Abstract
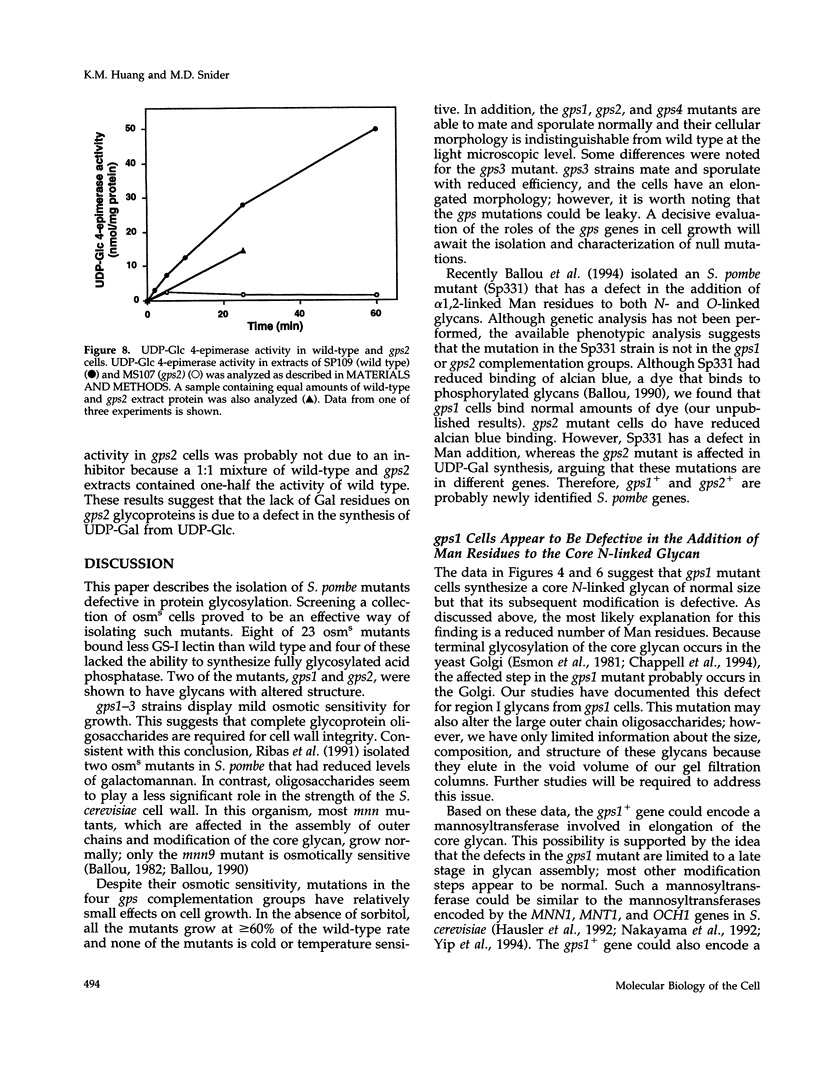
We have isolated mutants in the fission yeast Schizosaccharomyces pombe that are defective in protein glycosylation. A collection of osmotically sensitive mutants was prepared and screened for glycosylation defects using lectin staining as an assay. Mutants singly defective in four glycoprotein synthesis genes (gps1-4) were isolated, all of which bind less galactose-specific lectin. Acid phosphatase and other glycoproteins from the gps mutants have increased electrophoretic mobility, suggesting that these mutants make glycans of reduced size. N-linked glycan analysis revealed that terminal oligosaccharide modification is defective in the gps1 and gps2 mutants. Both mutants synthesize the Man9GlcNAc2 core glycan but have reduced amounts of larger structures. Modified core glycans from gps1 cells have normal amounts of galactose (Gal) residues, but reduced amounts of Man, consistent with a defect in a Golgi mannosyltransferase in this mutant. In contrast, N-linked oligosaccharides from gps2 mutants have much less Gal than wild type, because of reduced levels of the Gal donor, UDP-Gal. This reduction is caused by decreased activity of UDP-glucose 4-epimerase, which synthesizes UDP-Gal. Neither the gps1 or gps2 mutations are lethal, although the cells grow at reduced rates. These findings suggest that S. pombe cells can survive with incompletely glycosylated cell wall glycoproteins. In particular, these results suggest that Gal, which comprises approximately 30% by weight of cell wall glycoprotein glycans, is not crucial for cell growth or survival.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN J. M., GOCKERMAN J. ELECTROPHORETIC SEPARATION OF MULTIPLE FORMS OF PARTICLE ASSOCIATED ACID PHOSPHATASE. Ann N Y Acad Sci. 1964 Dec 28;121:616–633. doi: 10.1111/j.1749-6632.1964.tb14230.x. [DOI] [PubMed] [Google Scholar]
- Abeijon C., Orlean P., Robbins P. W., Hirschberg C. B. Topography of glycosylation in yeast: characterization of GDPmannose transport and lumenal guanosine diphosphatase activities in Golgi-like vesicles. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6935–6939. doi: 10.1073/pnas.86.18.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou C. E., Ballou L., Ball G. Schizosaccharomyces pombe glycosylation mutant with altered cell surface properties. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9327–9331. doi: 10.1073/pnas.91.20.9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou C. E. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 1990;185:440–470. doi: 10.1016/0076-6879(90)85038-p. [DOI] [PubMed] [Google Scholar]
- Bush D. A., Horisberger M., Horman I., Wursch P. The wall structure of Schizosaccharomyces pombe. J Gen Microbiol. 1974 Mar;81(1):199–206. doi: 10.1099/00221287-81-1-199. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Hajibagheri M. A., Ayscough K., Pierce M., Warren G. Localization of an alpha 1,2 galactosyltransferase activity to the Golgi apparatus of Schizosaccharomyces pombe. Mol Biol Cell. 1994 May;5(5):519–528. doi: 10.1091/mbc.5.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dibenedetto G., Cozzani I. Nonspecific acid phosphatase from Schizosaccharomyces pombe. Purification and physical chemical properties. Biochemistry. 1975 Jul;14(13):2847–2852. doi: 10.1021/bi00684a009. [DOI] [PubMed] [Google Scholar]
- Esmon B., Esmon P. C., Schekman R. Early steps in processing of yeast glycoproteins. J Biol Chem. 1984 Aug 25;259(16):10322–10327. [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993 Apr 1;7(6):540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Vonlanthen M., Rosset J. Localization of alpha-galactomannan and of wheat germ agglutinin receptors in Schizosaccharomyces pombe. Arch Microbiol. 1978 Nov 13;119(2):107–111. doi: 10.1007/BF00964260. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Häusler A., Ballou L., Ballou C. E., Robbins P. W. Yeast glycoprotein biosynthesis: MNT1 encodes an alpha-1,2-mannosyltransferase involved in O-glycosylation. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6846–6850. doi: 10.1073/pnas.89.15.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maley F., Trimble R. B., Tarentino A. L., Plummer T. H., Jr Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem. 1989 Aug 1;180(2):195–204. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Nagasu T., Shimma Y., Kuromitsu J., Jigami Y. OCH1 encodes a novel membrane bound mannosyltransferase: outer chain elongation of asparagine-linked oligosaccharides. EMBO J. 1992 Jul;11(7):2511–2519. doi: 10.1002/j.1460-2075.1992.tb05316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Warren G. Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus. Curr Opin Cell Biol. 1994 Aug;6(4):517–521. doi: 10.1016/0955-0674(94)90070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr S. F., Stevens T. H. Sorting of membrane proteins in the yeast secretory pathway. J Biol Chem. 1994 Apr 8;269(14):10185–10188. [PubMed] [Google Scholar]
- Pidoux A. L., Armstrong J. Analysis of the BiP gene and identification of an ER retention signal in Schizosaccharomyces pombe. EMBO J. 1992 Apr;11(4):1583–1591. doi: 10.1002/j.1460-2075.1992.tb05203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J. C., Roncero C., Rico H., Durán A. Characterization of a Schizosaccharomyces pombe morphological mutant altered in the galactomannan content. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):263–267. doi: 10.1016/0378-1097(91)90096-s. [DOI] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
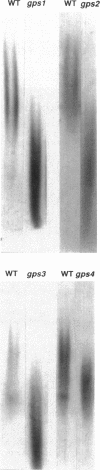
- Schweingruber A. M., Schoenholzer F., Keller L., Schwaninger R., Trachsel H., Schweingruber M. E. Glycosylation and secretion of acid phosphatase in Schizosaccharomyces pombe. Eur J Biochem. 1986 Jul 1;158(1):133–140. doi: 10.1111/j.1432-1033.1986.tb09730.x. [DOI] [PubMed] [Google Scholar]
- Schweingruber M. E., Schweingruber A. M. Modulation of a cell surface glycoprotein in yeast: acid phosphatase. Differentiation. 1981;19(1):68–70. doi: 10.1111/j.1432-0436.1981.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Smith D. G., Svoboda A. Golgi apparatus in normal cells and protoplasts of Schizosaccharomyces pombe. Microbios. 1972 May-Jun;5(19):177–182. [PubMed] [Google Scholar]
- Sáez M. J., Lagunas R. Determination of intermediary metabolites in yeast. Critical examination of the effect of sampling conditions and recommendations for obtaining true levels. Mol Cell Biochem. 1976 Nov 30;13(2):73–78. doi: 10.1007/BF01837056. [DOI] [PubMed] [Google Scholar]
- Yip C. L., Welch S. K., Klebl F., Gilbert T., Seidel P., Grant F. J., O'Hara P. J., MacKay V. L. Cloning and analysis of the Saccharomyces cerevisiae MNN9 and MNN1 genes required for complex glycosylation of secreted proteins. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2723–2727. doi: 10.1073/pnas.91.7.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler F. D., Gemmill T. R., Trimble R. B. Glycoprotein synthesis in yeast. Early events in N-linked oligosaccharide processing in Schizosaccharomyces pombe. J Biol Chem. 1994 Apr 29;269(17):12527–12535. [PubMed] [Google Scholar]