Abstract
In the closed structure of the P2X cation channel, three α-helical transmembrane domains cross the membrane obliquely: in rat P2X2 receptors, these intersect at Thr339. Replacing Thr339 by lysine in one, two or three subunits progressively increased chloride permeability and reduced unitary conductance. This implies that the closed-open transition involves a symmetrical separation of the three subunits, and that Thr339 from each contributes symmetrically to the open channel permeation pathway.
Keywords: ATP, concatemers, ion permeation, mutations, lysine, channel, T339
The conducting pore of ligand-gated ion channels is typically formed as a passage along the central axis of several subunits. ATP-gated channels (P2X receptors) are among the simplest such channels: in this case the central cation-selective channel is formed by the second of two transmembrane domains (TM2) from each of three subunits1. In the crystal structure of the closed zebrafish P2X4.1 receptor, these TM2 helices (from N334 to L361) cross the membrane at an oblique angle, such that the narrowest part of the channel is delimited by two helical turns from L340 to A3472. The corresponding region of the rat P2X2 receptor (I332 to T339) is a key determinant of the conducting properties of the open channel3-8. We introduced a lysine residue at this position (T339K) in one, two or three of the TM2 helices (Supplementary Methods) and determined that the open channel functions as a symmetrical trimer, in which each TM2 helix contributes equally to the permeation pathway.
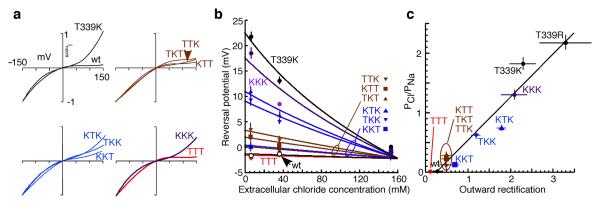
The current evoked by ATP (Supplementary Methods) at wild type P2X2 receptors shows marked inward rectification9: in contrast, outward currents through P2X2[T339K] receptors were larger at positive holding potentials7 (Fig. 1a). The concatemer with three wild type subunits (TTT) showed inward rectification similar to channels formed by the expression of single wild type subunits, and the rectification of the three-lysine concatemer (KKK) resembled that of the homotrimeric channel formed from single P2X2[T339K] subunits (Fig. 1a). Channels containing one or two lysine residues showed intermediate inward rectification (Fig. 1a and Supplementary Table). There was no obvious position dependence among forms KTT, TKT, and TTK. Concatemers containing two T339K subunits all showed enhanced outward currents, although this was less for KKT than for KTK and TKK (Fig. 1a): of all the constructs, only KKT showed evidence of partial breakdown (Supplementary Fig. 1) and it is possible that wild type monomers were also formed.
Figure 1.
Lysine at 339 progressively increase chloride permeability and outward current. (a) Current-voltage plots for ATP-induced currents in ten cells expressing concatenated trimeric P2X2 receptors with one, two or three lysines at position 339. Currents are normalized: scales apply to all panels (actual currents at -150 mV were (pA): wild type (wt) 2000, T339K 700, KTT 3100, TKT 2900, TTK 2700, KKT 230, KTK 800, TKK 1800 pA, TTT 3300 and KKK 1900). ATP concentrations used were 10 or 30 μM (close to EC50). (b) Reversal potential for ATP-evoked currents becomes dependent on the chloride concentration as lysines are introduced at position 339. Means ± s.e.m. (c) PCl/PNa (determined from experiments in b) increases according to the number of lysines at position 339 and outward rectification increases proportionately (Pearson's r = 0.97).
Wild type P2X2 channels have negligible chloride permeability1,10, but substitution by lysine at T339 converted the P2X receptor channel from cation-selective to anion-preferring: PCl/PNa increased from <0.1 for the wild type channel to ~2 for the T339K channel (Supplementary Table). For concatemeric channels, the increase in chloride permeability was progressive with the number of lysines at this position (Fig. 1b). There was a strong correlation between the increase in outward current measured at 150 mV and the increase in chloride permeability (Fig. 1c). In other words, the large outward currents in P2X2[T339K] results from the increased inward movement of chloride ions when the cell is strongly depolarized. This shows that the electrostatic environment around T339 is critical for the charge selectivity of the permeating ions.
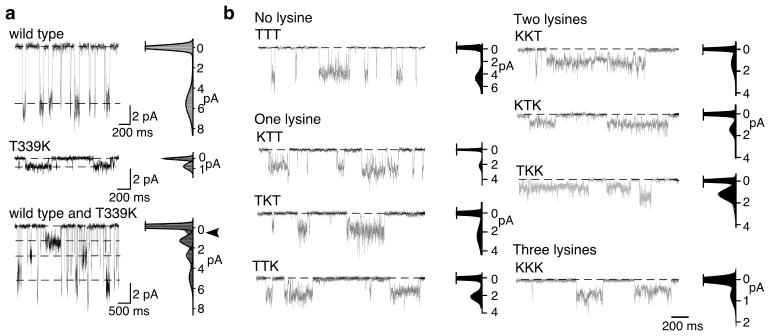
Single channel recording showed that wild type rat P2X2 receptors open to a single conducting level in ATP (Fig. 2a)(27.3 ± 1.3 pS, n = 12). P2X2[T339K] had much reduced unitary currents (6.1 ± 0.6 pS, n = 7). The corresponding values when potassium was the main internal ion were 41.1 ± 3.3 pS (n = 7) and 6.1 ± 0.3 pS (n = 8), so we used internal potassium in subsequent experiments to discriminate more easily levels intermediate between wild type and T339K. Outside-out patches from cells transfected with both wild type and T339K cDNAs usually showed multiple conductance levels (Fig. 2a). In 9 of 44 patches a single open level was observed at 44 ± 1.6 pS; in 8 of 44 patches a single open level occurred at 7.2 ± 0.1 pS. In 11 patches, we observed three open levels (i.e. four peaks in the all points histogram), which corresponded in amplitude to wild type level, and two new intermediate levels (II: 14.4 ± 0.9 pS; III: 24.5 ± 1.1 pS). In 16 patches, we observed a single intermediate conductance level, corresponding in amplitude to either II or III (Supplementary Fig. 2).
Figure 2.
Lysine at 339 reduces single channel currents. (a) ATP (0.3 μM or 1 μM) activates single channels in outside-out patches from cells expressing cDNAs encoding wild type P2X2 (top), P2X2[T339K] (middle), and both (bottom) subunits. Bottom trace shows the intermediate current amplitudes: zero current/closed channel peak is truncated, and open arrowhead indicates the position of the third level (< 1 pA). Holding potential –120 mV. (b) Outside-out recordings of single channel activity in patches from cells expressing concatenated cDNAs. The amino acid at position 339 in each subunit of the trimer is indicated above each trace. ATP concentrations were 1 to 10 μM. Rightside records are all-points histograms used to estimate unitary current amplitudes; zero level peaks are truncated.
Concatemers with only wild type or only T339K in each of the three subunits provided channels with unitary conductances similar to those observed with the corresponding monomers (Fig. 2b and Supplementary Fig. 2). Concatemers that contained one or two T339K subunits had unitary conductances not different from the intermediate levels observed with co-expression of monomers described above (Supplementary Fig. 2).
The progressive increase in positivity of the electrostatic field strength at this position by introduction of one, two or three positive charges converts the P2X2 receptor from ten-fold cation-selective to a channel that prefers anions, and this accounts for the rectification previously reported1,9. Similar conversion of ion selectivity from cationic to anionic by mutagenesis is reported for some other ion channels (e.g. nicotinic receptors11 and cyclic nucleotide-gated channels12). Introduction of a single lysine into the trimeric concatemer reduced the unitary current by about 50%, and further lysines caused further stepwise reductions (Fig. 2 and Supplementary Fig. 2). There was no obvious change in open probability in T339K as compared to wild type channels, indicating that the T339K substitution did not change gating (e.g. by endowing the protein with a new voltage-dependence).
It is currently thought that the P2X receptor pore opens by a separation13 and counter-clockwise rotation of each of the three TM2 helices, driven by forces transmitted through connecting rods passing through the ectodomain from three inter-subunit binding sites14. Our findings with concatenated channels strongly suggest that opening of P2X2 channels occurs by equivalent and symmetrical rearrangement of the TM2 helices. We conclude that the side chain of the residue that occludes the permeation pathway of the closed P2X receptor also contributes to the selectivity filter of the open channel (T339 in the P2X2 receptor)(Supplementary Fig. 3). This result is consistent with a symmetrical iris-like separation of the three TM2 helices: other recent evidence suggests that this is accompanied by a steepening and rotation of these helices14,15.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by The Wellcome Trust.
Footnotes
Note: Supplementary information is available on the Nature Neurosicence website.
AUTHOR CONTRIBUTIONS
R.A.N., L.E.B. and L.C. conceived and designed the experiments, and analyzed the data. H.E.B., L.B. and W.J.W. generated the constructs and did Western blotting. L.E.B. and L.C. performed the single channel and whole cell electrophysiology. L.E.B. constructed molecular models. R.A.N. wrote the paper, with contributions from all other authors.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.North RA. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 2.Kawate T, Michel JC, Birdsong WT, Gouaux E. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan TM, Haines WR, Voigt MM. J. Neurosci. 1998;18:2350–2359. doi: 10.1523/JNEUROSCI.18-07-02350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Chang TH, Silberberg SD, Swartz KJ. Nat. Neurosci. 2008;11:883–887. doi: 10.1038/nn.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rassendren F, Buell G, Newbolt A, North RA, Surprenant A. EMBO J. 1997;16:3446–3454. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migita K, Haines WR, Voigt MM, Egan TM. J. Biol. Chem. 2001;276:30934–30941. doi: 10.1074/jbc.M103366200. [DOI] [PubMed] [Google Scholar]
- 7.Cao L, Broomhead HE, Young MT, North RA. J. Neurosci. 2009;29:14257–14264. doi: 10.1523/JNEUROSCI.4403-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao L, Young MT, Broomhead HE, Fountain SJ, North RA. J. Neurosci. 2007;27:12916–12923. doi: 10.1523/JNEUROSCI.4036-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Hume RI. J. Physiol. 1998;507:353–364. doi: 10.1111/j.1469-7793.1998.353bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bo X, et al. Mol. Pharmacol. 2003;63:1407–1416. doi: 10.1124/mol.63.6.1407. [DOI] [PubMed] [Google Scholar]
- 11.Galzi JL, et al. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- 12.Qu W, et al. J. Gen. Physiol. 2006;127:375–389. doi: 10.1085/jgp.200509378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silberberg SD, Li M, Swartz KJ. Neuron. 2007;54:263–274. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Browne LE, Jiang LH, North RA. Trends Pharmacol. Sci. 2010;31:229–237. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kracun S, Chaptal V, Abramson J, Khakh BS. J. Biol. Chem. 2010;285:10110–10121. doi: 10.1074/jbc.M109.089185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.