Abstract
Background
Ethinyl estradiol (EE) and progestins have the ability to alter endothelial function. The type of progestin and the ratio of EE to progestin used in oral contraceptive pills (OCPs) may determine how they affect the arterial vasculature.
Study Design
In this study, we investigated endothelial function across a cycle in very low dose (VLD) and low dose (LD) combination EE and desogestrel (DSG) OCP users during two phases: active (VLD=20 mcg EE/150 mcg DSG; LD=30 mcg EE/150 mcg DSG) and pill-free. Endothelial function was also measured during an EE-only hormone phase (10 mcg EE) in group VLD.
Results
Endothelium-dependent vasodilation was greater during the active phase compared to the pill-free phase in group LD (9.02±0.72% vs. 7.33±0.84%; p=.029). This phase difference was not observed in group VLD (5.86±0.63% vs. 6.56±0.70%; p=.108). However, endothelium-dependent vasodilation was higher during the EE-only phase, compared to the active and pill-free phases (8.92±0.47% vs. 5.86±0.63%, and 6.56±0.70%; pb.001) in group VLD.
Conclusions
These data suggest DSG may antagonize the vasodilatory activity of EE and that this effect is further modulated by the EE-toDSG ratio.
Keywords: Progestin, Estrogen, Flow-mediated dilation, Birth control pills, Cardiovascular risk
1. Introduction
It is estimated that 78.5 million women worldwide currently use oral contraceptive pills (OCPs) and approximately 150 million women have used OCPs at some point in their lives [1]. OCP use has been associated with increased cardiovascular risk and, specifically, linked to a greater occurrence of adverse events in both the venous and arterial vasculature [2-7]. The increased risk of adverse venous events is thought to be primarily due to high ethinyl estradiol (EE) doses used in OCPs [8-10], but the link between OCPs and increased risk of adverse arterial events is still unclear.
Part of the confusion and complexity regarding this relationship stems from the grouping of OCPs into one general class. There are currently over 70 different combination OCPs offered in the United States, with many different formulations, doses and ratios of synthetic hormones being offered. These differences make each type of OCP a unique hormone treatment, and thus, the effect of different OCPs on cardiovascular health should be considered independently.
It has been well established that estrogen is beneficial to the health of the arterial system in women [11-14]. Endothelium-dependent vasodilation of the brachial artery, which has been shown to be a highly predictive measure of coronary artery health [15], increases significantly during the ovulatory phase of the menstrual cycle, when estrogen levels are highest in naturally cycling young women [16-18].It is also well documented that synthetic forms of estrogen, such as EE, have a cardioprotective effect on the arterial system [19]. Older formulations of OCPs contain ≥50 mcg of EE. This dose has been decreased substantially due to the association between high doses of EE and increased adverse events in the venous system [8-10]. Currently, the majority of combination OCPs contain either 30 or 20 mcg of EE and are referred to as “low dose” and “very low dose” OCPs, respectively. Unfortunately, when the standard dose of estrogen used in OCPs was lowered to reduce adverse events in the venous system and combined with second- and third-generation progestins, the incidence of adverse events in the arterial system remained elevated [2]. Based on this evidence and other studies showing that some progestins may antagonize the beneficial vascular effects of estrogen in postmenopausal women [20-23], it is logical that the type of progestin and the ratio of EE to progestin used in OCPs may determine how different OCPs affect the arterial vasculature in young women.
Each progestin can have dramatically different effects on the body based on the parent molecule from which it was created, its chemical structure, pharmacokinetics, activity and specificity to given receptors [24-27]. Some progestins antagonize the beneficial effects of estrogens [20-23], while others have no effect [28]. Newer third-generation progestins, including desogestrel (DSG), were specifically designed to be less androgenic. It is the androgenic properties inherent to certain progestins that have been blamed for antagonizing the beneficial cardiovascular effects of estrogen [29,30].To date, there have been no previous studies looking at endothelial function across the OC cycle in women using combination EE and DSG OCPs.
Therefore, the purpose of this study was twofold. The first goal was to evaluate endothelial function during the active (EE and DSG elevated) and pill-free (no exogenous hormones given) phases in young women taking very low dose (VLD) and low-dose (LD) combination EE and DSG OCPs. The dose of DSG prescribed in the active pills of the LD and VLD formulations was the same (150 mcg), but the dose of EE (20 vs. 30 mcg) was different. This design allowed us to gain insight as to how the EE-to-DSG ratio of commonly prescribed formulations of DSG-containing OCPs impacts endothelial function in reproductive-aged women. The second goal of this study was to assess endothelial function during an EE-only phase in group VLD in order to separate the independent effects of EE and DSG on endothelial function in young women. In addition to endothelial function, we also measured blood pressure and specific biomarkers of cardiovascular health across the cycle of DSG-containing OCP use, as this information is not currently available in the literature. We hypothesized that the ratio of DSG to EE would alter the magnitude of change in endothelial function in a dose-dependent manner. We further hypothesized that DSG would antagonize the beneficial vascular effects of EE on endothelial function in young women.
2. Methods
Twenty-two female subjects between the ages of 18 and 30 years completed this protocol. All subjects were currently taking a monophasic combination OCP with EE and DSG for ≥4 months as prescribed by their health care provider. All subjects were healthy, normally active (exercise ~1–3 days/week for ≤1 h), nonsmokers and were not taking any other medications. All subjects were screened to ensure they did not have any of the following health conditions: cardiovascular disease, obesity, hypertension, hypercholesterolemia, metabolic disorders, menstrual disorders, or a personal or family history of blood clots. Subjects were required to take a pregnancy test and show negative results immediately prior to the start of each study day. Approval of this study was granted by the Institutional Review Board of the University of Oregon. Each subject provided oral as well as written consent prior to participation.
The subject pool was divided into two groups of 11 based on the dose of exogenous hormones in their OCPs. The VLD group consisted of females using OCPs with 20 mcg EE+150 mcg DSG in each daily pill during Weeks 1, 2 and 3. Unlike most OCPs, the fourth week of pills in group VLD consists of 10 mcg EE (Mircette: Duramed Pharmaceuticals, Pomona, NY, USA; or Kariva: Barr Pharmaceuticals, Woodcliff, NY, USA). In contrast, the LD group consisted of females using OCPs with 30 mcg EE+150 mcg DSG in each daily pill during Weeks 1, 2 and 3, and no exogenous hormones during Week 4 (Apri: Barr Laboratories, Woodcliff, NY, USA; Desogen: Organon, West Orange, NJ, USA; or Ortho-Cept: Ortho-McNeil Pharmaceutical, Raritan, NJ, USA).
Subjects in group VLD participated in the experimental protocol on three different study days: once between Days 5 and 7 of Week 3, after taking 3 weeks of active pills (active phase); once between Days 5 and 7 of Week 4, after 1 week of EE-only pills (EE-only phase); and once between Days 5 and 7 of Week 4 after abstaining from taking any Week 4 pills (pill-free phase) of a different pill cycle (due to the 10-mcg dose of EE in the Week 4 pills in group VLD, these women simply abstained from taking these pills during Week 4 of a subsequent pill pack to allow us to study them during the pill-free phase). The ability to study group VLD across three different hormone profiles allowed us to tease apart independent effects of the EE and DSG components of these OCPs. Subjects in group LD participated in the experimental protocol on two different study days: once between Days 5 and 7 of Week 3, after taking 3 weeks of active pills (active phase), and once between Days 5 and 7 of Week 4, after 1 week of placebo pills containing no exogenous hormone (pill-free phase). Subjects gave verbal confirmation that they had taken their pills as directed. The order of experimental study days was randomized and counterbalanced. Subjects abstained from exercise, vitamins, alcohol and over-the-counter medications for 24 h and abstained from food and caffeine for 12 h prior to participating in each study day. All studies were conducted at the same time of day for each subject between the hours of 6:00 and 11:00 a.m. in a temperature-controlled room (22–24°C).
2.1. Protocol
Subjects were instrumented with electrocardiogram (ECG), and three cuffs: one blood pressure cuff on the ring finger of the left hand, one blood pressure cuff on the left upper arm and one occlusion cuff on the right forearm just below the antecubital fossa. Subjects were positioned supine with their right arm supported at heart level at an 80–90° angle from their torso. Subjects rested for 10–15 min in this position prior to the collection of venous blood samples. Next, subjects continued to rest in the supine position for 45–60 min before collection of endothelium-dependent vasodilation measurements, followed by a 20-min rest period and subsequent collection of endothelium-independent vasodilation measurements.
2.1.1. Endothelium-dependent vasodilation
After obtaining a clear image of the brachial artery with the transducer held in place above the subject's upper arm with a stereotactic clamp, 2 min of baseline data was recorded before rapidly inflating (E20 Rapid Cuff Inflater, D.E. Hokanson, Bellevue, WA, USA) the occlusion cuff (Zimmer, Dover, OH, USA) on the subject's forearm to 300 mmHg. The pressure in the occlusion cuff was held at 300 mmHg for 5 min and then rapidly deflated. Upon release of the occlusion cuff, arterial blood flow through the brachial artery increases and imposes a shear stress on the vascular endothelium, resulting in vasodilation. This vasodilation has been termed flow-mediated vasodilation (FMD) and is primarily dependent on the release of the potent vasodilator nitric oxide from endothelial cells [31]. The percent change in brachial artery diameter in response to FMD assesses endothelium-dependent vasodilation. Data collection continued for 2 min post-cuff release.
2.1.2. Endothelium-independent vasodilation
Subjects rested in the supine position for 20 min after completing the endothelium-dependent, flow-mediated vasodilation testing. Subsequently, 2 min of baseline data was collected for the endothelium-independent vasodilation test,followedbytheadministrationof0.04mgof sublingual nitroglycerin and 10 min of data recording. The percent change in brachial artery diameter in response to nitroglycerin administration assesses endothelium-independent vasodilation.
2.2. Measurement techniques
2.2.1. Blood samples
Venous blood samples were collected on each study day from an antecubital vein for a complete lipid panel analysis, consisting of low-density lipoproteins (LDL), high-density lipoproteins (HDL), total cholesterol (TC), triglycerides (TRG), TC-to-HLD ratio (TC/HDL ratio), high-sensitivity C-reactive protein (hs-CRP) and a coronary risk index. The coronary risk index is derived from the combination of risks of hs-CRP and TC/HDL ratio [32,33]. Blood samples were collected into 7.5-mL serum separator blood collection tubes (BD Vacutainer; Franklin, NJ, USA). The samples were centrifuged at 1300×g relative centrifugal force for 15 min at 4°C, separated and stored frozen at −70°C within 30 min until transport to Oregon Medical Laboratories (Eugene, OR, USA) for analysis. We choose not to analyze endogenous estrogen and progesterone on each study day as previous experiments from our laboratory have verified adequate suppression of these hormones during OCP use [34].
2.2.2. Heart rate and blood pressure
Heart rate and blood pressure were measured continuously throughout the protocol. Heart rate was measured using a five-lead ECG (CardioCap, Datex-Ohmeda, Louisville, CO, USA) dually interfaced with our data acquisition computer and Doppler ultrasound system. Blood pressure was measured using a finger blood pressure cuff (Portapres model-2, TNO-TPD, Biomedical Instrumentation, Amsterdam, Netherlands). Blood pressures from the finger blood pressure cuff were corrected against arm blood pressure measured noninvasively from the left arm via automated brachial auscultation (CardioCap, Datex-Ohmeda, Louisville, CO, USA).
2.2.3. Brachial artery diameter and blood velocity
Brachial artery diameter and blood velocity were assessed by imaging the brachial artery using a Doppler ultrasound machine (12XP, Acuson, New York, NY, USA) with a 7.0-MHz linear array transducer. The transducer was placed approximately 3–10 cm proximal to the antecubital fossa. Within this range, we selected the area with the greatest clarity of the near and far intimal–medial borders of the arterial wall and fixed the transducer in place over the subject's right arm with a stereotactic clamp. The arm and transducer remained in this position for the remainder of the study. Ultrasound parameters were set to optimize longitudinal, B-mode images of the lumen-arterial wall interface while insonating the lumen of the artery at an angle of 60° to determine blood velocity. Brachial artery diameter and blood velocity were recorded continuously throughout endothelium-dependent and endothelium-independent vasodilation.
2.3. Data analysis
2.3.1. Heart rate and blood pressure
Heart rate and blood pressure signals were digitized and stored on a computer at 20 Hz and saved for later analysis with signal processing software (WinDaq, DataQ Instruments, Akron, OH, USA).
2.3.2. Vascular function
Brachial artery diameter and blood velocity were recorded to a computer interfaced with custom-designed edge detection and wall-tracking analysis software (DICOM; Perth, Australia). The custom analysis software allows real-time video images of the brachial artery to be captured from the ultrasound machine, encoded and stored at 30 frames per second for later analysis of vessel diameter in synchrony with end diastole [35]. Endothelium-dependent, flow-mediated dilation was calculated as the percent change in brachial artery diameter from baseline to post-cuff release (FMD=(FMD peak diameter (mm)−baseline diameter (mm))/baseline diameter (mm))×100). Likewise, endothelium-independent, nitroglycerine-mediated vasodilation was calculated as the percent change in brachial artery diameter from baseline to post-nitroglycerin administration (nitroglycerin-mediated dilation=(nitroglycerin peak diameter (mm)−baseline diameter (mm))/baseline diameter (mm))×100). This software allows for more accurate and reproducible analysis measurements by decreasing observer error and eliminating observer bias. This software can detect a 1.5–2.0% change in endothelium-dependent dilation in six to eight subjects with a power of 80% and in seven to 11 subjects with a power of 90%, substantially decreasing the number of subjects needed to detect significant differences compared to other methods of analysis [35].
2.3.3. Flow-mediated vasodilation stimulus quantification
In order to assess the intensity of the FMD stimulus, shear rate was calculated by dividing blood velocity (centimeters per second) by diameter (millimeter) [36,37], and the time to peak (TTP) diameter during the endothelium-dependent vasodilation test was also determined [38]. Because the TTP vasodilation is highly variable [39,40], we plotted shear rate vs. time and determined the TTP shear rate area under the curve (TTP SR AUC) [38]. We measured and reported the peak endothelium-dependent vasodilation response of the brachial artery as a percent change from baseline independently and normalized to the shear rate stimulus (%FMD/TTP SR AUC) [38]. We observed no statistical differences when evaluating the independent and normalized endothelium-dependent vasodilation data in this study. However, we have reported the data in both forms for comparison.
2.4. Reproducibility
The intra-observer variability of brachial artery diameter measurements was assessed by comparing baseline diameter measurements prior to endothelium-dependent vasodilation in each of the subjects across study days. The coefficient of variation (SD/mean×100) was 1.9% for this study.
2.5. Statistical analysis
Subject characteristics between group VLD and group LD were compared using t tests. Within-group comparisons were made using one-way repeated measures ANOVAs and post hoc paired t tests for group VLD and group LD, respectively. Statistical significance was defined as pb.05. All data are expressed as mean±SE.
3. Results
3.1. Subject characteristics
Table 1 summarizes the physical characteristics of both subject groups. There was no difference in age, height, weight, BMI or the amount of time each group had been using EE/DSG OCPs. Table 2 displays the blood pressures and cardiovascular biomarker characteristics for each hormone phase of the OCP cycle for group LD and group VLD. There was no difference in HDL, TC, TRG, hs-CRP or the coronary risk index between hormone phases in group VLD. However, LDL was significantly greater in the pill-free phase compared to the active phase (p=.039), and the TC/HDL ratio was greater in the pill-free phase compared to the EE-only phase (p=.017) in this group (Table 2). In group LD, there were no differences in HDL, hs-CRP or the coronary risk index, but there were significant differences in LDL, TC, TRG and the TC/HDL ratio. LDL, TC and the TC/HDL ratio were significantly greater during the pill-free phase compared to the active phase (LDL: p=.004; TC: p=.006; TC/HDL ratio: p=.012), yet TRG were significantly greater in the active phase compared to the pill-free phase (p=.028) in group LD (Table 2). There were no significant differences in baseline heart rate, systolic blood pressure, diastolic blood pressure or mean arterial pressure between hormone phases in either group.
Table 1.
Baseline physical characteristics in group VLD and group LD
| Group VLD (n=11) |
Group LD (n=11) |
p value | |
|---|---|---|---|
| Age, year | 23±1 | 22±1 | .476 |
| Weight, kg | 61.2±2.9 | 65.8±3.1 | .292 |
| Height, cm | 165.6±2.1 | 167.5±1.7 | .492 |
| Body mass index, kg/m2 | 22.5±0.9 | 23.6±1.2 | .405 |
| Time on oral contraceptives, months | 18±3 | 15±3 | .453 |
Values are means±SE.
Table 2.
Cardiovascular markers across oral contraceptive phases in group VLD and group LD
| Group VLD (n=11) |
p value | Group LD (n=11) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Active | EE | Pill-free | Active | Pill-free | |||
| HDL, mg/dL | 56±4 | 63 ±4 | 58±5 | .082 | 56±2 | 58±3 | .129 |
| LDL, mg/dL | 90±6 | 99±9 | 106±7* | .039 | 100±6 | 117±6 | .004 |
| TC, mg/dL | 170±9 | 182± | 186±11 | .099 | 175±6 | 191±7 | .006 |
| TRG, mg/dL | 119±15 | 100±18 | 109±20 | .428 | 96±9 | 80±9 | .028 |
| TC/HDL ratio, mg/dL | 3.1±0.1 | 2.9±0.1 | 3.3±0.2† | .017 | 3.2±0.2 | 3.4±0.2 | .012 |
| hs-CRP, mg/L | 3.88±0.75 | 3.74±0.91 | 2.72±0.97 | .474 | 2.29±0.40 | 1.94±0.40 | .203 |
| Coronary risk index | 2.0±0.1 | 1.9±0.2 | 1.9±0.3 | .817 | 2.1±0.5 | 2.5±0.5 | .349 |
| Blood pressure, mmHg | |||||||
| Systolic | 116±2 | 118±3 | 117±2 | .648 | 109±2 | 111±2 | .151 |
| Diastolic | 75±2 | 76±2 | 78±2 | .074 | 69±1 | 70±2 | .503 |
| Mean arterial | 88±2 | 90±2 | 91±2 | .292 | 83±1 | 84±2 | .300 |
| Heart rate, bpm | 64±3 | 62±3 | 63±3 | .081 | 60±3 | 61±2 | .352 |
Values are means±SE.
p=.039 vs. active.
p=.017 vs. EE.
3.2. Endothelium-dependent vasodilation
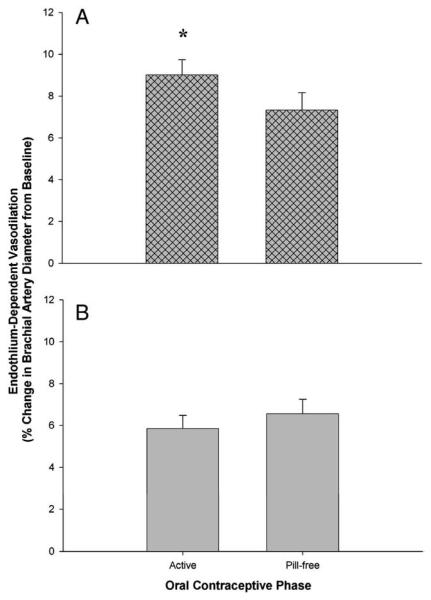
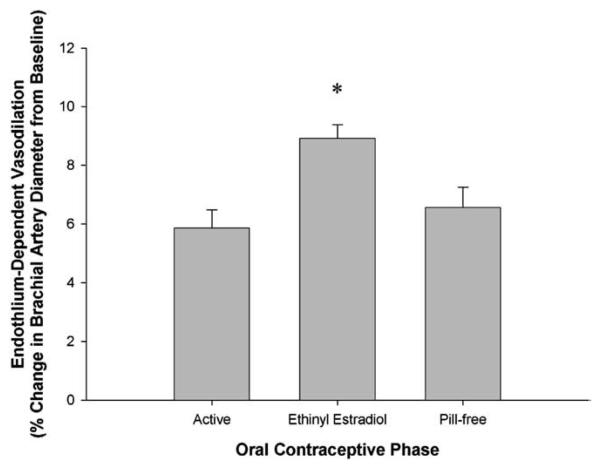
Table 3 displays the primary endothelial function values for each hormone phase of group LD and group VLD. In group LD, FMD was greater during the active phase than during the pill-free phase (p=.029; Fig. 1A). In contrast, there was no difference in FMD between the active and pill-free phase in group VLD (p=.108; Fig. 1B). However, FMD was greater during the EE-only phase than during the active and pill-free phases (p<.001; Fig. 2) in group VLD. There were no differences in baseline brachial artery diameters or shear rate between hormone phases in either group. This finding verifies that our flow-mediated stimulus for endothelium-dependent vasodilation was consistent across all study days in both groups.
Table 3.
Endothelial function across oral contraceptive phases in group VLD and group LD
| Group VLD (n=11) |
p value | Group LD (n=11) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Active | EE | Pill-free | Active | Pill-free | |||
| Baseline FMD diameter, mm | 3.15±0.14 | 3.19±0.12 | 3.21±0.11 | .560 | 3.16±0.13 | 3.18±0.13 | .193 |
| FMD, % change | 5.86±0.63 | 8.92±0.47* | 6.56±0.70 | .001 | 9.02±0.72 | 7.33±0.84 | .029 |
| TTP, s | 58±8 | 50±7 | 59±11 | .747 | 47±6 | 48±5 | .851 |
| Shear rate TTP AUC, velocity/diameter | 6275±479 | 6237±556 | 6761±652 | .533 | 6022±495 | 6604±544 | .151 |
| Normalized response, % FMD/shear rate TTP AUC | 0.0010±0.0017 | 0.0016±0.0002* | 0.0011±0.0002 | .006 | 0.0016±0.0001 | 0.0011±0.0001 | .030 |
| Baseline NTG diameter, mm | 3.14±0.13 | 3.21±0.13 | 3.21±0.12 | .219 | 3.13±0.13 | 3.17±0.13 | .115 |
| NTG, % change | 20.56±1.96 | 20.97±1.91 | 20.22±1.58 | .840 | 23.29±1.75 | 22.60±1.99 | .565 |
Values are means±SE.
Significantly different from active and pill-free phases.
Fig. 1.
Endothelium-dependent, flow-mediated vasodilation of the brachial artery during the active and pill-free OCP phases in (A) group LD and (B) group VLD. Values are means±SE. *p=.029 vs. pill-free phase.
Fig. 2.
Endothelium-dependent, flow-mediated vasodilation of the brachial artery during the active, EE and pill-free OCP phases in group VLD. Values are means±SE. * p<.001 vs. active and pill-free phases.
3.3. Endothelium-independent vasodilation
There were no differences in endothelium-independent vasodilation between the active phase, the estrogen phase and the pill-free phase of group VLD or between the active and pill-free phase of group LD.
4. Discussion
This is the first study to investigate vascular function across the cycle in young women taking LD and VLD combination EE and DSG OCPs. Studying these two groups of women during their active pill phase, when EE and DSG were elevated, and during the pill-free phase, allowed us to map the fluctuation patterns in endothelium-dependent vasodilation across the cycle in both of these groups and allowed us to gain insight as to the importance of the EE-to-progestin ratio used in OCPs containing DSG. In addition, this is the first study to investigate vascular function during an EE-only OCP phase in young women, which allowed us to tease out the individual effects of EE and DSG and better understand their interaction when given together in combination OCPs.
In support of our hypothesis, we found that endothelium-dependent vasodilation was higher in the active phase compared to the pill-free phase in group LD and, in contrast to our hypothesis, that there was no difference between the active and pill-free phase in group VLD. This indicates that the EE-to-progestin ratio used in OCPs determines how OCP formulations impact vascular function in young women. Furthermore, we found that endothelium-dependent vasodilation was greater during the EE phase, when EE was given unopposed by DSG, than during both the active and pill-free phases in group VLD. These data provide evidence that the commonly used progestin, DSG, antagonizes the vasodilatory properties of EE.
There was no difference in endothelium-dependent vasodilation between the active and pill-free phases in group VLD. However, we saw a profound and significant rise in endothelium-dependent vasodilation when EE was given unopposed by DSG in our subjects. Interestingly, the dose of EE taken unopposed was 10 mcg, half the dose of EE given in the active pill phase. This supports previous findings that EE appears to be beneficial to the arterial vasculature [19] even at relatively low doses and expands upon it to show that EE increases endothelium-dependent vasodilation in young women using combination OCPs. Furthermore, because endothelium-dependent vasodilation was not different between the active phase and the pill-free phase suggests that DSG antagonized EE during the active phase in group VLD. Although there is support that androgenic progestins have the ability to antagonize estrogens [29,30], this is the first study to provide evidence that DSG antagonizes EE in oral contraceptive users, despite its lower androgenic properties compared to earlier generations of progestins.
When compared to the second-generation progestin levonorgestrel, DSG has significantly fewer androgenic effects [41,42]. Torgrimson et al. [34] observed that endothelium-dependent vasodilation was lower during the active phase, when EE and levonorgestrel levels were high, compared to the pill-free phase, when no hormones were administered in women taking VLD combination EE and levonorgestrel OCPs. The authors concluded that levonorgestrel was antagonizing the beneficial effects of EE. Likewise, our data in group VLD suggest that DSG antagonizes EE, but if we compare the endothelium-dependent vasodilation fluctuation patterns from the active to the pill-free phase in our study to that of Torgrimson et al. [34], the patterns are different. Endothelium-dependent vasodilation was lower in the active phase than during pill-free phase in combination OCPs containing levonorgestrel, while there was no difference between these phases in our study on women taking combination OCPs containing DSG. Thus, it appears that levonorgestrel may have stronger antagonistic properties than DSG. This finding is supported by previous speculation that progestins with greater androgenic properties have greater antagonistic effects on estrogens [29,30].
In contrast, endothelium-dependent vasodilation was higher in the active phase compared to the pill-free phase in group LD. Recall that the doses of DSG in both the LD and the VLD groups were the same (150 mcg), but the dose of EE was different (LD=30 mcg and VLD=20 mcg). Consequently, endothelium-dependent vasodilation was greater in the active phase than in the pill-free phase in group LD, which had a higher EE dose, but endothelium-dependent vasodilation was not different between the active and pill-free phases in group VLD, which had a lower EE dose. This difference between the LD and VLD group calls attention to the importance of the EE dose, or more specifically, the ratio of EE to DSG used in OCPs. The ratio of EE to DSG in the active phase is 1:7.5 for group VLD and 1:5 for group LD. This suggests that endothelium-dependent vasodilation may have been greater in the active phase than in the pill-free phase in group LD due to the lower ratio of EE to DSG. Owing to our findings in the estradiol-only phase of group VLD, it is possible that DSG is still having an antagonistic effect on EE in group LD, but that the greater EE-to-progestin ratio allows the balance to be shifted, such that the vasodilatory properties of EE are great enough to outweigh the antagonistic properties of DSG in the LD formulation. This finding supports the concept that the ratio of EE to progestin used in OCPs determines the fluctuation patterns in endothelium-dependent vasodilation across an OCP cycle.
Many observational studies show that a relationship exists between levels of cholesterol and endothelial dysfunction [43-45]. OCPs have the ability to alter lipid profiles in women [46-49], but the direction and magnitude of these alterations are not consistent between studies. Interestingly, it has been suggested that the dose of EE and the type of progestin used in OCPs may determine how OCPs affect lipid profiles [48]. Therefore, the inconsistent observations in this area of research may be due to the grouping of different OCP formulations together. To date, the majority of literature regarding lipids and OCPs has focused on the differences in lipid profiles between nonOCP users and OCP users. These studies have found that estrogens generally tend to increase TG and HDL, and decrease LDL, while androgenic progestins tend to have an opposite effect [46]. Therefore, we performed a full lipid panel in our subjects and found statistically significant changes in some parameters across the OCP cycle. In group VLD, LDL and the TC/HDL ratio were greater in the pill-free phase compared to the active phase and the EE phase, respectively. In group LD, LDL, TC and the TC/HDL ratio were significantly greater during the pill-free phase compared to the active phase, yet TRG were significantly greater in the active phase compared to the pill-free phase. This finding is supported by multiple studies looking at lipid changes with the onset of combination EE and DSG OCP use in which similar changes were observed: LDL-C decreased while HDL-C and TRG increased [50,51].In addition, a study by Akerlund et al. [52] found that the ratio of EE to DSG in OCPs affected the type and magnitude of lipid changes observed 1 year after starting OCPs. Women taking a combination OCP with 150 mcg DSG+30 mcg EE had a significant increase in TC, HDL and TG over the course of a year, but the changes in TC and HDL were not observed in women taking a combination OCP with 150 mcg DSG+20 mcg EE. The group of women using an OCP with 150 mcg DSG+20 mcg EE had an increase in TG, no difference in TC and HDL, and a decrease in LDL, which suggests that the ratio of EE to DSG affects the long-term changes in lipids that occur with OCP use [52]. Although there are no data specifically looking at acute changes in lipids across the active and low hormone phases of combination EE and DSG OCP use, it is clear that EE and DSG affect lipid levels in young women, may have opposing effects on lipids and that the concentration and/or ratio of these synthetic hormones used in OCPs may determine their impact on circulating lipid concentrations in young women.
It is difficult to speculate as to how the small changes in cholesterol observed across the study affect endothelium-dependent vasodilation in our subjects, as the trends did not appear to mirror those observed in endothelium-dependent vasodilation, especially in group VLD. However, the lipid profile in group LD did appear to be slightly better during the active phase, in which endothelium-dependent vasodilation was also greater than the pill-free phase.
4.1. Technical considerations and limitations
There was no difference in shear rate across hormone phases in either group in the present study, which ensures that the magnitude of the FMD stimulus was the same for each study day [36,37]. Furthermore, we did not observe differences between baseline diameter, blood pressures or heart rates across phases in either group. These baseline data suggest that changes in endothelium-dependent vasodilation observed across hormone phases are likely not due to changes in blood pressure, sympathetic nerve activity, or vascular tone [37], but due to the direct effects of the different hormone profiles under which our subjects were studied. Moreover, there were no differences between endothelium-independent, nitroglycerin-mediated vasodilation between phases in either group. This solidifies that all observed changes in FMD across the OCP cycle were due to changes in the vascular endothelium and not due to the sensitivity of the vascular wall to nitric oxide.
We do not believe our primary findings would be altered by increasing the sample size in the present study. The differences we observed were highly significant and our sample size of 11 subjects per group is greater than the estimated sample size required to detect significant changes in endothelium-dependent vasodilation in a repeated measures study design using our custom edge-detection software [35]. Therefore, we feel confident that the statistically significant and important differences in endothelium-dependent vasodilation observed in each group are valid.
5. Conclusions and perspectives
This study, along with previous studies from our laboratory [34], demonstrates that when studying OCP users two things should be considered. First, depending on the study question, it may not always be appropriate to group women taking different formulations of OCPs together, because different OCPs can have dramatically different effects on a woman's physiology. Second, cycle phase should also be considered when studying OCP users, as it has been clearly demonstrated that the different hormone profiles inherent to OCPs can change physiological factors across the cycle [34,53,54].
It has been suggested that endothelial dysfunction may be the pathogenic link between OCP use and an increase in cardiovascular risk [55]. This study uncovered the importance of the ratio of EE to progestin used in OCPs on the fluctuations in endothelium-dependent vasodilation across the cycle. Although we do not know the direct link between fluctuation patterns during reproductive years and cardiovascular risk, we have speculated that fluctuations in endothelial function similar to those observed in naturally cycling women [16-18] may be advantageous [34].
In summary, the present study demonstrated that endothelium-dependent vasodilation was higher during the active phase than during the pill-free phase in group LD, but no difference was observed between these phases in group VLD. In addition, endothelium-dependent vasodilation was the greatest when EE was administered alone. Together, these data suggest that the progestin DSG antagonizes the vasodilatory properties of EE in young women taking combination OCPs. This finding highlights the potential significance of the type of progestin used in combination OCPs. Furthermore, these data bring to light the importance of the EE-to-progestin ratio to the acute function of the arterial vasculature when using a combination OCP with an antagonistic progestin. Because this study measured surrogate markers of arterial cardiovascular risk, future research comparing the long-term effects of contraceptives with different types of progestins and different EE-to-progestin ratios on the arterial vasculature is warranted.
Acknowledgments
The authors extend our appreciation to the research subjects who participated in this study. Additionally, we gratefully acknowledge the assistance of Sarah Williams and Sarah Luther in data collection.
Footnotes
This study was supported by the Northwest Health Foundation Grant #446400 and the Center for the Study of Women in Society Grant (to Jessica Meendering).
References
- 1.Rosenfield A. Oral and intrauterine contraception: a 1978 risk assessment. Am J Obstet Gynecol. 1978;132:92–106. doi: 10.1016/0002-9378(78)90806-2. [DOI] [PubMed] [Google Scholar]
- 2.Baillargeon JP, McClish DK, Essah PA, Nestler JE. Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: a meta-analysis. J Clin Endocrinol Metab. 2005;90:3863–70. doi: 10.1210/jc.2004-1958. [DOI] [PubMed] [Google Scholar]
- 3.Burkman RT. Oral contraceptives: current status. Clin Obstet Gynecol. 2001;44:62–72. doi: 10.1097/00003081-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Burkman RT, Collins JA, Shulman LP, Williams JK. Current perspectives on oral contraceptive use. Am J Obstet Gynecol. 2001;185:S4–S12. doi: 10.1067/mob.2001.117416. [DOI] [PubMed] [Google Scholar]
- 5.Carr BR, Ory H. Estrogen and progestin components of oral contraceptives: relationship to vascular disease. Contraception. 1997;55:267–72. doi: 10.1016/s0010-7824(97)00029-2. [DOI] [PubMed] [Google Scholar]
- 6.Dalen JE, Hickler RB. Oral contraceptives and cardiovascular disease. Am Heart J. 1981;101:626–39. doi: 10.1016/0002-8703(81)90231-3. [DOI] [PubMed] [Google Scholar]
- 7.Heinemann LA. Emerging evidence on oral contraceptives and arterial disease. Contraception. 2000;62(Suppl):29S–36S. doi: 10.1016/s0010-7824(00)00146-3. discussion 7S-8S. [DOI] [PubMed] [Google Scholar]
- 8.Böttiger LE, Boman G, Eklund G, Westerholm B. Oral contraceptives and thromboembolic disease: effects of lowering oestrogen content. Lancet. 1980;1:1097–101. doi: 10.1016/s0140-6736(80)91550-0. [DOI] [PubMed] [Google Scholar]
- 9.Gerstman BB, Piper JM, Tomita DK, Ferguson WJ, Stadel BV, Lundin FE. Oral contraceptive estrogen dose and the risk of deep venous thromboembolic disease. Am J Epidemiol. 1991;133:32–7. doi: 10.1093/oxfordjournals.aje.a115799. [DOI] [PubMed] [Google Scholar]
- 10.Vessey M, Mant D, Smith A, Yeates D. Oral contraceptives and venous thromboembolism: findings in a large prospective study. BMJ (Clin Res Ed) 1986;292:526. doi: 10.1136/bmj.292.6519.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cid MC, Schnaper HW, Kleinman HK. Estrogens and the vascular endothelium. Ann N Y Acad Sci. 2002;966:143–57. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- 12.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89:12E–8E. doi: 10.1016/s0002-9149(02)02405-0. [DOI] [PubMed] [Google Scholar]
- 13.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., III Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–91. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 14.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615–24. [PubMed] [Google Scholar]
- 15.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 16.Kawano H, Motoyama T, Kugiyama K, et al. Menstrual cyclic variation of endothelium-dependent vasodilation of the brachial artery: possible role of estrogen and nitric oxide. Proc Assoc Am Physicians. 1996;108:473–80. [PubMed] [Google Scholar]
- 17.Hashimoto M, Akishita M, Eto M, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–5. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 18.English JL, Jacobs LO, Green G, Andrews TC. Effect of the menstrual cycle on endothelium-dependent vasodilation of the brachial artery in normal young women. Am J Cardiol. 1998;82:256–8. doi: 10.1016/s0002-9149(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 19.Reis SE, Gloth ST, Blumenthal RS, Resar JR, Zacur HA, Gerstenblith G. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89:52–60. doi: 10.1161/01.cir.89.1.52. [DOI] [PubMed] [Google Scholar]
- 20.Faludi AA, Aldrighi JM, Bertolami MC, et al. Progesterone abolishes estrogen and/or atorvastatin endothelium dependent vasodilatory effects. Atherosclerosis. 2004;177:89–96. doi: 10.1016/j.atherosclerosis.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Kawano H, Motoyama T, Hirai N, et al. Effect of medroxyprogesterone acetate plus estradiol on endothelium-dependent vasodilation in postmenopausal women. Am J Cardiol. 2001;87:238–40. doi: 10.1016/s0002-9149(00)01329-1. A9. [DOI] [PubMed] [Google Scholar]
- 22.The Writing Group for the PEPI Trial Effects of estrogen or estrogen/ progestin regimens on heart disease risk factors in postmenopausal women. The postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 23.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation. 2001;104:1773–8. doi: 10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- 24.Benagiano G, Primiero FM, Farris M. Clinical profile of contraceptive progestins. Eur J Clin Chem Clin Biochem. 2004;9:182–93. doi: 10.1080/13625180400007736. [DOI] [PubMed] [Google Scholar]
- 25.Hammond GL, Rabe T, Wagner JD. Preclinical profiles of progestins used in formulations of oral contraceptives and hormone replacement therapy. Am J Obstet Gynecol. 2001;185(2 Suppl):S24–31. doi: 10.1067/mob.2001.117415. [DOI] [PubMed] [Google Scholar]
- 26.Sitruk-Ware R. New progestogens: a review of their effects in perimenopausal and postmenopausal women. Drugs Aging. 2004;21:865–83. doi: 10.2165/00002512-200421130-00004. [DOI] [PubMed] [Google Scholar]
- 27.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–90. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Squadrito F, Altavilla D, Crisafulli A, et al. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med. 2003;114:470–6. doi: 10.1016/s0002-9343(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 29.van Rooijen M, von Schoultz B, Silveira A, Hamsten A, Bremme K. Different effects of oral contraceptives containing levonorgestrel or desogestrel on plasma lipoproteins and coagulation factor VII. Am J Obstet Gynecol. 2002;186:44–8. doi: 10.1067/mob.2002.119179. [DOI] [PubMed] [Google Scholar]
- 30.Ganz P. Vasomotor and vascular effects of hormone replacement therapy. Am J Cardiol. 2002;90:11F–6F. doi: 10.1016/s0002-9149(01)02218-4. [DOI] [PubMed] [Google Scholar]
- 31.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-Reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 34.Torgrimson BT, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol. 2007;288:H103–10. doi: 10.1152/ajpheart.00762.2006. [DOI] [PubMed] [Google Scholar]
- 35.Woodman RJ, Playford DA, Watts GF, et al. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol. 2001;91:929–37. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- 36.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 37.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–9. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 39.Berry KL, Skyrme-Jones RA, Meredith IT. Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery after different circulatory occlusion conditions. Clin Sci. 2000;99:261–7. [PubMed] [Google Scholar]
- 40.Naylor LH, Weisbrod CJ, O'Driscoll G, Green DJ. Measuring peripheral resistance and conduit arterial structure in humans using Doppler ultrasound. J Appl Physiol. 2005;98:2311–5. doi: 10.1152/japplphysiol.01047.2004. [DOI] [PubMed] [Google Scholar]
- 41.Lammers P, Blumenthal PD, Huggins GR. Developments in contraception: a comprehensive review of desogen (desogestrel and ethinyl estradiol) Contraception. 1998;57(Suppl):1S–27S. doi: 10.1016/s0010-7824(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 42.Stone SC. Desogestrel. Clin Obstet Gynecol. 1995;38:821–8. doi: 10.1097/00003081-199538040-00017. [DOI] [PubMed] [Google Scholar]
- 43.Vogel RA. Cholesterol lowering and endothelial function. Am J Med. 1999;107:479–87. doi: 10.1016/s0002-9343(99)00261-2. [DOI] [PubMed] [Google Scholar]
- 44.Vogel RA, Corretti MC, Gellman J. Cholesterol, cholesterol lowering, and endothelial function. Prog Cardiovasc Dis. 1998;41:117–36. doi: 10.1016/s0033-0620(98)80008-x. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson IB, Cockcroft JR. Cholesterol, endothelial function and cardiovascular disease. Curr Opin Lipidol. 1998;9:237–42. doi: 10.1097/00041433-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Fotherby K. Oral contraceptives, lipids and cardiovascular disease. Contraception. 1985;31:367–94. doi: 10.1016/0010-7824(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 47.Hennekens CH, Evans DA, Castelli WP, Taylor JO, Rosner B, Kass EH. Oral contraceptive use and fasting triglyceride, plasma cholesterol and HDL cholesterol. Circulation. 1979;60:486–9. doi: 10.1161/01.cir.60.3.486. [DOI] [PubMed] [Google Scholar]
- 48.Knopp RH, Walden CE, Wahl PW, et al. Oral contraceptive and postmenopausal estrogen effects on lipoprotein triglycerides and cholesterol in an adult female population: relationships to estrogen and progestin potency. J Clin Endocrinol Metab. 1981;53:1123–32. doi: 10.1210/jcem-53-6-1123. [DOI] [PubMed] [Google Scholar]
- 49.Nessa A, Latif SA, Uddin M. Effects of low dose oral contraceptives on serum total cholesterol, TAG, HDL-C & LDL-C levels in contraceptive users. Mymensingh Med J. 2005;14:26–8. [PubMed] [Google Scholar]
- 50.Lobo RA, Skinner JB, Lippman JS, Cirillo SJ. Plasma lipids and desogestrel and ethinyl estradiol: a meta analysis. Fertil Steril. 1996;65:1100–9. [PubMed] [Google Scholar]
- 51.Penttila IM, Bergink EW, Holma P, et al. Serum lipids and proteins during treatment with a new oral contraceptive combination containing desogestrel. Eur J Obstet Gynecol Reprod Biol. 1983;16:275–81. doi: 10.1016/0028-2243(83)90145-4. [DOI] [PubMed] [Google Scholar]
- 52.Akerlund M, Alstrom E, Hogstedt S, Nabrink M. Oral contraceptive tablets containing 20 and 30 μg of ethinyl estradiol with 150 μg desogestrel: their influence on lipids, lipoproteins, sex hormone binding globulin and testosterone. Acta Obstet Gynecol Scand. 1994;73:136–43. doi: 10.3109/00016349409013416. [DOI] [PubMed] [Google Scholar]
- 53.Houghton BL, Holowatz LA, Minson CT. Influence of progestin bioactivity on cutaneous vascular responses to passive heating. Med Sci Sports Exerc. 2005;37:45–51. doi: 10.1249/01.mss.0000150075.81511.fe. [DOI] [PubMed] [Google Scholar]
- 54.Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Effects of menstrual cycle and oral contraceptive use on calf venous compliance. Am J Physiol Heart Circ Physiol. 2005;288:H103–10. doi: 10.1152/ajpheart.00691.2004. [DOI] [PubMed] [Google Scholar]
- 55.John S, Jacobi J, Schlaich MP, Delles C, Schmieder RE. Effects of oral contraceptives on vascular endothelium in premenopausal women. Am J Obstet Gynecol. 2000;183:28–33. doi: 10.1067/mob.2000.105739. [DOI] [PubMed] [Google Scholar]