Abstract
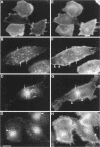
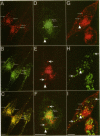
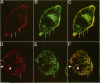
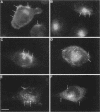
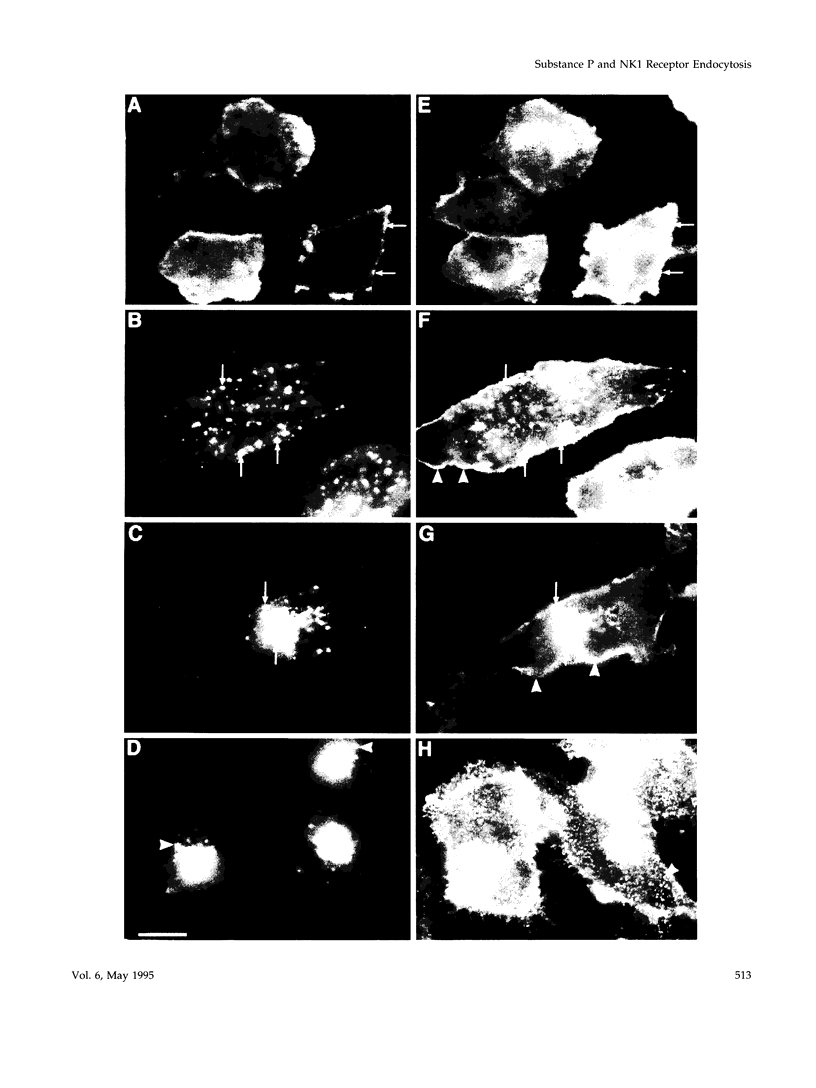
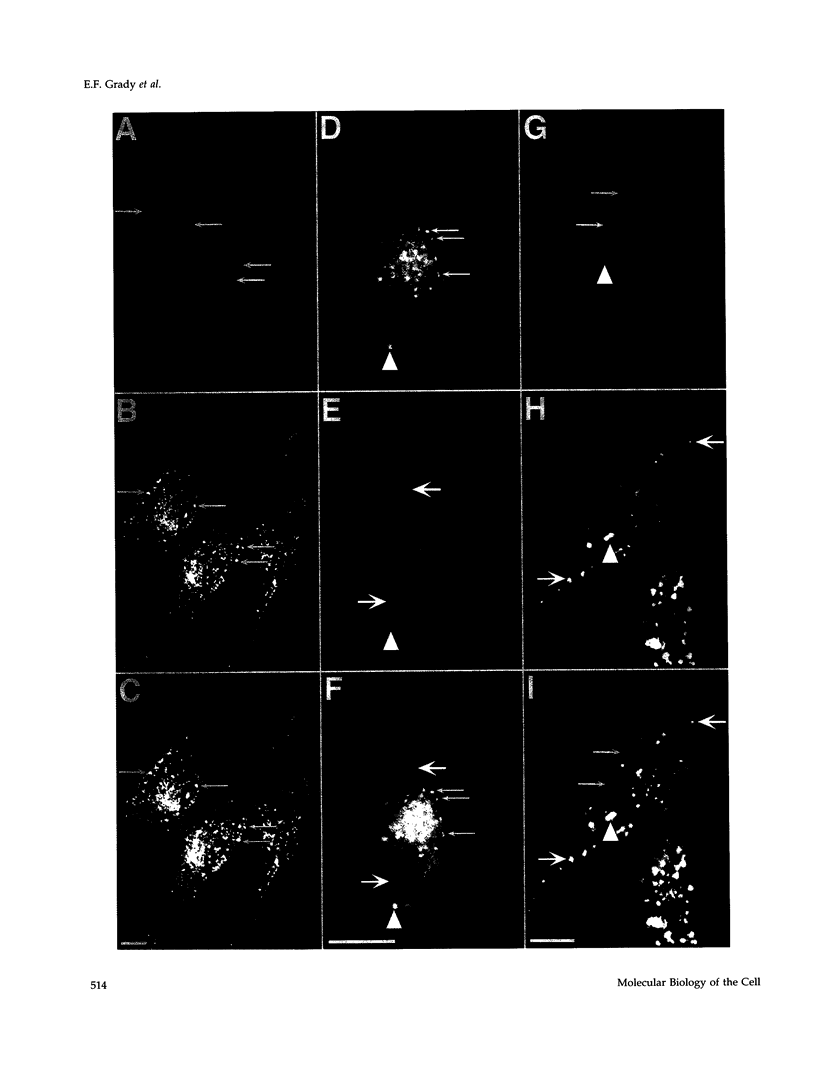
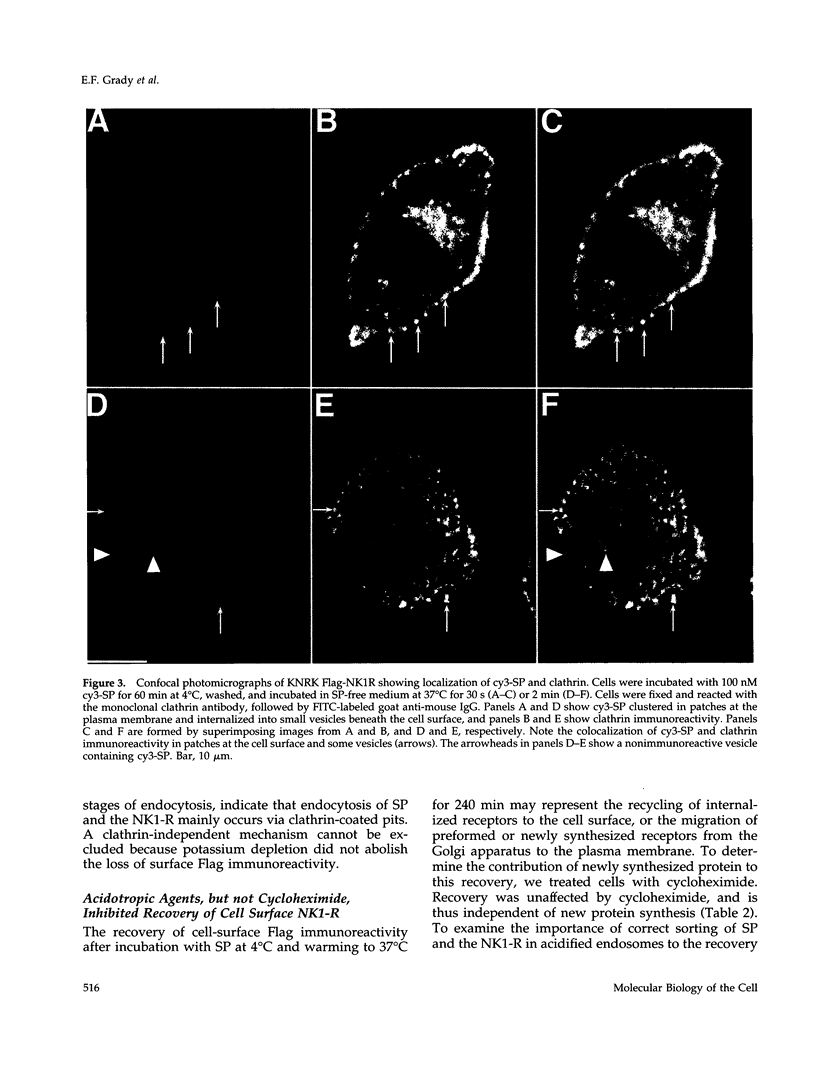
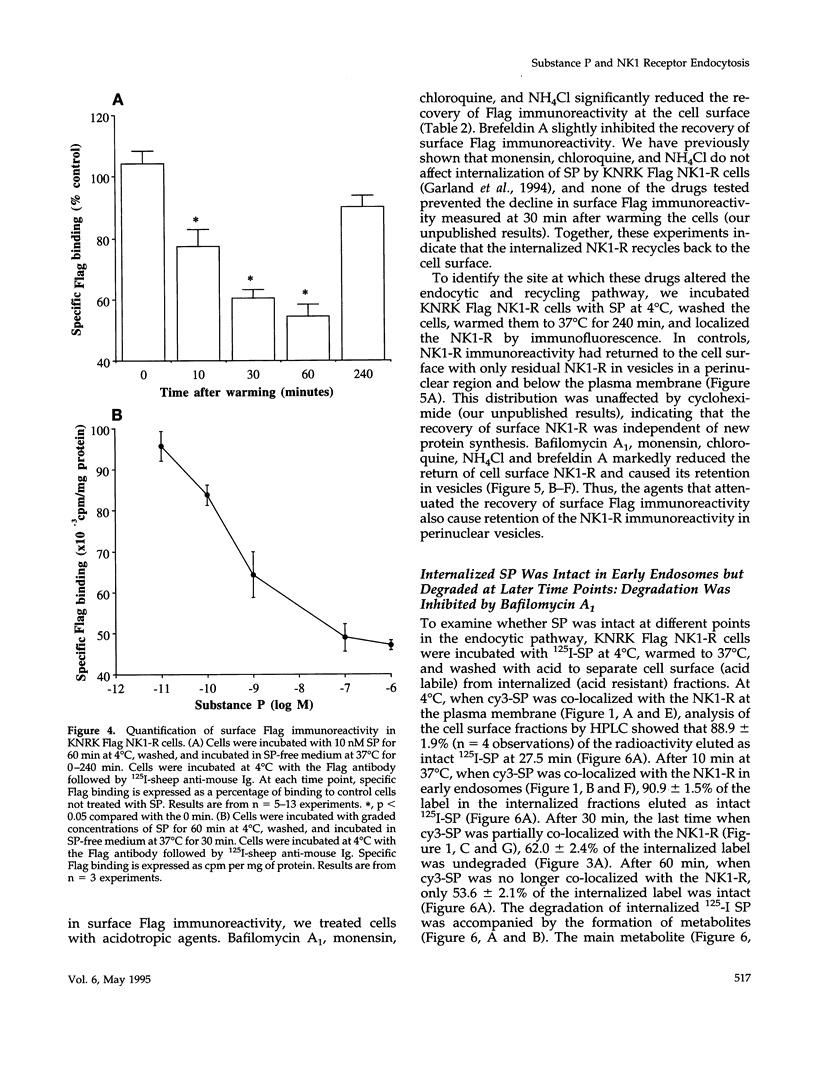
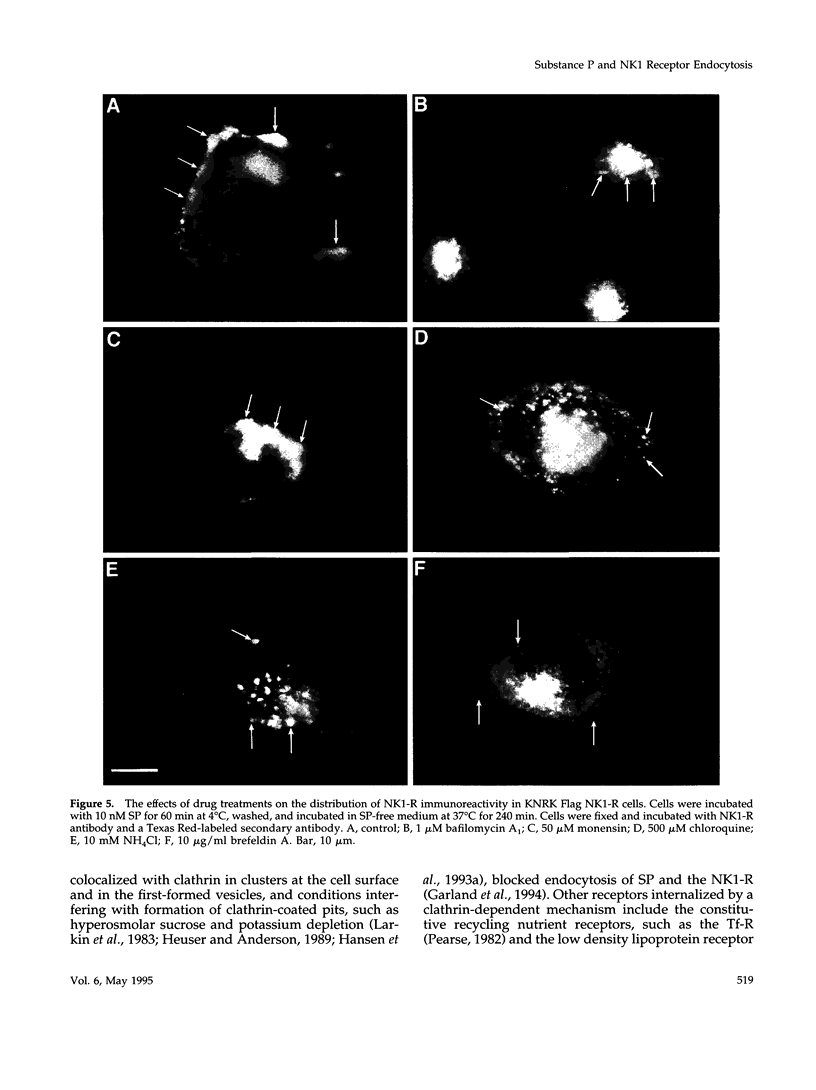
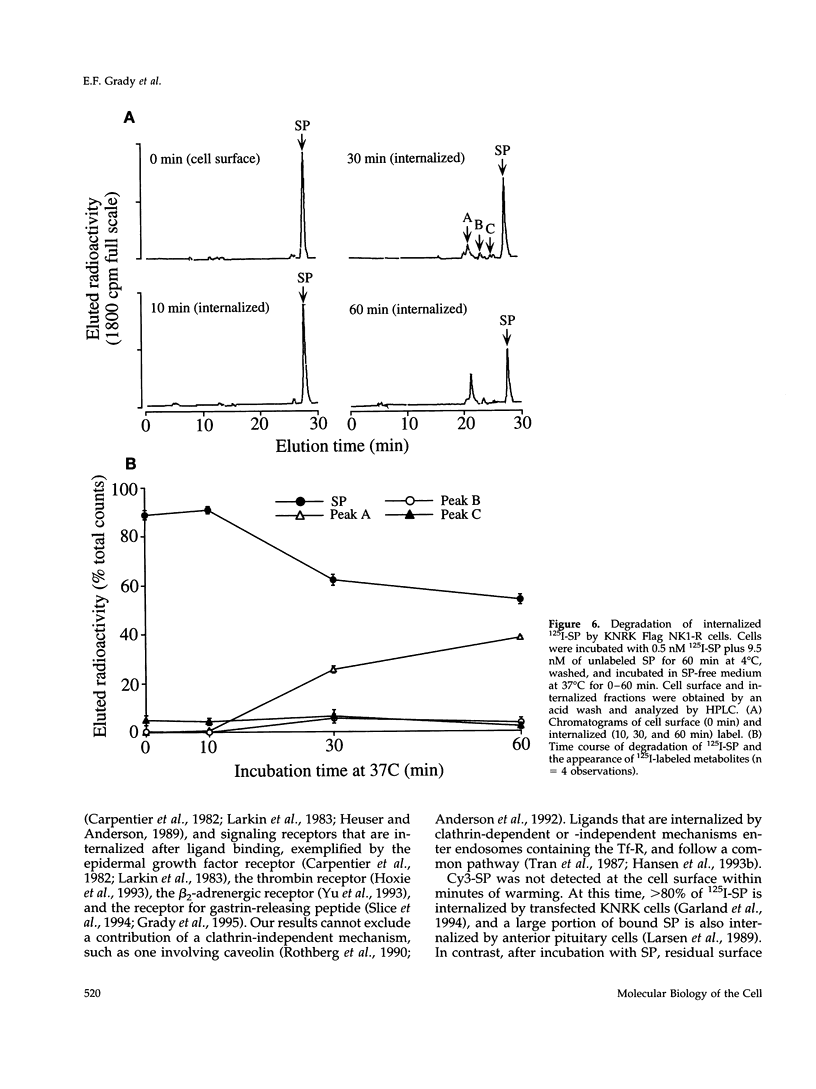
Many of the actions of the neuropeptide substance P (SP) that are mediated by the neurokinin 1 receptor (NK1-R) desensitize and resensitize, which may be associated with NK1-R endocytosis and recycling. We delineated this endocytic pathway in transfected cells by confocal microscopy using cyanine 3-SP and NK1-R antibodies. SP and the NK1-R were internalized into the same clathrin immunoreactive vesicles, and then sorted into different compartments. The NK1-R was colocalized with a marker of early endosomes, but not with markers of late endosomes or lysosomes. We quantified the NK1-R at the cell surface by incubating cells with an antibody to an extracellular epitope. After exposure to SP, there was a loss and subsequent recovery of surface NK1-R. The loss was prevented by hypertonic sucrose and potassium depletion, inhibitors of clathrin-mediated endocytosis. Recovery was independent of new protein synthesis because it was unaffected by cycloheximide. Recovery required endosomal acidification because it was prevented by an H(+)-ATPase inhibitor. The fate of internalized 125I-SP was examined by chromatography. SP was intact at the cell surface and in early endosomes, but slowly degraded in perinuclear vesicles. We conclude that SP induces clathrin-dependent internalization of the NK1-R. The SP/NK1-R complex dissociates in acidified endosomes. SP is degraded, whereas the NK1-R recycles to the cell surface.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992 Jan 24;255(5043):410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Barak L. S., Tiberi M., Freedman N. J., Kwatra M. M., Lefkowitz R. J., Caron M. G. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J Biol Chem. 1994 Jan 28;269(4):2790–2795. [PubMed] [Google Scholar]
- Benya R. V., Fathi Z., Battey J. F., Jensen R. T. Serines and threonines in the gastrin-releasing peptide receptor carboxyl terminus mediate internalization. J Biol Chem. 1993 Sep 25;268(27):20285–20290. [PubMed] [Google Scholar]
- Bowden J. J., Garland A. M., Baluk P., Lefevre P., Grady E. F., Vigna S. R., Bunnett N. W., McDonald D. M. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Pizarro S., Ahuja M., Belmonte E., Blanchard N., Stadel J. M., Hoxie J. A. Changes in the structure and function of the human thrombin receptor during receptor activation, internalization, and recycling. J Biol Chem. 1994 Jan 28;269(4):2943–2952. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Connolly T. P., Hubbard A. L. Receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes: receptor pathway. J Cell Biol. 1986 Jan;102(1):24–36. doi: 10.1083/jcb.102.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985 Mar 10;260(5):2646–2652. [PubMed] [Google Scholar]
- Garland A. M., Grady E. F., Payan D. G., Vigna S. R., Bunnett N. W. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem J. 1994 Oct 1;303(Pt 1):177–186. doi: 10.1042/bj3030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Grady E. F., Slice L. W., Brant W. O., Walsh J. H., Payan D. G., Bunnett N. W. Direct observation of endocytosis of gastrin releasing peptide and its receptor. J Biol Chem. 1995 Mar 3;270(9):4603–4611. doi: 10.1074/jbc.270.9.4603. [DOI] [PubMed] [Google Scholar]
- Green S. A., Kelly R. B. Low density lipoprotein receptor and cation-independent mannose 6-phosphate receptor are transported from the cell surface to the Golgi apparatus at equal rates in PC12 cells. J Cell Biol. 1992 Apr;117(1):47–55. doi: 10.1083/jcb.117.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Hoflack B., Simons K., Mellman I., Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988 Feb 12;52(3):329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Grimaldi K. A., Hutton J. C., Siddle K. Production and characterization of monoclonal antibodies to insulin secretory granule membranes. Biochem J. 1987 Jul 15;245(2):557–566. doi: 10.1042/bj2450557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993 Apr;121(1):61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J Cell Biol. 1993 Oct;123(1):89–97. doi: 10.1083/jcb.123.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Anderson R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989 Feb;108(2):389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Ahuja M., Belmonte E., Pizarro S., Parton R., Brass L. F. Internalization and recycling of activated thrombin receptors. J Biol Chem. 1993 Jun 25;268(18):13756–13763. [PubMed] [Google Scholar]
- Kay D. G., Lai W. H., Uchihashi M., Khan M. N., Posner B. I., Bergeron J. J. Epidermal growth factor receptor kinase translocation and activation in vivo. J Biol Chem. 1986 Jun 25;261(18):8473–8480. [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwatra M. M., Schwinn D. A., Schreurs J., Blank J. L., Kim C. M., Benovic J. L., Krause J. E., Caron M. G., Lefkowitz R. J. The substance P receptor, which couples to Gq/11, is a substrate of beta-adrenergic receptor kinase 1 and 2. J Biol Chem. 1993 May 5;268(13):9161–9164. [PubMed] [Google Scholar]
- Larkin J. M., Brown M. S., Goldstein J. L., Anderson R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983 May;33(1):273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- Larsen P. J., Mikkelsen J. D., Mau S., Saermark T. Binding and internalization of a iodinated substance P analog by cultured anterior pituitary cells. Mol Cell Endocrinol. 1989 Aug;65(1-2):91–101. doi: 10.1016/0303-7207(89)90169-x. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Caron M. G., Lefkowitz R. J. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990 Feb 25;265(6):3202–3211. [PubMed] [Google Scholar]
- Mayor S., Presley J. F., Maxfield F. R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993 Jun;121(6):1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Okamoto A., Lovett M., Payan D. G., Bunnett N. W. Interactions between neutral endopeptidase (EC 3.4.24.11) and the substance P (NK1) receptor expressed in mammalian cells. Biochem J. 1994 May 1;299(Pt 3):683–693. doi: 10.1042/bj2990683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993 Apr;73(2):229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci U S A. 1982 Jan;79(2):451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N. S., Campbell P. T., Caron M. G., Lefkowitz R. J., Lohse M. J. Comparative rates of desensitization of beta-adrenergic receptors by the beta-adrenergic receptor kinase and the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6201–6204. doi: 10.1073/pnas.88.14.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N. H., Maxfield F. R. Quantitative fluorescence techniques for the characterization of endocytosis in intact cells. Subcell Biochem. 1993;19:95–123. doi: 10.1007/978-1-4615-3026-8_4. [DOI] [PubMed] [Google Scholar]
- Slice L. W., Wong H. C., Sternini C., Grady E. F., Bunnett N. W., Walsh J. H. The conserved NPXnY motif present in the gastrin-releasing peptide receptor is not a general sequestration sequence. J Biol Chem. 1994 Aug 26;269(34):21755–21761. [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Valiquette M., Bonin H., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Involvement of tyrosine residues located in the carboxyl tail of the human beta 2-adrenergic receptor in agonist-induced down-regulation of the receptor. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5089–5093. doi: 10.1073/pnas.87.13.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigna S. R., Bowden J. J., McDonald D. M., Fisher J., Okamoto A., McVey D. C., Payan D. G., Bunnett N. W. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J Neurosci. 1994 Feb;14(2):834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Recycling of the hepatic asialoglycoprotein receptor in isolated rat hepatocytes. Receptor-ligand complexes in an intracellular slowly dissociating pool return to the cell surface prior to dissociation. J Biol Chem. 1984 Jan 25;259(2):1150–1154. [PubMed] [Google Scholar]
- Wikström L., Lodish H. F. Nonlysosomal, pre-Golgi degradation of unassembled asialoglycoprotein receptor subunits: a TLCK- and TPCK-sensitive cleavage within the ER. J Cell Biol. 1991 Jun;113(5):997–1007. doi: 10.1083/jcb.113.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T., Yamamoto A., Moriyama Y., Futai M., Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991 Sep 15;266(26):17707–17712. [PubMed] [Google Scholar]
- Yu S. S., Lefkowitz R. J., Hausdorff W. P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993 Jan 5;268(1):337–341. [PubMed] [Google Scholar]
- von Zastrow M., Kobilka B. K. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992 Feb 15;267(5):3530–3538. [PubMed] [Google Scholar]