Abstract
Shear stress is an important stimulus to arterial adaptation in response to exercise and training in humans. We recently observed significant reverse arterial flow and shear during exercise and different antegrade/retrograde patterns of shear and flow in response to different types of exercise. The purpose of this study was to simultaneously examine flow mediated dilation (FMD), a largely nitric oxide mediated vasodilator response, in both brachial arteries of healthy young men before and after 30-minute interventions consisting of bilateral forearm heating, recumbent leg cycling and bilateral handgrip exercise. During each intervention, a cuff inflated to 60mmHg was placed on one arm to unilaterally manipulate the shear rate stimulus. In the non-cuffed arm, antegrade flow and shear increased similarly in response to each intervention (ANOVA; P<0.001, no interaction between interventions; P=0.71). Baseline FMD (4.6, 6.9 and 6.7%) increased similarly in response to heating, handgrip and cycling (8.1, 10.4 and 8.9%, ANOVA; P<0.001, no interaction; 0.89). In contrast, cuffed arm antegrade shear rate was lower than in the non-cuffed arm for all conditions (P<0.05) and the increase in FMD was abolished in this arm (4.7, 6.7 and 6.1%) (2-way ANOVA: all conditions interacted P<0.05). These results suggest that differences in the magnitude of antegrade shear rate transduce differences in endothelial vasodilator function in humans, a finding which may have relevance for the impact of different exercise interventions on vascular adaptation in humans.
Keywords: Conduit Artery, Flow-Mediated Dilation, Exercise training
INTRODUCTION
Exercise training is a well-established and potent physiological stimulus which reduces primary,1–3 and secondary cardiovascular events.4, 5 Improvement in endothelial function induced by exercise training may contribute to these beneficial effects in cardiovascular risk.6 Data in animals and humans suggest that endothelial shear stress is a key stimulus responsible for vascular adaptation in both artery function and remodelling in response to repeated exercise.7–9 However, little is known about the exact shear stress stimulus responsible for the beneficial exercise-induced vascular adaptations.
We recently demonstrated that shear rate in the brachial artery differs markedly in response to different types of exercise. For example, handgrip exercise induces an elevation in antegrade shear rate, while cycling results in large increases in antegrade and retrograde blood flow and shear.10 The observation that different types of shear are present during various types of exercise raises the question of whether different patterns of shear rate are associated with different vascular adaptations. Whilst studies performed in vitro and in animals have suggested that different shear patterns induce different cellular events, varying between pro- and anti-atherogenic changes,9 limited information is available in humans.
The primary purpose of our study was to examine whether different flow and shear stimuli mediate different acute changes in vascular function, examined using the flow mediated dilation (FMD), a largely endothelium- and nitric oxide (NO)-dependent stimulus,11–15 in humans. We measured brachial artery FMD before and after 3 different 30-minute interventions (recumbent leg cycling, forearm heating and handgrip exercise) which were associated with significantly different shear rate patterns. To further elaborate on the impact of blood flow and shear rate patterns on endothelial function, we simultaneously performed identical interventions in the contralateral limb of each subject, which had a cuff inflated to 60 mmHg throughout the intervention period in order to attenuate shear levels within subjects.
METHODS
Subjects
Ten healthy recreationally active male volunteers were recruited (28±7 years, Table 1) and informed consent was obtained from all participants prior to the experimental procedures. Subjects were healthy; none reported having been diagnosed with cardiovascular disease, diabetes, insulin resistance or cardiovascular risk factors such as hypercholesterolaemia or hypertension. Subjects who smoked or were on medications of any type were excluded. The study procedures were approved by the Ethics Committee of Liverpool John Moores University and adhered to the Declaration of Helsinki.
Table 1.
Baseline characteristics of healthy young subjects before (pre) and after (post) each intervention.
| Forearm heating | Handgrip | Recumbent leg cycling | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Pre | Post | Pre | Post | Pre | Post | P-value |
| SBP, (mmHg) | 132±18 | 131±13 | 123±18 | 131±17* | 122±17 | 117±17 | 0.08 |
| DBP, (mmHg) | 56±10 | 55±9 | 51±9 | 53±12 | 54±30 | 48±14 | 0.76 |
| MAP, (mmHg) | 81±11 | 80±10 | 75±12 | 78±13 | 76±25 | 71±13 | 0.39 |
| HR (bpm) | 56±8 | 62±11 | 59±11 | 55±11 | 60±9 | 84±3* | 0.59 |
Values are means and ± SD. The P-value refers to one-way repeated measures ANOVA between the pre-intervention values. No differences were found between pre test values between the 3 testing days.
Significant from pre-exercise at P<0.05 (paired t-test). SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
Experimental Design
Subjects reported to the laboratory on 3 occasions to perform distinct 30-minute interventions consisting of bilateral forearm heating, recumbent leg cycling or bilateral handgrip exercise. Previous studies suggested that these interventions should result in different brachial artery blood flow patterns.16, 17 By adjusting the intensity of each exercise bout, we matched brachial artery mean shear rate, within subjects, to that observed during exposure to forearm heating. In addition, a cuff was inflated to 60 mmHg throughout the 30-minute intervention on one arm in order to induce a different shear rate pattern compared with the non cuffed arm. Before and 5 min after each intervention, brachial artery endothelial function was examined using flow-mediated dilatation (FMD) simultaneously in both arms. It has been repeatedly demonstrated that FMD is a largely NO-dependent dilator response in various conduit arteries of humans.11–15 On 3 additional occasions, endothelium-independent vasodilation was examined in a subgroup (n=6) before and after each intervention. This control experiment was performed to test whether possible changes in FMD relate to acute changes in smooth muscle cell sensitivity to NO. The experimental design therefore allowed comparison of different shear rate patterns under conditions where the mean shear rates were matched (interventional comparisons of the non-cuffed limb within subjects). In addition, our design allowed comparison of simultaneously derived FMD measures between limbs and within each subject, as cuff inflation altered shear rate during intervention on the cuffed side.
Experimental Procedures
Vascular function assessments were conducted in a quiet, temperature controlled environment. Repeated laboratory visits were conducted at the same time of day. Before each test, subjects were requested to fast for 4 hours, abstain from alcohol and caffeine for 12 hours and avoid exercise for 24 hours.
Assessment of endothelium-dependent vasodilator function
Before assessment of brachial artery FMD, subjects rested in an upright, seated position for a period of at least 15-minutes to facilitate baseline measurement of heart rate and blood flow. Heart rate and mean arterial pressure were determined from an automated sphygmomanometer (Dinamap; GE Pro 300V2, Tampa, FL) placed around the ankle. The hydrostatic column between heart level and the ankle were measured (in cm) and converted to units of mmHg to calculate the correct blood pressure at heart level.18
To examine brachial artery FMD, both arms were extended and positioned at an angle of ~80° from the torso. A rapid inflation and deflation pneumatic cuff (D.E. Hokanson, Bellevue, WA) was positioned on both forearms immediately distal to the olecranon process to provide a stimulus to forearm ischaemia.19 A 10-MHz multi-frequency linear array probe attached to a high resolution ultrasound machine (T3000; Terason, Burlington, MA) was used to image the brachial arteries in the distal 1/3rd of the upper arm. When an optimal image was obtained, the probe was held stable and the ultrasound parameters were set to optimize the longitudinal, B-mode images of lumen-arterial wall interface. Continuous Doppler velocity assessment was simultaneously obtained using the ultrasound machine, and was collected using the lowest possible insonation angle (always <60°), which did not vary during each study. Baseline images were recorded for 1-minute. The forearm cuff was then inflated (>200 mmHg) for 5-minutes. Diameter and blood flow recordings resumed 30 sec prior to cuff deflation and continued for 3 minutes thereafter. These procedures for bilateral and simultaneous FMD assessment were repeated immediately following each intervention and the same sonographers were involved in each assessment for a given subject.
Assessment of endothelium-independent vasodilator function using glyceryl trinitrate (GTN)
In a subset of 6 subjects, we examined brachial artery endothelium-independent vasodilation using GTN. Subjects were tested under the same conditions, using the same protocol, set-up, probe placement and equipment as that described above for the FMD tests. To examine brachial artery GTN%, baseline images were recorded for 1-minute. Subsequently, brachial artery smooth muscle dilator function was examined after administration of a single spray of sublingual glyceryl trinitrate (GTN, 400 µg), a NO donor. This was followed by 10 minute recording of the diameter images. GTN assessments were performed before and after each of the 30 minute interventions described below.
Shear stress interventions
Immediately after the initial bilateral FMD or GTN assessments, subjects performed a 30-minute intervention. During each intervention, brachial artery diameter and velocity were assessed using a high-resolution ultrasound machine (Aspen, Acuson, Mountain View, CA). We have developed custom-designed software which enables automated edge detection and wall tracking analysis of B-mode images for diameter calculation,20 combined with synchronized pulse wave velocity waveform detection and consequent real time (or post hoc) calculation of arterial blood flow, at ~30Hz.21 During each intervention, this system was used to provide real time feedback of instantaneously calculated blood flow in each arm. This enabled us to adjust exercise workloads such that, in the non cuffed arm, mean blood flows were matched to those recorded during the forearm heating intervention. Subjects performed the cycle and hand grip interventions on separate days, at the same time of day. Images were also recorded onto S-VHS video cassette recorder (SVO-9500 MDP, Sony, Tokyo, Japan) for post hoc analysis using the edge-detection and wall-tracking software. In addition, heart rate and mean arterial pressure were recorded every 5-min throughout the 30-min intervention using an automated sphygmomanometer around the ankle (Dinamap; GE Pro 300V2, Tampa, FL).
Forearm Heating
Forearm heating was used as the reference intervention for mean SR elevation, as the heating stimulus was not amenable to rapid adjustment. Forearm heating was performed using the same seated position as the FMD and GTN assessments. Both arms were placed in a separate water bath, with 40±1°C water for 30-min. Both water baths were maintained at constant temperature by a thermostatically controlled circulating heater pump.
Recumbent leg cycling
Subjects performed leg cycling exercise on a recumbent bike for 30-min at 60–70 rpm. Mean blood flow during recumbent leg cycling in the non cuffed arm was matched with that observed during forearm heating in that arm, by adjusting the workload which started at 80 W.
Handgrip Exercise
While seated in the upright position, subjects performed bilateral handgrip exercise using identical dynamometers at a cadence of 30 contractions/min for 30-min (assisted by a metronome). Subjects exercised using an initial weight of 1–2 kg, with this intensity subsequently adjusted to match the mean blood flow in the non cuffed arm recorded during forearm heating.
Comparison between cuffed/non cuffed arms
Both arms were simultaneously exposed to each of the interventions described above. However, a cuff was placed around one arm and inflated to 60 mmHg during each 30 minute intervention period. Placement of this cuff around the left or right arm was randomized between subjects, but always on the same arm for each intervention within subjects. Preliminary pilot studies revealed that 60 mmHg was sufficient to alter the mean shear rate and pattern of shear compared with the non cuffed, control arm. Mean shear rate and the pattern of shear rate (antegrade versus retrograde) in both the cuffed and non cuffed arms were recorded during each intervention. Pre- and post-intervention FMD or GTN measurements were examined simultaneously in both arms.
Brachial artery diameter, blood flow and shear rate analysis
Analysis of brachial artery diameters and shear rate before, during and after the 3 interventions was performed using custom-designed edge-detection and wall-tracking software which is largely independent of investigator bias.20 See recent papers for detailed descriptions of this analysis approach.22, 23 From this synchronised diameter and velocity data, blood flow (the product of lumen cross-sectional area and Doppler velocity (ν)) are calculated at 30 Hz. Shear rate (an estimate of shear stress without viscosity) was calculated as 4 times mean blood velocity / vessel diameter.24 Reproducibility of diameter measurements using this semi-automated software is significantly better than manual methods, reduces observer error significantly, and possesses an intra-observer CV of 6.7%.20, 21 This system was used for post hoc calculation of FMD and GTN responses as well as during each intervention to provide real time feedback for the matching of blood flows across the three trials.
Data analysis
FMD and GTN
FMD and GTN are presented as the absolute (mm) and relative (%) rise from the preceding baseline diameter and are calculated based on standardised algorithms applied to data which had undergone automated edge-detection and wall-tracking, and were therefore observer-independent.22 See previous studies for further detail.22
In accordance with recent findings,22, 25 we calculated the shear rate stimulus responsible for endothelium-dependent FMD following cuff deflation. The post-deflation shear rate data, derived from simultaneously acquired velocity and diameter measures at 30 Hz, was exported to a spreadsheet and the area under the shear rate curve (AUC) calculated for data up to the point of maximal post-deflation diameter (FMD),22 for each individual using the trapezoid rule.
Shear rate assessment during the interventions
The software described above was also used for analysis of shear rate, derived from simultaneously acquired velocity and diameter measures at 30 Hz, during the three interventions in the both the cuffed and non cuffed arms. The patterns of shear rate were also assessed by calculating the area under the curve for all antegrade blood flow and shear, and also the area under the retrograde blood flow and shear recordings.
Statistics
Statistical analyses were performed using SPSS 14.0 (SPSS, Chicago, Illinois) software. All data are reported as mean (SD) unless stated otherwise, while statistical significance was assumed at P<0.05. Assuming 80% power and an alpha of 0.05, 9 subjects are required to detect a clinically and physiologically relevant 1.5% difference in FMD% change between conditions. To correct for potential drop-out, we have included 10 subjects. One-way ANOVA with repeated measures (with intervention as independent factor) was used to assess differences between the 3 conditions for baseline characteristics and mean shear rate. A 2-way repeated measures ANOVA was used to compare the shear rate patterns between interventions, shear rate patterns between cuffed and non cuffed arms, pre- versus post-intervention FMDs between conditions, and pre- versus post-intervention FMDs between cuffed and non cuffed arms. Post-hoc tests with a least-square difference test were performed when the ANOVA reported a significant main or interaction effect.
RESULTS
Post-exercise FMD data from 1 subject after recumbent leg cycling and from another subject after forearm heating were excluded from the analysis because image quality was deemed inadequate.
Effect of interventions on shear rate responses in the non cuffed arm
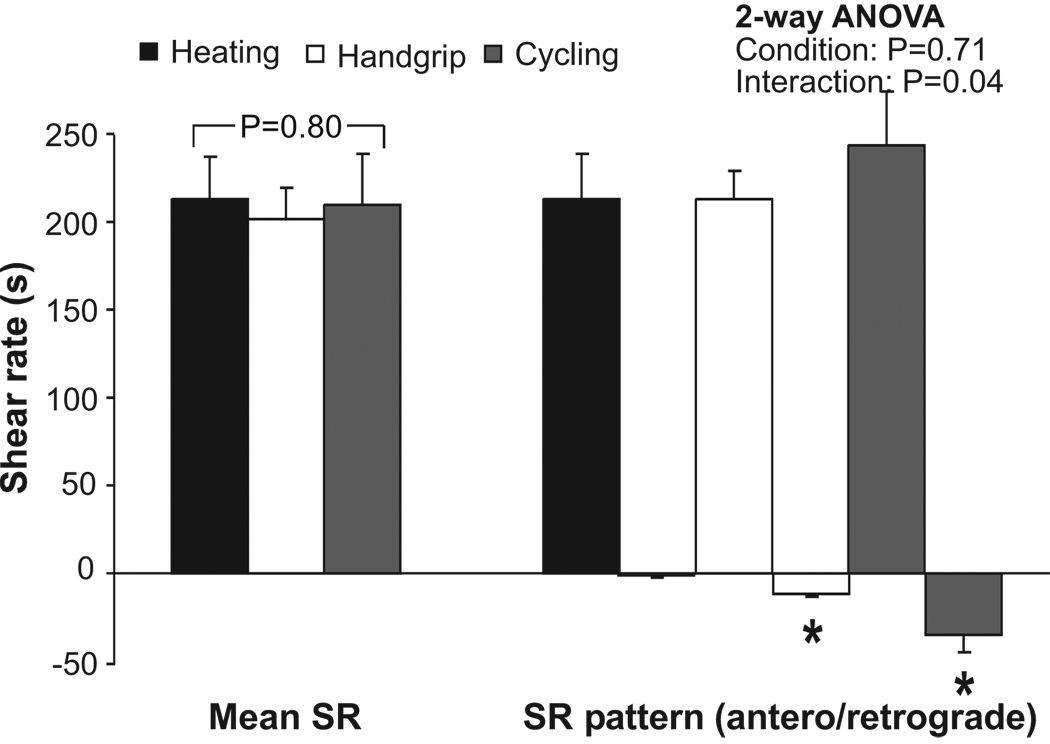
Baseline characteristics were not significantly different between the 3 exercise interventions (Table 1). A 2-way ANOVA revealed a significant difference in the pattern of shear rate (SR) between the 3 interventions (interaction; P=0.04; Figure 1). Post-hoc t-tests revealed a significantly lower retrograde SR during forearm heating compared with recumbent leg cycling and with handgrip exercise (P<0.05; Figure 1). Mean and antegrade SR were similar during forearm heating, recumbent leg cycling and handgrip exercise (Figure 1).
FIGURE 1.
Shear rate pattern (SR; mean, antegrade and retrograde shear rate) during heating (black bars), handgrip (white bars) and recumbent leg cycling (grey bars) in healthy young men (n=10) in the non cuffed arm. A 1-way ANOVA was used to examine differences between the 3 interventions for mean SR, while a 2-way ANOVA was utilized to examine the differences in blood flow pattern (antegrade – retrograde SR) between the 3 interventions. *Post hoc significantly different from forearm heating. Error bars represent SE.
Effect of shear rate interventions on change in FMD in the non cuffed arm
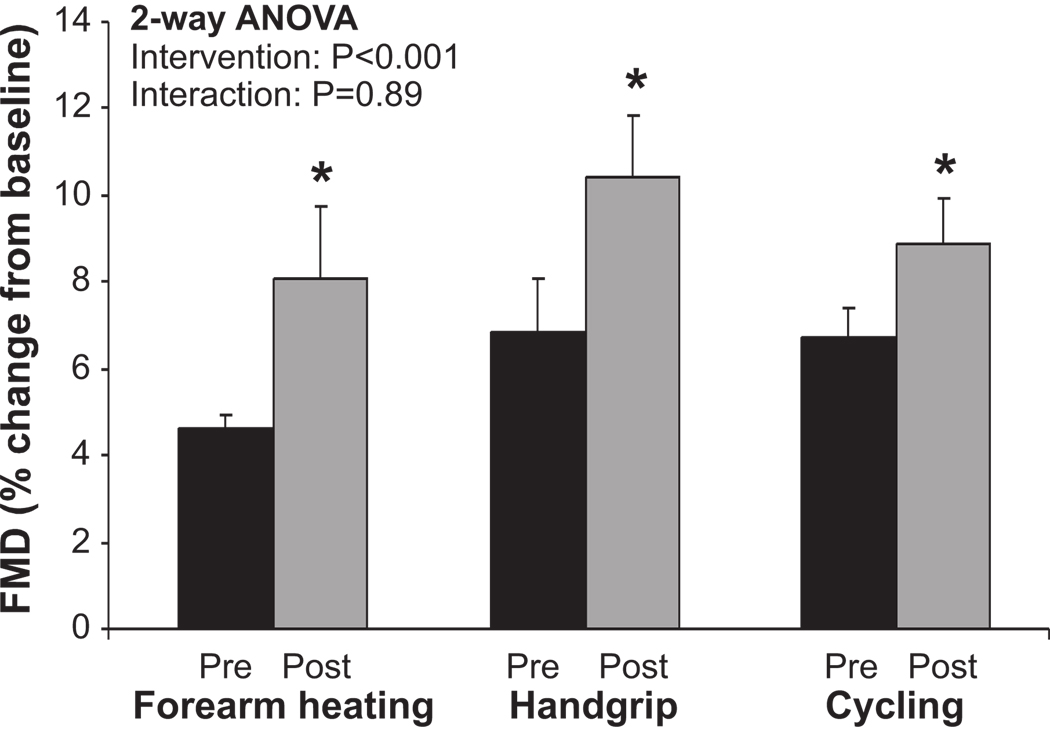
Pre-intervention FMD% was not significantly different between forearm heating, handgrip and recumbent leg cycling exercise (Figure 2). The interventions did not alter baseline artery diameter (Table 2). Post-intervention FMD% significantly increased, compared with pre-intervention, in response to each intervention (ANOVA; P<0.001; Figure 2). The 2-way ANOVA revealed no significant interaction effect, indicating a similar increase in FMD% for all interventions (Figure 2). Indeed, a similar magnitude of increase, expressed in relative (%, Figure 2) and absolute terms (mm, Table 2), was found across all 3 interventions. No differences were evident in AUCSR between pre- and post-intervention (Table 2), indicating that the eliciting shear rate stimulus for FMD following cuff deflation was not significantly altered by any of the interventions.
FIGURE 2.
Flow-mediated dilation (FMD) before (black) and after (grey) heating, handgrip and recumbent leg cycling in healthy young men (n=10) in the non cuffed arm. A 2-way repeated measures ANOVA was used to examine the impact of the interventions on FMD (main effect) and whether the change in FMD differed among the 3 interventions (interaction). *Post-hoc significant from pre-intervention. Error bars represent SE.
Table 2.
Brachial artery baseline characteristics of healthy young subjects before (pre) and after (post) each intervention in the non cuffed and cuffed arm.
| Forearm heating | Handgrip | Recumbent leg cycling | ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Brachial artery | Pre | Post | Pre | Post | Pre | Post | P-value |
| Non cuffed arm | |||||||
| Baseline (mm) | 4.0±0.8 | 4.3±0.8 | 4.1±1.0 | 3.9±0.6 | 3.9±0. 8 | 4.0±0.7 | 0.31 |
| FMD (mm) | 0.18±0.06 | 0.30±0.19* | 0.26±0.10 | 0.38± 0.12* | 0.26±0.10 | 0.34±0.08* | 0.07 |
| FMD (%) | 4.6±0.9 | 8.1±5.4* | 6.9±3.7 | 10.4±4.6* | 6.7±2.2 | 8.9±3.2* | 0.13 |
| AUCSR | 12679±3138 | 20121±13460 | 20121±13460 | 18768±12313 | 20733±16776 | 25470±11690 | 0.51 |
| Non cuffed arm | |||||||
| Baseline (mm) | 4.1±0.5 | 4.2±0.5 | 4.1±0.6 | 4.0±0.5 | 3.7±0.6 | 4.0±0.6 | 0.12 |
| FMD (mm) | 0.27±0.11 | 0.19±0.11 | 0.29±0.11 | 0.27±0.10 | 0.32±0.14 | 0.25±0.15 | 0.68 |
| FMD (%) | 5.8±2.2 | 4.7±2.2 | 7.2±3.1 | 6.7±2.5 | 8.8±2.7 | 6.1±3.4 | 0.11 |
| AUCSR | 15595±5805 | 16386±10861 | 18731±13004 | 20427±9454 | 19823±11875 | 25117±7397 | 0.67 |
Values are means and ± SD. The P-value refers to one-way repeated measuresANOVA between the pre-intervention values. No differences were found between pre test values between the 3 testing days.
Significant from pre-exercise at P<0.05 (paired t-test). SR, shear rate; BAFMD, brachial artery flow-mediated dilation; AUCSR, area under the shear rate curve.
Effect of cuff inflation on shear rate responses
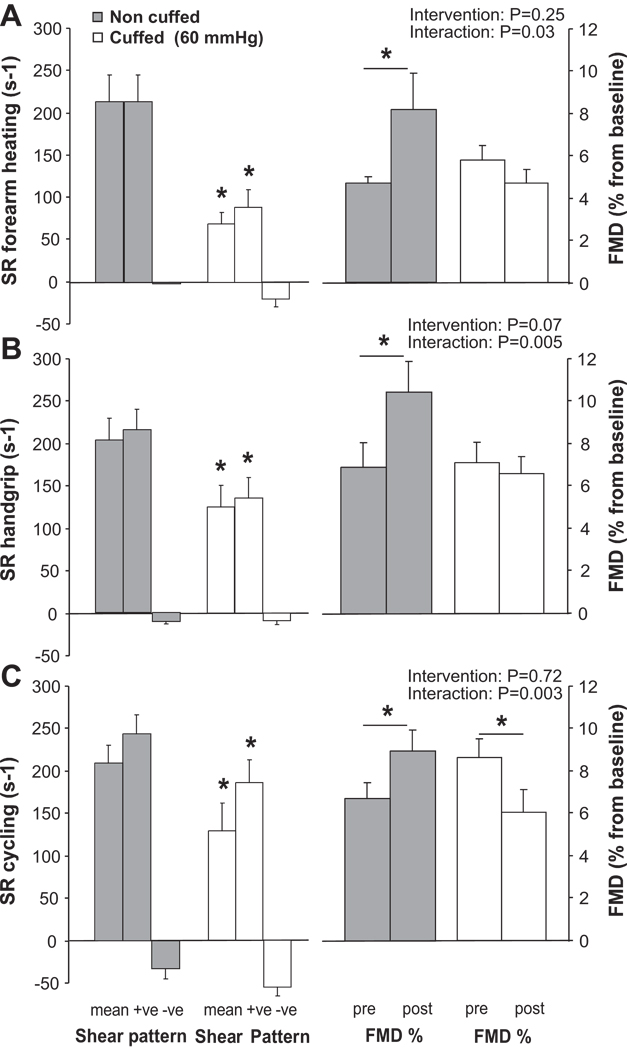
No differences in pre-intervention FMD% were evident between arms preceding any of the interventions (Figure 3). Mean SR was significantly lower in the cuffed arm, compared to the non cuffed arm, during each intervention (Figure 3, P<0.05). For each intervention, antegrade SR in the cuffed arm was significantly lower compared to the non cuffed arm (P<0.05), while retrograde shear did not significantly differ (P>0.05). Inflating a forearm cuff to 60 mmHg therefore decreased mean SR principally by altering the antegrade SR for each intervention; it did not significantly modify the retrograde shear rate pattern.
FIGURE 3.
Mean shear rate and shear rate pattern during the intervention (+ve; antegrade shear rate, −ve; retrograde shear rate) and flow-mediated dilation (FMD) before and after the intervention is presented for the non cuffed (grey bars) and cuffed arms (white bars). Data is presented for forearm heating (A), handgrip (B) and recumbent leg cycling (C) in healthy young men (n=10). Mean and pattern of shear between the non cuffed and cuffed arm during each intervention is tested with a paired t-test (*P<0.05). A 2-way ANOVA is used to examine whether cuff placement influenced the change in FMD upon the 30-min intervention (*Post-hoc significant between pre- and post-intervention). Error bars represent SE.
Effect of interventions on FMD between the cuffed and non cuffed arms
Forearm heating
A 2-way ANOVA revealed no main effect of the intervention on FMD%, but a significant interaction between both limbs (Figure 3). While forearm heating significantly increased brachial artery FMD% in the non cuffed arm (P<0.05), no change was found in the cuffed arm (Figure 3). Also, for the absolute change in brachial artery FMD (mm), a significant interaction between both limbs was present (ANOVA; P<0.05). The AUCSR was not different between limbs or pre- versus post-intervention (Table 2).
Handgrip
Handgrip exercise increased brachial artery FMD% (P<0.05) and FMDmm (P<0.05) in the non cuffed arm, but not in the cuffed arm (Figure 3, Table 2). Indeed, the 2-way ANOVA revealed a significant interaction effect of handgrip exercise on FMD% (P<0.05, Figure 3) as well as on FMDmm (P<0.05) between the cuffed and non cuffed arms. These effects were present, despite a similar AUCSR between pre- and post-exercise.
Recumbent leg cycling
A significant interaction effect was found between both arms regarding the effect of recumbent leg cycling on FMD% (P<0.005). An increase in brachial artery FMD% in the non cuffed arm was found (P<0.05), while a decrease in FMD% was observed in the cuffed arm (P<0.05) (Figure 3). Also, when presented as FMD (mm), a significant interaction between both arms (2-way ANOVA; P<0.01) was evident. The post-deflation AUCSR did not differ between limbs or change following recumbent leg cycling in both arms (Table 2).
Effect of interventions on GTN on the cuffed and non cuffed arms
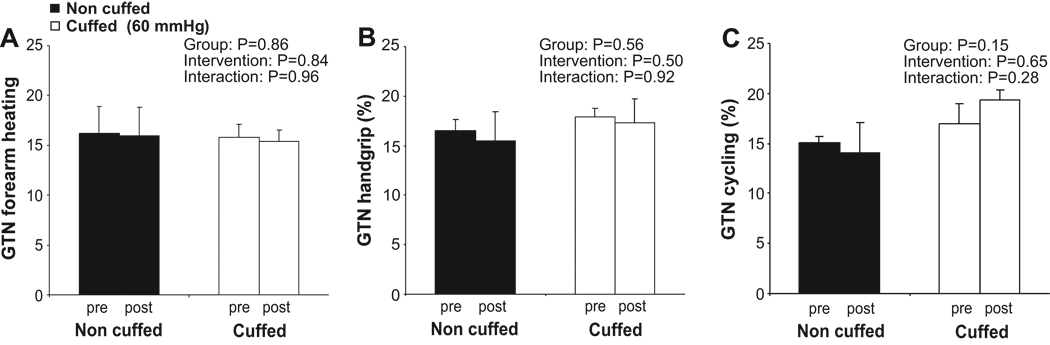
Pre-intervention GTN% was not significantly different between forearm heating, handgrip and recumbent leg cycling exercise in the non cuffed or in the cuffed arm (repeated measures ANOVA: P=0.72 and 0.49, respectively). Also, no differences in pre-intervention GTN% were present between the arms under each condition (Paired t-test: P=0.33).
None of the interventions elicited a significant change in brachial artery GTN% in either the non cuffed or cuffed arms (Figure 4). No interaction effect of each intervention on GTN was found between the non cuffed or cuffed arm (Figure 4).
FIGURE 4.
Endothelium-independent vasodilation examined using glyceryl trinitrate (GTN) before and after the intervention is presented for the non cuffed (black bars) and cuffed arms (white bars). Data is presented for forearm heating (A), handgrip (B) and recumbent leg cycling (C) in healthy young men (n=6). A 2-way ANOVA did not find an effect of the intervention of the GTN-response in the non cuffed or cuffed arm. Error bars represent SE.
DISCUSSION
In the present study we compared the effects of manipulating brachial artery blood flow and shear on FMD in humans. By monitoring real time blood flow and shear rate and subsequently modulating exercise intensities, we effectively matched mean shear rate and blood flow across our three interventions, while antegrade/retrograde patterns significantly differed. The use of bilateral and simultaneous measures also allowed us to manipulate shear rate using a cuff placed on one arm throughout each intervention, which effectively decreased the magnitude of antegrade flow and shear within subjects. The major findings are: 1. when antegrade shear rate is increased to a similar extent for 30-min using heating, handgrip or cycle exercise, FMD increased to a similar extent under all three conditions, 2. decreasing antegrade shear rate via sub-diastolic cuff inflation abolished this increase in FMD, 3. endothelium-independent NO mediated vasodilator function was unaffected by 30 minute period of shear rate modulation. These results indicate that FMD, a measure of largely NO-dependent endothelium-dependent vasodilation,11–15 is modulated by different shear rate interventions in vivo, primarily due to the differences in the magnitude of antegrade flow and shear.
In a previous study, we observed significant differences in the magnitude of antegrade and retrograde brachial artery flows between handgrip and leg cycling exercise.17 We speculated that these differences may importantly modulate vascular adaptations in response to exercise training. Indeed, as recently reviewed,9 studies performed in vitro and in animals suggest that different shear patterns induce different cellular events. Changes in unidirectional shear stress decrease expression of ET-1 and VCAM-1, whilst oscillatory shear increases ET-1,26 and adhesion molecules (VCAM-1),27, 28 decreases eNOS expression increases expression of enzymes that produce ROS (i.e., NADPH oxidase)29, 30 and increases superoxide release.31 The results of the present study provide some insight into the relative importance of antegrade versus retrograde patterns of blood flow and shear in humans. Differences in mean blood flow and shear rate between the cuffed and non cuffed arms, primarily due to differences in the antegrade shear rate, resulted in different impacts on FMD, while the endothelium-independent responses to GTN were unaffected. This suggests that changes in antegrade blood flow and shear rate provide an important stimulus to acute enhancement of endothelial function in vivo. Conversely, significant differences in the magnitude of retrograde blood flow between the interventions in the non cuffed arm did not significantly influence the change in FMD%. This suggests that, under the experimental conditions of the present study, retrograde blood flow and shear did not markedly influence endothelial function. This should not infer that changes in retrograde flow and shear are not important, as we recently found a dose-response relationship between increases in retrograde shear and impairment in FMD responses.32 Taken together, this data suggests that the magnitude of antegrade flow and shear provide an important stimulus to acutely increase endothelial function in humans and that increases in antegrade shear may also prevent any impairment in endothelial function associated with unopposed increases in retrograde flow and shear.
The implications of this study relate to the impact of exercise and physical conditioning on vascular adaptations. Whilst exercise training and physical activity are associated with substantial cardiovascular benefit, recent evidence suggest that exercise-mediated changes in traditional and novel risk factors fail to fully account for this benefit.6, 33 Exercise training can enhance endothelial function and induce endothelium-dependent arterial remodelling in humans.34 It has therefore been suggested that the direct anti-atherogenic effects of exercise on the vasculature, mediated through changes in shear stress and possibly perfusion pressure associated with each exercise bout,6, 9, 34 may account for the unexplained beneficial impact of training on CV risk.6 The present study indicates that changes in the magnitude of antegrade blood flow and shear rate, which are typically associated with exercise, are indeed associated with an acute improvement in brachial artery endothelial function. Future studies, involving exercise training are necessary to further establish the importance of blood flow and shear rate to induce vascular adaptations and the consequent cardiovascular benefit.
Many studies indicate that vascular adaptations to exercise training are not only localized to the active muscle bed, but may also be evident in arteries not directly feeding the active skeletal tissue.6 For example, changes in brachial artery endothelial function are evident following lower limb exercise training.34, 35 In the present study, cycle ergometer exercise had similar effects on brachial artery FMD as those evident in response to forearm handgrip exercise. This suggests that a sufficient increase in antegrade blood flow and shear rate, which occur as a consequence of hemodynamic responses associated with large muscle mass exercise, can enhance endothelial function in vascular beds feeding inactive skeletal muscle.
Several limitations of the present study are germane. Whilst the sample size was relatively modest, it is in keeping with physiological studies of this nature and the results were consistent between and within individuals. Recruiting a larger number of subjects would not have importantly changed our findings. We studied men to avoid the established cyclical effects of sex hormones on vascular function in women. Similarly, the subjects we recruited were young and healthy with normal endothelial function. One limitation relates to the fact that we did not perform blockade experiments to prove that our FMD changes were endothelium- or NO dependent. Nonetheless, FMD has consistently been demonstrated a NO-and endothelium-dependent stimulus in human conduit arteries.11–15 We suggest that the method presented here, involving clamping of mean shear rates by matching these between conditions, and simultaneous assessment of FMD bilaterally with unilateral manipulation, may provide a useful method for future investigation of the impact of various interventions or conditions on vascular function, as it minimizes the impact of between subject variability and repeated assessment effects.
Perspectives
In this study, we manipulated the antegrade versus retrograde patterns of blood flow and shear rate using three distinct stimuli. When the magnitude of increase in antegrade shear rate was similar across these interventions, we observed similar improvements in FMD. Conversely, when the rise in antegrade blood flow and shear rate was attenuated within subjects using unilateral cuff inflation, the increase in FMD was abolished. These data suggest that differences in the antegrade/retrograde pattern of shear rate, and in particular changes in antegrade shear and blood flow, have important modulating impacts on endothelial function in vivo. As exercise is typically associated with elevations in antegrade shear rate, this finding may have relevance for the impact of different exercise interventions on vascular adaptations in humans.
Acknowledgements
We would like to thank Chris Reed for his assistance with software development.
We also acknowledge the help of Marie-Louise Leijssen for her assistance in the analysis.
Funding sources:
D.H.J.T. is financially supported by The Netherlands Organization for Scientific Research (NWO-grant 82507010).
M.H.L. is financially supported by NIH-grant HL36088.
Footnotes
Disclosures:
None of the authors have conflict to disclosure
REFERENCES
- 1.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 2.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 3.Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation. 2000;102:975–980. doi: 10.1161/01.cir.102.9.975. [DOI] [PubMed] [Google Scholar]
- 4.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD001800. CD001800. [DOI] [PubMed] [Google Scholar]
- 5.Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:945–950. [PubMed] [Google Scholar]
- 6.Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. 2008;105:766–768. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Niebauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green D, Cheetham C, Mavaddat L, Watts K, Best M, Taylor R, O'Driscoll G. Effect of lower limb exercise on forearm vascular function: contribution of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;283:H899–H907. doi: 10.1152/ajpheart.00049.2002. [DOI] [PubMed] [Google Scholar]
- 11.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88:145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 12.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 13.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008;586:1137–1145. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001;101:629–635. [PubMed] [Google Scholar]
- 15.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93:210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 16.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Brachial Artery Blood Flow Responses to Different Modalities of Lower Limb Exercise. Med Sci Sports Exerc. 2009;41:1072–1079. doi: 10.1249/MSS.0b013e3181923957. [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. 2005;562:617–628. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groothuis JT, Poelkens F, Wouters CW, Kooijman M, Hopman MT. Leg intravenous pressure during head-up tilt. J Appl Physiol. 2008;105:811–815. doi: 10.1152/japplphysiol.90304.2008. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 20.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol. 2001;91:929–937. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- 21.Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol. 2002;93:361–368. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- 22.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension. 2008;51:203–210. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 23.Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow-mediated dilation?: a comparison between young and older humans. Am J Physiol Heart Circ Physiol. 2009;296:H57–H64. doi: 10.1152/ajpheart.00980.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker BA, Trehearn TL, Meendering JR. Pick Your Poiseuille: Normalizing the Shear Stimulus In Studies of Flow-Mediated Dilation. J Appl Physiol. 2008 Nov 13; doi: 10.1152/japplphysiol.91302.2009. 2008 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686–692. doi: 10.1161/01.atv.18.5.686. [DOI] [PubMed] [Google Scholar]
- 27.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H645–H653. doi: 10.1152/ajpheart.01087.2006. [DOI] [PubMed] [Google Scholar]
- 28.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 29.De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J. 1998;329(Pt 3):653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 32.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde Flow and Shear Rate Acutely Impair Endothelial Function in Humans. Hypertension. 2009 Apr 20; doi: 10.1161/HYPERTENSIONAHA.109.131508. 2009 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]