Abstract
The 12/15-lipoxygenase enzymes react with fatty acids producing active lipid metabolites that are involved in a number of significant disease states. The latter include type 1 and type 2 diabetes (and associated complications), cardiovascular disease, hypertension, renal disease, and the neurological conditions Alzheimer’s disease and Parkinson’s disease. A number of elegant studies over the last thirty years have contributed to unraveling the role that lipoxygenases play in chronic inflammation. The development of animal models with targeted gene deletions has led to a better understanding of the role that lipoxygenases play in various conditions. Selective inhibitors of the different lipoxygenase isoforms are an active area of investigation, and will be both an important research tool and a promising therapeutic target for treating a wide spectrum of human diseases.
Keywords: 12/15-lipoxygenase, arachidonic acid, pancreatic islet, adipose tissue, atherosclerosis, kidney, inflammation, obesity
1. INTRODUCTION
Twenty carbon fatty acids serve a variety of important physiological functions in humans, from providing cellular membrane structure to serving as substrates from which a number of important cell signaling molecules and secondary messengers are derived [1]. In particular, arachidonic acid serves as one major precursor for a number of molecules termed eicosanoids that have significant roles in human diseases, including type 1 and type 2 diabetes and atherosclerosis, as well as the neurological diseases Parkinson’s disease (PD) and Alzheimer’s disease (AD) [2-4]. The following review will focus on the 12- and 15-lipoxygenase enzymes (12-LOX, 15-LOX), their products, and the varied effects of those products in human metabolic, vascular, and neurological diseases.
Arachidonic acid (AA) is released from the cell membrane by phospholipases, such as phospholipase A1, in response to various cytokines, peptides, and growth factors that become active under inflammatory conditions [5, 6]. There are three families of enzymes involved in the oxidative metabolism of AA. These include the lipoxygenases, which produce leukotrienes (LT), hydroperoxyeicosatetraenoic acids (HPETEs), hydroxyeicosatetraenoic acids (HETEs), and hydroxyoctadecadienoic acids (HODEs); the cyclooxygenases (COX-1 and COX-2) which produce prostaglandins including G2 and H2 as well as thromboxanes ; and cytochrome P-450 monooxygenases which produce epoxides and HETEs [6, 7]. Of note, prostaglandin H2 is further metabolized to prostaglandins D2, F2α, and I2 (prostacyclin), as well as to thromboxane (TxA2) [8].
Lipoxygenases (LOXs) are found in both plants and in animals. The mouse has seven different ALOX genes (note that the LOX genes are termed by convention “ALOX”, for arachidonic acid lipoxygenase), while humans have five known genes [7]. The different LOX enzymes are named for the numbered carbon where they oxygenate their polyunsaturated fatty acid (PUFA) substrates, with the use of stereoisomer nomenclature (S and R) as appropriate (e.g., 12S-LOX and 12R-LOX) [7]. As shown in Table 1, the human LOX enzymes include 5-LOX (which produces LTs), 12-LOX (with platelet-type and leukocyte-type forms), and 15-LOX (which is further separated into the reticulocyte or leukocyte-type, 15-LOX-1, and the epidermis-type, 15-LOX-2) [9, 10]. The human leukocyte-type 12-LOX and the human reticulocyte-type 15-LOX-1 can form similar products from common substrates and are often referred to in the literature as 12/15-LOXs [6, 10]. Furthermore, there is significant species-specific variation in the products formed by the different 12- and 15-LOX isoforms. Mice do not express 15-LOX and only express the leukocyte-derived 12-LOX [11]. Rabbits express both reticulocyte-derived 15-LOX and leukocyte-derived 12-LOX [12]. These differences often make it difficult to translate data obtained in different animal models of disease to their human counterparts. This may, for instance, explain conflicting data on the effects of different 12- and 15-LOX isoforms on vascular function and on atherosclerosis [13].
Table 1. Lipoxygenase isoforms in humans. (From Entrez Gene, through the National Center for Biotechnology Information).
Humans have five different lipoxygenase (LOX) genes, termed by convention “ALOX” for arachidonic acid lipoxygenase. 5-LOX predominantly produces leukotrienes (LTs), while 12- and 15-LOX predominantly produce the eicosanoids hydroperoxyeicosatetraenoic acid (HPETE) and hydroxyeicosatetraenoic acid (HETE). All LOX genes are located on chromosome 17.
| Gene Name | Abbreviation | Alternative Nomenclature |
Predominant Enzyme Products |
|---|---|---|---|
| Arachidonate 12- lipoxygenase |
ALOX12 | 12S-LOX platelet-type lipoxygenase 12 |
12(S)-HPETE 12(S)-HETE |
| Arachidonate 12- lipoxygenase, 12R type |
ALOX12B | 12R-LOX, epidermis-type lipoxygenase 12 |
12(R)-HPETE 12(R)-HETE |
| Arachidonate 15- lipoxygenase |
ALOX15 | 15-LOX-1 | 15(S)-HPETE, 15HETE |
| Arachidonate 15- lipoxygenase, type B |
ALOX15B | 15-LOX-2; 15-LOX-B |
15(S)-HPETE, 15HETE |
| Arachidonate lipoxygenase 3 |
ALOXE3 | eLOX3, epidermis-type lipoxygenase 3 |
Epoxyalcohols (hepoxilins), from 12(R)- HPETE |
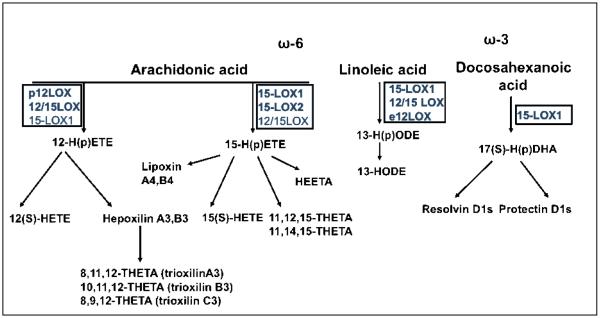
In humans, 12/15-LOXs act upon AA to create a number of important lipid mediators (Figure 1). These include 12- and 15HPETEs and 12- and 15HETEs [7]. The 15-LOX-1 enzyme also produces 13-S-hydroxyoctadecadienoic acid (HODE) from linoleic acid [14]. These lipid products have a variety of functions in human tissues. For example, 12(S)-HETE and 15(S)-HPETE are involved in monocyte binding in the vasculature, by stimulating protein kinase C (PKC) and various cellular adhesion molecules (CAMs) [6, 15]. Some products, including 13HPODE, are proinflammatory and act via various transcription factors including NF-κB [16]. HETEs are also involved in cell growth, acting through various mitogen-activated protein kinases (MAPKs) [17].
Figure 1. Major 12- and 15-lipoxygenase isoforms and their lipid substrates and products.
Several 12- and 15-LOX isoforms are known in mammalian cells, including 12-LOX in platelets, and 12/15LOX in vascular and immune cells (for a comprehensive list of human isoforms please refer to Table 1). When arachidonic acid is metabolized, all of the different LOX isoforms generate lipid hydroperoxides (HPETEs) as the primary product. The latter are rapidly reduced intracellularly to their corresponding hydroxides (HETEs). Alternatively, the LOX-derived hydroperoxides can serve as precursors for the generation of other classes of secondary lipid mediators such as lipoxins, hepoxilins and trioxilins. 12-HPETE generated by the action of 12/15-LOX could be converted to the bioactive 8-hydroxy-11,12-epoxyeicosatrienoic acid (hepoxilin A3) and the inactive 10-hydroxy-11,12-epoxyeicosatrienoic acid (hepoxilin B3); also, the the trihydroxy-containing trioxilin such as 8,9,12-trihydroxyeicosatrienoic acid (trioxilin C3) can be formed in some tissues. 15-HPETEs can be generated by the 12/15LOX isoform present in rodents and rabbits or by one of the two 15-LOX isoforms in humans. While 15-LOX1 produces 90% 15-HPETEs and 10% 12-HPETES, 15-LOX-2 produces exclusively 15-HPETEs and can only use arachidonic acid as a substrate. Like the 12-HPETE, the 15-HPETE can generate also a variety of secondary lipid mediators such as HEETAs (hydroxyepoxyeicosatrienoic acids) or THETAs (trioxilins). Another category of metabolites generated by the sequential action of 15-LOX and 5-LOX are the lipoxins (trihydroxytetraenes). Lipoxin generation may occur in the same cell or in a trans-cellular fashion, involving two different cell types expressing different LOX isoforms. 15-LOX-1 and epidermal 12-LOX (e12-LOX) can also metabolize linoleic acid generating 13-HPODE (hydroperoxyoctadecadienoic acid) which is further peroxidized to 13-HODE. Docosahexanoic acid is also a substrate for 15-LOX-1 which metabolizes the ω3 fatty acid to a hydroperoxy derivative which is rapidly transformed into two epoxy intermediates. Subsequently 5-LOX converts these intermediates into resolvin D series. Also, the epoxy intermediates can be directed towards formation of protectin D1 following the catalytic action of an epoxydase.
A number of interesting anti-inflammatory molecules have also been identified that are derived from AA or ω-3 fatty acids, including the lipoxins (for “lipoxygenase interaction products”), resolvins, and protectins [18, 19] (Figure 1). The lipoxins are synthesized from AA by 5-, 12-, and 15-LOX, as well as by COX-2 in the presence of aspirin [18]. These molecules are involved in actively limiting and resolving the inflammatory response. In particular, lipoxins derived from the 15-LOX product 15HETE (termed lipoxin A4 and B4) have been shown to stimulate vasodilation and inhibit neutrophil function [20]. The resolvins are derived from the omega-3 PUFAs docosahexanoic acid (the D-series resolvins) and eicosapentaenoic acid (the E-series resolvins), and their synthesis can involve aspirin and COX-2 (resolvin E1), as well as 5-LOX (resolvin E1) and 15-LOX (D-series resolvins) [19]. E-series resolvins are involved in granulocyte function and clearance, and reduce the release of various proinflammatory cytokines [19]. Synthesis of the protectins also involves the action of 15-LOX [19]. These agents appear to be involved in airway/mucosal injury in human asthma, and may also be protective after ischemic renal injury [21, 22].
An interesting class of 12- and 15-LOX-derived lipid products is the esterified eicosanoids formed by direct enzymatic oxidation of membrane phospholipids. First evidence for formation of these products was documented in eosinophils by Brinckman et al. [23]. Although the 12- and 15-lipoxygenases are regarded as cytosolic enzymes, upon an increase in intracellular calcium they can bind to membrane phospholipids in a reversible fashion [23, 24]. In hematopoietic cells and platelets, 15- and 12-LOX, respectively, have the ability to translocate to cellular membranes in the presence of agonists such as calcium ionophore, thrombin or collagen which increases the fatty acid oxygenase activity of the enzyme [25]. The esterified 15-HETEs are predominant in human peripheral monocytes, while 12-HETEs are predominant in human platelets [25, 26]. The esterified eicosanoids (either phosphatidylethanolamine (PE)- or phosphatidylcoline (PC) –HETEs), are retained in the cells and more recent evidence shows they play important roles as LOX-dependent signaling lipid mediators in the immune cells in inflammation as well as novel pro-thrombotic lipids promoting coagulation [24, 26]. Also, a Th2-dependent production of 12- HETE-PEs in mice and 15-HETE-PEs in humans was recently reported and interesting future studies will help determine the potential anti-inflammtory role of these HETEs in some forms of inflammatory disease in humans [27].
The complex array of metabolites formed as a result of 12- and 15-LOX catalytic activity are tissue and species-specific, and can have both pro- and anti-inflammatory effects. Targeted deletion studies in mouse models have helped identify the potential roles of these pathways. To further clarify the particular role of these products in disease, specific pharmacologic inhibitors for each of the LOX isoforms are needed. The development of highly specific pharmacological tools acting as isotype-specific LOX inhibitors is an imperative goal that is currently under intensive investigation [28]. In addition, adequate detection methods for LOX-derived lipid metabolites are crucial to obtaining accurate information on formation and tissue distribution in physiologic and pathologic states. The gold standard methods are liquid chromatography (LC)/electrospray ionization (ESI)/tandom mass spectrometry (MS/MS) and gas chromatography-mass spectrometry (GC/MS), but they represent a significant time and cost investment [29]. Antibody-based methods, such as enzyme-linked immunosorbent assay (ELISA), while much more accessible and cost-effective, should be carefully validated for the particular sample being analyzed. Also, the results should be interpreted with caution, due to the generally low specificity of immunodetection in differentiating structurally close lipid metabolites. An alternative to limit false positive results generated by antibody cross-reactivity would be to use an HPLC separation prior to immunochemical detection. For an excellent review on eicosanoid detection methodology and limitations please see O’Donnell, et al. [29].
Accumulating evidence indicates that 12-LOX and 15-LOX, and their products, play important roles in many tissues and organs, including the vasculature, kidney, adipose tissue, brain, and the pancreatic islet. To mechanistically dissociate the effects of the 12- and 15-LOX pathways and their respective metabolites is crucial to understanding how these pathways function in human disease. These areas will be further discussed in greater detail in the review.
2. THE 12/15-LIPOXYGENASE PATHWAY IN THE PANCREATIC ISLET AND IN DIABETES MELLITUS
Homeostasis of blood glucose is maintained by hormone secretion from the pancreatic islets of Langerhans. More specifically, insulin produced by the β cells of the islet plays a major role in proper maintenance of blood glucose. Normal levels of insulin are required to promote glucose uptake in muscle and in adipose tissue, and to suppress gluconeogenesis and promote glycogenesis in the liver. Therefore, either a loss of production of insulin or a defect in the release of insulin from the β cell creates dysregulation of overall glucose homeostasis in the body. Recent evidence indicates that both forms of diabetes (type 1 and type 2) are associated with a significant loss of β cells. A primary underlying cause of β cell loss arises as a consequence of inflammatory mechanisms. Expression and/or activity of 12-LOX in human islets is upregulated by hyperglycemia and by inflammatory cytokines. These observations indicate a role for 12-LOX in mediating the loss of functional insulin secretion and in the insulin resistance commonly associated with inflammation [30-34].
Early studies of the role of 12-LOX in islet function were guided by experiments using enzymatic inhibition. Initial studies led to incorrect conclusions due to the lack of specificity in the chemical inhibitors used. This has underscored the need to generate specific isotype-selective inhibitors. Gene-based knockout studies and targeted protein knockdown approaches have provided clarity in more recent studies of the important role of 12-LOX in islet function. Insulin resistance and impairment in islet function that develops on a high-fat diet were prevented in leukocyte- 12-LOX (12/15-LOX) knockout mice, suggesting that 12/15-LOX activity is relevant to type 2 diabetes, and to β cell dysfunction in obese states [35, 36]. Additionally, diabetic Zucker fatty rats that have a defect in insulin secretion have elevated 12-LOX, further supporting a role for 12-LOX in the pathogenesis of type 2 diabetes [34].
Bleich et al. reported on a mouse model deficient in the leukocyte-derived 12/15-LOX. In contrast to control C57BL/6J mice, the 12/15-LOX knockout mice (on the same genetic background) were resistant to the induction of diabetes by low-dose streptozotocin [37]. This streptozotocin protocol induced immune-mediated islet destruction similar to type 1 diabetes. The 12/15-LOX knockout mice lacked the cytokine-induced conversion of AA to 12HETE, implying that 12HETE generation was cytotoxic to β cells [37]. The role of 12-LOX as a key mediator in the development of autoimmune diabetes is further supported by the work of McDuffie et al., who developed a congenic 12/15-LOX knockout in non-obese diabetic (NOD) mice. The phenotype of the female NOD mouse includes the spontaneous development of autoimmune type 1 diabetes. 12/15-LOX knockout mice resulted in a significant reduction (2.5% vs > 60% in control animals) in the development of diabetes [38]. The mechanisms for how the deletion of 12-LOX protects against type 1 diabetes development are an active area of current investigation. Interestingly, 12-LOX activity mediates the expression of interleukin-12 (IL-12) [39-42]. IL-12 is a key cytokine driving the Th1 autoimmune response via STAT4 second-messenger signaling and the induction of interferon gamma (IFNγ).
A direct role of pro-inflamatory cytokines in stimulating 12-LOX activity is further supported by observations of cytokine-induced production of 12HETE in both islets and β cell lines [31, 43]. Moreover, the addition of 12-LOX products (12HETE and 12HPETE) to human islets resulted in a decrease in glucose-stimulated insulin secretion associated with a decrease in islet viability [30]. These studies also reported a partial restoration in glucose-stimulated insulin secretion if 12HETE was combined with lisofylline, an inhibitor of IL-12 signaling. Collectively these data support a predicted role of IL-12 in mediating the immune damage caused by the 12-LOX pathway.
Some of the mechanisms that involve 12-LOX as a potential pathogenic enzyme are illustrated in Figure 2. Direct β cell effects associated with the stimulation of 12-LOX activity include the activation of second messengers c-Jun N-terminal kinase and p38 MAPK, both of which show increased phosphorylation in response to 12-LOX activation [31, 44, 45]. Transient knockdown of 12/15-LOX expression in mice by in vivo siRNA resulted in reduced p38 MAPK activity and resistance to pro-inflammatory cytokine-induction [30]. Additionally, 12HETE may contribute to mitochondrial and oxidative stress, conditions to which β cells are highly sensitive, by increasing mitochondrial nitric oxide and intramitochondrial calcium [46]. Mitochondrial dysfunction reduces the ATP/ADP ratio, and thereby directly affects insulin secretion from the islet.
Figure 2. 12-Lipoxygenase associated pathways impacting islet function.
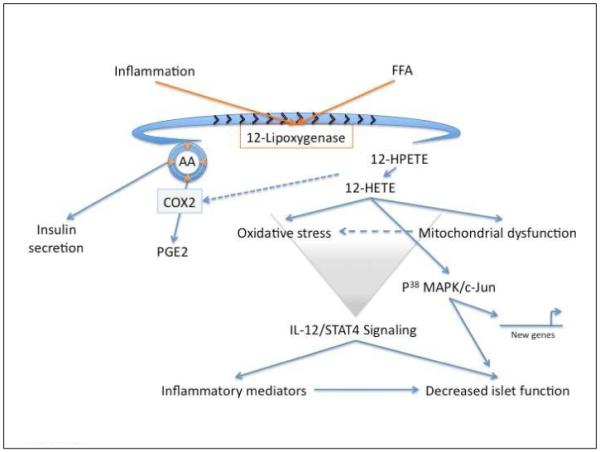
Activation of 12-lipoxygenase (12-LOX) either as a consequence of inflammation or elevated free fatty acids (FFA) leads to a reduction in the pool of arachidonic acid (AA; concentric circles with arrows). Since AA is important for insulin secretion this leads to impaired insulin release. The products of 12-LOX activity, 12-HPETE/12-HETE further contribute to a diminished AA pool by increased activation of the cyclooxygenase (COX2) enzyme that also uses AA as a substrate in the production of prostaglandin (PGE2). Lipid products of 12-LOX activity (12-HPETE/12-HETE) induce mitochondrial dysfunction which contribute to induced cellular oxidative stress in addition to the induction of second messenger signaling, including p38 MAPk/cJun that lead to new gene expression. The activity of 12-LOX and its lipid mediators are upstream of induced STAT4 signaling and interleukin-12 (IL-12) expression in islets. These are key mediators in the recruitment of inflammatory cells/mediators that compound islet dysfunction.
Other pathways, including ER stress, are current areas of investigation. Interestingly, 5-LOX has recently been shown to improve islet function in rodents [47]. The role of 5-LOX has not been extensively investigated to date, and there have been conflicting reports regarding the expression of 5-LOX in rodent islets. However, data from our lab (unpublished) indicates clear levels of ALOX 5 expression in human islets. Thus, unlike 12-LOX, the role of 5-LOX is not clear but could serve a beneficial role in preserving islet function in humans. This area is worthy of follow up investigation.
The activity of 12-LOX in the pancreatic islet is multifaceted. AA stimulates insulin secretion from pancreatic β cells, which is inhibited by 12-LOX activity, likely due to reduction in available AA via substrate metabolism by 12-LOX [48]. This may be further compounded by 12-LOX induction of COX-2, which converts AA to PGE2 [49]. Additionally, 12-LOX activity may trigger the activation of Toll-like receptor 4 (TLR4) in resident dendritic cells in islets, and thereby promote a pro-inflammatory/autoimmune environment through the upregulation of IL-12 [50, 51]. Cytokine-induction of 12-LOX activity and its lipid mediators in the β cell results in cell dysfunction mediated by second messenger activation. Targeting selective inhibitors of 12-LOX activity is a promising pharmacological strategy for the treatment of diabetes and has been validated in proof-of-concept studies using islet-specific 12/15-LOX knockout mice. It is important to note that the source of rodent 12/15-LOX expression in the islet is not completely clear. While β cells express the enzyme, resident macrophages or dendritic cells may play an important role in 12/15-LOX expression and activity or downstream effects on IL-12.
The predominant LOX enzyme identified in the non-diabetic human islet is the platelet-derived 12-LOX isoform (ALOX12), while leukocyte 12-LOX has been identified in rodent islets [37]. A dominant lipid product of 12-LOX activity, 12HETE, has been identified in rat and human islets, and in rodent β cell lines [30, 31, 43, 45, 50, 52-56]. The contribution of other LOX enzymes in the pancreatic islet is less clear. Turk and Shannon were unable to detect 5-LOX protein or enzymatic activity in rat islets [54, 55]. In contrast, mRNA for 5-LOX but not for LOX 15-1 has been reported in normal human islets [50]. Considering the four predominant endocrine cell types that are present in the islet (α, β, δ, and F cells), the insulin-producing β cell is reported to be the preferential site for 12-LOX expression [11, 31, 55]. However, immunohistochemical analysis of 12-LOX expression co-localized 12-LOX with glucagon-expressing α cells in the rat islet [56]. Kawajiri et al. reported that overexpression of 12-LOX in an α cell line doubled glucagon secretion.
It would be of interest to clarify the LOX isoforms expressed in islets from subjects with type 1 or type 2 diabetes mellitus. Studies evaluating the forms of LOX in islets from subjects with diabetes are ongoing. The results from these studies will be important in determining the therapeutic potential of targeting certain enzymes in the treatment of human diabetes. Table 2 summarizes the current evidence linking 12-LOX with pancreatic β cell function and diabetes.
Table 2.
Summary of current evidence for 12-lipoxygenase (12-LOX) involvement in diabetes
|
3. THE 12/15-LIPOXYGENASE PATHWAY AND MICROVASCULAR COMPLICATIONS OF DIABETES
3.1. Diabetic peripheral neuropathy
The study of 12/15-LOX and the peripheral nervous system is actively being investigated. A later section will cover additional aspects related to the nervous system. Obrosova et al. described elevated 12/15-LOX expression in the peripheral nerves and dorsal root ganglia of mice fed a high-fat diet for 16 weeks [57]. The animals became obese and developed impaired glucose tolerance, and had both motor and sensory peripheral nerve deficits. In an interesting follow up study, they showed that PMI-5011, an extract of the herb Artemisia dranunculus, reduced 12/15-LOX expression, normalized blood sugar, and improved peripheral neuropathy in their animal model [58]. Most recently this group has shown that 12/15-LOX is involved in the nitrosative stress seen in the diabetic peripheral nerves [59]. Increased 12/15-LOX expression was seen in mice made diabetic with streptozocin or through a high-fat diet. Human Schwann cells cultured in medium containing high concentrations of glucose also had increased 12/15-LOX expression. When 12/15-LOX was inhibited with cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate, the nitrosative stress in the spinal cord and sciatic nerve was reduced. Conduction deficits in small and large nerves were also improved (though intraepidermal nerve fiber loss was still seen). 12/15-LOX knockout mice fed a high-fat diet also had improvements in nitrosative stress and in conduction dysfunction. The authors concluded that 12/15-LOX might play a role in the nitrosative stress seen in human diabetic peripheral neuropathy [59]. It would be important to validate the expression of 12- or 15-LOX enzymes in human diabetic neuropathic samples.
3.2. Diabetic retinopathy
Augustin et al. demonstrated that 15HETE was expressed in the epiretinal membranes of patients with proliferative diabetic retinopathy, and suggested that it might play a role in the membrane growth seen in this diabetic complication [60]. In a later study of the lipid profile of non-diabetic and diabetic ocular vitreous, Schwartzman et al. found that the 5-LOX product 5HETE was significantly elevated in diabetic versus non-diabetic vitreous, especially in individuals with non-proliferative diabetic retinopathy [61]. However, there were no significant differences in concentrations of 15HETE. Gubitosi-Klug et al. found that the retinas of 5-LOX deficient diabetic mice had less superoxide production, leukostasis, and NF-κB expression compared with wild-type diabetic animals, and that the 12/15-LOX deficient diabetic mice had less leukostasis, but no changes in NF-κB expression or production of superoxide. They concluded that the 5-LOX pathway might be an appropriate pathway for inhibition in treating diabetic retinopathy [62]. Overall, the results to date indicate that the 5-LOX pathway likely plays a more significant role in the evolution of diabetic retinal disease than does the 12/15-LOX pathway.
4. THE 12/15-LIPOXYGENASE PATHWAY IN ADIPOSE TISSUE
Only recently has adipose tissue been recognized as a highly metabolically active endocrine organ imparting profound local and systemic inflammatory effects. Adipose tissue, either found as white or brown fat, is a complex organ comprised of fat cells (adipocytes) and the stromal vascular compartment containing a mixed population of pre-adipocytes, leukocytes, macrophages, fibroblasts, and endothelial cells. This organ is responsible for the secretion of inflammatory cytokines and numerous adipose-specific hormone-like proteins, called adipokines, that not only affect local adipocyte function, but also systemic bodily functions. There is emerging evidence that the LOX enzymes, expressed in both white and brown fat, are important for proper adipogenesis and ensuing adipocyte function in regulating whole-body energy homeostasis [63].
Study of adipogenesis is possible through the in vitro characterization of the 3T3-L1 fibroblastic pre-adipocyte cell line [64]. The addition of a differentiation cocktail media to these cells promotes full differentiation into a pure population of adipocytes characteristic of accumulated triacylglycerol content in lipid-droplets within eight days [65]. This adipogenesis is dependent on an exogenous supply of free fatty acids to facilitate activation of peroxisome proliferator-activated receptors (PPARs; nuclear receptor proteins that function as transcription factors). PPARγ is a strict requirement for early adipocyte differentiation, and several fatty acid metabolites of lipoxygenases appear to be necessary for PPARγ activation [63, 66-68]. Treatment of 3T3-L1 pre-adipocytes with either NDGA (nordihydroguaiaretic acid; a non-specific lipoxygenase inhibitor) or baicalein (a 12-LOX inhibitor) prevents adipogenesis. This phenotype is rescued upon treatment with rosiglitazone (a selective agonist of PPARγ in adipoctyes), consistent with the rise in PPARγ agonists during early adipocyte differentiation (marked by extensive mitotic clonal expansion) and observations that certain lipoxygenase metabolites activate PPARs [69-73]. In addition, during this period of differentiation, arachidonic acid is necessary for proper glucose uptake and is dependent on LOX activity [74]. These results demonstrate that certain LOXs are responsible for generating the endogenous PPARγ ligands necessary for adipogenesis. This role appears to be specific to the epidermal-derived 12-LOX, as the platelet- and leukocyte-derived 12-LOXs are expressed at very low levels in the pre-adipocytes and early differentiated adipocytes and adipogenic defects were not reported in leukocyte-12-LOX or platelet-12-LOX deficient mice [35, 63, 75-77]. Additionally, Hallenborg and colleagues recently demonstrated that overexpression of epidermal-12-LOX and its hepoxilin lipid products in 3T3-L1 preadipocytes stimulate adipogenesis, whereby epidermal-12-LOX knockdown prevents this differentiation [78]. The hepoxilins also accumulate during early 3T3-L1 differentiation and appear to directly activate PPARγ to promote adipogenesis [78]. It is possible that leukocyte-12-LOX may participate in lipogenesis during late stage adipogenesis since epidermal- and platelet-12-LOX expression are absent and leukocyte-12-LOX expression is maximal by day 8 of 3T3-L1 differentiation, which immediately follows the rise in activity of several key enzymes of fatty acid synthesis [76, 79] (also, our unpublished observations).
Leukocyte-12-LOX (12/15-LOX) appears to be a significant player in modulating adipocyte function in vivo in diet-induced mouse models of obesity. Comparison of 12/15-LOX knockout mice with C57BL6/J mice fed either a standard chow or high-fat “Western” type diet (a diet containing 0.2% cholesterol of which 42% calories are from fat, 15.3% calories are from protein, and 42.7% calories are from carbohydrate, primarily sucrose) revealed that 12/15-LOX is the primary enzyme generating the 12(S)-HETE products under obese conditions [35]. This increased 12/15-LOX activity coincides with increased inflammation both systemically and in epididymal adipose tissue [35, 36]. Although both C57BL6/J and 12/15-LOX knockout mice exhibited similar weight gain and increased adiopcyte size when fed the Western diet, fewer incidences of macrophage infiltration and activation were observed in the epididymal adipose fat pads from 12/15-LOX knockout mice when fed the Western diet. Additionally, MCP-1 staining was significantly decreased in adipose tissue from the 12/15-LOX knockout mice. Furthermore, mice were also protected from developing insulin resistance and maintained normal adiponectin (an adipokine that improves insulin sensitivity by increasing energy expenditure and fatty acid oxidation) levels during the high fat diet [80]. Thus 12/15-LOX activation under diet-induced obese conditions plays a significant role in mediating inflammation via ensuing adipocyte dysfunction. Preliminary data also suggests that the Zucker rat genetic model of obesity and insulin resistance shows higher 12/15-LOX in adipose tissue compared to lean controls (Chakrabarti, Wen, Dobrian, Cole, Ma, Pei, Williams, Bevard, Vandenhoff, Keller, Gu, and Nadler, unpublished observations).
Further validation for a role in 12/15-LOX pathway in mediating adipocyte function comes from studies revealing a regulatory role of lipocalin-2 on 12/15-LOX activity. Lipocalin-2, a glycoprotein member of the lipocalin superfamily, is a novel abundant adipokine implicated in obesity-mediated inflammation and insulin resistance. Reports demonstrate that lipocalin-2 expression is increased and correlates with increased inflammation in visceral adipose tissue from obese patients and obese rodent models [81-83]. Lipocalin-2 directly regulates adipose inflammation by activating 12/15-LOX activity [84]. Law and colleagues demonstrated that epididymal adipose tissue from lipocalin-2 deficient mice placed on a high-fat diet exhibited decreased macrophage infiltration, markers of oxidative stress, inflammatory markers, including TNF-α and MCP-1, with marked improvement in insulin action with increased insulin-stimulated glucose uptake compared to control C57BL6/J mice on a high fat diet [84]. This protection correlated with decreased expression of 12/15-LOX and its primary metabolite, 12(S)-HETE, and decreased metabolism of its main substrate, arachidonic acid, in adipose tissue. Addition of cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC; 12-LOX inhibitor) prevented lipocalin-2 induction of TNF-α and ensuing insulin resistance in adipose tissue of normal C57BL6/J mice fed a high-fat diet. Interestingly lipocalin-2 appears to exert adipose-specific effects on 12/15-LOX as expression of the latter was not altered and insulin sensitivity did not differ in other tissues examined between wild-type and lipocalin-2 deficient mice. Thus, these studies provide additional evidence implicating a critical role for 12/15-LOX in modulating adipocyte dysfunction with significant whole-body consequences.
Less investigation has been devoted to the role of epidermal- and platelet-12-LOX in obesity-induced adipocyte dysfunction. However, a recent paper from our lab has demonstrated that platelet-12-LOX is upregulated in adipocytes from C57BL6/J mice fed a Western diet for 12 weeks, and interestingly treatment with an angiotensin type 1 receptor (AT1R) blocker, valsartan, can abolish this effect [85]. It would be of interest to follow-up whether 12/15-LOX is also regulated by the renin-angiotensin system (RAS) in adipose tissue, as much evidence reveals that LOX products upregulate RAS components and in turn can be regulated by the RAS in several cell types [85].
A more detailed evaluation of the role of 12/15-LOX-derived products in adipocytes was performed by Chakrabarti and colleagues [76]. 12(S)-HETE and 12(S)-HPETE were added directly to differentiated 3T3-L1 adipocytes and shown to increase inflammatory cytokine expression of TNF-α, MCP-1, IL-6, and IL-12p40, and to decrease the expression of the anti-inflammatory adipokine, adiponectin. In addition, these products induced insulin resistance as measured by a decrease in insulin-mediated activation of key insulin-signaling proteins, such as Akt and IRS-1 (insulin receptor substrate-1). Furthermore, a free fatty acid component of high-fat diets, palmitic acid, was able to induce 12/15-LOX expression in 3T3-L1 adipocytes. These results demonstrate that products of 12/15-LOX pathway can directly impair adipocyte function in a fatty acid surplus environment.
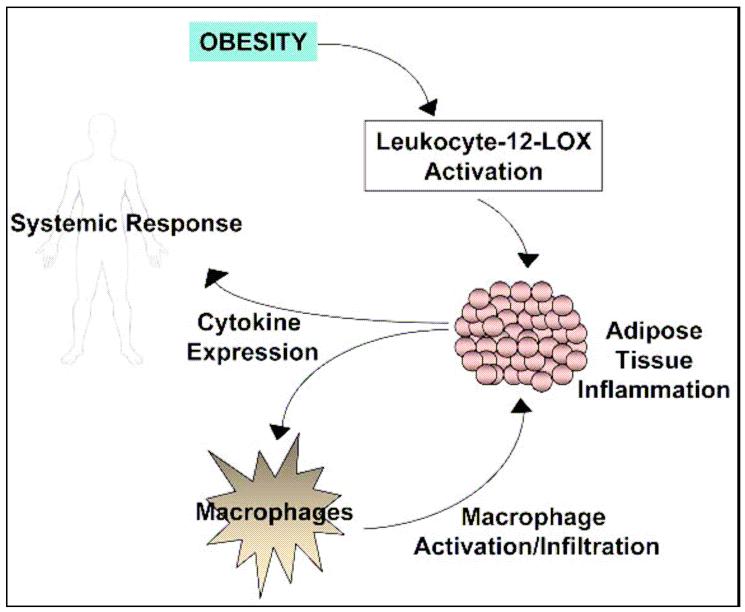
A model for a proposed role of leukocyte 12-LOX (12/15-LOX) in adipose tissue inflammation is shown in Figure 3. When this tissue is stressed, such as under diet-induced obesity, the adipocytes no longer function properly, and this leads to significant inflammatory consequences. Leukocyte-12-LOX appears to greatly contribute to this local and systemic decline (Figure 3). Thus further dissecting the role and regulation of leukocyte-12-LOX in adipose tissue will be of utmost importance and could lead to the development of novel therapeutic agents to reduce complications associated with obesity.
Figure 3. Proposed role of 12-lipoxygenase in obesity-induced inflammation in adipose tissue.
Excess consumption of energy leads to a situation where adipocytes become stressed due to the increasing demand for adipocyte storage of nutrients. The adipocytes become hypertrophic, leading to adipocyte dysfunction marked by ensuing inflammation. Secretion of inflammatory cytokines by adipocytes leads to activation and recruitment of macrophages into the fat bed, further propagating the inflammatory cascade. This inflammatory response is not confined to the adipose tissue, but also exerts systemic effects on other tissues in the body. A key player in the onset and progression of the inflammatory cascade is leukocyte-12-lipoxygenase (leukocyte-12-LOX). This enzyme is activated in a fatty-acid surplus environment whereby its products promote the onset of adipocyte dysfunction by inflammation.
5. THE 12/15-LIPOXYGENASE PATHWAY IN VASCULAR PHYSIOLOGY AND PATHOLOGY
The mammalian 12- and 15-LOXs have high substrate specificity oxidizing predominantly ω-6 (arachidonic and linoleic acids) but also the ω-3 (docosahexanoic) acid [86-88]. Different LOXs oxidize fatty acids both in the free form or in complex lipid-protein assemblies like membrane phospholipids and cholesterol esters in lipoproteins [87]. While having high substrate specificity, the positional selectivity of the oxygenation varies, leading in most cases to a mix of 12- and 15HPETEs which are subsequently reduced to their corresponding hydroxyl, more stable derivatives HETEs. In contrast, oxygenation of linoleic acid leads to the uniformly predominant formation of 13HPODEs and subsequently the reduced 13HODEs. Recently, 15-LOX1 was also implicated in oxygenation of ω-3 docosahexanoic acid leading to the resolvin D1 and protectin D1 classes of eicosanoids [89-91] (described in the Introduction and in Figure 1).
The complex array of metabolites formed as a result of 12- and 15-LOX catalytic action are tissue- and species-specific. Furthermore, the various metabolites generated in different pathways have pro- or anti-inflammatory actions and the net result varies according to the cell type and intracellular redox state. The evidence for protective vs. deleterious roles of different LOX isoforms and metabolites on vascular reactivity, atherosclerosis, and angiogenesis will be discussed.
5.1. The 12/15-lipoxygenase pathway in vascular reactivity and remodeling
5.1.1.Effects on vascular reactivity
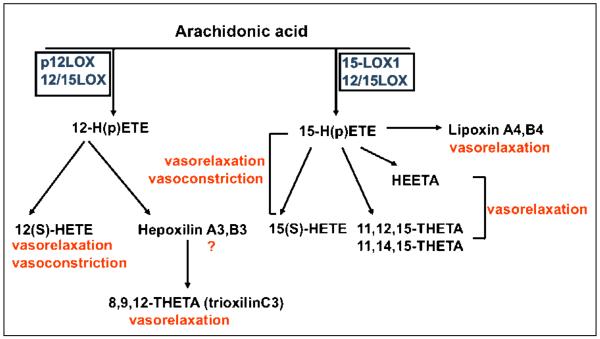
All of the 12- and 15-LOX isoforms illustrated in Figure 4 (blue boxes) reportedly have roles in modulation of vascular tone and remodeling via actions on vascular endothelium, smooth muscle cells, or both [92]. Expression of 15-LOX-1 was reported for human aortic endothelial cells [93] and 15-LOX-2 for pulmonary aortic and umbilical vein endothelial cells [94]. Also, in human vascular smooth muscle cells an isoform similar to the mouse 12/15-LOX was detected and is regulated by angiotensin II. Additionally, 15-LOX-1 is the major LOX expressed in rabbit aorta and other arteries [95, 96]. The vasoactive properties of 15HPETE were reported related to both vasodilation and vasoconstriction [92] (Figure 4). The net effect on vascular function is dose and species specific and also depends on other local regulators. For example, the contractile responses to 12/15 LOX products varied substantially between different vascular segments in guinea pig basilar arteries [97].
Figure 4. Roles of various lipoxygenase isoforms and lipid metabolites on vascular reactivity.
All major 12- and 15-LOX metabolites exhibit vasomotric properties. The same metabolite may exert either vasorelaxant or vasoconstrictive effects that are concentration-, vessel- or species-specific dependant. For example, 15-HETE and 15-HPETE cause slight relaxation at low concentrations while inducing vasoconstriction at higher concentrations. THETA and HEETA have an endothelium-dependent vasorelaxant effect on pre-constricted rabbit aorta and mesenteric arterioles. Evidence suggests that HEETAs and THETAs are major mediators of the action of 15-LOX-1 on vascular relaxation in conduit and resistance arteries. The 12-HETE generated from 12-LOX action has vasorelaxant effects in rat, human and porcine vessels. However, a vasoconstrictive effect of 12-HETE was reported for a similar concentration in dog renal arcuate arteries. Lipoxins reportedly produce vasorelaxation in aorta and in pulmonary arteries.
One important determinant of vasoconstrictive effect is production of bioactive nitric oxide (NO). Multiple interactions were uncovered between NO and the LOX pathway [98, 99]. LOX-expressing cells reduce NO bioavailability compared to LOX negative controls [100]. This process is guanylate cyclase dependent and results in vasoconstriction [100]. In vivo data confirmed that 12/15-LOX knockout mice have elevated biosynthesis of NO [101]. Also, in neo-natal rabbit pulmonary arteries increased expression of 12/15-LOX following chronic hypoxia induced vasoconstriction via formation of 15(S)-HETE [102]. On basal tone, 15HETE and 15HPETE cause slight relaxation at low concentrations while inducing constriction at high concentrations [92]. At least in rabbit aorta the vasomotric effect of 15-LOX metabolites 15HETE and 15HPETE are endothelium-dependent [103, 104]. In pre-constricted rabbit arterioles, treatment with AA led to vasorelaxation, the effect was endothelium dependent and the major mediators were the THETA and HEETA metabolites formed via the 15-LOX-1 pathway [104] (Figure 4). A recent report identified 13-H-14,15 EETA as the major vasodilator via K(+) channel activation and smooth muscle cell hyperpolarization in rabbit aorta and mesenteric arteries treated with AA [105]. Therefore, the HEETAs and THETAs may represent the major mediators of the actions of 15-LOX-1 on vascular relaxation in both conduit and resistance arteries (for a comprehensive review see [92]).
Like the 15-LOX pathway, various mediators generated via the 12-LOX pathway were found in arteries of different species. 12(S)-HETE is produced by arteries and the vascular endothelium [106, 107]. The endothelium-dependent vasodilatory effect of 12(S)-HETE was reported for rat basilar and mesenteric arteries [108, 109] and for human and porcine coronary vessels [107, 110]. Smooth muscle cells underwent hyperpolarization in response to addition of 12(S)-HETE through activation of large conductance K channels (BKca) [110]. However, vasoconstrictive responses were reported following treatment of dog renal arcuate arteries with 12(S)-HETE [111]. The alternative 12-LOX pathway generating hepoxilins and trioxilins has not been well studied in vasculature. One report showed production of trioxilin C3 by rat aorta treated with 12-LOX, which apparently mediates vasodilation [112]. Also, hepoxilin A3 while having no direct effect on vascular tone in rat aorta and portal vein, potentiated norepinephrine induced vascular contractions in a calcium-dependent fashion [113]. Lipoxins promote vasorelaxation in aorta and pulmonary arteries [114].
5.1.2. Effects on vascular remodeling
The 12- and 15-LOX pathways are also involved in vascular remodeling of conduit vessels and arterioles. Vascular remodeling is an active process that occurs in response to elevated shear stress or pressure. As a result of remodeling the vessel wall becomes thicker and involves active proliferation and migration of the smooth muscle cells. The lipoxygenase pathway is involved in smooth muscle cell migration, proliferation and apoptosis, processes involved in physiologic or pathogenic vessel remodeling [13]. 12/15-LOX metabolites have mitogenic and chemotactic effects on smooth muscle cells and signaling through MAP kinase has been implicated for the mitogenic effects [115, 116]. Similar data were obtained for 13HPODE effects on porcine vascular smooth muscle cells [16], and both the MAP kinase and NFkB and VCAM-1 were actively involved [117]. In addition, NFkB was involved in 13HPODE effect on inducing MCP-1 expression in smooth muscle cells [117]. Also, a recent report identified Rho-kinase pathway as the mediator of 15HETE remodeling effect induced by hypoxia in rat pulmonary arteries [118]. A unique effect for 12HETE and 13HODE was reported in porcine aortic smooth muscle cells on increasing intracellular calcium and cGMP which counteracted vasoconstriction [119]. Migration of porcine aortic smooth muscle cells in response to PDGFB was inhibited following silencing of 12/15LOX, suggesting that the LOX pathway is actively involved in smooth muscle cell migration [120]. Additionally, the effect of 15(S)-HETE on smooth muscle cell migration requires IL-6 expression via CREB activation [121] and is Src-dependent STAT3 mediated [122]. Furthermore, 12/15-LOX appears to be involved in cell cycle regulation and hence in control of proliferation, since vascular smooth muscle cells from 12/15LOX knockout mice displayed decreased S-phase entry [123]. Finally, a recent paper indicates that 15HETE protects rat pulmonary arterial smooth muscle cells from apoptosis via the PI3K/Akt pathway [124]. Collectively the data indicate that 12- and 15-LOX pathways have multiple effects on both endothelial and smooth muscle cells contributing to vasomotric properties and active remodeling of the large and small vessels.
5.2. Pro- and anti-atherogenic effects of the 12/15-lipoxygenase pathway
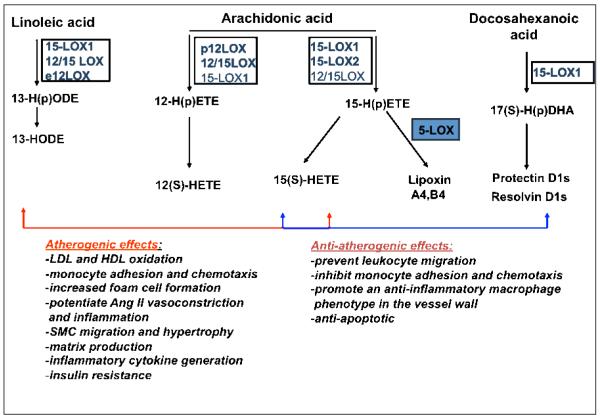
Atherosclerosis is associated with chronic inflammation at every stage and the progression of the disease may critically depend on the balance between the pro- and anti-inflammatory factors at any given time. The 12- and 15-LOX enzymes and associated metabolites are critical players in generation as well as resolution of inflammation [20, 125]. While the pro-atherogenic role of the 5-LOX pathway is generally better established in animal models and human studies, the role of the 12- and 15-LOX pathways is not yet clear. The pro-atherosclerotic effects of this latter pathway include effects on LDL oxidation, monocyte recruitment to the vessel wall, effects on proliferation of smooth muscle cells, and production of pro-inflammatory cytokines by various cells component or homing into the vessel wall [20]. Amongst the anti-atherosclerotic effects is inhibition of oxidative stress by 15-LOX metabolites as well as formation of anti-inflammatory lipids, such as lipoxins that have vasodilatory actions (Figures 1, 4 and 5) and protectins and resolvins that reduce endothelial-leukocyte adhesion, reduce monocyte chemotaxis and promote a more anti-inflammatory macrophage phenotype in the vessel wall.
Figure 5. Pro- and anti-atherogenic effects of various 12- and 15-lipoxygenase lipid metabolites.
The controversial effects of different 12- and 15-LOX isoforms on the development of atherosclerosis in different animal models or in human epidemiological studies may be due to both pro-and anti-atherogenic effects mediated via particular lipid metabolites. While 12-LOX - generated lipid products such as 13-HODEs and 12(S) HETEs have more unanimously atherogenic effects, both 15-LOX-1 and 15-LOX-2 may have pro- or anti-atherogenic effects depending on metabolites formed and the specific cell type and animal model. For example, 15-LOX-1 may generate lipoxins, protectins and resolvins that are associated with resolution of inflammation in vasculature. However, both 15-LOX-1 and 15-LOX-2 produce 15-HETEs that can exert anti-inflammatory effects by antagonizing the effects of leukotriene B4 on polymorphonuclear cells and inhibiting their trans-endothelial migration in response to cytokines.
Studies in various animal models susceptible to develop atherosclerosis are in line with both the anti- and pro-atherogenic effect of 12/15-LOX pathway (Figure 5). Mice overexpressing human 15-LOX in the endothelium are more susceptible to develop atherosclerotic lesions compared to littermate controls [126]. However, transgenic rabbits overexpressing human 15-LOX in the macrophages were protected against developing atherosclerosis [127]. Also, in rabbits with transient anemia that is characterized by overexpression of 15-LOX in reticulocytes, lipid deposition in the thoracic aorta was significantly diminished compared to controls [128]. Interestingly, apoE, LDLR and apobec/LDLR knockout mice lacking the 12-LOX gene consistently showed reduction of atherosclerosis [40, 129-131]. The apparent discordance in the results may be due in part to the species- and cell- specific pattern of expression of the 12- and 15-LOX enzymes and metabolites, as described earlier in the review. Also, the formation of anti-inflammatory lipid mediators via consecutive action of 15-LOX and 5-LOX may explain why in some 15-LOX deficient models an athero-protective effect is reported. For example, lipoxin A4 generation, with general vasorelaxant and anti-inflammatory properties, requires the sequential action of 15- and 5-LOX or of 5- and 12-LOX. Hence, formation of lipoxins may be trans-cellular involving, for instance, neutrophils and platelets [132]. In line with this comes a recent study by Funk et al. in which the double 15- and 5-LOX apoE knockout mice are protected from developing atherosclerosis [133].
Among the athero-relevant LOX isoforms, the 12/15-LOXs are unique due to their capability to oxidize lipids in membranes and lipoproteins. Formation of the oxidized LDL particles in the arterial wall is a hallmark for atheroma initiation and progression due to their ability to induce foam cell formation. The 15-LOX was shown to directly oxidize LDL in vitro [134, 135]. Also, fibroblasts transfected with 12-LOX cDNA have an enhanced capability to oxidize LDL in vitro [136]. In macrophages, deletion of 12-LOX led to a reduced ability to oxidize LDL [77], while LDL oxidation in macrophages treated with IL-13 or IL-6 was mediated via the 12-LOX pathway [137]. The mechanism by which cytoplasmic 15-LOX or 12-LOX causes LDL oxidation appears to require translocation of the enzymes from the cytosol to the plasma membrane where they directly oxidize the LDL particles [138]. The oxidation requires the binding of LDL particles to the low density lipoprotein receptor related protein (LRP) [139]. In addition to LDL oxidation, 12/15-LOX metabolites may contribute to foam cell formation via modulation of scavenger receptor CD36 on macrophages. 15-LOX, 15(S)-HETE and 13(S)-HODE were all shown to increase expression of CD36 in macrophages and the effect is mediated via PPARγ activation [71]. Also, recent findings show that 12/15-LOX activity increase degradation of ABCG1 transporter in murine macrophages [140] and the effects occur through p38 MAPK and JNK2-dependent pathways [141]. However, it is debatable whether the foam cell formation will be increased in vivo, since 13-HODE activation of PPARα also results in increased expression of ABCA1 transporter and increased cholesterol efflux from macrophages [142]. Besides LDL oxidation, 15-LOX oxidizes HDL particles, leading to impairment of their anti-atherogenic function. The 15-LOX oxidized HDL has a lower cholesterol accepting potential, probably by impaired binding to ABCA1 and SR-BI receptors [143, 144]. This may result in reduced cholesterol efflux from macrophages and increased development of the lesional lipid core. Also, HDL3 modification by 15-LOX results in a loss of anti-inflammatory mechanisms of HDL towards TNF-α in endothelial cells [145].
Another pro-atherogenic mechanism of the 12/15-LOX pathway is via interaction with angiotensin II. Angiotensin II plays multiple roles in early stages of atherosclerosis and has been shown to upregulate 12-LOX expression and activity in mouse macrophages and smooth muscle cells [146]. Enhanced expression of 12-LOX is critical to mediate the vasoconstrictive properties of angiotensin II, as shown by in vitro data and in 12-LOX deficient mice [101]. The 12-LOX effect on angiotensin II signaling appears to take place via AT1R, since valsartan treatment alleviated the inflammation induced by 12-LOX [85].
An additional key mechanism modulated by 12- and 15-LOX pathways is monocyte/endothelial interaction. Overexpression of 12-LOX in mice significantly increased monocyte adhesion to the endothelium and resulted in aortic fatty streak formation [147]. On the same line, addition of 12(S)-HETE and 13(S)-HODE to the endothelial cells of transgenic mice increased significantly monocyte adhesion [147]. Also, in hyperglycemic mice with increased expression of 12/15-LOX an augmented monocyte recruitment to endothelium was reported [147]. The mechanisms include an increase of monocyte chemotaxis via elevated MCP-1 expression and enhanced monocyte-endothelial adhesion via ICAM-1 and VCAM-1 [147-149]. 12HETE reportedly increased ICAM-1 expression via activation of protein kinase Cα [150]. 12/15-LOX may also exert atherogenic effects via modulation of the pro- inflammatory cytokine IL-12 in macrophages[40]. It has been shown that IL-12 has pro-atherogenic effects and 12/15-LOX enhances transcription of the IL-12p40 subunit in macrophages [40]. The effect is apparently not mediated via the 12/15-LOX pro-inflammatory lipid metabolites, but rather due to oxidative stress that changes the intracellular redox state [41].
An important pro-inflammatory role is played by 15-LOX-2, which is upregulated by hypoxia in human macrophages [151]. Increased expression of 15-LOX-2 in human macrophages induces chemokine secretion and T cell migration, both contributing to plaque inflammation and instability [152]. Finally, effects of 12- and 15-LOX pathway on vascular smooth muscle cell migration and induction of pro-inflammatory cytokines secretion (described in detail above) are contributors to atheroma formation.
While a wealth of evidence points towards a pro-atherogenic role of 12- and 15-LOX pathway, several reports suggest an anti-atherogenic role mainly through production of lipid mediators with anti-inflammatory and vasodilatory effects [20, 125]. In addition, besides the pro-inflammatory properties, both the 15(S)-HETE and 13(S)-HODE display anti-inflammatory effects on circulating and vascular cells. 15(S)-HETE antagonizes the action of leukotriene B4 on PMNs by inhibiting superoxide production and degranulation [153]; it also inhibits trans-endothelial migration of PMNs in vitro in response to cytokines [154]. The mechanism involves a substantial reduction in the affinity for LTB4 receptor following esterification of phospholipids in neutrophils by 15HETE [155]. Also, 13(S)-HODE inhibits leukocyte and platelet adhesion to the endothelium via binding of the lipid to the vitronectin receptor [156]. 13(S)-HODE also activates PPARγ and PPARα which results in reduction of pro-inflammatory TNF-α, IL-1β and IL-6 cytokine expression mainly by interfering with AP-1 and NFkB transcription factors [157].
The 12- and 15-LOX pathway has a particularly important role in cardiovascular complications related to insulin resistance and type 2 diabetes. An earlier study showed increased 12/15-LOX enzymes and metabolites in a diabetic pig model displaying accelerated atherosclerosis [158]. Also, activation of the 12-LOX pathway and the downstream STAT-4 signaling was reported during neointima formation in Zucker rats, a rodent model of metabolic syndrome [159]. Intimal hyperplasia due to carotid injury has been prevented by inhibition of rat 12-LOX achieved by ribozyme inactivation [160]. In addition, inactivation of the 12-LOX in porcine aortic smooth muscle cells significantly reduced the chemotactic effect of PDGFβ and reduced monocyte adhesion to transfected endothelial cells [120]. Finally, the 12-LOX pathway plays a role in cardiac enlargement via effect on cardiac fibroblasts hypertrophy [161].
The 15(S)-HETE could be further metabolized by 5-LOX to the anti-inflammatory lipoxins A4 and B4 (Fig 1). As described earlier, lipoxins have vasorelaxant actions on aorta and pulmonary arteries. In addition, they are counteracting the pro-inflammatory actions of leukotrienes and prostanoids. Lipoxins also limit neutrophil chemotaxis, adhesion, and transmigration [162, 163] and promote uptake of apoptotic neutrophis by macrophages [164, 165]. Interestingly, lipoxins are potent chemoattractants for monocytes but the latter show features of alternative activation after tissue recruitment and local differentiation [166]. Hence, the resulting non-phlogistic macrophages show reduced secretion of pro-inflammatory cytokines and increased scavenging activity of apoptotic cells [167]. This is an important mechanism in the resolution of plaque inflammation and clearance of accumulating apoptotic cells is important for plaque stability.
Other families of anti-inflammatory lipid mediators generated via the 15-LOX pathway are the resolvins and protectins (Figures 1 and 5). Recent studies have shown that some members of the resolvin and protectin families prevent neutrophil infiltration and cytokine secretion in models of inflammation, including atherosclerosis [125, 168]. Overexpression of 12/15-LOX in macrophages leads to formation of protectin D1 (PD1) and resolvin D1 (RvD1), along with lipoxin A4 (LXA4) during the resolution phase of inflammation [168]. These lipid mediators suppress the pro-inflammatory cytokine production by macrophages and down-regulate endothelial VCAM-1 and selectin-P [168]. Also, while not involving the 12- or 15- LOX pathways, resolvins of the E-series, derived from eicosapentaenoic acid (EPA) (in particular the most extensively studied resolvin E1) are important in limiting inflammation by antagonizing the BLT1 receptor and therefore dampening the effects of leukotrienes [169]. The potential beneficial role of protectins and resolvins in atherosclerosis is also of interest in the context of the abundant evidence on cardio-protective effects of ω-3 fatty acids that has otherwise limited mechanistic explanation [170].
Collectively, the studies in cell culture and animal models support a dual role of the 12- and 15-LOX pathways in atherosclerosis (Figure 5). However, to date, the relevance of these pathways for human atherosclerosis is not clear. The 15-LOX protein was found in macrophage-rich areas of human fatty streaks [171]. The presence of 15-LOX linoleic acid metabolite 13HPODE in early lesions, but not in advanced plaques, suggest a more important role of the pathway at early disease stages [172]. Also, 15-LOX-2 was found in human carotid plaques and associated to increased local hypoxia [173]. Human 15-LOX gene contains 11 polymorphisms, of which a −292C>T variant, associated to higher enzyme activity, showed a tendency towards protection against atherosclerosis in a case-control study involving 498 Caucasian heterozygotes [174]. In a study genotyping of atherosclerotic disease, vascular function and genetic epidemiology (ADVANCE) heterozygote carriers of a near null T560M allele (associated to a 20-fold reduction in enzymatic activity) had an increased risk of clinical coronary artery disease [175]. While these two studies may suggest an atheroprotective role of 15-LOX pathway in humans, a recent study indicates that polymorphisms in ALOX12 gene are associated to sub-clinical atherosclerosis and biomarkers of disease in families with type 2 diabetes [176]. Larger genetic association studies are clearly imperative to determine whether the pro- or anti-atherosclerotic effects of 12/15-LOX pathway prevail in human atherogenesis. It is possible the the 12- and 15-LOX pathways will play a more prominent role in atherosclerosis associated with insulin resistant or diabetic states.
Developing conditional knockout models for vascular or immune cells for each of the LOX isoforms is of utmost importance. Also, to complement the knockout approach and extend the studies to species other than rodents, it will be crucial to conduct studies using specific pharmacologic inhibitors for each of the LOX isoforms. Unfortunately, this has been a limitation in the field, since there are no specific LOX inhibitors that do not also have non-specific anti-oxidant properties. Also, specific pharmacologic inhibitors for different LOX isoforms could be used in the future for therapeutic intervention in human disease. Development of highly specific pharmacological tools acting as isotype specific LOX inhibitors is therefore an imperative goal that is currently under investigation.
5.3. The 12/15-lipoxygenase pathway in angiogenesis
There is limited evidence that LOX pathways are involved in the control of pathogenic angiogenesis. Evidence for both promoting and limiting angiogenesis has been published in different animal models and in humans. The 12/15-LOX pathway was involved in vascular, retinal, and tumor angiogenesis. A human cell line of prostate cancer over-expressing 15-LOX-1 displayed increased VEGF secretion and enhanced angiogenesis [177]. In contrast, in a rabbit skeletal muscle system, co-administration of 15-LOX-1 significantly blunted all angiogenic effects induced by VEGF-A including capillary number and perfusion and vascular permeability [178]. A similar effect was noticed in two xenograft models, where tumor angiogenesis is inhibited in mice overexpressing 12/15-LOX [179]. One possible mechanism described for the anti-angiogenic effect of the 15-LOX pathway is via reduction of NO production and availability in endothelial cells [178]. An anti-angiogenic effect of adenoviral transfection of 15-LOX-1 gene prevented corneal neovascularization induced by VEGF-A in rabbits [180]. Moreover, substantial alteration in vascular morphology was reported in subcutaneously implanted mouse tumors overexpressing 12/15-LOX, and the vascular phenotype was successfully reversed following 12/15-LOX pharmacological inhibition [181]. 12-LOX, via the 12(S)-HETE metabolite, enhanced angiogenesis and HIF-1α expression in hypoxic tumor cells of the prostate [182]. 15(S)-HETE has also been shown to increase pathologic angiogenesis associated with atherosclerosis and re-stenosis via Src-mediated Egr-1 dependent induction of FGF-2 expression [183]. Finally, recent evidence suggests that LXA4 and lipoxin A4 receptors have anti-angiogenic effects in a model of corneal vascularization following injury [184]. LXA4 seems to be a key metabolite that is responsible for the anti-angiogenic effect of 15-LOX-1 observed in certain angiogenic model systems [184]. In conclusion, the role of 12/15-LOX pathway in angiogenesis remains controversial and requires careful future examination.
6. THE 12/15-LIPOXYGENASE PATHWAY IN REGULATION OF RENAL FUNCTION AND PATHOLOGY
A variety of AA metabolites formed via the three major enzymatic pathways (COX, CYP450, and LOX) have significant effects on regulation of renal hemodynamics, and disturbances in any of these pathways can contribute to renal injury, progression to renal nephropathy and renal function alterations [185-187]. Generation of eicosanoids in the kidney is altered in a variety of conditions such as hypertension, diabetic nephropathy, and acute renal failure. Therefore there is evidence that altered vascular production of AA metabolites could be both cause and effect in various renal pathologic conditions. In this chapter we will focus solely on evidence for the physiologic and pathogenic role of the 12- and 15-LOX pathway in the kidney.
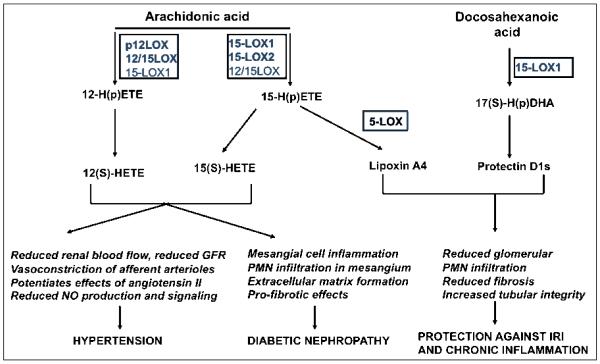
Several studies showed key renal hemodynamic effects of 12- and 15-LOX pathway in the kidney (Figure 6). 12(S)-HETE and 15(S)-HETE were shown to have vasoconstrictive actions on renal vessels and glomerular mesangial cells [185, 188]. 12HETE infusion in the renal artery of rats resulted in decreased renal blood flow and glomerular filtration rate (GFR) [189]. Also, 12(S)-HETE was shown to contribute to vasoconstrictive response of the renal afferent arteriole to angiotensin II [188]. Likewise, 12(S)-HETE enhanced the vasoconstrictive effect of angiotensin II in the aorta of SHR through an increase in intracellular calcium [190]. Recent studies showed that interaction between 12/15-LOX and angiotensin II is mediated in part by AT1 receptor. 12-LOX enhanced AT1R expression in diabetic glomeruli and 12-LOX inhibition could ameliorate diabetic nephropathy progression through downregulation of AT1R expression [191]. Also, valsartan reduced platelet 12-LOX in mice in adipocytes [85]. Of importance, as described under vascular actions of 12/15-LOX in this review, there is mutual interaction with the NO production and signaling. 12/15-LOX induces catalytic consumption of NO and can prevent NO-dependent soluble guanylate cyclase activation [192]. In accordance to above reported effects, 12/15 LOX knockout mice had reduced vasoconstriction responses to angiotensin II and increased eNOS expression and NO bioavailability [101].
Figure 6. Effects of 12- and 15-lipoxygenases and their metabolites on renal function and pathology.
Arachidonic acid-derived 12-HETEs and 15-HETEs generated by platelet 12-LOX or 15-LOX-1 or 15-LOX-2 are associated with hypertension and may have a causative effect by reducing renal blood flow, reducing GFR and NO production and by potentiating angiotensin II-related vasoconstriction. In addition, 12- and 15-HETEs may contribute to progression of diabetic nephrophathy via profibrotic effect, matrix proliferation and inflammation. Also, 15-LOX may generate lipoxinA4, via sequential action of 5-LOX or the protectin D1 lipid mediators. The latter were shown to protect against ischemic renal injury (IRI) and chronic renal inflammation by reducing local PMN infiltration and attenuating fibrosis and tubulo-sclerosis.
Of importance, the 12- and 15-LOX pathways also act as mediators of the interaction between angiotensin II and aldosterone in the adrenal cortex with important implications on renal function and on cardiac hypertrophy. Angiotensin II stimulated aldosterone production in both rat and human granulosa cells [193, 194]. The major lipid mediator responsible for this effect is the 12(S)-HETE but not the 15(S)-HETE. Basal aldosterone secretion is not mediated via the 12-LOX products, however angiotensin-dependent aldosterone secretion is critically dependent on the the LOX pathway metabolite. A recent report emphasizes the reciprocal effect of aldosterone on up-regulation of 12- and 15-LOX expression and LDL oxidation in human vascular smooth muscle cells [195]. Increased production of 12(S)-HETE and 15(S)-HETE induced increased smooth muscle cell contractility, hypertrophy and migration as well as enhanced LDL oxidation [195]. Via these effects, 12- and 15-LOX could contribute to vascular reactivity and atherogenesis, as well as blood pressure regulation.
Since all the above vascular and glomerular effects contribute to blood pressure regulation, it is expected for 12/15-LOX pathway to play a role in pathogenesis of hypertension (Figure 6). Indeed, there is evidence for alterations of the 12- and 15LOX enzymes and metabolites both in humans with essential hypertension and in various animal models of hypertension. In patients with essential hypertension urinary 12HETE excretion was found to be increased [196]. Also, a polymorphism in the human 12-LOX gene (encoding for the platelet form) is associated with essential hypertension [197]. Increased 12(S)-HETE production and 12-LOX mRNA expression was reported in the vasculature of SHR as well as other animal models of both angiotensin-dependent and renovascular hypertension [198-200]. Also, blood pressure was decreased in 12/15-LOX knockout mice that were chronically infused with angiotensin II [101]. In addition, 12-LOX inhibitors have also been shown to ameliorate hypertension in different animal models [200, 201]. Altogether the data supports a key role for 12- and 15-LOX pathway in animal and human hypertension; additional studies are needed to clearly substantiate a causative effect for this pathway in different forms of hypertension.
The 12- and 15-LOX pathway is the primary LOX pathway implicated in vascular and renal injury associated with diabetes [6, 186] (Figure 6). 12/15-LOX was detected in renal microvessels, glomeruli mesangial cells, and podocytes [188, 202-204]. Importantly, 12(S)-HETE is increased in urine of diabetic patients with early kidney disease [6, 205] and 12- and 15-LOX mRNA and protein expression increases in parallel with established markers of diabetic nephropathy [205]. 12/15-LOX expression has been increased in glomeruli of diabetic animals and glucose was shown to directly increase 12/15-LOX expression in cultured mesangial cells [203, 205, 206]. The LOX pathway is also involved in high glucose-induced monocyte adhesion to endothelial cells [120, 207]. In addition, the 12/15-LOX pathway is a critical mediator of mesangial cell hypertrophy and matrix accumulation induced by TGF-β and angiotensin II [101, 208, 209] and the effect could be blocked by LOX pharmacological inhibition or by targeted 12/15-LOX gene deletion [6, 101, 208, 209]. Cultured rat mesangial cells treated with TGFβ and angiotensin II displayed increased 12-LOX mRNA expression and formation of 12(S)-HETE [6].
There is evidence for a dual role of the LOX pathway in acute renal failure. While LOX enzymes and metabolites were shown to act as vasoconstrictive and pro-inflammatory mediators in acute and chronic renal disease, there is also evidence for a protective role of the 15-LOX pathway in acute renal failure mediated by the lipoxin family of metabolites [210] (Figure 6). The spectrum of bioactivities reported for lipoxins suggests that they may be protective in various human renal diseases. LXA4 has been demonstrated to oppose the reduction in renal blood flow and GFR induced by LTD4 infusion, and the effect is owed to the activation of peptide-LT receptors [185, 211]. Lipoxin generation can also shift the glomerular response from inflammation to resolution and inhibition of monocyte recruitment [211].
Also lipoxins reportedly reduced PMN chemotaxis, adhesion, and migration across glomerular endothelial cells [212] and increased clearance of apoptotic PMNs from inflamed glomeruli [164]; they also inhibited mesangial cell proliferation in response to PDGF and reduced pro-inflammatory cytokine production [213-215]. Overexpression of 15-LOX in rat kidney has demonstrated a protective role in immune-mediated glomerulonephritis and is paralleled by lipoxin formation [216]. Of importance, structural analogs of LXA4 show therapeutic potential for the treatment of acute renal failure. Also, in a murine model of ischemia-reperfusion injury (IRI) the lipoxin analog 15-epi-16(FPhO)-LXA4-Me reduced PMN infiltration, preserved tubular integrity, and normalized serum creatinine levels [217]. Also, SOCS-1 and -2 were increased in IRI animals treated with the LXA4 analogue and several pro-inflammatory cytokine expression was reduced [217]. Finally, the role of LXA4 in its ability to downregulate the mesangial cell receptor tyrosine kinases in primary cultures of human mesangial cells is suggestive of a protective role of lipoxins against renal chronic inflammatory response [213, 214]. A very recent report indicates a protective role of protectins (PD1) in IRI [218]. Therapeutic or dietary amplification of PD1 formation via increase in dietary ω-3 PUFA dramatically impacts renal lipid autacoid formation and positive outcome of IRI [218].
In conclusion, the 12- and 15-LOX enzymes and metabolites have a dual role in renal disease and are key regulators of renal function. Better understanding of this pathway in different renal pathologies will lead to newer therapeutic options in the future for treatment of hypertension and chronic renal disease and renal injury. This is another example where development of targeted pharmacologic inhibitors will be valuable for research and as therapeutic tools.
7. THE 12/15-LIPOXYGENASE PATHWAY IN DISEASES OF THE NERVOUS SYSTEM
7.1. Cerebrovascular disease
Concentrations of AA in neuronal tissue rise (up to 30 times normal) after an ischemic insult, and are a source of the neurotoxic free radicals involved in cell death and damage following a stroke [219]. While earlier data suggested a role for cycloxygenase and its products in cerebrovascular disease, more recent studies have demonstrated a role for the 12/15-LOXs [220, 221]. Van Leyen et al. demonstrated increased concentrations of 12/15-LOX in the neurons surrounding an infarct in a murine model of transient middle cerebral artery occlusion, and showed that intraperitoneal injection of the 12/15-LOX inhibitor baicalein prior to the ischemic event led to reductions in infarct size [221]. Similar reductions in infarct size were seen in a 12/15-LOX knockout mouse model. In a rabbit model of embolic stroke, baicalein given within one hour of a stroke reduced post-stroke deficits in behavior [222].
Accumulation of the oxidant peroxynitrite has been suggested as having an important role in neuron damage after ischemia. Zhang et al., in studies involving rat neurons in culture, showed that 12-LOX mediated peroxynitrite toxicity, perhaps after activation by increased intracellular zinc release [223]. 12-LOX activation led to activation of p38 MAPK and caspase-3 proteins involved in neuronal cell death. The non-selective lipoxygenase inhibitor AA-861 reduced the reactive oxygen species generation seen after neuronal exposure to zinc, and also blocked the activation of p38 MAPK. Pallast et al. have recently associated murine 12/15-LOX with apoptosis-inducing factor (AIF), a mitochondrial protein involved in a caspase-independent pathway of neuron death after ischemic stroke [224]. They found that 12/15-LOX and AIF co-localized in peri-infarct areas of the mouse cortex following cerebral ischemia, and demonstrated that 12/15-LOX was activated after glutathione depletion, leading to AIF movement to the nucleus of the neuron. This translocation was inhibited by baicalein. This nuclear translocation step may be necessary for the apoptotic effect of AIF, which may promote condensation of nuclear chromatin [225]. Jin et al. evaluated the role that 12/15-LOX plays in the cerebral vasculature [226]. The 12/15-LOX inhibitors AA-861 and baicalein were each able to reduce the cell injury seen after transformed human brain endothelial cells were exposed to hydrogen peroxide. 12/15-LOX was expressed in both neurons and vascular endothelial cells in peri-ischemic areas of mouse brain after ninety minutes of middle cerebral artery occlusion, but was not present in astrocytes. Jin et al. also studied the role of 12/15-LOX in the blood-brain-barrier [226]. They found that baicalein reduced the loss of the endothelial tight junction protein claudin-5 seen after ischemia. 12/15-LOX knockout animals had less leakage of immunoglobulin IgG into the brain after ischemia, as did animals treated with baicalein, both evidence for a role for 12/15-LOX in the disruption of the blood brain barrier seen after an ischemic event. By studying the water content of ischemic brains, the group was able to show that baicalein-treated animals and 12/15-LOX knockout mice had less water, and therefore less cerebral edema, after an ischemic insult.
7.2. Alzheimer’s disease
A role for 12/15-LOX has been suggested in Alzheimer’s disease (AD). Lebeau et al. demonstrated that 12/15-LOX and 12HETE were involved in the over-expression of c-Jun, a protein necessary for the apoptosis associated with the beta-amyloid peptide found in AD [227]. Praticò et al. demonstrated by Western blot analysis elevated concentrations of 12/15-LOX in the frontal and temporal brain regions of patients that had died from AD when compared to controls without AD [4]. 12HETE and 15HETE concentrations were elevated in both of these brain regions as well. In an extension of this work, Yao et al. showed that 12HETE and 15HETE concentrations were elevated in the cerebrospinal fluid of individuals with both mild cognitive impairment and AD [228]. Elevated HETE concentrations correlated with isoprostane F2α, a marker of lipid peroxidation that is elevated in AD. A recent study in a murine model of AD that develops neuro-amyloidosis and cognitive deficits revealed that deleting 12/15-LOX reduced amyloid formation and improved memory, and that this effect involved the beta-secretase proteolytic pathway [229].
7.3. Parkinson’s disease
12/15-LOXs may also play a role in the neuromotor disorder Parkison’s disease (PD). Li et al. demonstrated that decreased neuronal concentrations of the antioxidant glutathione, an early finding in (PD), were associated with 12-LOX activation (as assessed by 12HETE production) in vitro [230]. Canals et al. have suggested that NO, under glutathione-depleted conditions, becomes neurotoxic, particularly to the dopaminergic neurons of the midbrain that are damaged in PD [231]. Later work by this group demonstrated that inhibition of 12-LOX with nordihydrogualaretic acid and baicalein prevented this neurotoxic effect of NO [3]. The addition of AA to cells that had been depleted of glutathione was found to be neurotoxic, as was the separate addition of the 12-LOX product, 12HETE. The authors hypothesized that the glutathione-depletion seen in PD led to neurotoxicity via a NO/12-LOX pathway [3].
8. CONCLUSIONS AND FUTURE PERSPECTIVES
Arachidonic acid and other polyunsaturated fatty acids, and their lipid metabolites, play very important roles in human health and disease. This review has outlined the functions of 12- and 15-lipoxygenases, enzymes that are present in multiple systems and organs of the body, including pancreatic islet, adipose, vascular, immune, renal, and nervous tissues. As a result of their widespread expression in the body, 12- and 15-LOX and their metabolites are important in a variety of disease states, including diabetes (both type 1 and type 2), atherosclerosis, renal disease, obesity, and various diseases of the central and peripheral nervous system. In all of these areas, the development of isoform specific LOX-inhibitors will be necessary to fully establish the therapeutic opportunity to treat these disorders by reducing expression or activity of 12/15-LOXs. A recent paper has indicated promising new 15-LOX-1 inhibitors have been developed [232]. Much interesting and important work is still needed and underway in this exciting field. Nevertheless, the existing data indicate promise of the 12/15-LOX pathway as a target in a number of disorders, particularly related to diabetes and its complications and in states of insulin resistance.
9. ACKNOWLEDGEMENTS
The authors wish to thank all of the collaborators, investigators, postdoctoral fellows, research associates, and graduate students that have contributed to advances in this field. In particular, we wish to thank Drs. Lynn Hedrick and Rama Natarajan for their significant contributions.
Work in the authors’ laboratory was supported by the Juvenile Diabetes Research Foundation, the Iacocca Foundation, and the National Institutes of Health (Grants: NIDDK R01 DK 55240 and NHLBI P01 HL55798).
Abbreviations
- AD
Alzheimer’s disease
- AIF
apoptosis-inducing factor
- AA
arachidonic acid
- CAM
cellular adhesion molecule
- CDC
cinnamyl-3,4-dihydroxy-α-cyanocinnamate
- COX
cyclooxygenase
- EPA
eicosapentaenoic acid
- ESI
electrospray ionization
- ELISA
enzyme-linked immunosorbent assay
- GC/MS
gas chromatography-mass spectrometry
- HPETE
hydroperoxyeicosatetraenoic
- HETE
hydroxyeicosatetraenoic acid
- HODE
hydroxyoctadecadienoic acid
- HPODE
hydroperoxyoctadecadienoic acid
- LT
leukotrienes
- LRP
lipoprotein receptor-related protein
- LXA4
lipoxin A4
- LOX
lipoxygenase
- 12-LOX
12-lipoxygenase
- 15-LOX
15-lipoxygenase
- LC
liquid chromatography
- MS/MS
tandom mass spectrometry
- MAPK
mitogen-activated protein kinase
- NO
nitric oxide
- NDGA
nordihydroguaiaretic acid
- PD
Parkinson’s Disease
- PPAR
peroxisome proliferators-activated receptor
- PC
phosphatidylcoline
- PE
phosphatidylethanolamine
- PUFA
polyunsaturated fatty acid
- PD1
protectin D1
- PKC
protein kinase C
- RAS
renin-angiotensin system
- RvD1
resolvin D1
- TxA2
thromboxane
- TLR4
Toll-like receptor 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Lieb and Dobrian contributed equally to the work, and should be designated as co-first authors for publication
REFERENCES
- [1].Simopoulos AP. Human requirement for N-3 polyunsaturated fatty acids. Poult Sci. 2000;79:961–70. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- [2].Williams M, Nadler J. Hypertension Primer: The Essentials of High Blood Pressure: Basic Science, Population Science, and Clinical Management. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2007. Lipoxygenase products. [Google Scholar]
- [3].Canals S, Casarejos MJ, de Bernardo S, Rodriguez-Martin E, Mena MA. Nitric oxide triggers the toxicity due to glutathione depletion in midbrain cultures through 12-lipoxygenase. J Biol Chem. 2003;278:21542–9. doi: 10.1074/jbc.M213174200. [DOI] [PubMed] [Google Scholar]
- [4].Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, et al. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–62. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- [6].Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–8. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- [7].Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–82. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- [8].Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- [9].Chen XS, Funk CD. Structure-function properties of human platelet 12-lipoxygenase: chimeric enzyme and in vitro mutagenesis studies. Faseb J. 1993;7:694–701. doi: 10.1096/fasebj.7.8.8500694. [DOI] [PubMed] [Google Scholar]
- [10].Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68-69:263–90. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- [11].Chen XS, Kurre U, Jenkins NA, Copeland NG, Funk CD. cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J Biol Chem. 1994;269:13979–87. [PubMed] [Google Scholar]
- [12].Berger M, Schwarz K, Thiele H, Reimann I, Huth A, Borngraber S, et al. Simultaneous expression of leukocyte-type 12-lipoxygenase and reticulocyte-type 15-lipoxygenase in rabbits. J Mol Biol. 1998;278:935–48. doi: 10.1006/jmbi.1998.1737. [DOI] [PubMed] [Google Scholar]
- [13].Kuhn H, Chaitidis P, Roffeis J, Walther M. Arachidonic Acid metabolites in the cardiovascular system: the role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. J Cardiovasc Pharmacol. 2007;50:609–20. doi: 10.1097/FJC.0b013e318159f177. [DOI] [PubMed] [Google Scholar]
- [14].Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61:6307–12. [PubMed] [Google Scholar]
- [15].Sultana C, Shen Y, Rattan V, Kalra VK. Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte-like HL-60 cells is linked to protein kinase C activation. J Cell Physiol. 1996;167:477–87. doi: 10.1002/(SICI)1097-4652(199606)167:3<477::AID-JCP12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- [16].Natarajan R, Reddy MA, Malik KU, Fatima S, Khan BV. Signaling mechanisms of nuclear factor-kappab-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:1408–13. doi: 10.1161/hq0901.095278. [DOI] [PubMed] [Google Scholar]
- [17].Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J Biol Chem. 2002;277:9920–8. doi: 10.1074/jbc.M111305200. [DOI] [PubMed] [Google Scholar]
- [18].Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–59. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Levy BD. Resolvins and protectins: natural pharmacophores for resolution biology. Prostaglandins Leukot Essent Fatty Acids. 2010;82:327–32. doi: 10.1016/j.plefa.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wittwer J, Hersberger M. The two faces of the 15-lipoxygenase in atherosclerosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [21].Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–11. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- [23].Brinckmann R, Schnurr K, Heydeck D, Rosenbach T, Kolde G, Kuhn H. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 1998;91:64–74. [PubMed] [Google Scholar]
- [24].Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–56. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [25].Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW, et al. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem. 2007;282:20151–63. doi: 10.1074/jbc.M611776200. [DOI] [PubMed] [Google Scholar]
- [26].Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL, et al. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem. 2010;285:6891–903. doi: 10.1074/jbc.M109.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morgan AH, Dioszeghy V, Maskrey BH, Thomas CP, Clark SR, Mathie SA, et al. Phosphatidylethanolamine-esterified eicosanoids in the mouse: tissue localization and inflammation-dependent formation in Th-2 disease. J Biol Chem. 2009;284:21185–91. doi: 10.1074/jbc.M109.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kenyon V, Chorny I, Carvajal WJ, Holman TR, Jacobson MP. Novel human lipoxygenase inhibitors discovered using virtual screening with homology models. J Med Chem. 2006;49:1356–63. doi: 10.1021/jm050639j. [DOI] [PubMed] [Google Scholar]
- [29].O’Donnell VB, Maskrey B, Taylor GW. Eicosanoids: generation and detection in mammalian cells. Methods Mol Biol. 2009;462:5–23. [PubMed] [Google Scholar]
- [30].Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets. J Clin Endocrinol Metab. 2010;95:887–93. doi: 10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48:486–95. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- [32].Laybutt DR, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC. Genetic regulation of metabolic pathways in beta-cells disrupted by hyperglycemia. J Biol Chem. 2002;277:10912–21. doi: 10.1074/jbc.M111751200. [DOI] [PubMed] [Google Scholar]
- [33].Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, et al. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1993;90:4947–51. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tokuyama Y, Sturis J, DePaoli AM, Takeda J, Stoffel M, Tang J, et al. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–57. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- [35].Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, et al. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab. 2008;295:E1065–75. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, et al. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103:1431–6. doi: 10.1172/JCI5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57:199–208. doi: 10.2337/db07-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- [40].Zhao L, Cuff CA, Moss E, Wille U, Cyrus T, Klein EA, et al. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J Biol Chem. 2002;277:35350–6. doi: 10.1074/jbc.M205738200. [DOI] [PubMed] [Google Scholar]
- [41].Middleton MK, Rubinstein T, Pure E. Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J Immunol. 2006;176:265–74. doi: 10.4049/jimmunol.176.1.265. [DOI] [PubMed] [Google Scholar]
- [42].Middleton MK, Zukas AM, Rubinstein T, Kinder M, Wilson EH, Zhu P, et al. 12/15-lipoxygenase-dependent myeloid production of interleukin-12 is essential for resistance to chronic toxoplasmosis. Infect Immun. 2009;77:5690–700. doi: 10.1128/IAI.00560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bleich D, Chen S, Gu JL, Thomas L, Scott S, Gonzales N, et al. Interleukin-1 beta regulates the expression of a leukocyte type of 12-lipoxygenase in rat islets and RIN m5F cells. Endocrinology. 1995;136:5736–44. doi: 10.1210/endo.136.12.7588331. [DOI] [PubMed] [Google Scholar]
- [44].Bleich D, Chen S, Wen Y, Nadler JL. The stress-activated c-Jun protein kinase (JNK) is stimulated by lipoxygenase pathway product 12-HETE in RIN m5F cells. Biochem Biophys Res Commun. 1997;230:448–51. doi: 10.1006/bbrc.1996.5981. [DOI] [PubMed] [Google Scholar]
- [45].Prasad KM, Thimmalapura PR, Woode EA, Nadler JL. Evidence that increased 12-lipoxygenase expression impairs pancreatic beta cell function and viability. Biochem Biophys Res Commun. 2003;308:427–32. doi: 10.1016/s0006-291x(03)01418-9. [DOI] [PubMed] [Google Scholar]
- [46].Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C, et al. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys. 2007;468:114–20. doi: 10.1016/j.abb.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Radmark O. 5-lipoxygenase-derived leukotrienes: mediators also of atherosclerotic inflammation. Arterioscler Thromb Vasc Biol. 2003;23:1140–2. doi: 10.1161/01.ATV.0000082460.58448.01. [DOI] [PubMed] [Google Scholar]
- [48].Persaud SJ, Muller D, Belin VD, Kitsou-Mylona I, Asare-Anane H, Papadimitriou A, et al. The role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. Diabetes. 2007;56:197–203. doi: 10.2337/db06-0490. [DOI] [PubMed] [Google Scholar]
- [49].Han X, Chen S, Sun Y, Nadler JL, Bleich D. Induction of cyclooxygenase-2 gene in pancreatic beta-cells by 12-lipoxygenase pathway product 12-hydroxyeicosatetraenoic acid. Mol Endocrinol. 2002;16:2145–54. doi: 10.1210/me.2001-0300. [DOI] [PubMed] [Google Scholar]
- [50].Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–8. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- [51].Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–9. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- [52].Metz S, VanRollins M, Strife R, Fujimoto W, Robertson RP. Lipoxygenase pathway in islet endocrine cells. Oxidative metabolism of arachidonic acid promotes insulin release. J Clin Invest. 1983;71:1191–205. doi: 10.1172/JCI110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Metz SA. Glucose increases the synthesis of lipoxygenase-mediated metabolites of arachidonic acid in intact rat islets. Proc Natl Acad Sci U S A. 1985;82:198–202. doi: 10.1073/pnas.82.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Turk J, Colca JR, McDaniel ML. Arachidonic acid metabolism in isolated pancreatic islets. III. Effects of exogenous lipoxygenase products and inhibitors on insulin secretion. Biochim Biophys Acta. 1985;834:23–36. doi: 10.1016/0005-2760(85)90172-9. [DOI] [PubMed] [Google Scholar]
- [55].Shannon VR, Ramanadham S, Turk J, Holtzman MJ. Selective expression of an arachidonate 12-lipoxygenase by pancreatic islet beta-cells. Am J Physiol. 1992;263:E828–36. doi: 10.1152/ajpendo.1992.263.5.E828. [DOI] [PubMed] [Google Scholar]
- [56].Kawajiri H, Zhuang D, Qiao N, Yoshimoto T, Yamamoto M, Iseki S, et al. Expression of arachidonate 12-lipoxygenase in rat tissues: a possible role in glucagon secretion. J Histochem Cytochem. 2000;48:1411–9. doi: 10.1177/002215540004801011. [DOI] [PubMed] [Google Scholar]
- [57].Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- [58].Watcho P, Stavniichuk R, Ribnicky DM, Raskin I, Obrosova IG. High-fat diet-induced neuropathy of prediabetes and obesity: effect of PMI-5011, an ethanolic extract of Artemisia dracunculus L. Mediators Inflamm. 2010;2010:268547. doi: 10.1155/2010/268547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Stevens MJ, Nadler JL, et al. Role of 12/15-lipoxygenase in nitrosative stress and peripheral prediabetic and diabetic neuropathies. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Augustin AJ, Grus FH, Koch F, Spitznas M. Detection of eicosanoids in epiretinal membranes of patients suffering from proliferative vitreoretinal diseases. Br J Ophthalmol. 1997;81:58–60. doi: 10.1136/bjo.81.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schwartzman ML, Iserovich P, Gotlinger K, Bellner L, Dunn MW, Sartore M, et al. Profile of Lipid and Protein Autacoids in Diabetic Vitreous Correlates with the Progression of Diabetic Retinopathy. Diabetes. 2010;59:1780–8. doi: 10.2337/db10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57:1387–93. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Madsen L, Petersen RK, Sorensen MB, Jorgensen C, Hallenborg P, Pridal L, et al. Adipocyte differentiation of 3T3-L1 preadipocytes is dependent on lipoxygenase activity during the initial stages of the differentiation process. Biochem J. 2003;375:539–49. doi: 10.1042/bj20030503. [DOI] [PubMed] [Google Scholar]
- [64].Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–33. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- [65].Hausman GJ, Campion DR, Martin RJ. Search for the adipocyte precursor cell and factors that promote its differentiation. J Lipid Res. 1980;21:657–70. [PubMed] [Google Scholar]
- [66].Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- [67].Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- [68].Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- [69].Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–83. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- [70].Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–40. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- [71].Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–82. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- [72].Shappell SB, Gupta RA, Manning S, Whitehead R, Boeglin WE, Schneider C, et al. 15S-Hydroxyeicosatetraenoic acid activates peroxisome proliferator-activated receptor gamma and inhibits proliferation in PC3 prostate carcinoma cells. Cancer Res. 2001;61:497–503. [PubMed] [Google Scholar]
- [73].Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, et al. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–86. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- [74].Nugent C, Prins JB, Whitehead JP, Wentworth JM, Chatterjee VK, O’Rahilly S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2001;276:9149–57. doi: 10.1074/jbc.M009817200. [DOI] [PubMed] [Google Scholar]
- [75].Johnson EN, Nanney LB, Virmani J, Lawson JA, Funk CD. Basal transepidermal water loss is increased in platelet-type 12-lipoxygenase deficient mice. J Invest Dermatol. 1999;112:861–5. doi: 10.1046/j.1523-1747.1999.00595.x. [DOI] [PubMed] [Google Scholar]
- [76].Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-Lipoxygenase Products Induce Inflammation and Impair Insulin Signaling in 3T3-L1 Adipocytes. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sun D, Funk CD. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J Biol Chem. 1996;271:24055–62. [PubMed] [Google Scholar]
- [78].Hallenborg P, Jorgensen C, Petersen RK, Feddersen S, Araujo P, Markt P, et al. Epidermis-Type Lipoxygenase 3 Regulates Adipocyte Differentiation and Peroxisome Proliferator-Activated Receptor {gamma} Activity. Mol Cell Biol. 2010;30:4077–91. doi: 10.1128/MCB.01806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mackall JC, Student AK, Polakis SE, Lane MD. Induction of lipogenesis during differentiation in a “preadipocyte” cell line. J Biol Chem. 1976;251:6462–4. [PubMed] [Google Scholar]
- [80].Dyck DJ. Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 2009;34:396–402. doi: 10.1139/H09-037. [DOI] [PubMed] [Google Scholar]
- [81].Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Silva C, Rotellar F, et al. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J Mol Med. 2009;87:803–13. doi: 10.1007/s00109-009-0486-8. [DOI] [PubMed] [Google Scholar]
- [82].Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- [83].Zhang J, Wu Y, Zhang Y, Leroith D, Bernlohr DA, Chen X. The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol Endocrinol. 2008;22:1416–26. doi: 10.1210/me.2007-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–82. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high-fat diet in mice. Hypertension. 2010;55:715–21. doi: 10.1161/HYPERTENSIONAHA.109.148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kuhn H, Thiele BJ. The diversity of the lipoxygenase family. Many sequence data but little information on biological significance. FEBS Lett. 1999;449:7–11. doi: 10.1016/s0014-5793(99)00396-8. [DOI] [PubMed] [Google Scholar]
- [87].Noguchi N, Yamashita H, Hamahara J, Nakamura A, Kuhn H, Niki E. The specificity of lipoxygenase-catalyzed lipid peroxidation and the effects of radical-scavenging antioxidants. Biol Chem. 2002;383:619–26. doi: 10.1515/BC.2002.064. [DOI] [PubMed] [Google Scholar]
- [88].Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Top Med Chem. 2007;7:311–40. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- [89].Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–15. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;297:H495–507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Aggarwal NT, Holmes BB, Cui L, Viita H, Yla-Herttuala S, Campbell WB. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: role in arachidonic acid-induced relaxation. Am J Physiol Heart Circ Physiol. 2007;292:H1033–41. doi: 10.1152/ajpheart.00624.2006. [DOI] [PubMed] [Google Scholar]
- [94].Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–52. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-I is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arterioscler Thromb Vasc Biol. 2006;26:78–84. doi: 10.1161/01.ATV.0000191640.73313.ad. [DOI] [PubMed] [Google Scholar]
- [96].Sigal E, Grunberger D, Craik CS, Caughey GH, Nadel JA. Arachidonate 15-lipoxygenase (omega-6 lipoxygenase) from human leukocytes. Purification and structural homology to other mammalian lipoxygenases. J Biol Chem. 1988;263:5328–32. [PubMed] [Google Scholar]
- [97].Uski TK, Hogestatt ED. Effects of various cyclooxygenase and lipoxygenase metabolites on guinea-pig cerebral arteries. Gen Pharmacol. 1992;23:109–13. doi: 10.1016/0306-3623(92)90056-p. [DOI] [PubMed] [Google Scholar]
- [98].Rubbo H, O’Donnell V. Nitric oxide, peroxynitrite and lipoxygenase in atherogenesis: mechanistic insights. Toxicology. 2005;208:305–17. doi: 10.1016/j.tox.2004.11.019. [DOI] [PubMed] [Google Scholar]
- [99].Holzhutter HG, Wiesner R, Rathmann J, Stosser R, Kuhn H. A kinetic model for the interaction of nitric oxide with a mammalian lipoxygenase. Eur J Biochem. 1997;245:608–16. doi: 10.1111/j.1432-1033.1997.00608.x. [DOI] [PubMed] [Google Scholar]
- [100].Coffey MJ, Natarajan R, Chumley PH, Coles B, Thimmalapura PR, Nowell M, et al. Catalytic consumption of nitric oxide by 12/15- lipoxygenase: inhibition of monocyte soluble guanylate cyclase activation. Proc Natl Acad Sci U S A. 2001;98:8006–11. doi: 10.1073/pnas.141136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Anning PB, Coles B, Bermudez-Fajardo A, Martin PE, Levison BS, Hazen SL, et al. Elevated endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. Am J Pathol. 2005;166:653–62. doi: 10.1016/S0002-9440(10)62287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res. 2003;92:992–1000. doi: 10.1161/01.RES.0000070881.65194.8F. [DOI] [PubMed] [Google Scholar]
- [103].Pfister SL, Spitzbarth N, Edgemond W, Campbell WB. Vasorelaxation by an endothelium-derived metabolite of arachidonic acid. Am J Physiol. 1996;270:H1021–30. doi: 10.1152/ajpheart.1996.270.3.H1021. [DOI] [PubMed] [Google Scholar]
- [104].Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of the 11,14,15- and 11,12, 15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J Biol Chem. 1998;273:30879–87. doi: 10.1074/jbc.273.47.30879. [DOI] [PubMed] [Google Scholar]
- [105].Chawengsub Y, Gauthier KM, Nithipatikom K, Hammock BD, Falck JR, Narsimhaswamy D, et al. Identification of 13-hydroxy-14,15-epoxyeicosatrienoic acid as an acid-stable endothelium-derived hyperpolarizing factor in rabbit arteries. J Biol Chem. 2009;284:31280–90. doi: 10.1074/jbc.M109.025627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Moore SA, Spector AA, Hart MN. Eicosanoid metabolism in cerebromicrovascular endothelium. Am J Physiol. 1988;254:C37–44. doi: 10.1152/ajpcell.1988.254.1.C37. [DOI] [PubMed] [Google Scholar]
- [107].Zink MH, Oltman CL, Lu T, Katakam PV, Kaduce TL, Lee H, et al. 12-lipoxygenase in porcine coronary microcirculation: implications for coronary vasoregulation. Am J Physiol Heart Circ Physiol. 2001;280:H693–704. doi: 10.1152/ajpheart.2001.280.2.H693. [DOI] [PubMed] [Google Scholar]
- [108].Miller AW, Katakam PV, Lee HC, Tulbert CD, Busija DW, Weintraub NL. Arachidonic acid-induced vasodilation of rat small mesenteric arteries is lipoxygenase-dependent. J Pharmacol Exp Ther. 2003;304:139–44. doi: 10.1124/jpet.102.041780. [DOI] [PubMed] [Google Scholar]
- [109].Faraci FM, Sobey CG, Chrissobolis S, Lund DD, Heistad DD, Weintraub NL. Arachidonate dilates basilar artery by lipoxygenase-dependent mechanism and activation of K(+) channels. Am J Physiol Regul Integr Comp Physiol. 2001;281:R246–53. doi: 10.1152/ajpregu.2001.281.1.R246. [DOI] [PubMed] [Google Scholar]
- [110].Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–9. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ma YH, Harder DR, Clark JE, Roman RJ. Effects of 12-HETE on isolated dog renal arcuate arteries. Am J Physiol. 1991;261:H451–6. doi: 10.1152/ajpheart.1991.261.2.H451. [DOI] [PubMed] [Google Scholar]
- [112].Pfister SL, Spitzbarth N, Nithipatikom K, Falck JR, Campbell WB. Metabolism of 12-hydroperoxyeicosatetraenoic acid to vasodilatory trioxilin C3 by rabbit aorta. Biochim Biophys Acta. 2003;1622:6–13. doi: 10.1016/s0304-4165(03)00097-7. [DOI] [PubMed] [Google Scholar]
- [113].Laneuville O, Corey EJ, Couture R, Pace-Asciak CR. Hepoxilin A3 (HxA3) is formed by the rat aorta and is metabolized into HxA3-C, a glutathione conjugate. Biochim Biophys Acta. 1991;1084:60–8. doi: 10.1016/0005-2760(91)90056-n. [DOI] [PubMed] [Google Scholar]
- [114].Serhan CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- [115].Setty BN, Graeber JE, Stuart MJ. The mitogenic effect of 15- and 12-hydroxyeicosatetraenoic acid on endothelial cells may be mediated via diacylglycerol kinase inhibition. J Biol Chem. 1987;262:17613–22. [PubMed] [Google Scholar]
- [116].Rao GN, Baas AS, Glasgow WC, Eling TE, Runge MS, Alexander RW. Activation of mitogen-activated protein kinases by arachidonic acid and its metabolites in vascular smooth muscle cells. J Biol Chem. 1994;269:32586–91. [PubMed] [Google Scholar]
- [117].Dwarakanath RS, Sahar S, Reddy MA, Castanotto D, Rossi JJ, Natarajan R. Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-kappa B (NF-kappa B) J Mol Cell Cardiol. 2004;36:585–95. doi: 10.1016/j.yjmcc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [118].Ma J, Liang S, Wang Z, Zhang L, Jiang J, Zheng J, et al. ROCK pathway participates in the processes that 15-hydroxyeicosatetraenoic acid (15-HETE) mediated the pulmonary vascular remodeling induced by hypoxia in rat. J Cell Physiol. 2010;222:82–94. doi: 10.1002/jcp.21923. [DOI] [PubMed] [Google Scholar]
- [119].Stoll LL, Morland MR, Spector AA. 13-HODE increases intracellular calcium in vascular smooth muscle cells. Am J Physiol. 1994;266:C990–6. doi: 10.1152/ajpcell.1994.266.4.C990. [DOI] [PubMed] [Google Scholar]
- [120].Patricia MK, Natarajan R, Dooley AN, Hernandez F, Gu JL, Berliner JA, et al. Adenoviral delivery of a leukocyte-type 12 lipoxygenase ribozyme inhibits effects of glucose and platelet-derived growth factor in vascular endothelial and smooth muscle cells. Circ Res. 2001;88:659–65. doi: 10.1161/hh0701.088838. [DOI] [PubMed] [Google Scholar]
- [121].Chava KR, Karpurapu M, Wang D, Bhanoori M, Kundumani-Sridharan V, Zhang Q, et al. CREB-mediated IL-6 expression is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2009;29:809–15. doi: 10.1161/ATVBAHA.109.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Potula HS, Wang D, Quyen DV, Singh NK, Kundumani-Sridharan V, Karpurapu M, et al. Src-dependent STAT-3-mediated expression of monocyte chemoattractant protein-1 is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. J Biol Chem. 2009;284:31142–55. doi: 10.1074/jbc.M109.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Reddy MA, Kim YS, Lanting L, Natarajan R. Reduced growth factor responses in vascular smooth muscle cells derived from 12/15-lipoxygenase-deficient mice. Hypertension. 2003;41:1294–300. doi: 10.1161/01.HYP.0000069011.18333.08. [DOI] [PubMed] [Google Scholar]
- [124].Wang S, Wang Y, Jiang J, Wang R, Li L, Qiu Z, et al. 15-HETE protects rat pulmonary arterial smooth muscle cells from apoptosis via the PI3K/Akt pathway. Prostaglandins Other Lipid Mediat. 2010;91:51–60. doi: 10.1016/j.prostaglandins.2009.12.007. [DOI] [PubMed] [Google Scholar]
- [125].Hersberger M. Review: Potential role of the lipoxygenase derived lipid mediators in atherosclerosis: leukotrienes, lipoxins and resolvins. Clin Chem Lab Med. 2010 doi: 10.1515/CCLM.2010.212. [DOI] [PubMed] [Google Scholar]
- [126].Harats D, Shaish A, George J, Mulkins M, Kurihara H, Levkovitz H, et al. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2100–5. doi: 10.1161/01.atv.20.9.2100. [DOI] [PubMed] [Google Scholar]
- [127].Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, et al. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–8. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Trebus F, Heydeck D, Schimke I, Gerth C, Kuhn H. Transient experimental anemia in cholesterol-fed rabbits induces systemic overexpression of the reticulocyte-type 15-lipoxygenase and protects from aortic lipid deposition. Prostaglandins Leukot Essent Fatty Acids. 2002;67:419–28. doi: 10.1054/plef.2002.0452. [DOI] [PubMed] [Google Scholar]
- [129].Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, et al. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cyrus T, Pratico D, Zhao L, Witztum JL, Rader DJ, Rokach J, et al. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation. 2001;103:2277–82. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- [131].Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, et al. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–31. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- [132].Chiang N, Arita M, Serhan CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005;73:163–77. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- [133].Poeckel D, Zemski Berry KA, Murphy RC, Funk CD. Dual 12/15- and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in ApoE knock-out mice. J Biol Chem. 2009;284:21077–89. doi: 10.1074/jbc.M109.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Belkner J, Wiesner R, Rathman J, Barnett J, Sigal E, Kuhn H. Oxygenation of lipoproteins by mammalian lipoxygenases. Eur J Biochem. 1993;213:251–61. doi: 10.1111/j.1432-1033.1993.tb17755.x. [DOI] [PubMed] [Google Scholar]
- [135].Rankin SM, Parthasarathy S, Steinberg D. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J Lipid Res. 1991;32:449–56. [PubMed] [Google Scholar]
- [136].Sigari F, Lee C, Witztum JL, Reaven PD. Fibroblasts that overexpress 15-lipoxygenase generate bioactive and minimally modified LDL. Arterioscler Thromb Vasc Biol. 1997;17:3639–45. doi: 10.1161/01.atv.17.12.3639. [DOI] [PubMed] [Google Scholar]
- [137].Folcik VA, Aamir R, Cathcart MK. Cytokine modulation of LDL oxidation by activated human monocytes. Arterioscler Thromb Vasc Biol. 1997;17:1954–61. doi: 10.1161/01.atv.17.10.1954. [DOI] [PubMed] [Google Scholar]
- [138].Zhu H, Takahashi Y, Xu W, Kawajiri H, Murakami T, Yamamoto M, et al. Low density lipoprotein receptor-related protein-mediated membrane translocation of 12/15-lipoxygenase is required for oxidation of low density lipoprotein by macrophages. J Biol Chem. 2003;278:13350–5. doi: 10.1074/jbc.M212104200. [DOI] [PubMed] [Google Scholar]
- [139].Takahashi Y, Zhu H, Xu W, Murakami T, Iwasaki T, Hattori H, et al. Selective uptake and efflux of cholesteryl linoleate in LDL by macrophages expressing 12/15-lipoxygenase. Biochem Biophys Res Commun. 2005;338:128–35. doi: 10.1016/j.bbrc.2005.07.182. [DOI] [PubMed] [Google Scholar]
- [140].Nagelin MH, Srinivasan S, Lee J, Nadler JL, Hedrick CC. 12/15-Lipoxygenase activity increases the degradation of macrophage ATP-binding cassette transporter G1. Arterioscler Thromb Vasc Biol. 2008;28:1811–9. doi: 10.1161/ATVBAHA.108.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nagelin MH, Srinivasan S, Nadler JL, Hedrick CC. Murine 12/15-lipoxygenase regulates ATP-binding cassette transporter G1 protein degradation through p38- and JNK2-dependent pathways. J Biol Chem. 2009;284:31303–14. doi: 10.1074/jbc.M109.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Hersberger M, von Eckardstein A. Low high-density lipoprotein cholesterol: physiological background, clinical importance and drug treatment. Drugs. 2003;63:1907–45. doi: 10.2165/00003495-200363180-00003. [DOI] [PubMed] [Google Scholar]
- [143].Norata GD, Pirillo A, Catapano AL. Modified HDL: biological and physiopathological consequences. Nutr Metab Cardiovasc Dis. 2006;16:371–86. doi: 10.1016/j.numecd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- [144].Pirillo A, Uboldi P, Kuhn H, Catapano AL. 15-Lipoxygenase-mediated modification of high-density lipoproteins impairs SR-BI- and ABCA1-dependent cholesterol efflux from macrophages. Biochim Biophys Acta. 2006;1761:292–300. doi: 10.1016/j.bbalip.2006.03.009. [DOI] [PubMed] [Google Scholar]
- [145].Pirillo A, Uboldi P, Bolego C, Kuhn H, Catapano AL. The 15-lipoxygenase-modified high density lipoproteins 3 fail to inhibit the TNF-alpha-induced inflammatory response in human endothelial cells. J Immunol. 2008;181:2821–30. doi: 10.4049/jimmunol.181.4.2821. [DOI] [PubMed] [Google Scholar]
- [146].Kim JA, Gu JL, Natarajan R, Berliner JA, Nadler JL. A leukocyte type of 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Biol. 1995;15:942–8. doi: 10.1161/01.atv.15.7.942. [DOI] [PubMed] [Google Scholar]
- [147].Reilly KB, Srinivasan S, Hatley ME, Patricia MK, Lannigan J, Bolick DT, et al. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem. 2004;279:9440–50. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- [148].Wolle J, Welch KA, Devall LJ, Cornicelli JA, Saxena U. Transient overexpression of human 15-lipoxygenase in aortic endothelial cells enhances tumor necrosis factor-induced vascular cell adhesion molecule-1 gene expression. Biochem Biophys Res Commun. 1996;220:310–4. doi: 10.1006/bbrc.1996.0402. [DOI] [PubMed] [Google Scholar]
- [149].Viita H, Sen CK, Roy S, Siljamaki T, Nikkari T, Yla-Herttuala S. High expression of human 15-lipoxygenase induces NF-kappaB-mediated expression of vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and T-cell adhesion on human endothelial cells. Antioxid Redox Signal. 1999;1:83–96. doi: 10.1089/ars.1999.1.1-83. [DOI] [PubMed] [Google Scholar]
- [150].Bolick DT, Orr AW, Whetzel A, Srinivasan S, Hatley ME, Schwartz MA, et al. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:2301–7. doi: 10.1161/01.ATV.0000186181.19909.a6. [DOI] [PubMed] [Google Scholar]
- [151].Rydberg EK, Krettek A, Ullstrom C, Ekstrom K, Svensson PA, Carlsson LM, et al. Hypoxia increases LDL oxidation and expression of 15-lipoxygenase-2 in human macrophages. Arterioscler Thromb Vasc Biol. 2004;24:2040–5. doi: 10.1161/01.ATV.0000144951.08072.0b. [DOI] [PubMed] [Google Scholar]
- [152].Danielsson KN, Rydberg EK, Ingelsten M, Akyurek LM, Jirholt P, Ullstrom C, et al. 15-Lipoxygenase-2 expression in human macrophages induces chemokine secretion and T cell migration. Atherosclerosis. 2008;199:34–40. doi: 10.1016/j.atherosclerosis.2007.10.027. [DOI] [PubMed] [Google Scholar]
- [153].Smith RJ, Justen JM, Nidy EG, Sam LM, Bleasdale JE. Transmembrane signaling in human polymorphonuclear neutrophils: 15(S)-hydroxy-(5Z,8Z,11Z,13E)-eicosatetraenoic acid modulates receptor agonist-triggered cell activation. Proc Natl Acad Sci U S A. 1993;90:7270–4. doi: 10.1073/pnas.90.15.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Takata S, Papayianni A, Matsubara M, Jimenez W, Pronovost PH, Brady HR. 15-Hydroxyeicosatetraenoic acid inhibits neutrophil migration across cytokine-activated endothelium. Am J Pathol. 1994;145:541–9. [PMC free article] [PubMed] [Google Scholar]
- [155].Takata S, Matsubara M, Allen PG, Janmey PA, Serhan CN, Brady HR. Remodeling of neutrophil phospholipids with 15(S)-hydroxyeicosatetraenoic acid inhibits leukotriene B4-induced neutrophil migration across endothelium. J Clin Invest. 1994;93:499–508. doi: 10.1172/JCI116999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Buchanan MR, Bertomeu MC, Haas TA, Orr FW, Eltringham-Smith LL. Localization of 13-hydroxyoctadecadienoic acid and the vitronectin receptor in human endothelial cells and endothelial cell/platelet interactions in vitro. Blood. 1993;81:3303–12. [PubMed] [Google Scholar]
- [157].Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- [158].Natarajan R, Gerrity RG, Gu JL, Lanting L, Thomas L, Nadler JL. Role of 12-lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia. 2002;45:125–33. doi: 10.1007/s125-002-8253-x. [DOI] [PubMed] [Google Scholar]
- [159].Pei H, Gu J, Thimmalapura PR, Mison A, Nadler JL. Activation of the 12-lipoxygenase and signal transducer and activator of transcription pathway during neointima formation in a model of the metabolic syndrome. Am J Physiol Endocrinol Metab. 2006;290:E92–E102. doi: 10.1152/ajpendo.00133.2005. [DOI] [PubMed] [Google Scholar]
- [160].Gu JL, Veerapanane D, Rossi J, Natarajan R, Thomas L, Nadler J. Ribozyme-mediated inhibition of expression of leukocyte-type 12-lipoxygenase in porcine aortic vascular smooth muscle cells. Circ Res. 1995;77:14–20. doi: 10.1161/01.res.77.1.14. [DOI] [PubMed] [Google Scholar]
- [161].Wen Y, Gu J, Peng X, Zhang G, Nadler J. Overexpression of 12-lipoxygenase and cardiac fibroblast hypertrophy. Trends Cardiovasc Med. 2003;13:129–36. doi: 10.1016/s1050-1738(03)00027-6. [DOI] [PubMed] [Google Scholar]
- [162].Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- [163].Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–7. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- [165].Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–46. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [167].Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- [168].Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. Faseb J. 2008;22:3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–7. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- [170].Lee JH, O’Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–32. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- [171].Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, et al. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991;87:1146–52. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kuhn H, Heydeck D, Hugou I, Gniwotta C. In vivo action of 15-lipoxygenase in early stages of human atherogenesis. J Clin Invest. 1997;99:888–93. doi: 10.1172/JCI119253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Hulten LM, Olson FJ, Aberg H, Carlsson J, Karlstrom L, Boren J, et al. 15-Lipoxygenase-2 is expressed in macrophages in human carotid plaques and regulated by hypoxia-inducible factor-1alpha. Eur J Clin Invest. 2010;40:11–7. doi: 10.1111/j.1365-2362.2009.02223.x. [DOI] [PubMed] [Google Scholar]
- [174].Wittwer J, Bayer M, Mosandl A, Muntwyler J, Hersberger M. The c.-292C>T promoter polymorphism increases reticulocyte-type 15-lipoxygenase-1 activity and could be atheroprotective. Clin Chem Lab Med. 2007;45:487–92. doi: 10.1515/CCLM.2007.103. [DOI] [PubMed] [Google Scholar]
- [175].Assimes TL, Knowles JW, Priest JR, Basu A, Borchert A, Volcik KA, et al. A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis. 2008;198:136–44. doi: 10.1016/j.atherosclerosis.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Burdon KP, Rudock ME, Lehtinen AB, Langefeld CD, Bowden DW, Register TC, et al. Human lipoxygenase pathway gene variation and association with markers of subclinical atherosclerosis in the diabetes heart study. Mediators Inflamm. 2010;2010:170153. doi: 10.1155/2010/170153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Kelavkar UP, Nixon JB, Cohen C, Dillehay D, Eling TE, Badr KF. Overexpression of 15-lipoxygenase-1 in PC-3 human prostate cancer cells increases tumorigenesis. Carcinogenesis. 2001;22:1765–73. doi: 10.1093/carcin/22.11.1765. [DOI] [PubMed] [Google Scholar]
- [178].Viita H, Markkanen J, Eriksson E, Nurminen M, Kinnunen K, Babu M, et al. 15-lipoxygenase-1 prevents vascular endothelial growth factor A- and placental growth factor-induced angiogenic effects in rabbit skeletal muscles via reduction in growth factor mRNA levels, NO bioactivity, and downregulation of VEGF receptor 2 expression. Circ Res. 2008;102:177–84. doi: 10.1161/CIRCRESAHA.107.155556. [DOI] [PubMed] [Google Scholar]
- [179].Harats D, Ben-Shushan D, Cohen H, Gonen A, Barshack I, Goldberg I, et al. Inhibition of carcinogenesis in transgenic mouse models over-expressing 15-lipoxygenase in the vascular wall under the control of murine preproendothelin-1 promoter. Cancer Lett. 2005;229:127–34. doi: 10.1016/j.canlet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- [180].Viita H, Kinnunen K, Eriksson E, Lahteenvuo J, Babu M, Kalesnykas G, et al. Intravitreal adenoviral 15-lipoxygenase-1 gene transfer prevents vascular endothelial growth factor A-induced neovascularization in rabbit eyes. Hum Gene Ther. 2009;20:1679–86. doi: 10.1089/hum.2009.069. [DOI] [PubMed] [Google Scholar]
- [181].Schneider M, Wortmann M, Mandal PK, Arpornchayanon W, Jannasch K, Alves F, et al. Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia. 2010;12:254–63. doi: 10.1593/neo.91782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Krishnamoorthy S, Jin R, Cai Y, Maddipati KR, Nie D, Pages G, et al. 12-Lipoxygenase and the regulation of hypoxia-inducible factor in prostate cancer cells. Exp Cell Res. 2010;316:1706–15. doi: 10.1016/j.yexcr.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Kundumani-Sridharan V, Niu J, Wang D, Van Quyen D, Zhang Q, Singh NK, et al. 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression. Blood. 2010;115:2105–16. doi: 10.1182/blood-2009-09-241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010;176:74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Imig JD. Eicosanoids and renal vascular function in diseases. Clin Sci (Lond) 2006;111:21–34. doi: 10.1042/CS20050251. [DOI] [PubMed] [Google Scholar]
- [186].Hao CM, Breyer MD. Roles of lipid mediators in kidney injury. Semin Nephrol. 2007;27:338–51. doi: 10.1016/j.semnephrol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- [187].Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007;71:1105–15. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- [188].Yiu SS, Zhao X, Inscho EW, Imig JD. 12-Hydroxyeicosatetraenoic acid participates in angiotensin II afferent arteriolar vasoconstriction by activating L-type calcium channels. J Lipid Res. 2003;44:2391–9. doi: 10.1194/jlr.M300183-JLR200. [DOI] [PubMed] [Google Scholar]
- [189].Katoh T, Takahashi K, DeBoer DK, Serhan CN, Badr KF. Renal hemodynamic actions of lipoxins in rats: a comparative physiological study. Am J Physiol. 1992;263:F436–42. doi: 10.1152/ajprenal.1992.263.3.F436. [DOI] [PubMed] [Google Scholar]
- [190].Saito F, Hori MT, Ideguchi Y, Berger M, Golub M, Stern N, et al. 12-Lipoxygenase products modulate calcium signals in vascular smooth muscle cells. Hypertension. 1992;20:138–43. doi: 10.1161/01.hyp.20.2.138. [DOI] [PubMed] [Google Scholar]
- [191].Xu ZG, Miao LN, Cui YC, Jia Y, Yuan H, Wu M. Angiotensin II type 1 receptor expression is increased via 12-lipoxygenase in high glucose-stimulated glomerular cells and type 2 diabetic glomeruli. Nephrol Dial Transplant. 2009;24:1744–52. doi: 10.1093/ndt/gfn703. [DOI] [PubMed] [Google Scholar]
- [192].Coffey MJ, Coles B, O’Donnell VB. Interactions of nitric oxide-derived reactive nitrogen species with peroxidases and lipoxygenases. Free Radic Res. 2001;35:447–64. doi: 10.1080/10715760100301471. [DOI] [PubMed] [Google Scholar]
- [193].Nadler JL, Goodson S, Tuck M, Stern N. Role of lipoxygenase metabolites in angiotensin-induced aldosterone synthesis. Adv Prostaglandin Thromboxane Leukot Res. 1987;17B:701–3. [PubMed] [Google Scholar]
- [194].Nadler JL, Natarajan R, Stern N. Specific action of the lipoxygenase pathway in mediating angiotensin II-induced aldosterone synthesis in isolated adrenal glomerulosa cells. J Clin Invest. 1987;80:1763–9. doi: 10.1172/JCI113269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Limor R, Kaplan M, Sharon O, Knoll E, Naidich M, Weisinger G, et al. Aldosterone up-regulates 12- and 15-lipoxygenase expression and LDL oxidation in human vascular smooth muscle cells. J Cell Biochem. 2009;108:1203–10. doi: 10.1002/jcb.22352. [DOI] [PubMed] [Google Scholar]
- [196].Gonzalez-Nunez D, Claria J, Rivera F, Poch E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 2001;37:334–8. doi: 10.1161/01.hyp.37.2.334. [DOI] [PubMed] [Google Scholar]
- [197].Quintana LF, Guzman B, Collado S, Claria J, Poch E. A coding polymorphism in the 12-lipoxygenase gene is associated to essential hypertension and urinary 12(S)-HETE. Kidney Int. 2006;69:526–30. doi: 10.1038/sj.ki.5000147. [DOI] [PubMed] [Google Scholar]
- [198].Chang WC, Su GW. Increase in 12-lipoxygenase activity in platelets of spontaneously hypertensive rats. Biochem Biophys Res Commun. 1985;127:642–8. doi: 10.1016/s0006-291x(85)80209-6. [DOI] [PubMed] [Google Scholar]
- [199].Sasaki M, Hori MT, Hino T, Golub MS, Tuck ML. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens. 1997;10:371–8. [PubMed] [Google Scholar]
- [200].Nozawa K, Tuck ML, Golub M, Eggena P, Nadler JL, Stern N. Inhibition of lipoxygenase pathway reduces blood pressure in renovascular hypertensive rats. Am J Physiol. 1990;259:H1774–80. doi: 10.1152/ajpheart.1990.259.6.H1774. [DOI] [PubMed] [Google Scholar]
- [201].DelliPizzi A, Guan H, Tong X, Takizawa H, Nasjletti A. Lipoxygenase-dependent mechanisms in hypertension. Clin Exp Hypertens. 2000;22:181–92. doi: 10.1081/ceh-100100071. [DOI] [PubMed] [Google Scholar]
- [202].Gonzalez-Nunez D, Sole M, Natarajan R, Poch E. 12-Lipoxygenase metabolism in mouse distal convoluted tubule cells. Kidney Int. 2005;67:178–86. doi: 10.1111/j.1523-1755.2005.00068.x. [DOI] [PubMed] [Google Scholar]
- [203].Xu ZG, Li SL, Lanting L, Kim YS, Shanmugam N, Reddy MA, et al. Relationship between 12/15-lipoxygenase and COX-2 in mesangial cells: potential role in diabetic nephropathy. Kidney Int. 2006;69:512–9. doi: 10.1038/sj.ki.5000137. [DOI] [PubMed] [Google Scholar]
- [204].Kang SW, Natarajan R, Shahed A, Nast CC, LaPage J, Mundel P, et al. Role of 12-lipoxygenase in the stimulation of p38 mitogen-activated protein kinase and collagen alpha5(IV) in experimental diabetic nephropathy and in glucose-stimulated podocytes. J Am Soc Nephrol. 2003;14:3178–87. doi: 10.1097/01.asn.0000099702.16315.de. [DOI] [PubMed] [Google Scholar]
- [205].Kang SW, Adler SG, Nast CC, LaPage J, Gu JL, Nadler JL, et al. 12-lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int. 2001;59:1354–62. doi: 10.1046/j.1523-1755.2001.0590041354.x. [DOI] [PubMed] [Google Scholar]
- [206].Antonipillai I, Nadler J, Vu EJ, Bughi S, Natarajan R, Horton R. A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab. 1996;81:1940–5. doi: 10.1210/jcem.81.5.8626861. [DOI] [PubMed] [Google Scholar]
- [207].Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, et al. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:2615–22. doi: 10.1161/01.atv.19.11.2615. [DOI] [PubMed] [Google Scholar]
- [208].Kim YS, Xu ZG, Reddy MA, Li SL, Lanting L, Sharma K, et al. Novel interactions between TGF-{beta}1 actions and the 12/15-lipoxygenase pathway in mesangial cells. J Am Soc Nephrol. 2005;16:352–62. doi: 10.1681/ASN.2004070568. [DOI] [PubMed] [Google Scholar]
- [209].Kim YS, Reddy MA, Lanting L, Adler SG, Natarajan R. Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney Int. 2003;64:1702–14. doi: 10.1046/j.1523-1755.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- [210].McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol Renal Physiol. 2004;286:F189–201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- [211].Kieran NE, Maderna P, Godson C. Lipoxins: potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–54. doi: 10.1111/j.1523-1755.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- [212].Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol. 1996;156:2264–72. [PubMed] [Google Scholar]
- [213].McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C. Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. Faseb J. 2002;16:1817–9. doi: 10.1096/fj.02-0416fje. [DOI] [PubMed] [Google Scholar]
- [214].McMahon B, Stenson C, McPhillips F, Fanning A, Brady HR, Godson C. Lipoxin A4 antagonizes the mitogenic effects of leukotriene D4 in human renal mesangial cells. Differential activation of MAP kinases through distinct receptors. J Biol Chem. 2000;275:27566–75. doi: 10.1074/jbc.M001015200. [DOI] [PubMed] [Google Scholar]
- [215].Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, et al. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–92. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- [216].Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, et al. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci U S A. 1999;96:13375–80. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Leonard MO, Hannan K, Burne MJ, Lappin DW, Doran P, Coleman P, et al. 15-Epi-16-(para-fluorophenoxy)-lipoxin A(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13:1657–62. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- [218].Hassan IR, Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J Immunol. 2009;182:3223–32. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- [219].Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- [220].Nakagomi T, Sasaki T, Kirino T, Tamura A, Noguchi M, Saito I, et al. Effect of cyclooxygenase and lipoxygenase inhibitors on delayed neuronal death in the gerbil hippocampus. Stroke. 1989;20:925–9. doi: 10.1161/01.str.20.7.925. [DOI] [PubMed] [Google Scholar]
- [221].van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–8. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- [222].Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–91. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- [223].Zhang Y, Wang H, Li J, Jimenez DA, Levitan ES, Aizenman E, et al. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24:10616–27. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, et al. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–67. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004;23:2785–96. doi: 10.1038/sj.onc.1207517. [DOI] [PubMed] [Google Scholar]
- [226].Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–43. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Lebeau A, Terro F, Rostene W, Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ. 2004;11:875–84. doi: 10.1038/sj.cdd.4401395. [DOI] [PubMed] [Google Scholar]
- [228].Yao Y, Clark CM, Trojanowski JQ, Lee VM, Pratico D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann Neurol. 2005;58:623–6. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- [229].Yang H, Zhuo JM, Chu J, Chinnici C, Pratico D. Amelioration of the Alzheimer’s Disease Phenotype by Absence of 12/15-Lipoxygenase. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- [230].Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–63. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- [231].Canals S, Casarejos MJ, de Bernardo S, Rodriguez-Martin E, Mena MA. Glutathione depletion switches nitric oxide neurotrophic effects to cell death in midbrain cultures: implications for Parkinson’s disease. J Neurochem. 2001;79:1183–95. doi: 10.1046/j.1471-4159.2001.00635.x. [DOI] [PubMed] [Google Scholar]
- [232].Rai G, Kenyon V, Jadhav A, Schultz L, Armstrong M, Jameson JB, et al. Discovery of Potent and Selective Inhibitors of Human Reticulocyte 15-Lipoxygenase-1. J Med Chem. 2010 doi: 10.1021/jm1008852. [DOI] [PMC free article] [PubMed] [Google Scholar]