PI3K or PI3K/mTOR inhibitors), it is difficult to visualize a scenario in which these mono-selective inhibitors would provide a therapeutic advantage over dual inhibitors.
If combination therapy with EGFR and PI3K/mTOR inhibitors represents the future for glioma therapeutics, is there an additional preclinical rationale for use of Akt inhibitors and/or ATP-competitive inhibitors of mTOR in this disease? In our preclinical studies, inhibition of Akt had little effect on proliferation in glioma. These observations do not preclude the importance of Akt inhibition as a regulator of apoptosis however. A second issue is whether Akt blockade can be accomplished using agents that inhibit kinases upstream of Akt. The ability of PI3K/mTOR inhibitors to block Akt is well-established and raises questions as to whether additional benefit will be derived from use of Akt-selective inhibitors (either instead of or in combination with dual inhibitors of PI3K/mTOR). Further, the two ATP-competitive inhibitors of mTOR studied to date also quite effectively shut down Akt signaling, consistent with the idea that phosphorylation of Akt at S-473 (by mTORC2) is a priming phosphorylation required in order to secondarily phosphorylate T-308 (Feldman et al. 2009; Thoreen et al. 2009). Further studies are needed to determine whether this class of agents shows activities equivalent to or distinct from those displayed by PI3K/mTOR inhibitors, and comparing the efficacy of these agents to that of Akt inhibitors.
10 Inhibitors of PKC
Is there a role for inhibitors of PKC in PTEN-mutant glioma and will such agents be more active that EGFR inhibitors in PTEN-wild-type glioma? There are currently few clinical agents in development against individual PKC isoforms, although enzastaurin, a potent inhibitor of both PKC alpha and PKC beta (Graff et al. 2005), is currently being tested clinically in this disease. Whether this and future agents show efficacy in glioma, and whether this class of targeted therapy offer any potential advantage over inhibitors of mTOR remains to be determined.
11 Future Directions
11.1 Therapeutic Strategies to Promote Cytotoxicity in Glioma
The PI3K signaling pathway has risen to prominence as a key regulator of survival in cancer cells, with downstream components Akt and mTOR having well-established anti-apoptotic activities. However, inhibiting components of the PI3K-Akt pathway generally fails to induce appreciable apoptosis in glioma. This is exemplified in our recently study using the dual PI3K/mTOR inhibitor PI-103. PI-103 efficiently inhibited phosphorylation of Akt and mTOR, concomitantly blocking proliferation of glioma cell lines and xenografts (Fan et al. 2006). Even when used in combination with EGFR inhibitors however, PI-103 blocked proliferation without the induction of apoptosis (Fan et al. 2007). NVP-BEZ235, a dual PI3K/mTOR inhibitor currently in phase I clinical trials, also showed strong antiproliferative activity with no obvious affect on viability of U87MG cells (Maira et al. 2008). Further, clinical trials using inhibitors of EGFR in GBM have shown only limited clinical responses, even in those patients whose tumors are driven by EGFR and wild-type for PTEN. Why does efficient inhibition of survival signaling in a number of cancers generally fail to induce apoptosis in cancer cells? One possibility is that PI3K inhibition is inducing one or more alternative survival pathways, enabling cancer cells to evade pro-apoptotic signals mediated by PI3K/Akt/mTOR blockade.
Inhibition of PI3K/mTOR pathway typically induces autophagy, raising the possibility that autophagy represents a candidate survival signal leading to cytostasis rather than cytotoxicity in response to inhibitors targeting the PI3K-Akt-mTOR pathway. Autophagy drives a pathway that enables cells to survive periods of catabolic stress by cannibalizing themselves to preserve adenosine triphosphate (ATP) stores. During autophagy, cells degrade their own proteins and organelles through formation of a double-membraned vesicle (autophagosome) that fuses with a lysosome, leading to self-digestion of cytoplasmic organelles and other constituents in the lysosomal compartments. Although autophagy may not always allow cells to survive prolonged periods of stress – ultimately resulting in cell death – this process allows cells to recycle proteins and organelles as a source of new macromolecules, thereby evading apoptosis at least for a limited time. Inhibiting autophagy can thus either promote or impair cell death depending on the conditions and reagents used (Kroemer and Levine 2008). Can blockade of autophagy in the setting of PI3K or PI3K/mTOR enhance the cytotoxicity of these agents in glioma? In support of this model, inhibition of Akt activity by siRNA-mediated knockdown or using Akt inhibitors did not induce significant apoptosis, but rather markedly increased autophagy in the PTEN-null human prostate cancer cell line PC3 and in the glioma cell line U87MG (Degtyarev et al. 2008). A number of lysosomotropic agents such as chloroquine or bafilomycin A1 cooperated with Akt siRNA or Akt inhibitors to precipitate PC3 cell death in vitro and in vivo, although details of signaling required to achieve cell death in this descriptive study remain unclear. Nevertheless, these results suggest that blockade of autophagy preclinically may enhance the anticancer efficacy of at least some PI3K-Akt-mTOR pathway inhibitors.
PI3K inhibition has also been shown to sensitize cells to chemically induced cell death. Apoptotic cell death proceeds through one of two pathways: the extrinsic-death ligand/receptor-mediated pathway; and the intrinsic-mitochondrial-mediated pathway. Sensitivity of glioma cells to apoptosis induced through either of these pathways can be enhanced by PI3K inhibition (Kao et al. 2007; Opel et al. 2008). PI-103 a dual PI3K/mTOR inhibitor has been shown synergistically or additively to promote the cytotoxic effects of either chemotherapy (BCNU and temozolomide) or radiation therapy (Chen et al. 2008; Prevo et al. 2008). Take together, these results suggest that inhibitors of PI3K may be useful in concert with traditional therapies to enhance apoptosis in GBM.
12 Biomarkers to Stratify Patients and to Measure Responses
A second challenge in glioma is to improve our ability in identifying subgroups of patients most likely to benefit from inhibition of the RTK-PI3K-Akt-mTOR axis, and to develop biomarkers of response. While essentially all patients with GBM have tumors resected as standard of care, additional surgeries are not always performed at relapse, and routine sampling of tumors during therapy is essentially not done. A potential model for future therapy in this disease was recently presented by Cloughesy and colleagues who gave an allosteric mTOR inhibitor to GBM patients with PTEN deficient tumors who had suffered a radiological relapsed after standard treatment, and then biopsied relapsed tumors 1 week after starting rapamycin (Cloughesy et al. 2008). This strategy allowed investigators to assess the biochemical response to mTOR inhibition, while also limiting the trial to patients with PI3K-driven tumors. While such an approach is clearly a move in the right direction, future studies in glioma will likely be improved by development and routine incorporation of non-invasive methods of assessment (metabolic PET imaging, MRI with MR spectroscopy or serum markers) in order to clarify those patients most likely to respond to these agents, and to monitor response to targeted therapies.
13 Conclusion
Advances in neurosurgical techniques and in radiation therapy continue to benefit patients with glioma. In contrast, the comparatively modest effects of medical therapy pose a challenge. In light of the relative failure of medical therapy in this disease, the need for effective targeted therapy in GBM is formidable. Agents only recently introduced into clinical use, which mainly inhibit one component of the EGFR-PI3K-Akt-mTOR pathway, have shown some activity, however agents that significantly improve survival are sorely needed in this disease. As GBM at recurrence is generally associated with enhanced therapeutic resistance, it is critical to identify optimal effective medical therapies that up-front, can induce durable responses in appropriate patient populations. Our ability to identify appropriate patients, to target multiple components within the RTK-PI3K-Akt-mTOR axes, and to combine these therapies with cytotoxic chemotherapy, radiation (Table 1), and potentially with inhibitors of survival signaling including inhibitors of autophagy, all represent challenges for the future. As the cocktail of agents grows increasingly complex, a parallel challenge is to identify effective combinations that yield cytotoxicity specifically in cancer cells, and that are tolerated by patients.
Table 1.
Combination strategies through EGFR-PI3K-mTOR axis to improve therapeutic efficacy
| Agent | Targets | Biological Effects |
|---|---|---|
| Erlotnib + mTOR | EGFR, mTOR | Antiproliferative |
| Erlotnib + PI3K/mTOR | EGFR, p110α, mTOR | Antiproliferative |
| shAkt123 + hyroxychloroquine | Akt, lysosomotropic | Antiproliferative, apoptosis? |
| PI3K/mTOR + temozolomide | p110α, mTOR, nonspecifc | Antiproliferative, apoptosis? |
| PI3K/mTOR + radiation | p110α, mTOR, nonspecifc | Antiproliferative, apoptosis? |
Combination strategies to target multiple nodes within the EGFR-PI3K-mTOR network. Combining kinase inhibitors with cytotoxic chemotherapy, radiation, and with inhibitors off autophagy may improve the therapeutic efficacy. Whether these approaches show improved efficacy and whether their use is associated with acceptable levels of toxicity are areas of active basic and clinical investigation. A number of allosteric inhibitors of mTORC1 and dual inhibitors of PI3K and mTOR are currently being tested clinically. See text for details
Acknowledgments
We are grateful to Kevan Shokat for useful discussions and to Theo Nicolaides for critical review. We acknowledge support from NIH P50CA097257, Burroughs Wellcome Fund, American Brain Tumor Association, The Brain Tumor Society, Accelerate Brain Cancer Cure; Alex’s Lemonade Stand, Children’s National Brain Tumor, Wallace H. Coulter, Katie Dougherty, Pediatric Brain Tumor, Samuel G. Waxman and V Foundations.
Abbreviations
- CML
Chronic myelongenous leukemia
- EGFR
Epidermal growth factor receptor
- GBM
Glioblastoma multiforme
- mTOR
Mammalian target of rapamycin
- PKC
Protein kinase C
- PI3K
Phosphatidylinositol 3′kinase
- RTK
Receptor tyrosine kinase
Contributor Information
Qi-Wen Fan, Departments of Neurology, University of California, 1450 3rd St, MC0520, San Francisco, CA 94158-9001, USA.
William A. Weiss, Email: weiss@cgl.ucsf.edu, Departments of Neurology, University of California, 1450 3rd St, MC0520, San Francisco, CA 94158-9001, USA. Pediatrics, University of California, 1450 3rd St, MC0520, San Francisco, CA 94158-9001, USA, Neurological Surgery and Brain Tumor Research Center, University of California, 1450 3rd St, MC0520, San Francisco, CA 94158-9001, USA. Helen Diller Family Comprehensive Cancer Center, University of California, 1450 3rd St, MC0520, San Francisco, CA 94158-9001, USA
References
- Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R. Epidermal growth factor receptor inhibitors in neuro-oncology: hopes and disappointments. Clin Cancer Res. 2008;14:957–960. doi: 10.1158/1078-0432.CCR-07-1810. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, De Angelis L, Raizer J, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- Chen JS, Zhou LJ, Entin-Meer M, Yang X, Donker M, Knight ZA, Weiss W, Shokat KM, Haas-Kogan D, Stokoe D. Characterization of structurally distinct, isoform-selective phosphoinositide 3′-kinase inhibitors in combination with radiation in the treatment of glioblastoma. Mol Cancer Ther. 2008;7:841–850. doi: 10.1158/1535-7163.MCT-07-0393. [DOI] [PubMed] [Google Scholar]
- Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma–animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, et al. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JF, Gilbert MR, Aldape K, Hess KR, Hanna TA, Ictech S, Groves MD, Conrad C, Colman H, Puduvalli VK, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90:89–97. doi: 10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty L, Gigas DC, Kesari S, Drappatz J, Kim R, Zimmerman J, Ostrowsky L, Wen PY. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology. 2006;67:156–158. doi: 10.1212/01.wnl.0000223844.77636.29. [DOI] [PubMed] [Google Scholar]
- Fan QW, Specht KM, Zhang C, Goldenberg DD, Shokat KM, Weiss WA. Combinatorial efficacy achieved through two-point blockade within a signaling pathway-a chemical genetic approach. Cancer Res. 2003;63:8930–8938. [PubMed] [Google Scholar]
- Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, Weiss WA. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, McCormick F, Shokat KM, Weiss WA. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-Site Inhibitors of mTOR Target Rapamycin-Resistant Outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi E, Cavallo G, Lonardi S, Magrini E, Tosoni A, Grosso D, Scopece L, Blatt V, Urbini B, Pession A, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96:1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, Peralba JM, Jenkins RB, Dakhil SR, Morton RF, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, Banks C, Capen A, Goode R, Lewis JE, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin ( LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- Haas-Kogan DA, Prados MD, Tihan T, Eberhard DA, Jelluma N, Arvold ND, Baumber R, Lamborn KR, Kapadia A, Malec M, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007;282:21206–21212. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- Opel D, Westhoff MA, Bender A, Braun V, Debatin KM, Fulda S. Phosphatidylinositol 3-kinase inhibition broadly sensitizes glioblastoma cells to death receptor- and drug-induced apoptosis. Cancer Res. 2008;68:6271–6280. doi: 10.1158/0008-5472.CAN-07-6769. [DOI] [PubMed] [Google Scholar]
- Persson A, Fan Q-W, Phillips J, Weiss WA. Glioma. Elsevier; San Diego: 2007. [Google Scholar]
- Prados MD, Lamborn KR, Chang S, Burton E, Butowski N, Malec M, Kapadia A, Rabbitt J, Page MS, Fedoroff A, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006;8:67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados MD, Chang SM, Butowski N, DeBoer R, Parvataneni R, Carliner H, Kabuubi P, Ayers-Ringler J, Rabbitt J, Page M, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27:579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevo R, Deutsch E, Sampson O, Diplexcito J, Cengel K, Harper J, O’Neill P, McKenna WG, Patel S, Bernhard EJ. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res. 2008;68:5915–5923. doi: 10.1158/0008-5472.CAN-08-0757. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Vredenburgh JJ, Gururangan S, Friedman AH, Desjardins A, Sathornsumetee S, Herndon JE, II, Dowell JM, McLendon RE, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–868. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mTOR inhibitor reveals rapamycin-insensitive functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Lu KV, Zhu S, Dia EQ, Vivanco I, Shackleford GM, Cavenee WK, Mellinghoff IK, Cloughesy TF, Sawyers CL, Mischel PS. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]