Abstract
As efficacy trials of antiretroviral pre-exposure prophylaxis (PrEP) continue, a growing literature has begun anticipating the potential challenges of implementing PrEP for HIV prevention. These efforts coincide with a shift toward combination interventions for preventing HIV, which integrate biomedical, behavioral, and structural components. The optimal implementation of PrEP would exemplify this combination model, incorporating not only PrEP drugs, but also HIV testing, safety screening, behavioral interventions addressing adherence and risk behavior, and long-term monitoring. Efforts to plan for PrEP implementation therefore present an opportunity to advance the science of implementation and delivery in HIV prevention, in order to better address the challenges of scaling up combination approaches. We review the published and unpublished literature on PrEP implementation, organizing themes into five categories: scientific groundwork, regulatory and policy groundwork, stakeholder and infrastructure groundwork, delivery, and long-term monitoring. The lessons from PrEP planning can benefit the scale-up of future combination interventions.
Keywords: Pre-exposure prophylaxis, HIV prevention, antiretroviral therapy, implementation, dissemination
Introduction
Nearly 60 million people have been infected with HIV since the start of the epidemic [1], and projected estimates suggest that as many as 60 million more infections could occur during a 15 to 20 year wait for an effective vaccine [2]. Advances in biomedical HIV prevention have focused on alternatives to vaccines, including prophylactic uses of antiretroviral drugs (ARVs) [3]. Recent research has examined the potential for ARVs to reduce infectiousness among HIV-positive persons, to prevent infection among HIV-negative persons when used as post-exposure prophylaxis (PEP), and to prevent infection when given as pre-exposure prophylaxis (PrEP) [3, 4]. We focus here on antiretroviral PrEP, which requires HIV-uninfected individuals to take ARVs, such as tenofovir with or without emtricitabine, before engaging in HIV risk behavior [5•]. Ongoing trials of PrEP are evaluating both oral and topical formulations, studying efficacy among samples including men who have sex with men, injection drug users, high-risk heterosexual women, and serodiscordant couples [6]. Although PrEP is unlikely to be completely efficacious for prevention, mathematical modeling suggests that even a partially efficacious PrEP drug may lead to meaningful declines in HIV incidence at a population level [7, 8•, 9, 10•]. While the field waits for trial results, efforts are underway to anticipate challenges in the implementation and rollout of PrEP, should trial results be favorable [5•, 11–15]. A consistent theme of this literature is that PrEP must be part of a comprehensive HIV prevention package, which should include support services such as safety screening, HIV testing, behavioral interventions to support adherence and reduce risk behaviors, and the treatment of side effects and HIV infection in the event of PrEP failure [16]. As an integrated strategy, PrEP exemplifies a growing shift in HIV prevention paradigms toward combination approaches—packages that include behavioral, biomedical, and structural elements to maximize preventive impact [3, 17–19].
This trend in combination HIV prevention programming has far-reaching implications for the science of implementation and intervention delivery. We suggest that the process of planning for PrEP scale-up presents an unmatched opportunity to shift implementation paradigms to address combination interventions. Implementation science in the HIV prevention field has been underdeveloped, and it centers largely on the replication of evidence-based interventions (EBIs) to reduce sexual risk behavior [17]. Interventions that include both biomedical and behavioral components will demand new approaches to planning for scale-up, requiring collaboration among behavioral and clinical scientists. PrEP presents an important moment to make these advances for several reasons. First, there is already widespread awareness of the need to combine PrEP prescription with behavioral and structural intervention. Next, PrEP can benefit directly from lessons from the scale-up of antiretroviral treatment, as well as initial planning for the scale-up of male circumcision [20], which can help predict implementation needs and structural implications. PrEP is also emerging at a time when intervention science has recognized the need for combination packages, but before the emergence of proven combination strategies. Finally, PrEP is not the only combination prevention strategy in development; integrated approaches for delivering other multicomponent interventions such as non-ARV microbicides, cervical barriers, HSV-2 treatment, and the next generation of vaccines [3] can benefit from the PrEP planning process.
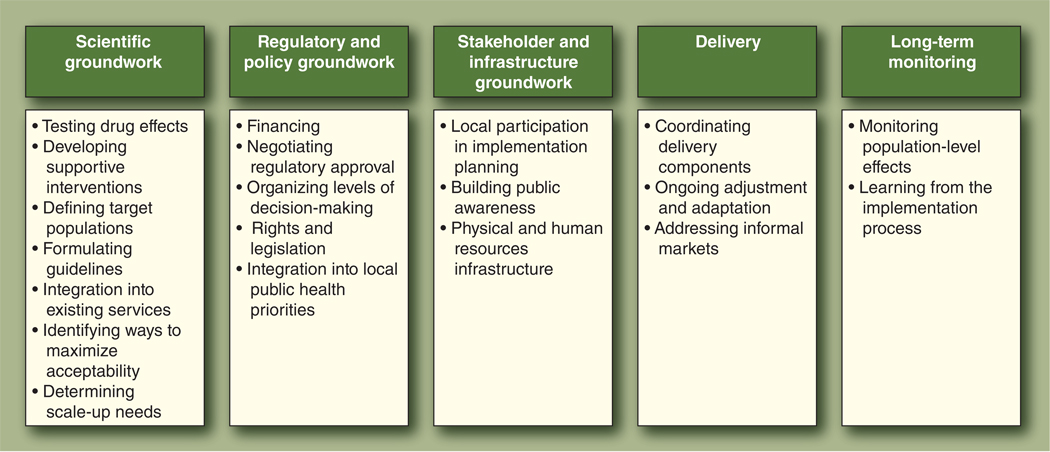
This review summarizes the available literature on the implementation of PrEP, drawing on published and unpublished sources to describe possible challenges. We have identified five implementation processes that may influence the effectiveness of PrEP for public use, and which might also underlie the implementation of future combination HIV interventions: 1) scientific groundwork, 2) regulatory and policy groundwork, 3) stakeholder and infrastructure groundwork, 4) actual delivery, and 5) the long-term process of monitoring and adjustment (Fig. 1). The field is still at the initial stage of scientific groundwork for PrEP and other combination packages, and this list of implementation challenges is neither exhaustive nor chronological. However, consideration of these five broad processes may be useful in planning for PrEP and future combination prevention programs.
Figure 1.
Implementation processes identified for pre-exposure prophylaxis.
Scientific Groundwork: Constructing the PrEP Package
Testing drug effects
Ongoing trials will answer many of the outstanding questions about the effects of PrEP drugs, including optimal dosing schedule (eg, daily, intermittent, coitally associated), route of administration (eg, topical or oral), and efficacy for different types of HIV exposure (eg, vaginal sex, anal sex, parenteral exposure). A phase 2 trial of tenofovir PrEP in West Africa (n = 936 women, exposed to HIV primarily through vaginal sex) found eight seroconversions over 476 person-years of HIV testing, of which six occurred in the placebo group and two in the group taking daily oral tenofovir [21••]. The CAPRISA 004 study in South Africa, recently released at the XVIII International AIDS Conference, assessed the effectiveness over 30 months of a vaginal gel containing tenofovir (n = 889 analyzed); this formulation reduced HIV infection by approximately 39% overall, and 54% among women with high adherence to dosage schedules [22••]. Several population-level models have suggested that PrEP could have a substantial impact in the US [8•, 9] and in resource-limited settings [7], including Africa and India [10•]. PrEP’s level of efficacy will shape plans for implementation, with key implications for cost-effectiveness, population targeting, acceptability, and the development of supportive interventions [5•, 8•, 9, 12, 14]. Efforts to plan for implementation must also address PrEP’s potential to produce side effects and increase the incidence of drug-resistant HIV infection. Possible side effects of tenofovir or tenofovir plus emtricitabine include renal effects, loss of bone mineral density, gastrointestinal effects, and flares of hepatitis B after discontinuing use [5•, 13]. The West Africa trial discussed above found that tenofovir was well-tolerated and did not cause hepatitis B flare-ups [21••]; the CAPRISA 004 trial found that a vaginal gel containing tenofovir was well-tolerated and did not cause renal toxicity or hepatitis B flare-ups [22••]; and a trial of tenofovir in Bangkok found no serious safety concerns after several years of enrollment [23]. An extended safety study of daily oral tenofovir PrEP among 400 men who have sex with men in the US also found no serious safety concerns after over 2 years of assessment [24]. However, monitoring and treating side effects will be an integral part of implementation, which will require provider training, user education, and resource mobilization. Acquisition of drug-resistant HIV and the development of secondary resistance are also potential risks; although using a combination of ARVs for PrEP may minimize this concern [5•], and modeling suggests that resistance may be rare [25], the possibility of resistance has prompted some questioning of whether certain classes of ARV drugs should be reserved for treatment purposes only [11]. The possibility of resistance will require routine HIV testing among PrEP users, so that PrEP use may be discontinued as soon as possible after infection.
Developing supportive interventions
Optimal PrEP use will require behavioral interventions to maximize drug adherence and minimize risk compensation behaviors by PrEP users. Adherence behaviors will be critical for maximizing PrEP’s public health impacts [7, 8•, 9, 10•] and minimizing drug resistance [7], and modeling suggests that the population-level impact of PrEP is sensitive to even moderate risk compensation behavior [7, 9, 10•]. Trial data have thus far shown promising behavioral results. Adherence was imperfect but relatively high among Ghanaian women enrolled in the West Africa trial [21••, 26•], and trials in Nigeria, Bangkok, Peru, and Ecuador have also reported relatively strong adherence [23, 27, 28] that may improve over time [28]. Adherence is demonstrably related to PrEP’s protective benefit; the CAPRISA 004 study found that approximately 40% of participants had adherence below 50%, and the protective effect of tenofovir gel was less pronounced among low adherers [22••]. Risk compensation behavior was not observed in the West Africa trial of oral PrEP discussed above, which instead showed overall declines in risk behavior among enrolled Ghanaian women [29•]; this pattern was mirrored in early data from a PrEP study among injection drug users in Bangkok [30], as well as in the CAPRISA 004 trial [22••]. However, adherence and risk compensation behaviors may be more pronounced outside clinical trials; for example, behavioral models by Sood and Goldman [31] suggest that population-level sexual risk behaviors have increased following breakthroughs in HIV biomedicine. Although PrEP adherence research will benefit from previous work on ARV treatment, treatment adherence models may not match the behavior of HIV-uninfected individuals [3, 11, 32]. New behavioral interventions are needed to optimize adherence and risk compensation among PrEP users, and research is especially needed on ways to communicate with users about PrEP’s partial efficacy [5•, 11, 13].
Given the possibility of side effects and PrEP failures, supportive services must also include screening to monitor for wild-type and resistant HIV infection, renal impairment, and hepatitis B flare-ups [11]; however, questions remain about the necessary frequency of testing [14] and the effect of testing on PrEP’s acceptability. Practical challenges for these testing mechanisms will include providing linkages to care for individuals who test positive; negotiating confidentiality concerns; addressing the differential impact of testing on at-risk populations [33]; accommodating the costs and human resource needs of testing and counseling [34]; addressing limited insurance coverage for testing and treatment [35, 36]; maximizing the accessibility of testing services for hard-to-reach populations; and identifying appropriate and cost-effective testing strategies for PrEP users, including potential opportunities for task-shifting [37], provider-initiated or routine (opt-out) testing [35], and alternative testing strategies such as home-based tests [38, 39]. Some implementation challenges may be heightened in resource-limited settings, such as human resource shortages, uncertain access to treatment, limited health services utilization by at-risk individuals, ongoing stigma, and insufficient protections against involuntary status disclosure or discrimination [40].
Defining target populations
A universal concern in implementation planning efforts concerns ways to identify PrEP users. The cost of PrEP may not support widespread use by the general population. Targeting PrEP to individuals with greatest sexual activity may make the intervention more cost-effective, although greater coverage (eg, 75% of susceptible sexually active individuals, in one model) may result in greater overall reductions in HIV infections [7]. Ongoing trials have included populations at high risk for HIV; however, important groups are underrepresented or not represented in these trials, including pregnant women [5•, 41], adolescents [5•, 41], and racial and ethnic minority populations [42]. Additional research may be necessary to create PrEP formulations that are safe for these groups, and behavioral interventions will be necessary to address social and epidemiological factors influencing PrEP use in these populations.
Formulating guidelines
Implementation will depend on the development of guidelines for PrEP implementation in practice settings, which should address many of the questions discussed above [43]. Guidelines must specify the necessary personnel qualifications to prescribe PrEP, conduct safety screening, and provide supportive services, and guidelines may also address the types of settings in which PrEP may be delivered safely and effectively. The process of constructing an optimal package for PrEP implementation will vary by setting [44], depending on factors such as local epidemiology, capacity, and prevention priorities. There will be pressure for rapid guideline development—the US Centers for Disease Control and Prevention (CDC) may aim to issue guidelines within 6 months of test results [45]—but guidelines should remain sensitive to research on long-term effects and supportive interventions to optimize PrEP use.
Developing strategies for integration into existing services
Another common theme in PrEP planning reflects the need to identify an optimal mix of HIV prevention interventions, balancing factors such as efficacy and cost-effectiveness [43]. Strategies for combining PrEP with other HIV prevention approaches, such as sexually transmitted infection treatment or circumcision, are an area for future development, which will expand as new biomedical strategies emerge. An important concern in this area is the possible effect of identifying PrEP as the best current standard for HIV prevention; if PrEP is the best available technology for HIV prevention, future HIV prevention trials may be ethically obliged to offer PrEP to all participants, just as HIV prevention counseling and condoms are offered today. This may complicate future efforts to compare PrEP against other intervention approaches, as well as to isolate the effects of new biomedical strategies [3, 18, 41, 46]. Further research is also necessary to ascertain how PrEP may be integrated into other services needed by populations at risk for HIV, such as family planning services, substance abuse treatment, mental health services, and basic health care needs.
Identifying ways to maximize acceptability
Research efforts should also identify ways to maximize PrEP’s acceptability to potential users, since PrEP’s public health impact will depend in part on uptake [7, 8•, 9, 10•]. Different formulations of PrEP have been found acceptable to women enrolled in the West Africa trial [26•], to women enrolled in the CAPRISA 004 study [22••], and to serodiscordant couples seeking to conceive [47]. Additional studies have found willingness to use PrEP among groups at elevated risk for HIV in Peru, Ecuador, Bangkok, the US, and India [48–55]. Acceptability may depend on perceived efficacy, side effects, and cost [48, 54], and several studies have found greater intentions to use PrEP or willingness to pay more for PrEP among individuals with higher levels of risk behavior [52, 53, 55]. PrEP acceptability will likely also be complicated by attitudes toward medical providers, pill-taking, vaginal or rectal products, ARVs, PEP, and other psychosocial factors. Importantly, acceptability of PrEP drugs may differ from acceptability of a larger package of safety screening, long-term use, behavioral intervention, HIV testing, and potential costs, so acceptability research must continue throughout implementation. PrEP use may also carry a stigma, given social attitudes toward populations at highest risk for HIV [12, 41]. If PrEP use is stigmatized, research should address strategies for marketing PrEP acceptably, packaging PrEP pills discreetly [12, 41], and addressing PrEP in stigma reduction campaigns.
Determining setting-specific implementation needs
To plan for PrEP rollout, it will be critical to understand the scale-up needs of new settings [56], including needs for physical infrastructure and supplies, monitoring and surveillance infrastructure, human resources, PrEP financing, provider training, and resources needed to mobilize local support for PrEP implementation. This operational research should focus especially on the need for providing outreach, education, and support to potential PrEP users, who may be underrepresented in clinical care populations. Formulating and testing potential outreach strategies will be an important component of PrEP success [14].
Regulatory, Policy, and Financial Groundwork
Financing
If PrEP is determined effective for use, new strategies for financing PrEP delivery to at-risk individuals will be imperative [5•, 12, 14, 15, 57, 58]. The costs of PrEP have been estimated variously at between $523 and $900 per month, depending on the drug regimen and location [9, 58]. The cost of providing PrEP to the 100,000 most at-risk people in the US could exceed $1 billion each year, which would exceed the CDC’s current HIV prevention budget [43]. Finances will be necessary not only for PrEP drug delivery, but also for infrastructure and human resources development, training clinical providers, outreach and community education, monitoring and surveillance, safety screening, long-term HIV testing and referrals, supportive behavioral interventions, and an ongoing research program to identify optimal strategies for PrEP intervention and integration with existing services. The costs of PrEP may conflict with funding priorities for behavioral HIV prevention [5•]; inadequate financing and capacity limitations are already primary factors limiting the scale-up of effective HIV prevention strategies [59].
Shifting costs to insurers or consumers is unlikely to meet the need for PrEP financing. Insurers, including governments, may prefer behavioral alternatives such as risk reduction counseling or provision of condoms [58]. For example, an anticipatory planning process for PrEP rollout in Malawi found that stakeholders favored safer sex promotion and would not support widespread PrEP rollout; instead, these decision-makers viewed PrEP as a temporary strategy to be embedded in behavior change programming [44]. Insurance coverage is also likely to be complicated by regulatory issues (eg, if PrEP drugs are not approved for prophylaxis [15]), fears of risk compensation, and stigma—particularly if PrEP financing is interpreted as expressing approval of HIV risk behaviors [5•]. Cost-shifting to consumers is unlikely, as out-of-pocket costs could be a major barrier to use [48]. Many groups at high risk for HIV are less likely to have health insurance coverage [14, 15], and one study among US women found that willingness to use PrEP was inversely correlated with personal income, and was greater among women who were unemployed [53].
Negotiating regulatory approval
Plans for PrEP implementation must also incorporate regulatory concerns, specifically approval for using ARVs for PrEP indications. Off-label use may make PrEP marketing and financing difficult, as insurers are less likely to cover prescriptions for unapproved uses [15, 57], and clinicians may be less willing to prescribe drugs for off-label purposes [14, 15]. In the US, the Food and Drug Administration would require a New Drug Application to approve Viread (tenofovir) or Truvada (tenofovir with emtricitabine) for use as PrEP; Gilead, which manufactures both drugs, has not yet announced an intention to seek this approval [57]. Processes of seeking and gaining approval may be further complicated by concerns about legal liability for PrEP failures [14], as well as questions about risk compensation and the possibility that PrEP will be deemed a “lifestyle drug” (which may increase costs) [57]. Specific regulatory challenges will differ across jurisdictions, and one advocacy group, the AIDS Vaccine Advocacy Coalition, has already called for transparency in describing global and country-specific scenarios for manufacturing PrEP, accessing PrEP drugs, and navigating regulatory regimes [20].
Organizing levels of decision-making
PrEP implementation will require organization among various levels of leadership, including international bodies such as the World Health Organization (WHO) and UNAIDS [20], donors and private payers, national and local government agencies, professional organizations, scientific leaders, and advocacy groups including PrEP users. The roles of these different groups will require clarification, and the extent to which PrEP guidance is centralized will affect the speed at which PrEP protocols can be adjusted to reflect new data. A key challenge for PrEP, as a combination intervention, is that different levels of centralization may be appropriate for different components of the overall package. For example, although guidelines for prescribing PrEP may be set by groups such as the CDC or WHO, behavioral interventions to support adherence or minimize risk compensation may require more specific tailoring to local populations; in this way, centralized guidance may be more appropriate for biomedical components such as safety screening and dosage, but less appropriate for PrEP’s behavioral components. The organization of PrEP leadership should establish processes for addressing unanticipated implementation concerns, ideally in ways that incorporate feedback from PrEP clinicians and users along with scientific and policy personnel.
Identifying implications for rights and legislation
Implementation planning groups have identified possible effects of PrEP on policy and legislation, including laws related to HIV testing, partner notification, and criminalization of HIV transmission [46]. An additional question may concern arguments about PrEP and human rights. Access to antiretroviral therapy for treatment has been framed as a human right, and several national courts have interpreted the right to health to include a right to ARV treatment provided by the state [60]. Access to prevention services, including prevention technologies, has also been described as a fundamental human right [61]. However, the universe of people at risk for HIV is vast, particularly in settings where the epidemic is generalized. Reconciling rights-based reasoning with available resources for PrEP delivery will be challenging, and implementation plans should anticipate these tensions.
Integration with local public health priorities
A final policy challenge will be determining how to prioritize PrEP in the larger context of international, national, and local health concerns. Ethical questions will arise from financing PrEP when many HIV-infected individuals still lack access to ARVs for treatment [3, 14]. Similar issues may arise from allocating additional resources for HIV prevention compared to other health priorities, which may again be influenced by stigma against PrEP users [5•]. Scientific development, especially cost-effectiveness research, will help to locate PrEP in HIV/AIDS and public health programming, but questions about funding will necessarily bridge scientific and ethical concerns.
Stakeholder and Infrastructure Groundwork
Local participation in implementation planning
The history of PrEP research and early implementation planning has illuminated the need to involve local stakeholders early in planning [44, 62]. The time before trial results are available presents opportunities to establish communication channels with a variety of stakeholder groups, including clinical personnel and paraprofessionals, HIV prevention service providers, potential PrEP users, policymakers, governments, advocacy groups, and the media. PrEP scientists should emphasize caution and nuance in media messages [12, 14, 32], and the need for clear communication will be acutely important if trials show lack of efficacy or mixed results [14]. The involvement of clinical providers and HIV prevention service personnel at an early stage will also be key; one planning group has noted that providers may resist PrEP if they perceive it as a threat to existing prevention services, if they believe it will remove HIV prevention from behavioral approaches entirely to medicine, or if they serve populations that may have difficulty accessing PrEP [15]. Policymakers and scientists planning for PrEP implementation should plan to address these concerns through direct engagement with group representatives, as well as in provider training, statements through the media, and PrEP delivery guidelines.
Building public awareness
Securing adequate levels of PrEP coverage will rely on ways to inform potential PrEP users about this new technology. Active outreach outside clinical settings will be particularly crucial for populations that are stigmatized or hidden, such as sex workers, injection drug users, non–gay-identifying men who have sex with men (MSM), racial and ethnic minorities, and individuals in the criminal justice system [14]. Several population-level surveys have assessed PrEP awareness among MSM in the US, finding that between 16% and 25% had heard of using ARVs for PrEP [52, 54, 63, 64]; after PrEP was described, a majority of men in two studies expressed willingness to use PrEP if it is proven safe and effective [52, 54]. Awareness campaigns should also include the general public, focusing on incorporating PrEP and PrEP users into campaigns to reduce HIV-related stigma. These campaigns have the potential to raise both awareness and the acceptability of PrEP.
Physical and human resources infrastructure
PrEP implementation will require substantial physical and human resources. Infrastructure investments needed for PrEP delivery will likely include training and supervision for clinical and nonclinical providers, structures for drug delivery and distribution, personnel and laboratory facilities for safety screening and additional HIV testing, mechanisms for monitoring and surveillance, outreach and education for potential users, and personnel and infrastructure for behavioral interventions [5•, 11]. Personnel shortages are a common barrier to implementing HIV prevention and treatment services, particularly in resource-limited settings, and many countries still lack systems for HIV-related surveillance [59]. Guidelines for PrEP use and decisions about who will receive PrEP will inform the types of development necessary to support optimal PrEP delivery, and the initial phase of preparations will require tremendous financial support. Although integrating PrEP into pre-existing comprehensive HIV prevention services may help reduce costs [11], access to comprehensive prevention remains rare [5•].
Delivering PrEP: Bringing a Combination Intervention into Practice
Coordinating delivery components
The PrEP delivery process will be the culmination of the implementation challenges described above, and the coordination of PrEP’s different components—including outreach, safety screening, prescription, drug delivery, HIV testing, behavioral intervention, and long-term follow-up—will require collaboration among a variety of providers and practice settings. Mechanisms may be necessary to coordinate different aspects of PrEP implementation for individual users, to ensure that each user receives the necessary HIV testing, follow-up care, and behavioral support in addition to PrEP drugs. The delivery of PrEP may have additional implications for health care services, as PrEP-related outreach may bring more people to health clinics [41], and PrEP users may require additional services [16].
Ongoing adjustment and adaptation
As the focus of PrEP implementation moves from planning to delivery, it will be important to engage users, providers, and other stakeholders in a long-term process of adjusting PrEP implementation practices. It may be necessary to modify PrEP protocols to reflect changes in behavioral risks, local epidemiology, prevention priorities, and available resources. This feedback loop will also need to accommodate research findings on PrEP’s long-term effects, including population-level indicators such as PrEP coverage and incidence of resistant HIV. The process of adjusting PrEP practices will involve all levels of PrEP decision-making, as identified in the previous section.
Addressing informal markets
Ensuring the safety and sustainability of PrEP delivery will also require efforts to monitor and limit informal PrEP markets [13, 20]. HIV treatment providers and bodies such as the WHO may need to issue guidance statements to discourage the use of ARVs without clinical monitoring, as well as to deter HIV-positive individuals from sharing or selling their treatment drugs for prophylactic purposes. The sharing of ARVs by HIV-positive individuals has already been a supply route for informal PrEP use [52, 54, 64]; this can diminish adherence to ARV treatment regimens [13, 18], and may result in the use of drugs that are untested or unapproved for prophylaxis indications. Taking PrEP without clinical monitoring could also result in adverse events for PrEP users, as well as the development of resistance among PrEP users who seroconvert (or who are already HIV-positive) and HIV-positive individuals who share medications. As PrEP use becomes more widespread, attention to this informal market will be imperative.
Long-Term Monitoring
Monitoring population-level effects
The long-term implementation of PrEP should include ongoing phase 4 studies and the measurement of population-level effects, including drug safety, adverse events, PrEP failures, PrEP uptake, acceptability, spread of resistant HIV, and available data on risk compensation and adherence [11, 14, 46]. These data should inform adjustment of PrEP guidelines, outreach strategies, user education, and behavioral interventions. The integration of PrEP monitoring into national monitoring and evaluation systems will also require resources and expertise. Despite improvements in these systems, persistent limitations include a lack of funding for monitoring and evaluation, division and duplication of monitoring efforts, a lack of human resources, ambiguity in performance frameworks and indicators, and limited data availability (particularly for high-risk populations) [65]. Collecting behavioral surveillance data may require the institution of new mechanisms, and tailoring data collection to address PrEP use will require adjustments to existing behavioral surveillance programs [66]. Monitoring and evaluating PrEP’s cost-effectiveness will also present resourcing and information infrastructure needs, including financial data collection and storage, safeguarding confidentiality, transferring and analyzing information, and instituting procedures to ensure that cost-effectiveness data inform policy [67]. The sustainability of PrEP as a population-level prevention strategy will depend in part on the success of these monitoring efforts, and the expansion of existing monitoring programs will be imperative to track PrEP’s long-term effects.
Learning from the implementation process
The process of addressing PrEP implementation challenges is also an opportunity to monitor implementation planning efforts, and to extract lessons for scaling up future combination approaches to preventing HIV. The scientific community should take advantage of this opportunity to document and analyze processes of PrEP planning, delivery, and subsequent adjustment.
Conclusions
This review aimed not only to describe the implementation challenges that have been identified throughout the literature on PrEP, but also to organize these challenges in a way that may help the field think systematically about implementation science of combination HIV interventions. As an intervention composed of biomedical, behavioral, and structural components, PrEP illustrates a growing trend in HIV prevention toward multicomponent prevention strategies. The state of implementation and delivery science in HIV prevention is underdeveloped, and PrEP presents an ideal opportunity to build this field. In particular, HIV prevention science may benefit from a conceptual framework to identify challenges to the scale-up of interventions with behavioral and biomedical components. Although specific challenges will vary among these new interventions and settings, a broad classification of implementation characteristics may help to guide plans for rollout. Ongoing efforts to plan for PrEP implementation have raised a variety of implementation issues, and a review of this literature suggests that a comprehensive taxonomy of implementation concerns may be useful for guiding PrEP planning efforts in particular. We have made a start toward an organizational scheme; however, future work should reexamine and expand on these categories and subcategories, drawing on lessons from biomedical and behavioral prevention successes. As anticipation builds for the results of PrEP efficacy trials, interim efforts to plan for PrEP scale-up already have much to contribute to implementation science.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
Contributor Information
Kristen Underhill, Postdoctoral Research Fellow, Department of Community Health, Brown University, Box G-S121-4, Providence, RI 02912, USA. kristen_underhill@brown.edu.
Don Operario, Associate Professor of Medical Sciences, Department of Community Health, Brown University, Box G-S121-5, Providence, RI 02912, USA. don_operario@brown.edu.
Matthew J. Mimiaga, Instructor, Harvard Medical School/Massachusetts General Hospital, Departments of Psychiatry; Harvard School of Public Health, Department of Epidemiology; Research Scientist, The Fenway Institute, Fenway Health; 1 Bowdoin Square, 7th floor, Boston, MA 02114, USA. mmimiaga@fenwayhealth.org
Margie R. Skeer, Postdoctoral Research Fellow, Department of Community Health, Brown University; Research Scientist, The Fenway Institute, Fenway Health; Box G-S121-4, Providence, RI 02912, USA. margie_skeer@brown.edu
Kenneth H. Mayer, Professor of Medicine and Community Health, Brown University, Miriam Hospital; Medical Research Director, The Fenway Institute, Fenway Health; Infectious Diseases Division, Miriam Hospital, 164 Summit Avenue, Providence, RI 02906, USA. kenneth_mayer@brown.edu
References
- 1.UNAIDS. Global Facts & Figures. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2009. [Google Scholar]
- 2.Lagakos SW, Gable AR. Challenges to HIV prevention - seeking effective measures in the absence of a vaccine. N Engl J Med. 2008;358:1543–1545. doi: 10.1056/NEJMp0802028. [DOI] [PubMed] [Google Scholar]
- 3.Padian NS, Buvé A, Balkus J, et al. Biomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet. 2008;372:585–599. doi: 10.1016/S0140-6736(08)60885-5. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Gay C, Kashuba ADM, et al. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 5. Paxton LA, Hope T, Jaffe HW. Pre-exposure prophylaxis for HIV infection: what if it works? Lancet. 2007;370:89–93. doi: 10.1016/S0140-6736(07)61053-8. This article outlines possible implications of PrEP trial results, including implementation challenges.
- 6.AIDS Vaccine Advocacy Coalition. PrEP Clinical Trials. [Accessed March 24, 2010]; Available at: http://www.avac.org/ht/d/sp/i/326/pid/326. [Google Scholar]
- 7.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS ONE. 2007;2:e875. doi: 10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desai K, Sansom SL, Ackers ML, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS. 2008;22:1829–1839. doi: 10.1097/QAD.0b013e32830e00f5. This article constructs a mathematical model of PrEP’s potential public health impact in the US, focusing on urban MSM, and concludes that PrEP may be cost-effective within a range of efficacy.
- 9.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–815. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vissers DCJ, Voeten HACM, Nagelkerke NJD, et al. The impact of pre-exposure prophylaxis (PrEP) on HIV epidemics in Africa and India: a simulation study. PLoS ONE. 2008;3:e2077. doi: 10.1371/journal.pone.0002077. This article models the impact of PrEP in several settings of Africa and India, concluding that PrEP could have a substantial impact, but that this impact could be diminished by changes in risk behavior.
- 11.Abdool Karim SS. Antiretroviral chemoprophylaxis for the prevention of HIV infection: future implementation challenges. HIV Ther. 2009;3:3–6. [Google Scholar]
- 12.Coates TJ, Szekeres G. Think tank: Policy and practice implications of HIV pre-exposure prophlaxis (PrEP) in the United States - Meeting proceedings. Los Angeles, CA: UCLA Program in Global Health; 2006. [Google Scholar]
- 13.Liu AY, Grant RM, Buchbinder SP. Preexposure prophylaxis for HIV: unproven promise and potential pitfalls. JAMA. 2006;296:863–865. doi: 10.1001/jama.296.7.863. [DOI] [PubMed] [Google Scholar]
- 14.Szekeres G, Coates TJ. Policy and practice implications of HIV pre-exposure prophylaxis (PrEP) in the United States. Los Angeles, CA: UCLA Program in Global Health, AIDS Policy Development Center; 2006. [Google Scholar]
- 15.Szekeres G, Coates TJ, Frost S, et al. Anticipating the efficacy of HIV pre-exposure prophylaxis (PrEP) and the needs of at-risk Californians. Oakland, CA: AIDS Partnership California; 2004. [Google Scholar]
- 16.Underhill K, Operario D, Skeer M, et al. Packaging PrEP to prevent HIV: An integrated framework to plan for pre-exposure prophylaxis in clinical practice. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/qai.0b013e3181e8efe4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009:143–167. doi: 10.1146/annurev.clinpsy.032408.153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J. Prevention cocktails: combining tools to stop HIV's spread. Science. 2005;309:1002–1005. doi: 10.1126/science.309.5737.1002. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. Program Announcement: Methods for Prevention Packages Program (MP3 II) [Accessed May 27, 2010]; Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-AI-10-005.html.
- 20.AIDS Vaccine Advocacy Coalition. Part of the solution: setting expectations for WHO and UNAIDS. New York, NY: AVAC; AVAC Report. 2009:54–63.
- 21. Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: A phase 2, doubleblind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2:e27. doi: 10.1371/journal.pctr.0020027. This article evaluates efficacy and safety of oral tenofovir PrEP among 936 HIV-negative women.
- 22. Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 July 19; doi: 10.1126/science.1193748. 10.1126/science.1193748. This article evaluates effectiveness of a topical PrEP formulation among 889 HIV-negative women.
- 23.Martin M, Vanichseni S, Suntharasamai P, et al. Screening, enrollment, and follow-up of injecting drug users in an HIV pre-exposure prophylaxis trial in Bangkok. Presented at the 5th IAS Conference on HIV Pathogenesis and Treatment; Cape Town, South Africa. 2009. [abstract WEPEC081] [Google Scholar]
- 24.Grohskopf L, Gvetadze R, Pathak S, et al. Preliminary analysis of biomedical data from the phase II clinical safety trial of tenofovir disoproxil fumarate (TDF) for HIV-1 pre-exposure prophylaxis (PrEP) among U.S. men who have sex with men (MSM). Presented at the XVIII International AIDS Conference; Vienna, Austria. 2010. [abstract FRLBC102] [Google Scholar]
- 25.Smith D, Kebaabetswe P, Disasi K, et al. Antiretroviral resistance is not an important risk of the oral tenofovir prophylaxis trial in Botswana: a simple mathematical modelling approach. Presented at the XVI International AIDS Conference; Toronto, Canada. 2006. [abstract THAX0105] [Google Scholar]
- 26. Guest G, Shattuck D, Johnson L, et al. Acceptability of PrEP for HIV prevention among women at high risk for HIV. J Womens Health. 2010 doi: 10.1089/jwh.2009.1576. In press. This article presents data on PrEP acceptability and adherence among a subset of women enrolled in a phase 2 PrEP trial.
- 27.Lawoyin T, Alleman P, Guest G, MacQueen K. Factors influencing adherence to pills for pre-exposure prophylaxis: lessons learned from a phase 2 trial. Presented at the XVI International AIDS Conference; Toronto, Canada. 2006. [abstract TUPE0423] [Google Scholar]
- 28.Lama JR, Guanira JV, Casapia M, et al. iPrEx: successful initiation of a HIV pre exposure prophylaxis trial in high risk men. Presented at the XVII International AIDS Conference; Mexico City, Mexico. 2008. [abstract WEPE0256] [Google Scholar]
- 29. Guest G, Shattuck D, Johnson L, et al. Changes in sexual risk behavior among participants in a PrEP HIV prevention trial. Sex Transm Dis. 2008;35:1002–1008. This article presents qualitative and quantitatve data on sexual risk behavior among a subset of women enrolled in a phase 2 PrEP trial.
- 30.Vanichseni S, Martin M, Suntharasamai P, et al. HIV-associated risk behavior among injecting drug users participating in an HIV pre-exposure prophylaxis trial in Bangkok. Presented at the 5th IAS Conference on HIV Pathogenesis and Treatment; Cape Town, South Africa. 2009. [abstract WEPDC103] [Google Scholar]
- 31.Sood N, Goldman D. HIV breakthroughs and risky sexual behavior. Q J Econ. 2006;121:1063–1102. [Google Scholar]
- 32.Grant RM. Antiretroviral agents used by HIV-uninfected persons for prevention: pre- and postexposure prophylaxis. Clin Infect Dis. 2010;50 Suppl 3:S96–S101. doi: 10.1086/651479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klosinski LE. HIV testing from a community perspective. J Acquir Immune Defic Syndr. 2000;25:S94–S96. doi: 10.1097/00042560-200012152-00002. [DOI] [PubMed] [Google Scholar]
- 34.Pinkerton SD, Bogart LM, Howerton D, et al. Cost of rapid testing at 45 U.S. hospitals. AIDS Patient Care STDs. 2010;24:409–413. doi: 10.1089/apc.2009.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartlett JG, Branson BM, Fenton K, et al. Opt-out testing for human immunodeficiency virus in the United States. JAMA. 2008;300:945–951. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 36.Kates J, Levi J. Insurance coverage and access to HIV testing and treatment: Considerations for individuals at risk for infection and for those with undiagnosed infection. Clin Infect Dis. 2007;45:S255–S260. doi: 10.1086/522547. [DOI] [PubMed] [Google Scholar]
- 37.McCollum ED, Preidis GA, Kabue MM, et al. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PLoS One. 2010;5:e9626. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bateganya M, Abdulwadud OA, Kiene SM. Home-based HIV voluntary counselling and testing (VCT) for improving uptake of HIV testing. Cochrane Database Syst Rev. 2010;7 doi: 10.1002/14651858.CD006493.pub4. CD006493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaguli I, Bassett IV, Dong KL, Walensky RP. Home testing for HIV infection in resource-limited settings. Curr HIV/AIDS Rep. 2009;6:217–223. doi: 10.1007/s11904-009-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monjok E, Smesny A, Mgbere O, Essien EJ. Routine HIV testing in health care settings: The deterrent factors to maximal implementation in Sub-Saharan Africa. J Int Assoc Physicians AIDS Care. 2010;9:23–29. doi: 10.1177/1545109709356355. [DOI] [PubMed] [Google Scholar]
- 41.AIDS Vaccine Advocacy Coalition. Anticipating the results of PrEP trials: a powerful new HIV prevention tool may be on the horizon. Are we prepared? New York, NY: AVAC; 2008. [Google Scholar]
- 42.Smith DK, Sutton M. What we don’t know and when should we know it: anticipating disparities in implementing biomedical prevention methods in the U.S. Presented at the XVII International AIDS Conference; Mexico City, Mexico. 2008. [abstract MOPE0448] [Google Scholar]
- 43.CDC. Pre-exposure prophylaxis (PrEP) for HIV prevention: Planning for potential implementation in the U.S. [Fact Sheet] Atlanta, GA: CDC; 2009. [Google Scholar]
- 44.Mack N, Mwale B, Lanham M, et al. Anticipatory planning for PrEP rollout in Malawi based on country priorities. Presented at the XVII International AIDS Conference; Mexico City, Mexico. 2008. [abstract WEPE0261] [Google Scholar]
- 45.Bennett S, Randall T. AIDS "next big thing" rests on study of Gilead prevention pill. [Accessed August 2010]; http://www.bloomberg.com/apps/news?pid=20601124&sid=aMqiWbk3JC34. [Google Scholar]
- 46.CDC. Summary report from the expert consultation on the implications of tenofovir as HIV chemoprophylaxis. Atlanta, GA: CDC; 2004. [Google Scholar]
- 47.Vernazza P, Brenner I, Graf I. Pre-exposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. Presented at the 4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; Sydney, Australia. 2007. [abstract MOPDC01] [Google Scholar]
- 48.Cunningham W, Galea J, Kinsler J, et al. The acceptability of pre-exposure prophylaxis (PrEP) for HIV prevention in Lima, Peru. Presented at the XVII International AIDS Conference; Mexico City, Mexico. 2008. [abstract WEPE0260] [Google Scholar]
- 49.Lama JR, Guanira J, Goicochea P, et al. Willingness to participate in a HIV pre-exposure prophylaxis efficacy trial among high risk men who have sex with men in the Andean region. Presented at the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. 2005. [abstract MOPEC038] [Google Scholar]
- 50.Jommaroeng R, Chaitiamrus S, Apornpong T, et al. Willingness to participate in HIV prevention trials is high among FSW, MSM, youth, and IDU in Bangkok, Thailand. Presented at the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. 2007. [abstract TUPDC05] [Google Scholar]
- 51.Nodin N, Carballo-Dieguez A, Ventuneac AM, et al. Knowledge and acceptability of alternative HIV prevention bio-medical products among MSM who bareback. AIDS Care. 2008;20:106–115. doi: 10.1080/09540120701449096. [DOI] [PubMed] [Google Scholar]
- 52.Liu AY, Kittredge PV, Vittinghoff E, et al. Limited knowledge and use of HIV post- and pre-exposure prophylaxis among gay and bisexual men. J Acquir Immune Defic Syndr. 2008;47:241–247. [PubMed] [Google Scholar]
- 53.Dunkle K, Wingood G, Camp C, DiClemente R. Intention to use pre-exposure prophylaxis among African-American and white women in the United States: results from a national telephone survey. Presented at the XVII International AIDS Conference; Mexico City, Mexico. 2008. [abstract WEPE0258] [Google Scholar]
- 54.Mimiaga MJ, Case P, Johnson CV, et al. Preexposure antiretroviral prophylaxis attitudes in high-risk Boston area men who report having sex with men: limited knowledge and experience but potential for increased utilization after education. J Acquir Immune Defic Syndr. 2009;50:77–83. doi: 10.1097/QAI.0b013e31818d5a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider J, Oruganti G, Pasupneti S, et al. Comparison of acceptability towards circumcision (Circ), pre-exposure prophylaxis (PREP) and herpes simplex suppression (HSV-S) as novel HIV prevention strategies amongst truck drivers in India. Presented at the 5th IAS Conference on HIV Pathogenesis and Treatment; Cape Town, South Africa. 2009. [abstract WEPEC105] [Google Scholar]
- 56.Imrie J, Elford J, Kippax S, Hart GJ. Biomedical HIV prevention -- and social science. Lancet. 2007;370:10–11. doi: 10.1016/S0140-6736(07)61026-5. [DOI] [PubMed] [Google Scholar]
- 57.AIDS Vaccine Advocacy Coalition. Summary from the AVAC think tank on PrEP financing in the US. San Francisco, CA: AVAC; 2009. [Google Scholar]
- 58.Fisher K, Bass E, Feuer C, et al. Who pays if PrEP works? Potential challenges to PrEP delivery in the United States. Presented at the 5th IAS Conference on HIV Pathogenesis and Treatment; Cape Town, South Africa. 2009. [abstract CDC049] [Google Scholar]
- 59.Global HIV Prevention Working Group. Bringing HIV prevention to scale: An urgent global priority. Bill & Melinda Gates Foundation, Henry J. Kaiser Family Foundation. 2007 [Google Scholar]
- 60.Novogrodsky N. The duty of treatment: Human rights and the HIV/AIDS pandemic. Yale Human Rights and Development Law Journal. 2009;12:1–61. [Google Scholar]
- 61.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–684. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills EJ, Singh S, Singh JA, et al. Designing research in vulnerable populations: lessons from HIV prevention trials that stopped early. BMJ. 2005;331:1403–1406. doi: 10.1136/bmj.331.7529.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kellerman SE, Hutchinson AB, Begley EB, et al. Knowledge and use of HIV pre-exposure prophylaxis among attendees of minority gay pride events, 2004. J Acquir Immune Defic Syndr. 2006;43:376–377. doi: 10.1097/01.qai.0000234085.18914.d5. [DOI] [PubMed] [Google Scholar]
- 64.Voetsch AC, Heffelfinger JD, Begley EB, et al. Knowledge and use of preexposure and postexposure prophylaxis among attendees of minority gay pride events, 2005 through 2006. J Acquir Immune Defic Syndr. 2007;46:378–380. doi: 10.1097/QAI.0b013e3181576874. [DOI] [PubMed] [Google Scholar]
- 65.Peersman G, Rugg D, Erkkola T, et al. Are the investments in national HIV monitoring and evaluation systems paying off? J Acquir Immune Defic Syndr. 2009;52:S87–S96. doi: 10.1097/QAI.0b013e3181baede7. [DOI] [PubMed] [Google Scholar]
- 66.Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral surveillance among people at risk for HIV infection in the U.S.: The National HIV Behavioral Surveillance System. Public Health Rep. 2007;122:32–38. doi: 10.1177/00333549071220S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beck EJ, Santas XM, DeLay PR. Why and how to monitor the cost and evaluate the cost-effectiveness of HIV services in countries. AIDS. 2008;22:S75–S85. doi: 10.1097/01.aids.0000327626.77597.fa. [DOI] [PubMed] [Google Scholar]