Abstract
The retinoblastoma protein (Rb) inhibits both cell division and apoptosis, but the mechanism by which Rb alternatively regulates these divergent outcomes remains poorly understood. Cyclin dependent kinases (Cdks) promote cell division by phosphorylating and reversibly inactivating Rb by a hierarchical series of phosphorylation events and sequential conformational changes. The stress-regulated mitogen activated protein kinase (MAPK) p38 also phosphorylates Rb, but it does so in a cell cycle-independent manner that is associated with apoptosis rather than with cell division. Here, we show that p38 phosphorylates Rb by a novel mechanism that is distinct from that of Cdks. p38 bypasses the cell cycle-associated hierarchical phosphorylation and directly phosphorylates Rb on Ser567, which is not phosphorylated during the normal cell cycle. Phosphorylation by p38, but not Cdks, triggers an interaction between Rb and the human homologue of murine double minute 2 (Hdm2), leading to degradation of Rb, release of E2F1 and cell death. These findings provide a mechanistic explanation for how Rb regulates cell division and apoptosis through different kinases, and reveal how Hdm2 may functionally link the tumor suppressors Rb and p53.
Keywords: apoptosis, p38, phosphorylation, retinoblastoma protein, Hdm2
Introduction
Rb functions as a tumor suppressor in part by inhibiting cell division (Goodrich et al, 1991; Knudsen et al, 1998). In order for cells to divide, Rb is temporarily inactivated by Cdks, which phosphorylate Rb at up to 16 Ser/Thr-Pro phospho-acceptor sites, thereby allowing cells to traverse from G1 into S phase (Chen et al, 1989; Hinds et al, 1992; Lin et al, 1991; Lundberg & Weinberg, 1998; Serrano et al, 1993). Most tumors co-opt this mechanism and maintain Rb in a partially hyperphosphorylated state, often by constitutive activation of Cdks or inactivation of Cdk inhibitors such as p16Ink4a (Sherr & McCormick, 2002). Rb also inhibits apoptosis (Chau & Wang, 2003; Harbour & Dean, 2000), with loss of Rb leading to p53-dependent and independent apoptosis in the nervous system, lens and melanocytes and other tissues (Jacks et al, 1992; Lee et al, 1992; Macleod et al, 1996; Morgenbesser et al, 1994; Wu et al, 2003).
The anti-proliferative and anti-apoptotic functions of Rb can be biochemically uncoupled and are both mediated largely through interactions with E2F transcription factors (E2Fs) (Chau et al, 2006). E2Fs activate genes involved in cell division and apoptosis (Stevaux & Dyson, 2002). Rb inhibits the E2F transactivation function by directly binding and masking the transactivation domain and by recruiting chromatin remodeling factors to alter local chromatin structure to an inhibitory state (Almasan et al, 1995; Chellappan et al, 1991; Dyson, 1998; Hsieh et al, 1997; Irwin et al, 2000; Morgenbesser et al, 1994; Nevins, 1998; Qin et al, 1994; Shan & Lee, 1994; Tsai et al, 1998). Phosphorylation of Rb was originally thought to drive cell cycle progression simply by releasing E2Fs to activate cell cycle genes. However, this model fails to explain how cells inactivate Rb and release E2Fs in order to divide without also triggering apoptosis by activating E2F-bound pro-apoptotic genes. Towards a potential explanation, recent observations suggest that Rb exists not only in fully active and fully inactive forms, but rather, it can exist in multiple phosphorylation states that have differing abilities to bind E2Fs and other proteins, and to inhibit cell division and apoptosis (Lundberg & Weinberg, 1998; Ma et al, 2003; Ianari et al, 2009). Partially phosphorylated forms of Rb bind E2Fs (Ezhevsky et al, 2001; Ezhevsky et al, 1997), and Rb continues to co-localize with E2Fs at certain promoters in vivo beyond the G1/S transition despite being sufficiently phosphorylated to allow for cell cycle progression (Wells et al, 2000; Wells et al, 2003). The different phosphorylated forms of Rb appear to allow the cell to differentially control cell cycle genes and apoptotic genes, the former being derepressed by partial phosphorylation of Rb and the latter being activated by more complete phosphorylation of Rb (Young & Longmore, 2004; Young et al, 2003; Zhang et al, 2000). Taken together, these observations indicate that the decision between proliferation and apoptosis is made, at least in part, by the manner in which Rb is phosphorylated.
These findings raise a key question: if Cdks do not completely phosphorylate nor fully inactivate Rb during the normal cell cycle, then under what physiologic conditions and by what mechanism does complete inactivation of Rb and concomitant activation of apoptotic genes occur? In recent years other kinases capable of phosphorylating Rb have been identified, such as p38, which is activated during cellular stress and promotes apoptosis (Wang et al, 1999; Nath et al, 2003; Hou et al, 2002). p38 has been shown to phosphorylate Rb in response to stress and death receptor signaling in multiple cell types, such as endothelial cells, cerebellar neurons, Jurkat lymphocytic cells, colon cancer cells, and melanoma cells (Hou et al, 2002; Kishore et al, 2003; Lee et al, 2003; Nath et al, 2003; Yeste-Velasco et al, 2009; Wang et al, 1999). Rather than promoting cell division, phosphorylation of Rb by p38 appears to promote apoptosis under physiologic conditions of cellular stress (Bowen et al, 2002). While the ability of p38 to phosphorylate Rb has been firmly established, it remains unclear why p38 favors apoptosis whereas Cdks promote cell division. One possibility is that p38 preferentially phosphorylates a different site or sites on Rb that confer different biochemical and, thus, physiologic effects.
In this study, we sought to determine mechanistically how phosphorylation of Rb by p38 differs from that of Cdks and why this results in such a dramatically different physiologic effect (apoptosis versus cell division, respectively). We show that residue Ser567 on Rb is preferentially phosphorylated by p38, but not Cdk2, in response to genotoxic stress. This phosphorylation triggers an interaction between Rb and Hdm2, which in turn leads to degradation of Rb, release of E2Fs, and induction of apoptosis. This response to genotoxic stress can be prevented by inhibition of p38. Our findings provide a potential mechanism linking Hdm2 with the two tumor suppressors p53 and Rb in a tightly controlled cellular response to genotoxic stress.
Results
Loss of Rb leads to apoptosis
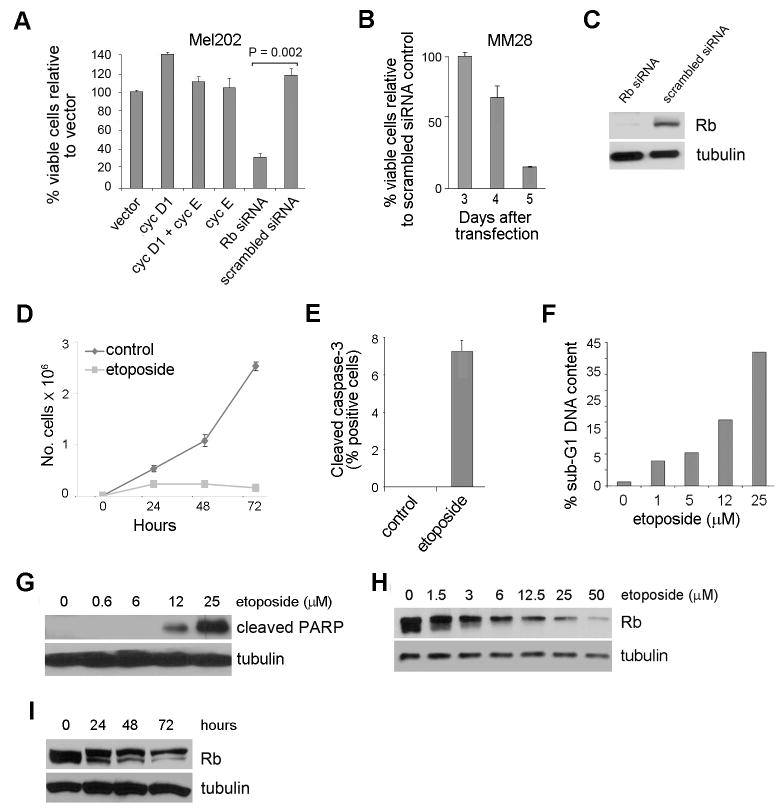
Functional and reversible inactivation of Rb by Cdk-mediated hyper-phosphorylation, as occurs during normal cell division and in most proliferating cancer cells, did not induce cell death in melanoma cells (Figure 1A). In contrast, complete and irreversible inactivation of Rb by RNAi-mediated depletion of the protein induced cell death both in the Mel202 melanoma cell line and in primary melanoma cells (Figure 1A-C). This is consistent with previous work demonstrating that specifically knocking out Rb from melanocytes led to melanocyte cell death (Yu et al, 2003). To explore why these mechanisms for inactivating Rb that have been assumed to be functionally equivalent result in such strikingly different phenotypic outcomes, we developed an experimental model system in which Rb degradation and apoptosis were induced by genotoxic stress in Mel202 cells, which have been extensively characterized and shown to be wildtype for Rb and p53 (Sun et al, 2005). At low concentrations, the DNA damaging agent etoposide resulted in stabilization of p53, hypophosphorylation of Rb, and cell cycle arrest as expected (data not shown) (Strobeck et al, 2000; Valentini et al, 2007), but at slightly higher concentrations, etoposide induced apoptosis in a dose-dependent manner (Figure 1D-G). This apoptosis was accompanied by hyperphosphorylation and degradation of Rb, with over half of the cellular Rb depleted by 72 h (Figure 1H-I). This effect was also seen in other cells such as primary human melanocytes, OCM1A and 92.1uveal melanoma cells, A375 cutaneous melanoma cells, MCF-7 and F-MB-231 breast cancer cells, U2OS osteosarcoma cells, and 10T/2 mouse fibroblasts (data not shown).
Figure 1.
Loss of Rb leads to apoptosis. (A) Mel202 cells were transfected with scrambled siRNA or Rb siRNA, or with vectors expressing cyclin D1 and/or cyclin E to activate endogenous Cdks for 4 days and cell viability assays were performed. (B) Cell viability assays were performed in MM28 primary melanoma cells using scrambled siRNA or Rb siRNA. (C) Immunoblot analysis confirming effective knock down of Rb with siRNA in Mel202s after 4 days. Tubulin was used as a loading control in all immunoblot experiments. (D) 12.5 μM of etoposide treatment halted cell growth in Mel202 cells as shown by growth curves. (E) Cleaved caspase 3 staining of Mel202 cells treated with or without 12.5 μM of etoposide for 48 h. (F) Flow cytometry was performed using Mel202 cells treated with 0, 1, 5, 12, 25 μM etoposide for 48 h. (G) Mel202 cells were treated with 0, 0.6, 6, 12, 25 μM etoposide for 48 h and immunoblotted for cleaved PARP. (H) Mel202 cells were treated with 0, 1.5, 3, 6, 12.5, 25, 50 μM etoposide for 48 h and immunoblotted for Rb. (I) Mel202 cells were treated with 12.5 μM etoposide for 0, 24, 48, 72 h and immunoblotted for Rb.
Genotoxic stress triggers phosphorylation of Rb on Ser567 by p38
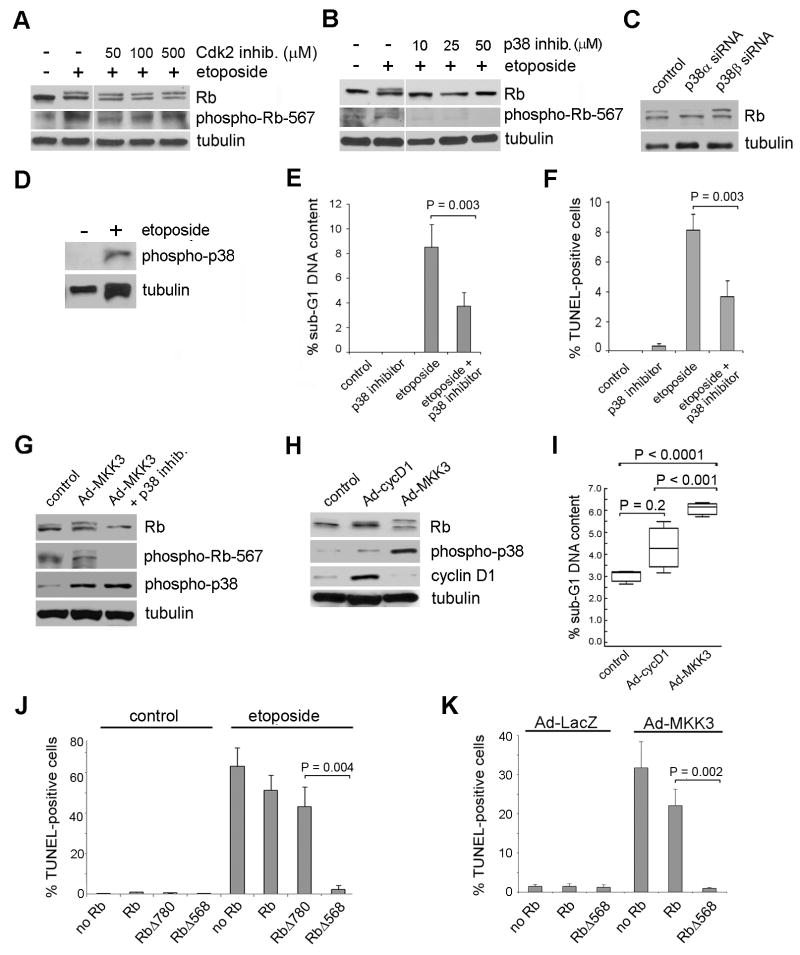
To determine which kinase catalyzes phosphorylation of Rb in response to this genotoxic stress, we started by evaluating Cdks that phosphorylate Rb during cell division. Inhibitors of Cdk2 and Cdk4 did not block Rb hyperphosphorylation induced by etoposide (Figure 2A and Supplemental Figure 1A). This led us to hypothesize that p38, which is activated by etoposide, might mediate this phosphorylation (Pillaire et al, 2000). Indeed, inhibition of p38 with a specific inhibitor potently blocked Rb phosphorylation (Figure 2B) in response to etoposide. Further, knock down of p38α, but not p38β, by RNAi blocked the etoposide-induced Rb phosphorylation (Figure 2C). Consistent with these findings, p38 underwent activating phosphorylation in response to etoposide treatment (Figure 2D). Importantly, chemical inhibition of p38 substantially reduced etoposide-induced apoptosis (Figure 2E-F). Direct activation of endogenous p38 by adenoviral expression of the upstream effector MKK3 (Ad-MKK3) triggered phosphorylation and degradation of Rb and induced apoptosis in a manner that was indistinguishable from etoposide treatment (Figure 2G-I, 2K). In contrast, activation of endogenous Cdk by adenoviral expression of cyclin D1 (Ad-cycD1) did not induce phosphorylation or degradation of Rb and it did not result in a significant increase in apoptosis (Figure 2H-I). A Ser567-phospho-specific Rb antibody showed that this site was phosphorylated in cells in response to either etoposide treatment (Figure 2A-B) or Ad-MKK3 (Figure 2G). Moreover, this phosphorylation could be blocked by a p38 inhibitor but not by Cdk inhibitors. We next wished to determine whether a mutant defective in Ser567 phosphorylation would be resistant to stress-induced apoptosis. The Ser567 phosphoacceptor site is unique in that it mimics, rather than blocks, Ser567 phosphorylation, so this mutant could not be used to assess how blocking Ser567 phosphorylation affects Rb function. Instead, we previously generated an Rb mutant in which proline568 was substituted with an alanine (RbΔ568), and showed that this mutant remained partially active while blocking Ser567 phosphorylation (Harbour et al, 1999; Ma et al, 2003). Ectopic expression of RbΔ568, but not wildtype Rb or an Rb mutant in which Ser780 was substituted with an alanine preventing phosphorylation of Ser780 (RbΔ780), inhibited apoptosis in cells treated with etoposide (Figure 2J) and cells treated with Ad-MKK3 (Figure 2K), indicating that phosphorylation of Ser567 is specifically required for apoptosis.
Figure 2.
Genotoxic stress triggers phosphorylation of Rb on Ser567 by p38. (A) Mel202 cells were treated with 12.5 μM of etoposide and 0, 50, 100, or 500 μM of the Cdk2 inhibitor, Roscovitine, for 48 h and then immunoblotted for total Rb and phospho-Rb-567. See Supplemental Figure 1B for confirmation of Roscovitine activity. (B) Mel202 cells were treated with p38 inhibitor SB203580 for 48 h and immunoblotted for total Rb and phospho-Rb-567. See Supplemental Figure 1C for confirmation of p38 inhibitor activity. (C) siRNA against p38α or p38β was transfected into Mel202 cells at a final concentration of 50 nM for 72 h, all samples were treated with 12.5 μM of etoposide for 48 h, and immunoblotted for Rb. (D) Mel202 cells were treated with or without 12.5 μM of etoposide for 48 h and immunoblotted for phospho-p38. (E) Mel202 cells were treated with 4 μM etoposide, 30 μM of p38 inhibitor, or both for 48 h. Propidium iodide was used in order to determine the sub-G1 DNA content by flow cytometry. (F) Mel202 cells were treated with 12.5 μM etoposide, 30 μM of p38 inhibitor, or both for 48 h. TUNEL staining was performed to determine the percent of apoptotic cells. (G) Mel202 cells were treated with Ad-LacZ or Ad-MKK3 at an MOI of 100 with or without 30 μM of p38 inhibitor and immunoblotted for Rb, phospho-Rb-567 or phospho-p38. (H) Mel202 cells were treated with Ad-GFP, Ad-cycD1, or Ad-MKK3 at an MOI of 30 for 48 h and immunoblotted for total Rb, phospho-p38, or cyclin D1. (I) Flow cytometry was performed using propidium iodide staining to determine the percent of cells in sub-G1. Mel202 cells were treated with Ad-cycD1 or Ad-MKK3 at an MOI of 30 for 48 h. (J) Mel501 cells were transfected with SKTT control, Rb, RbΔ780, or RbΔ568 for 72 h and treated with 12.5 μM etoposide for 48 h. TUNEL staining was performed. (K) Mel501 cells were transfected with SKTT control, Rb, or RbΔ568 for 72 h and treated with Ad-MKK3 or Ad-LacZ MOI 15 for 48 h. TUNEL staining was performed. Data were normalized to RbΔ568 for each virus.
p38 phosphorylates Rb on Ser567 by a mechanism distinct from Cdks
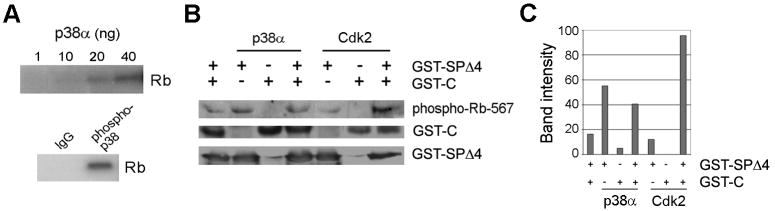
While p38 has been shown to phosphorylate Rb (Wang et al, 1999), it remains unclear whether p38 phosphorylates Rb by the same mechanism previously shown for Cdks involving a hierarchical series of conformational changes (Harbour et al, 1999). We performed in vitro kinase experiments to confirm the ability of p38 to phosphorylate Rb directly. Both recombinant p38α and endogenous p38, immunoprecipitated from melanoma cells treated with etoposide, resulted in robust phosphorylation of Rb in vitro (Figure 3A). As we showed previously (Harbour et al, 1999), cyclin E-Cdk2 can phosphorylate Rb on Ser567 only in the presence of the phosphorylated Rb C-terminus, which provides a docking site for Cdk2 and brings it into proximity of Ser567 following a series of hierarchical conformational changes. In contrast, p38 did not require the C-terminus for efficient phosphorylation of Ser567; instead, p38 phosphorylated this residue more efficiently in the absence of the C-terminus (Figure 3B-C). This indicated that p38 phosphorylates Rb on Ser567 through a unique mechanism unlike that of Cdks.
Figure 3.
p38 phosphorylates Rb directly and by a different mechanism than cyclin E-Cdk2. (A) In vitro radioactive kinase assays revealed that p38 directly phosphorylated Rb. Top Panel: Purified p38α phosphorylated 0.5 μg of purified full length Rb. Bottom Panel: Endogenous phospho-p38 immunoprecipitated from Mel202 cells phosphorylated 0.5 μg of purified full length Rb. (B) Non-radioactive in vitro kinase assays were performed using 20 ng of purified p38 or cyclin E-Cdk2 kinase. GST-SPΔ4 (small pocket of Rb, amino acids 379-772 with all phosphoacceptor sites except Ser567 mutated to an alanine) and GST-C (C-terminus of Rb, amino acids 773-928) were used as substrates. Lanes 1, 4, and 7 had 3.2 μg of GST-C, lanes 2 and 5 had no GST-C, lanes 3 and 6 had 1.6 μg of GST-C. 1 μg of GST- SPΔ4 was used. Samples were immunoblotted with an antibody against phospho-Rb-567 which recognizes the only phosphorylation site on GST-SPΔ4, Ser567, or an antibody against GST which recognizes both GST-SPΔ4 and GST-C. The GST-C and GST-SPΔ4 Rb fragments interact (Harbour et al, 1999). GST-C is necessary for efficient phosphorylation of the small pocket by Cdk2, but is not required for phosphorylation by p38. (C) A graphical representation of the band intensity of the phospho-Rb-567 antibody in (B).
Phosphorylation of Rb by p38 disrupts the E2F1-Rb interaction and triggers E2F1-mediated apoptosis
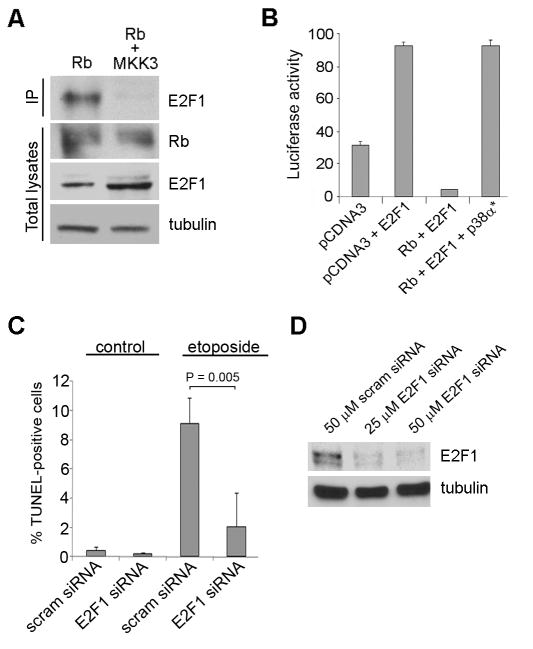
We reasoned that stress-related phosphorylation of Rb is unique in its ability to release E2Fs that can then activate apoptotic genes, and that this could account for the apoptosis that occurs when p38 phosphorylates Rb. To test this, we activated endogenous p38 in cells by expressing MKK3, and used co-immunoprecipitation experiments to evaluate the interaction between E2F1, one of the E2F family members involved in apoptosis, and ectopically expressed Rb. As expected, phosphorylation of Rb by p38 disrupted the interaction between Rb and E2F1 (Figure 4A). Concomitantly, the release of E2F1 from Rb repression by p38α allowed it to activate E2F1-responsive promoters (Figure 4B). Further, RNAi-mediated knock down of E2F1 inhibited Ad-MKK3-associated apoptosis, confirming that p38 induced apoptosis, at least in part, through release of E2F1 (Figure 4C-D).
Figure 4.
Phosphorylation of Rb by p38 leads to E2F1-mediated apoptosis. (A) Mel202 cells were transfected with Rb alone or with MKK3 for 48 h and immunoprecipitation for Rb was performed. The immunoprecipitation was immunoblotted for E2F1. Total lysates were immunoblotted for total Rb and E2F1. (B) An E2F1 promoter with three E2F-responsive repeats was used in a luciferase assay. Empty vector (pCDNA3), Rb, E2F1, and constitutively active p38α expression constructs were transfected into Mel202 cells. The star represents constitutively active p38α. (C) Scrambled or E2F1 siRNA was used at a final concentration of 50nM for 72 h in Mel501 cells. The cells were treated with 12.5 μM of etoposide for 48 h and TUNEL staining was performed. (D) Knock down of E2F1 by siRNA was confirmed in Mel501 cells. 50 nM of scrambled siRNA, 25 nM of E2F1 siRNA, or 50 nM of E2F1 siRNA were transfected for 72 h.
p53 plays a role in p38-mediated apoptosis, but not Rb phosphorylation
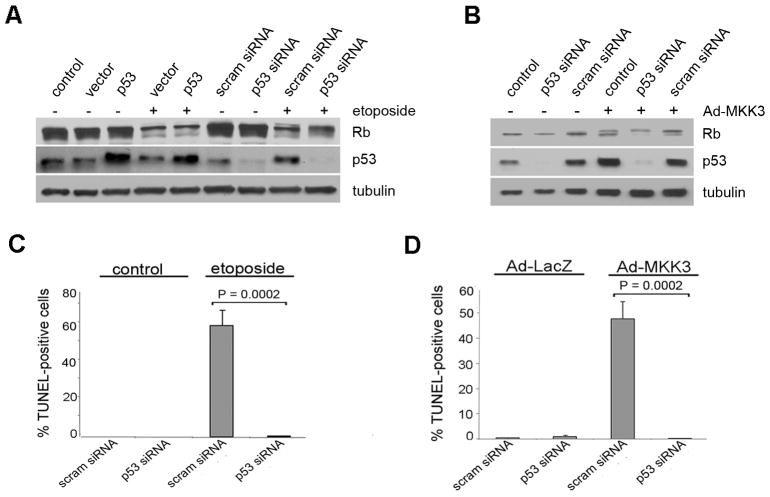
Recognizing that p53 is activated by etoposide as part of the DNA damage response (Lowe et al, 1993), we wished to determine whether p53 was required for p38-mediated Rb phosphorylation and/or apoptosis. Knock down of p53 by RNAi had no effect on etoposide-induced Rb hyperphosphorylation or degradation (Figure 5A), but it did inhibit etoposide-induced cell death (Figure 5C). Similar results were observed in Ad-MKK3 treated cells (Figure 5B, 5D). These data indicate that p53 is not required for p38-mediated hyperphosphorylation or degradation of Rb, but it is required to cooperate with p38 to induce apoptosis in this setting (Figure 5C-D).
Figure 5.
p53 is involved in p38 induced apoptosis, but not in p38-mediated phosphorylation and degradation of Rb. (A) Mel202s were treated with 12.5 μM of etoposide for 48 h. Scrambled or p53 siRNA was used at a final concentration of 50 nM for 72 h. p53 expression vector or empty vector were transfected for 72 h. Samples were immunoblotted with antibodies against Rb and p53. (B) Mel202s were transfected with siRNA against p53 or scrambled siRNA for 72 h and treated with Ad-MKK3 MOI of 15 for 48 h. Samples were immunoblotted with antibodies against Rb and p53. (C) Mel202s were transfected with p53siRNA or scrambled siRNA for 72 h. Cells were treated with 12.5 μM of etoposide for 48 h and TUNEL staining was performed. (D) Mel202s were transfected with p53 siRNA or scrambled siRNA for 72 h and treated with Ad-LacZ or Ad-MKK3 at an MOI of 15. TUNEL staining was performed.
Phosphorylation of Rb on Ser567 by p38 triggers Hdm2-mediated Rb degradation and apoptosis
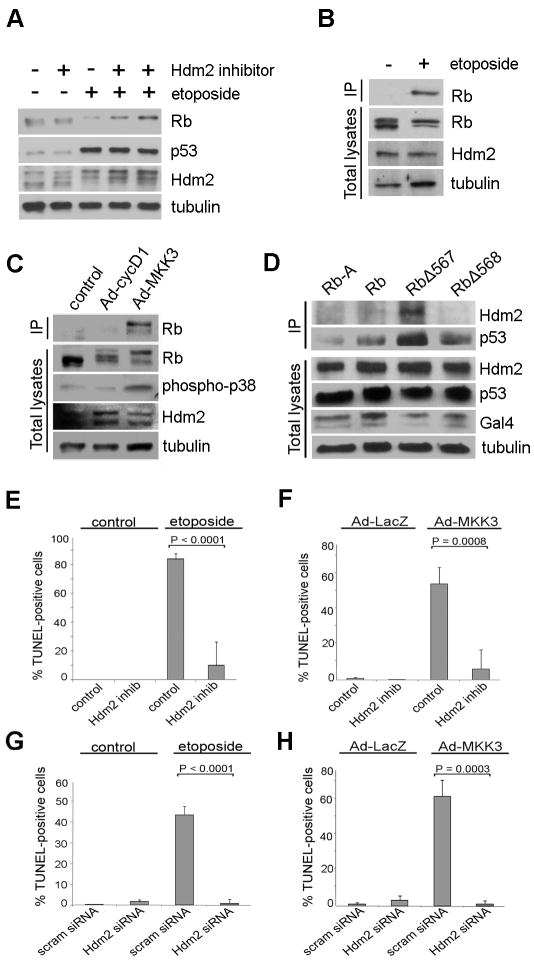
In our experiments, genotoxic stress or activation of endogenous p38 with Ad-MKK3 resulted not only in hyperphosphorylation of Rb but also in a precipitous decline in total Rb protein levels, suggesting that p38-mediated phosphorylation causes degradation of Rb (Figure 1H-I). Although Rb has been shown previously to be cleaved by caspases under certain conditions (Fattman et al, 2001; Fattman et al, 1997), we found that the pan-caspase inhibitor Z-VAD-FMK could not block etoposide-induced Rb degradation (Supplemental Figure 2). Other studies have shown that Rb can interact with the E3 ligase Hdm2, which targets proteins such as Rb and p53 for proteosomal degradation, such that it can act as an oncogene when overexpressed (Xiao et al, 1995; Sdek et al, 2004; Sdek et al, 2005). The critical role played by Hdm2 in maintaining p53 at low cellular levels during normal conditions has been firmly established (Momand et al, 1992; Oliner et al, 1993), but the physiologic significance and mechanism of the Hdm2-Rb interaction remains less certain. We hypothesized that Hdm2 mediates the degradation of Rb that occurs in response to p38-mediated phosphorylation. Indeed, degradation of Rb was efficiently inhibited by a small molecule inhibitor of Hdm2 E3 ligase activity (Figure 6A), and in co-immunoprecipitation experiments, the interaction between Rb and Hdm2 was enhanced by activating endogenous p38 either through etoposide or Ad-MKK3 treatment, but not by activating endogenous Cdks through Ad-cycD1 treatment (Figure 6B-C). More specifically, Ad-MKK3, but not Ad-cycD1, led to an increased interaction between Hdm2 hyperphosphorylated Rb (Figure 6C), and knock down of Hdm2 by RNAi led to an accumulation of hyperphosphorylated Rb (Supplemental Figure 3A). Interestingly, treating cells with a proteosome inhibitor also resulted in increased levels of phosphorylated Rb (Supplemental Figure 3B) revealing that a low level of Rb degradation occurs in melanoma cells even at baseline.
Figure 6.
Phosphorylation of Rb on Ser567 by p38 enhances the Rb-Hdm2 interaction and leads to Hdm2-mediated apoptosis. (A) Mel202 cells were treated for 48 h with or without12.5 μM of etoposide. 50 μM of Hdm2 inhibitor was used in lane 2, 5 μM in lane 4, and 10 μM in lane 6. No Hdm2 inhibitor was used in Lanes 1 and 3. (B) Mel202 cells were treated with 12.5 μM etoposide for 48 h. Immunoprecipitation for endogenous Hdm2 was performed and samples were immunoblotted for endogenous Rb. Total lysates were immunoblotted for Rb and Hdm2. (C) Mel202 cells were treated with Ad-cycD1 or Ad-MKK3 at an MOI of 15 for 48 h, immunoprecipitated for endogenous Hdm2, and immunoblotted for endogenous total Rb. Total lysates were immunoblotted for Rb, phospho-p38, and Hdm2. (D) Mel202 cells were transfected with Gal4-tagged Rb constructs. Rb-A (the A domain of Rb, amino acids 379-572, serves as a negative control), Rb, RbΔ567, or RbΔ568. Cells were immunoprecipitated for Gal4 to pull down Rb, and immunoblotted for endogenous Hdm2 and endogenous p53. Total lysates were immunoblotted for Hdm2, p53, and Gal4. (E) Mel202 cells were treated with 10 μM Hdm2 inhibitor and 12.5 μM etoposide for 48 h and TUNEL staining was performed. (F) Mel202 cells were treated with 10 μM Hdm2 inhibitor for 72 h. Cells were treated Ad-LacZ or Ad-MKK3 at a MOI of 100 for 48 h and TUNEL staining was performed. (G) Mel202 cells were transfected with Hdm2 siRNA for 72 h and treated with 12.5 μM etoposide for 48 h and TUNEL staining was performed. (H) Mel202 cells were transfected with Hdm2 siRNA for 72 h and treated with Ad-LacZ or Ad-MKK3 at an MOI of 50 for 48 h and TUNEL staining was performed.
These data suggest that Hdm2 preferentially interacts with and degrades a phosphorylated form of Rb produced by p38- rather than Cdk-mediated phosphorylation. Since we showed earlier that Ser567 phosphorylation by p38 occurs through a mechanism different from that of Cdks, we speculated that the ability of p38 to induce an Rb-Hdm2 interaction may be mediated by phosphorylation specifically on Ser567. We previously generated an Rb mutant in which serine567 was substituted with an alanine (RbΔ567), and showed that RbΔ567 mimics phosphorylation of Ser567 by disrupting the pocket and abrogating binding to E2Fs (Harbour et al, 1999; Ma et al, 2003). Since RbΔ567 destabilizes the Rb protein, it has the same impact as phosphorylation of Ser567 and thus could be used to study how phosphorylation-induced disruption of Rb tertiary structure might affect its interaction with Hdm2. The RbΔ567 mutant interacted strongly with Hdm2 in co-immunoprecipitation experiments, whereas wildtype Rb or RbΔ568 which blocks Ser567 phosphorylation interacted only weakly with Hdm2 (Figure 6D). Inhibition or knock down of Hdm2 blocked etoposide and Ad-MKK3 induced apoptosis (Figure 6E-H). Taken together, these results suggest that p38-mediated phosphorylation of Rb on Ser567 leads to Rb degradation and cell death by modulating its interaction with and degradation by Hdm2.
Discussion
In this study, we provide evidence for a pro-apoptotic signaling pathway induced by genotoxic stress, in which p38 phosphorylates Rb in a manner that triggers Hdm2-mediated Rb degradation, and E2F1-dependent cell death (Figure 7). The Rb residue Ser567 is implicated as a key target of this p38-mediated phosphorylation, providing a molecular explanation for why Rb phosphorylation by p38 differs mechanistically and physiologically from that catalyzed by Cdks. This p38-mediated pathway could provide a rapid, cell cycle-independent means for overriding the cell cycle and triggering apoptosis in response to genotoxic stress.
Figure 7.
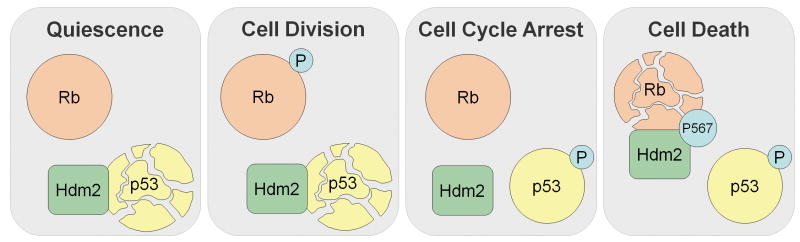
Hypothesis for how p38-mediated phosphorylation of Rb on Ser567 provides a death signal, rather than the Cdk-mediated proliferative signal, and how shuttling of Hdm2 between p53 and Rb may tightly regulate the cellular response to stress. In quiescent cells, Rb is hypophosphorylated and active, preventing cell cycle progression. In parallel, Hdm2 maintains p53 at low levels by proteosomal degradation. In normal cycling cells, Rb is phosphorylated and partially inactivated by Cdks, and Hdm2 continues to maintain p53 at low levels. When cells are exposed to low levels of genotoxic and perhaps other forms of stress, p53 is phosphorylated and released from Hdm2, allowing cellular levels of p53 to increase. A major effect of this increase in p53 is transactivation of p21 which, in turn, promotes hypophosphorylation and activation of Rb. Rb is available because it has not been degraded by activation of p38. The net result is stress-induced cell cycle arrest. When cells are exposed to higher levels of stress, p53 accumulates and, in addition, p38 is activated to phosphorylate Rb on Ser567, which promotes interaction with Hdm2, degradation of Rb, and E2F1-mediated apoptosis. With Rb degraded and unavailable to cooperate with p53 to induce cell cycle arrest, the apoptotic genes under basal inhibition by Rb are derepressed, leading to an apoptotic response.
When initial models were being developed to explain how Rb is regulated, Cdks were the only kinases known to phosphorylate Rb. However more recently other kinases such as p38 have been shown to phosphorylate Rb during stress (Hou et al, 2002; Kishore et al, 2003; Lee et al, 2003; Nath et al, 2003; Yeste-Velasco et al, 2009; Wang et al, 1999). Phosphorylation of Rb by Cdks during cell division does not lead to Rb degradation or apoptosis. This normal cycling depends on Rb remaining in a reversible, partially inactivated state such that it can still repress pro-apoptotic E2Fs (Wells et al, 2003; Young et al, 2003). In contrast, p38-mediated phosphorylation leads to degradation of Rb, release of E2Fs and cell death.
The ability of p38, but not Cdks, to phosphorylate Rb on Ser567 efficiently may provide a key to understanding the different effect that p38 has on Rb compared to Cdks. Phosphorylation of Rb by Cdks occurs through a hierarchical series of phosphorylation events each triggering successive conformational changes that enable further phosphorylation in a stepwise manner (Brown et al, 1999; Harbour et al, 1999; Lundberg & Weinberg, 1998; Zhang et al, 2000). Previous work has demonstrated that the site that is least efficiently phosphorylated by Cdks is Ser567. Phosphorylation of this site by Cdks requires prior phosphorylation of the C-terminus, which contains Cdk docking sites that bring cyclin-Cdk complexes into proximity of Ser567 through an intramolecular interaction between the C-terminus and the pocket (Harbour et al, 1999). Ser567 has not been reported to be phosphorylated during normal cell cycle progression (Lees et al, 1991). We show here that p38 efficiently phosphorylates Ser567 under conditions favoring apoptosis, and this phosphorylation does not depend on the intramolecular interactions and conformational changes associated with Cdk-mediated phosphorylation. This would allow p38 to bypass the Cdks and directly regulate Rb in a cell cycle-independent manner during apoptotic signaling. The ability of p38 to phosphorylate Ser567 may be due to its small size, allowing it to access Ser567, which is located in a recess at the interface of the A and B boxes of the pocket, where it would likely be inaccessible to the much larger cyclin-Cdk complexes under normal circumstances (Harbour et al, 1999; Lee et al, 2002). Taken together, these findings suggest that p38 phosphorylates Rb in a cell cycle-independent manner and that it is the ability of p38 to phosphorylate Ser567 efficiently that distinguishes the pro-apoptotic effect of p38 phosphorylation from the proliferative effect of Cdk phosphorylation. Consistent with this hypothesis, Nath and colleagues showed that an Rb mutant with all available phosphorylation sites mutated to prevent their phosphorylation except Ser567 was unable to rescue p38-mediated inactivation of Rb (Nath et al, 2003). Similarly, von Willebrand and colleagues showed that a phosphorylation defective Rb mutant was not resistant to ubiquitination despite elimination of most of the phosphorylation sites; Ser567 was one of the few sites that were not mutated (von Willebrand et al, 2003).
Several other lines of evidence point to Ser567 as playing an important role in Rb regulation. The Ser567-Pro568 phospho-acceptor motif is highly conserved across species (Ma et al, 2003), suggesting that it is the ability of Ser567 to be phosphorylated that is important in its function. Ser567 is the only phosphorylation site on Rb that is a target of naturally occurring mutations in human retinoblastoma (Templeton et al, 1991; Yilmaz et al, 1998), suggesting that it plays a different role than the other 15 phosphorylation sites. Ser567 is the only phosphorylation site on Rb that, when phosphorylated or mutated, disrupts the A-B box interaction, destabilizes the pocket structure, and abrogates interaction with E2Fs (Harbour et al, 1999; Ma et al, 2003). Crystallographic studies have shown that Ser567 mediates critical contacts between the A and B boxes, and that phosphorylation of this site would be predicted to destabilize the pocket structure and eliminate the E2F binding site (Lee et al, 2002; Lee et al, 1998).
Our finding that genotoxic stress promotes interaction between Rb and Hdm2 parallels recent findings that a p53 activating compound promotes Mdm2-mediated degradation of p21, both ultimately leading to cell death (Enge et al, 2009). Here, we provide new insights into the relationship between Hdm2, an E3 ligase and oncoprotein, and the tumor suppressors p53 and Rb. It has long been known that Hdm2 interacts with and triggers the degradation of both p53 and Rb (Momand et al, 1992; Uchida et al, 2005; von Willebrand et al, 2003; Xiao et al, 1995; Sdek et al, 2005). In particular, Hdm2 binds and ubiquitinates Rb, targeting it for proteosome-dependent and - independent degradation (Sdek et al, 2004; von Willebrand et al, 2003; Xiao et al, 1995). Here we show that phosphorylation of Ser567 and consequent disruption of the Rb pocket may be a critical event that triggers interaction of Rb with Hdm2. This represents a rare circumstance in which phosphorylation of Rb enhances, rather than abolishes, its interaction with a binding partner. Further, it is tempting to speculate that the dual role of Hdm2 in regulating protein levels of two major tumor suppressors – Rb and p53 – is physiologically relevant, but this has not been confirmed experimentally. In this model, lower stress levels would favor cycle arrest by disruption of the Hdm2-p53 interaction and subsequent accumulation of activated p53. But under these conditions, p38 would not phosphorylate Rb, and Hdm2 would thus not interact with Rb. The result would be active Rb that could cooperate with p53 to trigger cell cycle arrest. At higher stress levels, apoptosis would be favored as a mechanism for eliminating a potentially deleterious cell. Disruption of the Hdm2-p53 interaction would activate p53, and release Hdm2; however, in this setting, activated p38 would phosphorylate Rb on Ser567, allowing Hdm2 to interact with Rb triggering its degradation. Furthermore, it has been shown that Rb-Hdm2 binding stabilizes p53 and promotes p53-dependent apoptosis (Hsieh et al, 1999; Yap et al, 1999). MK2, a direct transcriptional target of p38, phosphorylates Hdm2 and enhances its activity (Weber et al, 2005), thus p38 may promote the degradation of Rb through multiple complementary mechanisms. Loss of Rb and the consequent release of pro-apoptotic E2Fs, coupled with activation of p53, then tips the balance of cellular events in favor of apoptosis over cell cycle arrest (Sun et al, 2010; Kitagawa et al, 2008). Without such a mechanism, activation of p53 might indiscriminately trigger both cell cycle arrest and apoptotic programs, leading to a chaotic and inefficient response to stress. Consistent with this model, p53 and Rb compete for binding to Hdm2 (Uchida et al, 2005), p53 requires Rb for efficient induction of cell cycle arrest (Harrington et al, 1998), and activation of p53 triggers apoptosis in the absence of Rb (Macleod et al, 1996).
We describe a genotoxic stress-induced signaling pathway in which p38 phosphorylates Rb on Ser567, leading to Hdm2-mediated degradation of Rb, release of E2F1, and induction of apoptosis. This mechanism is not mutually exclusive with other strategies that the cell may employ for differentially regulating cell proliferation and apoptosis, such as through PI3K/Akt signaling to block the E2F1 apoptotic program during proliferation (Dick & Dyson, 2003; Hallstrom et al, 2008; Hallstrom et al, 2003) or through binding of phosphorylated Rb to E2Fs at pro-apoptotic targets and the recruitment of coactivators that drive expression of these genes (Ianari et al, 2009). It will be important in future studies to determine how these complementary mechanisms coordinate cell death. Further work will be necessary to determine whether other E2F family members or other phospho-acceptor sites on Rb play a role in this cell death response (Dick & Dyson, 2003; Xu et al, 2007).
Insights gleaned from this work could have important therapeutic implications, not only in cancer, where one may wish to promote cancer cell death or sensitize cancer cells to therapy by stimulating or mimicking p38 signaling to Rb (Zagorski et al, 2007), but also in degenerative diseases, where inhibiting this process may have therapeutic benefit. For example, Rb hyperphosphorylation and degradation mediates neuronal cell death in multiple neurodegenerative disorders such as Parkinsons disease, Alzheimers disease and stroke (Giovanni et al, 2000; Hayashi et al, 2000; Hoglinger et al, 2007; Liu & Greene, 2001; O'Hare et al, 2000; Park et al, 2000; Ramalho et al, 2004; Thakur et al, 2008). Further, p38 can regulate Rb and modulate cell death in neurons, and pharmacologic blockade of p38 has a neuroprotection effect (Hou et al, 2002; Yeste-Velasco et al, 2009). Similar mechanisms may govern the development of atherosclerosis and cardiomyocyte apoptosis that occur in association with myocardial infarction (Hauck et al, 2002; Proctor et al, 2008). These findings may provide new insights for therapeutic intervention, both to inhibit cell death in degenerative diseases and to promote cell death in cancer where manipulation of this mechanism could kill cells directly or sensitize them to radiation or chemotherapy.
Materials and methods
Cell cultures
Mel202 uveal melanoma cells were provided by B. Ksander (Harvard Medical School, Boston, MA, USA) and Mel501 cutaneous melanoma cells provided by L. Cornelius (Washington University, St. Louis, MO, USA). Transfections of expression plasmids were performed using Effectene or Attractene (Qiagen, Germantown, MD, USA). Transfections of siRNA were performed using HiPerFect (Qiagen). When indicated, cells were treated with etoposide (Sigma Aldrich, St. Louis, MO, USA), p38 inhibitor (SB 203580, Millipore, Billerica, MA, USA), Cdk2 inhibitor (Roscovitine, Sigma Aldrich), Cdk4 inhibitor (EMD, Darmstadt, Germany), Hdm2 E3 Ligase Inhibitor 373225 (EMD), Z-VAD-FMK (Sigma Aldrich) or MG132 (Sigma Aldrich). Cell viability assays were performed with methyltetrazolium sulfate (MTS) using the CellTiter 96 AQueous kit (Promega, Madison, WI, USA). TUNEL staining was performed using the In Situ Cell Death Detection Kit (Roche Diagnostics Corporation, Basel, Switzerland). Luciferase assays were performed using Luciferase Assay System (Promega).
Expression constructs
Validated siRNA against p38α, p38β, Hdm2, E2F1 (Ambion, Austin, TX, USA), and p53 (Thermo Fisher Scientific, Waltham, MA, USA), were used. siRNA against Rb was designed using an algorithm previously described (Elbashir et al, 2001). pCMV, pcDNA3, RC-CMV, RC-Cyclin D, RC-Cyclin E, Hdm2, p53, Rb-A, Rb, RbΔ567, RbΔ568, RbΔ780, and E2F1, expression plasmids were used (Ma et al, 2003; Harbour et al, 1999). RSV2-MKK3 was provided by R. Davis (UMass Medical School, Worcester, MA, USA). The constitutively active p38 expression plasmid was provided by D. Engelberg (Hebrew University of Jerusalem, Jerusalem, Israel) (Askari et al, 2009). E2F1 luciferase promoter was from Panomics (Fremont, CA). Adenovirus expressing GFP (Ad-GFP) was provided by A. Samarel (Cardiovascular Institute, Stritch School of Medicine, Maywood, Illinois, USA) (Heidkamp et al, 2005). Adenovirus expressing LacZ (Ad-LacZ) was provided by T. Kuroki (Showa University, Tokyo, Japan) (Ohba et al, 1998). Ad-MKK3 was provided by Y. Wang (University of California, Los Angelos, CA, USA) (Wang et al, 1998). Ad-cycD1 was provided by H. Piwnica-Worms (Washington University).
Antibodies
Immunoblots, immunoprecipitations and immunofluorescence experiments were performed as previously described (Ma et al, 2003). Antibodies against Rb (IF8), E2F1, Hdm2, Gal4, and p53 were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Antibodies against Rb-phospho-567 were obtained from Santa Cruz Biotechnology, Inc. and Bethyl Laboratories (Montgomery, TX, USA) (Ma et al, 2003). Antibodies against cleaved poly(ADP-ribose) polymerase (PARP), phospho-p38, p38 total, cyclin D1, Rb-phospho-807-811, Rb-phospho-780, Rb-phospho-795 and cleaved caspase 3 were from Cell Signaling Technology (Danvers, MA, USA). Antibody against α-tubulin was from Sigma Aldrich.
Flow Cytometry
Cells were washed in PBS, fixed in 70% ethanol overnight at 4 degrees, washed in PBS and incubated in DNA staining buffer (PBS pH 7.4 Triton X-100 0.1%, EDTA pH 7.4 0.5 mM, RNAse A 0.05 mg/ml) and propidium iodide 50 μg/ml for 30 min at room temperature in the dark. Cells were analyzed on a flow cytometer (Beckman Coulter FC500, Brea, CA, USA).
In Vitro Kinase Assays
Assays were performed as previously described (Harbour et al, 1999) using purified phospho-p38α, cyclin E-Cdk2, or cyclin D-Cdk4 kinase (Cell Signaling), IgG (Sigma Aldrich), GST-SPΔ4, GST-C (Harbour et al, 1999) and purified full length Rb (QED Bioscience Inc, San Diego, CA, USA).
Supplementary Material
Supplemental Figure 1. Inhibition of p38, but not Cdks, inhibits etoposide induced phosphorylation of Rb. (A) Mel202 cells were treated with Cdk4 inhibitor and 12.5 μM etoposide for 48 h and immunoblotted for Rb. (B-D) Kinase assays confirmed that the Cdk2, p38 and Cdk4 inhibitors were active.
Supplemental Figure 2. Caspase inhibition fails to block etoposide induced phosphorylation of Rb. ZVAD-FMK and etoposide were used at 50 μM and 12.5 μM respectively for 72 h. Lysates were immunoblotted for Rb.
Supplemental Figure 3. Rb is ubiquitinated and degraded in the proteosome. (A) Mel202 cells were treated with 50 nM of scrambled or Hdm2 siRNA for 72 h and immunoblotted for Rb or Hdm2. (B) Mel202 cells were treated with 50 μM of MG132 for 3 h and immunoblotted for Rb.
Acknowledgments
We thank Doug Dean and members of the Harbour Lab for helpful discussions.
This work was supported by the Vision Training Grant 5T32EY013360-09, and grants from the NIH (EY013169-09), the Barnes-Jewish Hospital Foundation, the Horncrest Foundation, The Kling Family Foundation, and a Research to Prevent Blindness David F. Weeks Professorship. This work was also supported by awards to the Department of Ophthalmology and Visual Sciences at Washington University from a Research to Prevent Blindness, Inc. Unrestricted grant, and the NIH Vision Core Grant P30 EY 02687.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Almasan A, Yin Y, Kelly RE, Lee EY, Bradley A, Li W, Bertino JR, Wahl GM. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc Natl Acad Sci U S A. 1995;92:5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari N, Beenstock J, Livnah O, Engelberg D. p38alpha is active in vitro and in vivo when monophosphorylated at threonine 180. Biochemistry. 2009;48:2497–2504. doi: 10.1021/bi900024v. [DOI] [PubMed] [Google Scholar]
- Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung Y, Komarov AP, Keyomarsi K, Yarden Y, Seger R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene. 2001;20:147–155. doi: 10.1038/sj.onc.1204062. [DOI] [PubMed] [Google Scholar]
- Bowen C, Birrer M, Gelmann EP. Retinoblastoma protein-mediated apoptosis after gamma-irradiation. J Biol Chem. 2002;277:44969–44979. doi: 10.1074/jbc.M202000200. [DOI] [PubMed] [Google Scholar]
- Brown VD, Phillips RA, Gallie BL. Cumulative effect of phosphorylation of pRB on regulation of E2F activity. Mol Cell Biol. 1999;19:3246–3256. doi: 10.1128/mcb.19.5.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BN, Pan CW, Wang JY. Separation of anti-proliferation and anti-apoptotic functions of retinoblastoma protein through targeted mutations of its A/B domain. PLoS One. 2006;1:e82. doi: 10.1371/journal.pone.0000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BN, Wang JY. Coordinated regulation of life and death by RB. Nat Rev Cancer. 2003;3:130–138. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chen PL, Scully P, Shew JY, Wang JY, Lee WH. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989;58:1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Dick FA, Dyson N. pRB contains an E2F1-specific binding domain that allows E2F1-induced apoptosis to be regulated separately from other E2F activities. Mol Cell. 2003;12:639–649. doi: 10.1016/s1097-2765(03)00344-7. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. Embo J. 2001;20:6877–6888. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M, Bao W, Hedstrom E, Jackson SP, Moumen A, Selivanova G. MDM2-dependent downregulation of p21 and hnRNP K provides a switch between apoptosis and growth arrest induced by pharmacologically activated p53. Cancer Cell. 2009;15:171–183. doi: 10.1016/j.ccr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Ezhevsky SA, Ho A, Becker-Hapak M, Davis PK, Dowdy SF. Differential regulation of retinoblastoma tumor suppressor protein by G(1) cyclin-dependent kinase complexes in vivo. Mol Cell Biol. 2001;21:4773–4784. doi: 10.1128/MCB.21.14.4773-4784.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhevsky SA, Nagahara H, Vocero AA, Gius DR, Wei MC, Dowdy SF. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc Natl Acad Sci U S A. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattman CL, Delach SM, Dou QP, Johnson DE. Sequential two-step cleavage of the retinoblastoma protein by caspase-3/-7 during etoposide-induced apoptosis. Oncogene. 2001;20:2918–2926. doi: 10.1038/sj.onc.1204414. [DOI] [PubMed] [Google Scholar]
- Fattman CL, An B, Sussman L, Dou QP. p53-independent dephosphorylation and cleavage of retinoblastoma protein during tamoxifen-induced apoptosis in human breast carcinoma cells. Cancer Lett. 1998;130:103–113. doi: 10.1016/s0304-3835(98)00121-9. [DOI] [PubMed] [Google Scholar]
- Fattman CL, An B, Dou QP. Characterization of interior cleavage of retinoblastoma protein in apoptosis. J Cell Biochem. 1997;67:399–408. [PubMed] [Google Scholar]
- Giovanni A, Keramaris E, Morris EJ, Hou ST, O'Hare M, Dyson N, Robertson GS, Slack RS, Park DS. E2F1 mediates death of B-amyloid-treated cortical neurons in a manner independent of p53 and dependent on Bax and caspase 3. J Biol Chem. 2000;275:11553–11560. doi: 10.1074/jbc.275.16.11553. [DOI] [PubMed] [Google Scholar]
- Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991;67:293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Hallstrom TC, Mori S, Nevins JR. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13:11–22. doi: 10.1016/j.ccr.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom TC, Nevins JR. Specificity in the activation and control of transcription factor E2F-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:10848–10853. doi: 10.1073/pnas.1831408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–67. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Luo RX, Dei Sante A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bruce JL, Harlow E, Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc Natl Acad Sci U S A. 1998;95:11945–11950. doi: 10.1073/pnas.95.20.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck L, Hansmann G, Dietz R, von Harsdorf R. Inhibition of hypoxia-induced apoptosis by modulation of retinoblastoma protein-dependent signaling in cardiomyocytes. Circ Res. 2002;91:782–789. doi: 10.1161/01.res.0000041030.98642.41. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Sakai K, Sasaki C, Zhang WR, Abe K. Phosphorylation of retinoblastoma protein in rat brain after transient middle cerebral artery occlusion. Neuropathol Appl Neurobiol. 2000;26:390–397. doi: 10.1046/j.1365-2990.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Scully BT, Vijayan K, Engman SJ, Szotek EL, Samarel AM. PYK2 regulates SERCA2 gene expression in neonatal rat ventricular myocytes. Am J Physiol Cell Physiol. 2005;289:C471–482. doi: 10.1152/ajpcell.00130.2005. [DOI] [PubMed] [Google Scholar]
- Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Breunig JJ, Depboylu C, Rouaux C, Michel PP, Alvarez-Fischer D, Boutillier AL, Degregori J, Oertel WH, Rakic P, Hirsch EC, Hunot S. The pRb/E2F cell-cycle pathway mediates cell death in Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:3585–3590. doi: 10.1073/pnas.0611671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou ST, Xie X, Baggley A, Park DS, Chen G, Walker T. Activation of the Rb/E2F1 pathway by the nonproliferative p38 MAPK during Fas (APO1/CD95)-mediated neuronal apoptosis. J Biol Chem. 2002;277:48764–48770. doi: 10.1074/jbc.M206336200. [DOI] [PubMed] [Google Scholar]
- Hsieh JK, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- Hsieh JK, Chan FS, O'Connor DJ, Mittnacht S, Zhong S, Lu X. RB regulates the stability and the apoptotic function of p53 via MDM2. Mol Cell. 1999;3:181–193. doi: 10.1016/s1097-2765(00)80309-3. [DOI] [PubMed] [Google Scholar]
- Ianari A, Natale T, Calo E, Ferretti E, Alesse E, Screpanti I, Haigis K, Gulino A, Lees JA. Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell. 2009;15:184–194. doi: 10.1016/j.ccr.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W, Flores ER, Tsai KY, Jacks T, Vousden KH, Kaelin WG., Jr Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Kishore R, Luedemann C, Bord E, Goukassian D, Losordo DW. Tumor necrosis factor-mediated E2F1 suppression in endothelial cells: differential requirement of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signal transduction pathways. Circ Res. 2003;93:932–940. doi: 10.1161/01.RES.0000102400.22370.20. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Aonuma M, Lee SH, Fukutake S, McCormick F. E2F-1 transcriptional activity is a critical determinant of Mdm2 antagonist-induced apoptosis in human tumor cell lines. Oncogene. 2008;40:5303–5314. doi: 10.1038/onc.2008.164. [DOI] [PubMed] [Google Scholar]
- Knudsen ES, Buckmaster C, Chen TT, Feramisco JR, Wang JY. Inhibition of DNA synthesis by RB: effects on G1/S transition and S- phase progression. Genes Dev. 1998;12:2278–2292. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Chang JH, Lee HS, Cho Y. Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor. Genes Dev. 2002;16:3199–3212. doi: 10.1101/gad.1046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Park HG, Kang HS. Sodium salicylate induces apoptosis in HCT116 colorectal cancer cells through activation of p38MAPK. Int J Oncol. 2003;23:503–508. [PubMed] [Google Scholar]
- Lee EY, Chang CY, Hu N, Wang YC, Lai CC, Herrup K, Lee WH, Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Lee JO, Russo AA, Pavletich NP. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature. 1998;391:859–865. doi: 10.1038/36038. [DOI] [PubMed] [Google Scholar]
- Lees JA, Buchkovich KJ, Marshak DR, Anderson CW, Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. Embo J. 1991;10:4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C, Godefroy N, Costina-Parvu I, Rincheval V, Renaud F, Trotot P, Bouleau S, Mignotte B, Vayssiere JL. Caspase-9 can antagonize p53-induced apoptosis by generating a p76(Rb) truncated form of Rb. Oncogene. 2005;24:3297–3308. doi: 10.1038/sj.onc.1208493. [DOI] [PubMed] [Google Scholar]
- Lin BT, Gruenwald S, Morla AO, Lee WH, Wang JY. Retinoblastoma cancer suppressor gene product is a substrate of the cell cycle regulator cdc2 kinase. Embo J. 1991;10:857–864. doi: 10.1002/j.1460-2075.1991.tb08018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 2001;305:217–228. doi: 10.1007/s004410100396. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Zhou P, Harbour JW. Distinct mechanisms for regulating the tumor suppressor and antiapoptotic functions of Rb. J Biol Chem. 2003;278:19358–19366. doi: 10.1074/jbc.M301761200. [DOI] [PubMed] [Google Scholar]
- Macleod KF, Hu Y, Jacks T. Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. Embo J. 1996;15:6178–6188. [PMC free article] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Morgenbesser SD, Williams BO, Jacks T, Depinho RA. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371:72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- Nath N, Wang S, Betts V, Knudsen E, Chellappan S. Apoptotic and mitogenic stimuli inactivate Rb by differential utilization of p38 and cyclin-dependent kinases. Oncogene. 2003;22:5986–5994. doi: 10.1038/sj.onc.1206843. [DOI] [PubMed] [Google Scholar]
- Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- O'Hare MJ, Hou ST, Morris EJ, Cregan SP, Xu Q, Slack RS, Park DS. Induction and modulation of cerebellar granule neuron death by E2F-1. J Biol Chem. 2000;275:25358–25364. doi: 10.1074/jbc.M001725200. [DOI] [PubMed] [Google Scholar]
- Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh NH, Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- Park DS, Morris EJ, Bremner R, Keramaris E, Padmanabhan J, Rosenbaum M, Shelanski ML, Geller HM, Greene LA. Involvement of retinoblastoma family members and E2F/DP complexes in the death of neurons evoked by DNA damage. J Neurosci. 2000;20:3104–3114. doi: 10.1523/JNEUROSCI.20-09-03104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaire MJ, Nebreda AR, Darbon JM. Cisplatin and UV radiation induce activation of the stress-activated protein kinase p38gamma in human melanoma cells. Biochem Biophys Res Commun. 2000;278:724–728. doi: 10.1006/bbrc.2000.3877. [DOI] [PubMed] [Google Scholar]
- Proctor BM, Jin X, Lupu TS, Muglia LJ, Semenkovich CF, Muslin AJ. Requirement for p38 mitogen-activated protein kinase activity in neointima formation after vascular injury. Circulation. 2008;118:658–666. doi: 10.1161/CIRCULATIONAHA.107.734848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XQ, Livingston DM, Kaelin WJ, Adams PD. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc Natl Acad Sci U S A. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho RM, Ribeiro PS, Sola S, Castro RE, Steer CJ, Rodrigues CM. Inhibition of the E2F-1/p53/Bax pathway by tauroursodeoxycholic acid in amyloid beta-peptide-induced apoptosis of PC12 cells. J Neurochem. 2004;90:567–575. doi: 10.1111/j.1471-4159.2004.02517.x. [DOI] [PubMed] [Google Scholar]
- Sdek P, Ying H, Chang DL, Qiu W, Zheng H, Touitou R, Allday MJ, Xiao ZX. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20:699–708. doi: 10.1016/j.molcel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Sdek P, Ying H, Zheng H, Margulis A, Tang X, Tian K, Xiao ZX. The central acidic domain of MDM2 is critical in inhibition of retinoblastoma-mediated suppression of E2F and cell growth. J Biol Chem. 2004;279:53317–53322. doi: 10.1074/jbc.M406062200. [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Shan B, Lee WH. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Stevaux O, Dyson NJ. A revised picture of the E2F transcriptional network and RB function. Curr Opin Cell Biol. 2002;14:684–691. doi: 10.1016/s0955-0674(02)00388-5. [DOI] [PubMed] [Google Scholar]
- Strobeck MW, Fribourg AF, Puga A, Knudsen ES. Restoration of retinoblastoma mediated signaling to Cdk2 results in cell cycle arrest. Oncogene. 2000;19:1857–1867. doi: 10.1038/sj.onc.1203510. [DOI] [PubMed] [Google Scholar]
- Sun B, Wingate H, Swisher SG, Keyomarsi K, Hunt KK. Absence of pRb facilitates E2F1-induced apoptosis in breast cancer cells. Cell Cycle. 2010;9 doi: 10.4161/cc.9.6.10990. [DOI] [PubMed] [Google Scholar]
- Sun Y, Tran BN, Worley LA, Delston RB, Harbour JW. Functional analysis of the p53 pathway in response to ionizing radiation in uveal melanoma. Invest Ophthalmol Vis Sci. 2005;46:1561–1564. doi: 10.1167/iovs.04-1362. [DOI] [PubMed] [Google Scholar]
- Templeton DJ, Park SH, Lanier L, Weinberg RA. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc Natl Acad Sci U S A. 1991;88:3033–3037. doi: 10.1073/pnas.88.8.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Siedlak SL, James SL, Bonda DJ, Rao A, Webber KM, Camins A, Pallas M, Casadesus G, Lee HG, Bowser R, Raina AK, Perry G, Smith MA, Zhu X. Retinoblastoma Protein Phosphorylation at Multiple Sites is Associated with Neurofibrillary Pathology in Alzheimer Disease. Int J Clin Exp Pathol. 2008;1:134–146. [PMC free article] [PubMed] [Google Scholar]
- Tsai KY, Hu Y, Macleod KF, Crowley D, Yamasaki L, Jacks T. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol Cell. 1998;2:293–304. doi: 10.1016/s1097-2765(00)80274-9. [DOI] [PubMed] [Google Scholar]
- Uchida C, Miwa S, Kitagawa K, Hattori T, Isobe T, Otani S, Oda T, Sugimura H, Kamijo T, Ookawa K, Yasuda H, Kitagawa M. Enhanced Mdm2 activity inhibits pRB function via ubiquitin-dependent degradation. Embo J. 2005;24:160–169. doi: 10.1038/sj.emboj.7600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini A, Gravina P, Federici G, Bernardini S. Valproic acid induces apoptosis, p16INK4A upregulation and sensitization to chemotherapy in human melanoma cells. Cancer Biol Ther. 2007;6:185–91. doi: 10.4161/cbt.6.2.3578. [DOI] [PubMed] [Google Scholar]
- von Willebrand M, Zacksenhaus E, Cheng E, Glazer P, Halaban R. The tyrphostin AG1024 accelerates the degradation of phosphorylated forms of retinoblastoma protein (pRb) and restores pRb tumor suppressive function in melanoma cells. Cancer Res. 2003;63:1420–1429. [PubMed] [Google Scholar]
- Wang S, Nath N, Minden A, Chellappan S. Regulation of Rb and E2F by signal transduction cascades: divergent effects of JNK1 and p38 kinases. Embo J. 1999;18:1559–1570. doi: 10.1093/emboj/18.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Hullinger RL, Andrisani OM. Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis. J Biol Chem. 2008;283:25455–25467. doi: 10.1074/jbc.M801934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- Weber HO, Ludwig RL, Morrison D, Kotlyarov A, Gaestel M, Vousden KH. HDM2 phosphorylation by MAPKAP kinase 2. Oncogene. 2005;24:1965–1972. doi: 10.1038/sj.onc.1208389. [DOI] [PubMed] [Google Scholar]
- Wells J, Yan PS, Cechvala M, Huang T, Farnham PJ. Identification of novel pRb binding sites using CpG microarrays suggests that E2F recruits pRb to specific genomic sites during S phase. Oncogene. 2003;22:1445–1460. doi: 10.1038/sj.onc.1206264. [DOI] [PubMed] [Google Scholar]
- Wells J, Boyd KE, Fry CJ, Bartley SM, Farnham PJ. Target gene specificity of E2F and pocket protein family members in living cells. Mol Cell Biol. 2000;20:5797–5807. doi: 10.1128/mcb.20.16.5797-5807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, Yang Y, Opavska J, Wilson P, Thompson JC, Ostrowski MC, Rosol TJ, Woollett LA, Weinstein M, Cross JC, Robinson ML, Leone G. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature. 2003;421:942–947. doi: 10.1038/nature01417. [DOI] [PubMed] [Google Scholar]
- Xiao ZX, Chen J, Levine AJ, Modjtahedi N, Xing J, Sellers WR, Livingston DM. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995;375:694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- Xu X, Bieda M, Jin VX, Rabinovich A, Oberley MJ, Green R, Farnham PJ. A comprehensive ChIP-chip analysis of E2F1, E2F4, and E2F6 in normal and tumor cells reveals interchangeable roles of E2F family members. Genome Res. 2007;17:1550–1561. doi: 10.1101/gr.6783507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap DB, Hsieh JK, Chan FS, Lu X. mdm2: a bridge over the two tumour suppressors, p53 and Rb. Oncogene. 1999;18:7681–7689. doi: 10.1038/sj.onc.1202954. [DOI] [PubMed] [Google Scholar]
- Yeste-Velasco M, Folch J, Pallas M, Camins A. The p38(MAPK) signaling pathway regulates neuronal apoptosis through the phosphorylation of the retinoblastoma protein. Neurochem Int. 2009;54:99–105. doi: 10.1016/j.neuint.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Yilmaz S, Horsthemke B, Lohmann DR. Twelve novel RB1 gene mutations in patients with hereditary retinoblastoma. Mutations in brief no. 206. Hum Mutat. 1998;12:434. doi: 10.1002/(SICI)1098-1004(1998)12:6<434::AID-HUMU16>3.0.CO;2-7. Online. [DOI] [PubMed] [Google Scholar]
- Young AP, Longmore GD. Differential regulation of apoptotic genes by Rb in human versus mouse cells. Oncogene. 2004;23:2587–2599. doi: 10.1038/sj.onc.1207330. [DOI] [PubMed] [Google Scholar]
- Young AP, Nagarajan R, Longmore GD. Mechanisms of transcriptional regulation by Rb-E2F segregate by biological pathway. Oncogene. 2003;22:7209–7217. doi: 10.1038/sj.onc.1206804. [DOI] [PubMed] [Google Scholar]
- Yu BD, Becker-Hapak M, Snyder EL, Vooijs M, Denicourt C, Dowdy SF. Distinct and nonoverlapping roles for pRB and cyclin D:cyclin-dependent kinases 4/6 activity in melanocyte survival. Proc Natl Acad Sci U S A. 2003;100:14881–14886. doi: 10.1073/pnas.2431391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorski WA, Knudsen ES, Reed MF. Retinoblastoma deficiency increases chemosensitivity in lung cancer. Cancer Res. 2007;67:8264–8273. doi: 10.1158/0008-5472.CAN-06-4753. [DOI] [PubMed] [Google Scholar]
- Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, Harbour JW, Dean DC. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Inhibition of p38, but not Cdks, inhibits etoposide induced phosphorylation of Rb. (A) Mel202 cells were treated with Cdk4 inhibitor and 12.5 μM etoposide for 48 h and immunoblotted for Rb. (B-D) Kinase assays confirmed that the Cdk2, p38 and Cdk4 inhibitors were active.
Supplemental Figure 2. Caspase inhibition fails to block etoposide induced phosphorylation of Rb. ZVAD-FMK and etoposide were used at 50 μM and 12.5 μM respectively for 72 h. Lysates were immunoblotted for Rb.
Supplemental Figure 3. Rb is ubiquitinated and degraded in the proteosome. (A) Mel202 cells were treated with 50 nM of scrambled or Hdm2 siRNA for 72 h and immunoblotted for Rb or Hdm2. (B) Mel202 cells were treated with 50 μM of MG132 for 3 h and immunoblotted for Rb.