Abstract
Sphingolipids including glycosphingolipids have myriad effects on cell functions and affect cancer in aspects of tumorigenesis, metastasis and tumor response to treatments. Bioactive ones like ceramide, sphingosine 1-phosphate and globotriaosylceramide initiate and process cellular signaling to alter cell behaviors immediately responding to oncogenic stress or treatment challenges. Recent studies pinpoint that sphingolipid-mediated gene expression has long and profound impacts on cancer cells, and these play crucial roles in tumor progression and treatment outcome. More than ten sphingolipids and glycosphingolipids selectively mediate expressions of approximate fifty genes including c-myc, p21, c-fos, telomerase reverse transcriptase, caspase-9, Bcl-x, cyclooxygenase-2, matrix metalloproteinases, integrins, Oct-4, glucosylceramide synthase and multidrug-resistant gene 1. By diverse functions of these genes, sphingolipids enduringly affect cellular processes of mitosis, apoptosis, migration, stemness of cancer stem cells and cellular resistance to therapies. Mechanistic studies indicate that sphingolipids regulate particular gene expression by modulating phosphorylation and acetylation of proteins that serve as transcription factors (β-catenin, Sp1), repressor of transcription (histone H3), and regulators (SRp30a) in RNA splicing. Disclosing molecular mechanisms by which sphingolipids selectively regulate particular gene expression, instead of other relevant ones, requires understanding of the exact roles of individual lipid instead of a group, the signaling pathways that are implicated in and interaction with proteins or other lipids in details. These studies not only expand our knowledge of sphingolipids, but can also suggest novel targets for cancer treatments.
Keywords: gene expression, ceramide, sphingosine 1-phosphate, cancer, drug resistance, apoptosis
1. Introduction
Sphingolipids are a class of lipids derived from the aliphatic amino alcohol sphingosine and present mainly in eukaryote membranes [1-2]. All sphingolipids consist of sphingoid base (phytoceramide or sphinganine) linked to a fatty acid, and ceramide is the simplest one in structure (Fig. 1). Diverse sphingolipids result from different hydrophobic sphingoid bases combined with fatty acids; both vary in chain length, and degrees of saturation and hydroxylation. Complex sphingolipids possess additional hydrophilic regions, such as phosphate, phosphorylcholine and sugar moieties attached to sphingoid base in the R position (Fig. 1) [3-4]. Glucose or galactose replaces “H” in the R position, attached to the 1-hydroxy group of ceramide, and generates the simple glycosphingolipid, glucosylceramide by UDP-glucose:ceramide glucosyltransferase (UGCG; glucosylceramide synthase, GCS) or galactosylceramide by UDP-galacose:ceramide galacosyltransferase (CGT; galactosylceramide synthase), respectively. From these, more complex glycosphingolipids, such as lactosylceramide, globotriaosylceramide (Gb3) and monosialoganlioside GM3 can be synthesized by incorporation of additional glycose subunits in the Golgi [1, 5] (Fig. 2). Biosynthesis of sphingolipids is intertwined and regulated by two different enzymes, respectively. Ceramide is in the centre of metabolism, and predominantly synthesized by the de novo pathway from serine and palmitoyl-CoA in the endoplasmic reticulum (ER) and ER-associated membrane. Ceramide can be produced from sphingomyelin breakdown catalyzed by sphingomylinases (SMase) in the inner leaflet of plasma membrane (neutral SMase) or outer leaflet of lysosome membrane (acid SMase) [1, 6]. The generic “ceramide” is a family of more than 50 distinct molecular species that are synthesized by 6 ceramide synthases (CerS1-6 or longevity assurance genes, LASS1-6) that catalyze dihydro-sphingosine acylation in the de novo biosynthetic pathway [7-8]. CerS1-6 selectively utilize variant acyl-CoA (CerS1, C18; CerS2, C22-24; CerS3, C16-26; CerS5, C18/C20; CerS5, C16; CerS6, C14-16) to produce different ceramides. However, C18-ceramide is the major one. Ceramide can be metabolized to diverse derivatives like glucosylceramide, ceramide 1-phosphate, 1-O-acylceramide and sphingosine by respective kinases and synthases [9-10] (Fig. 2).
Fig. 1.
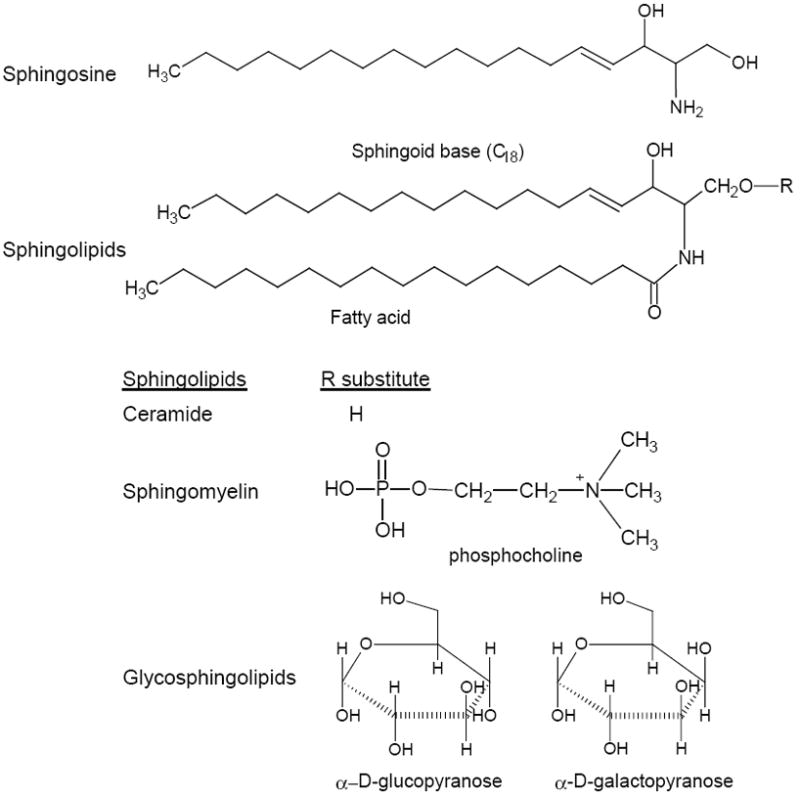
Basic structures and classification of sphingolipids. In mammals, the prevalent sphingoid base is sphingosine which has a chain length of 18 carbon atoms and E-double bond between C4 and C5.
Fig. 2.
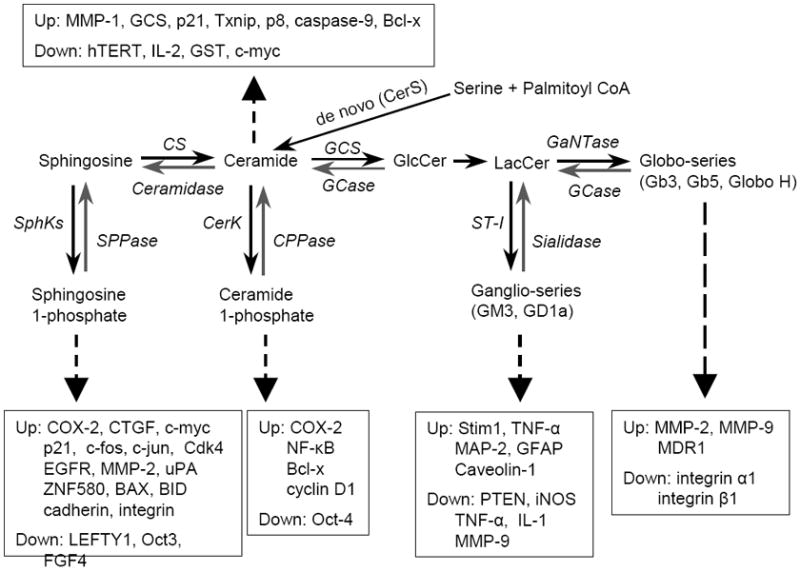
Biosynthetic pathway of sphingolipids that mediate expressions of genes associated with cellular processes in cancer. Dotted arrows indicate particular sphingolipids that up- or down-regulate gene expression (Up, Down). CerS, ceramide synthase; GCS, glucosylceramide synthase; LacCerS, lactosylceramide synthase; Gb3S, globotriaosylceramide synthase; GCase, glucosylceramide β-glucosidase; GLA, α-galactosidase A; GALC, galactosylceramidase; SphK, sphingosine kinase; SPPase, sphingosine phosphate phosphatase; CerK, ceramide kinase; CPPase, ceramide phosphate phosphatase; GM3S, GM3 synthase.
Sphingolipids are important biological molecules and highly associated with several diseases including cancer. Besides providing structural integrity in cell membranes, sphingolipids play crucial roles in signal transduction and gene regulation. Through these, sphingolipids actively modulate various aspects of cells including apoptosis, proliferation, endocytosis, transport, migration, senescence, and inflammation [1]. These sphingolipid-modulated processes, in turn are crucial in tumorigenesis, cancer progression, and the efficacies of cancer therapies [11-14]. The balance between different types of sphingolipids can make cells undergo malignant growth or rescue cancerous cells to normal. The rate-limiting enzymes in sphingolipid metabolism actively participate in cancer biology by shifting reactions and favoring metabolites in a particular direction [13, 15]. Sphingolipids can influence cellular processes directly through interactions with effectors, example in ceramide-induced mitochondria activation to apoptosis [1, 16]. Sphingolipids, particularly glycosphingolipids (GSLs) form different rafts or GSL-enriched microdomains (GEM) in the plasma membrane and thus supporting or modulating definite signaling cascades [1, 17]. Several comprehensive reviews have summarized the progress on dys-regulated sphingolipid metabolism and cancers [10, 13-14, 18]. Compared to other aspects of sphingolipid studies, little is known about the role of sphingolipids in gene regulation. In this review, we discuss the evidence that ceramide, S1P, GSLs and others are involved in modulating expression of genes contributing to cell proliferation, apoptosis, metastasis, cancer stem cells and drug resistance [17, 19-24]. To face the challenge of understanding mechanisms by which sphingolipids regulate gene expression, we examine these findings with relation to the promoter activation, the epigenetic effects of histone acetylation and DNA methylation, and post-transcriptional processing. We also consider the treatment of tumors through the inhibition of enzymes in sphingolipid metabolism that mediate the processes of gene regulation.
2. Progress in Research on Sphingolipid-Mediated Gene Expression
The production of particular sphingolipids can be activated through various stimuli including growth factors, oncogenic proteins, irradiation and anticancer drugs [10, 13-14, 25]. The bioactive sphingolipids generated may affect cancer cells at two levels: directly modifying the functions of effectors involved in cell processing, and mediating the expression levels of these effectors. Sphingolipids activate signaling pathways, which in turn immediately affect cell functions through modifying the functions of effectors. This may have temporary effects on cancer. Sphingolipid-mediated expression of genes may have profound impacts in the long-term and play more important roles in cancer development and progression. An increasing body of evidence indicates that sphingolipids mediate the expression of genes and broadly affect almost all aspect of cancer cell biology. As illustrated in Fig. 2, sphingosine, S1P, ceramide, C1P, ganglio-series (GM3, GD1a) and Globo-series of GLSs (Gb3, Gb5, SSEA3, globo-H) can modulate numerous genes involved in cell proliferation, apoptosis, metastasis, cancer stem cells and subsequent response to therapies.
2.1. Sphingolipids regulate genes associated with cell proliferation
Among sphingolipids, ceramide and sphingosine have been reported to regulate genes involved in cell proliferation. It is widely accepted that alterations of COX-2 and its product prostaglandin E2 (PGE2) are associated with cell proliferation in inflammation and cancers. COX-2 is a therapeutic target for cancers. Neutral sphingomylinase (SMase) hydrolyzes sphingomyelin to ceramide in the inner leaflet of plasma membrane. In human mammary epithelial cells (184B5/HER), endogenous ceramide generated after neutral SMase treatments (5 to 100 μunit/ml, 4.5 hr) and cell-permeable ceramides (C2-ceramide or C6-ceramide at 5 μM and 10 μM) activated the COX-2 promoter 4-fold and significantly increased the levels of COX-2 mRNA, and PGE2 production [26]. Induction of COX-2 by ceramide was inhibited by calphostin C, an inhibitor of protein kinase C. Triggering the ceramide-pathway also leads to an increase in extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK) activities. Overexpression of ERK1, JNK and p38 leads to several-fold increases in COX-2 promoter activity [26]. By comparison, overexpression of dominant negatives for ERK1/2, JNK, or p38 blocked the activation of COX-2 promoter activity by SMase [26]. C1P exerts its mitogenic kinase effects and implicates stimulation of the ERK1/2, phosphatidylinositol 3-kinase (PI3-K)/protein kinase B (PKB, also known as Akt), and JNK pathways [27-28]. In this manner, ceramide mediates COX-2 expression via C1P and MAPK signaling. Possible in the same way, C1P increases the expression of c-myc, cyclin D1 and NF-κB [27]. S1P generated by SphK1 is necessary for TNF-α and IL-1β to induce COX-2 [29-30]. S1P induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in vascular smooth muscle cells [31].
It has been reported that ceramide as an important mediator of cell growth arrest, upregulates the cyclin dependent kinase inhibitor p21 to induce the dephosphorylation of phosphorylated Rb and G1 arrest [32-34]. C6-Ceramide (15 μM) can induce p21 expression by either p53-dependent or independent pathway in SK-Hep-1 and Hep3B hepatocarcinoma cells and, thus inhibit cyclin-dependent kinase 2 (CDK 2) resulting in a G1 arrest [33, 35]. Increased hepatic p21 levels are highly associated with elevated C16-ceramide in CerS2 null mouse which has defects in synthesizing long acyl chain ceramides (C22-24) [34]. Nuclear S1P generated by SphK2 induces p21 expression in response to doxorubicin stress which is independent of p53 in MCF-7 breast cancer and HCT116 colon cancer cell lines [36-37]. Increased C2-ceramide, but not C2-dihydroceramide (20 μM) represses glutathione S-transferase (GST) expression by inhibiting the transactivation of CCAAT/enhancer binding protein-β (C/EBPβ) and NF-E2-related factor-2 (Nrf2) on GST promoter. Thus ceramide inhibits the effects of hepatic nuclear factor-1 (HNF1) on cell survival of rat H4IIE hepatocytes [38]. Ceramide is an important mediator of the effect of tumor necrosis factor-α (TNF-α) on growth inhibition and apoptosis. TNF-α, like other extracellular agents, such as 1,25-dyhydroxyvitamine D3, γ-interferon and interleukin-1 activates neutral sphingomyelinase resulting in hydrolysis of membrane sphingomyelin to generate ceramide and inhibit cell proliferation [39-40]. Both endogenous ceramide formed by TNF-α induction and C2 ceramide (10 μM, 2 hr) cause 80% inhibition of c-myc mRNA levels in HL-60 myeloid leukemia cells through ceramide-activated protein phosphates (CAPP) and blocks transcription elongation through the first exon of c-myc [39]. Endogenous C16-ceramide or C6-ceramide also shows the same effect on c-myc [40]. Ceramide represses the expression of human telomerase reverse transcriptase (hTERT) that synthesizes telomere to protect cancer cell immortality [19, 41-42]. It has been found that C6-ceramide (20 μM, 24 hr) and endogenous C16-ceramide activate the transactivation and down-regulated expression of hTERT by rapid proteolysis of the ubiquitin-conjugated c-myc transcription factor in A549 lung cancer cells [19, 21]. De novo-generated endogenous C18-ceramide formed by ceramide synthase 1 (CerS1) represses the hTERT promoter activity via deacytylation of Sp3 by histone deacetylase 1 (HDAC1) in A549 human lung adenocarcinoma cells [41-42].
Cell proliferation is also modulated by GSLs through expression alterations of genes involved in the cell cycle. A synthetic glycosphingolipid 7, Manβ(1-4)[Fucα(1-3)]Glcβ1-Cer, (50 μM, 15 hr) significantly suppresses the expressions of cyclin D1 and cycline-dependent kinase 4 (CDK4), but has no effect on p21 expression, through the FAK-Akt pathway and Erk 1/2 in B16F10 melanoma cells [43]. The monoganglioside GM3 has been shown to have anti-proliferative effects in several in vitro and in vivo cancer models. GM3 (30 μM, 24 hr) inhibits cell proliferation in HCT116 colon cancer cells, since it induces the expressions of tumor suppressor PTEN, p53 and p21 by PI3K/AKT/MDM2 signaling [44]. Transcription factor AP-2α is required for the GM3-stimulated transcription of PTEN gene and this is not associated with p53 in HCT116 cells [45].
S1P, a zwitterionic lysophospholipid shows pro-proliferative effects. S1P is generated by the enzyme ceramide and the subsequent conversion of sphingosine to S1P which is catalyzed by sphingosine kinase (SphK). S1P binds to a family of five G protein-coupled receptors termed S1PR1~5. S1PRs which are coupled to heterotrimeric G proteins and Rac or Rho to control various effects, such as MAPKs [18]. It has been found that S1P induces the expression of connective tissue growth factor (CTGF) that regulates cell proliferation, fibrosis, angiogenesis and apoptosis [46]. In WiT49 Wilms tumor cells, S1P (200 nM, 24 hr) induces CTGF expression through activation of RhoA/Rock and C-Jun NH2-terminal pathway [46]. The specific S1P receptor 2 (S1PR2) antagonist JTE-013 (100 nM) can completely prevent S1P induction in CTGF [46]. S1P can also elicit intracellular actions and these may be strongly associated with its role in mediation of gene expression, even though the intracellular targets are not fully defined [47]. Extracellular S1P signaling via its receptors on plasma membranes is not involved in adenoma cell proliferation in APCMin/+ mice; S1P via intracellular signaling induce the expression of c-myc and CDK4 [48]. After sphingosine treatment (10 μM), S1P generated by SphK1 increases Cdk4 expression through Rb phosphorylation in rat RIE intestinal epithelial cells [48]. S1P induces c-Jun and c-fos expression in cancer cells [37, 49]. Addition of S1P (100~1000 nM, 30 min~2 hr) transactivates c-jun and c-fos in rat HTC4 hepatoma cells [49]. Silencing or overexpression of SphK2 that located in chromatin indicates that S1P induces c-fos expression by enhanced H3 acetylation in MCF-7 breast cancer cells [37]. S1P induces the expression of epidermal growth factor receptor (EGFR) via Akt/NF-kB and ERK/AP-1 pathways as shown in a time- and concentration-dependent manner in rat vascular smooth muscle cells (VSMCs) [50].
Briefly, ceramide, sphingosine, GM3 and S1P mediate expression of gene regulating cell proliferation. Through upregulation of COX-2, CTGF, c-myc, c-jun, c-fos, EGFR and CDK4 expression, these sphingolipids can permanently promote cell proliferation. The dominant effects of ceramide and GM3 are anti-proliferation, as ceramide upregulates p21 expression and represses hTERT and c-myc expression. GM3 upregulates PTEN, p53 and p21 expression meanwhile it suppresses cyclin D1 and CDK4 expression.
2.2. Sphingolipids regulate genes associated with apoptosis
Ceramide is a crucial sphingolipids in cell death signaling molecule. Ceramide produced from sphingomyelin cycling or the de novo synthesis can directly inhibit mitochondrial respiratory chain complex III, mediate the permeability transition pore complex (PTPC) to release cytochrome-c and promote apoptosis via ceramide activated serine/threonine protein phosphatase (CAPP1, CAPP2) and proline-directed protein kinase/protein kinase C [51-52]. Sphingosine acts in a similar manner as ceramide and induces apoptosis by activating Bax-mediated cytochrome-c from mitochondria advancing to activation of caspase-2 and caspase-9 [53]. In addition to this transcription-independent manner, ceramide and other sphingolipids can mediate the expression of genes modulating apoptosis. Endogenous ceramide after chemotherapy or C2-ceramide (40 μM, 6 hr) upregulate the expression of thioredoxin interacting protein (Txnip), a tumor suppressor gene and cause cells to apoptose; this processing is through activation of ASK1, p38 and JNK pathway in mouse 10I T hybridoma and human Jurkat T-cell lines [54]. Ceramide generated by antitumoral agent tetrahydrocannabinol (THC) in the de novo synthesis upregulates the expression of stress-regulated protein p8, that in turn mediates activating transcription factor 4 (ATF-4), C/EBP homologous protein (CHOP) and (endoplasmic reticulum stress-related genes) TRB3 and leads MiaPaCa2 and Panc1 pancreatic cancer cells and others to apoptosis in vitro and in vivo [55-56]. Exogenous C2-ceramide up-regulates p8 expression, but cannot induce ATF-4, CHOP and TRB3 in U87MG astrocytoma cells after p8 silencing [56]. Interestingly, ceramide generated from the de novo pathway in response to anticancer drug gemcitabine regulates the alterative splicing of caspase-9 and Bcl-x in A549 lung adenocarcinoma [57-59]. Gemcitabine (1 μM, 24 hr) or C6-ceramide (20 μM, 24 hr) down-regulate the levels of Bcl-x(L) and caspase-9b mRNA with a concomitant increase in the mRNA levels of Bcl-x(s) and caspase-9 [58-59]. C1P (30 μM, 30 hr) induces the expression of anti-apoptotic Bcl-x expression detected by Western blotting in bone marrow-derived macrophage (BMDM) [60]. GCS catalyzes ceramide glycosylation and is the rate-limiting step for ceramide levels and GSLs synthesis. Interestingly, we have found that ceramide up-regulates the expression of GCS that catalyzes ceramide glycosylation and this positive feedback regulation depends on Sp1 transcription factor [61]. In this way, upregulated GCS confers cell resistance to ceramide induced apoptosis when MCF-7 breast cancer cells exposed to TNF-α and doxorubicin [61-63]. Gangliosides derived from spontaneous T cell lymphoma (2-30 μM, 24 hr) suppress the expressions of inducible nitric oxide synthase (iNOS), TNF-α and IL-1β at the protein level, and consequently inhibit nitric oxide production and apoptosis of macrophages and microglia [64-65]. Gangliosides activate macrophage and microglia via PKC and NFκB [65]. It suggests that by modulation of macrophages and microglia, gangliosides play important roles in tumor immune-surveillance.
Ceramide upregulates the expression of pro-apoptosis genes including Txnip, p8, caspase-9, Bcl-x and prompts cancer cells to apoptosis. This transcription-dependent apoptosis consequently follows the transcription-independent one that ceramide directly acts on mitochondria and has long-term and profound effects, particularly in chemotherapy or radiation therapy of cancer.
2.3. Sphingolipids regulate genes associated with tumor metastasis
Sphingolipids, particular of GSLs mediate the expression of genes involved in cell migration, invasion and angiogenesis contributing to tumor metastasis. Matrix metalloproteinasese (MMP) are zinc-dependent proteolytic enzymes, which are involved in degradation of the extracellular matrix and play critical roles in cell migration and matrix remodeling during angiogenesis. S1P upregulates the expression of urokinase plasminogen activator (uPA), a protein known to stimulate cancer cells invasiveness in via S1P1 receptor in human U-118 glioblastoma multiforme cells [66]. S1P up-regulates the expression of MMP-2 expression via ERK-NFκB and calcium influx dependent signaling pathways in HUVECs and EAby925 endothelial hybridoma cells [67]. Exogenous S1P treatments (1 μM, 4 hr) significantly increase the levels of MMP-2 mRNA and protein and its gelatinolytic activities in dose- and time-dependent manner [67]. In this study, lysophospholipids (LPA) shares the same effects on MMP-2 with S1P. Endogenous ceramide by SMase (1-100 mU/ml, 24 hr) or C2 and C6 ceramide (10-100 μM) induces collagenase-1 or MMP-1 expression in mRNA and protein in human ski fibroblasts [68]. Ceramide activates MMP-1 promoter via AP-1 in fibroblasts and this induction is via MAPK pathways including ERK1/2, SAPK/JNK, and p38 [68]. S1P mediate cell migration and proliferation of endothelial cells that are critical for tumor angiogenesis [69]. C2H2-zinc finger (ZNF) proteins usually play an essential role in altering gene expression and regulating angiogenesis. It has been reported that S1P (0.5-10 μM, 1-12 hr) upregulates both ZNF580 mRNA and protein levels through S1P receptors (S1P1, S1P3, S1P5) and p38-MARK pathway in a concentration- and time-dependent manner in human EAhy926 endothelial cell hybridoma cells [70]. ZNF580 further regulates vascular endothelial growth factor (VEGF) and MMP-2 expression in EAhy926 cells [70].
Disialoganglioside GD1a is responsible for regulating cell motility, cell adhesiveness to vitronectin, phosphorylation of c-Met and metastatic ability of cancer cells. GD1a upregulates caveolin-1 and Stim1 (transformation suppression genes) and may reduce cancer cell metastasis [71]. Caveolin-1 and Stiml are highly expressed in mouse FBJ-S1 cells enriched GA1a, compared to FBJ-LL cells. Introduction of 1-4GalNAcT-1 (GM2/GD2 synthase) into FBJ-LL cell significantly increase caveolin-1 and Stim1, and silencing of St3Gal5 by siRNA repression of caveolin-1 and Stim1 in FBJ-S1 cells. Exposure of mouse melanoma B16 cells and human HepG2 hepatoma cells to GD1a elevates caveolin-1 and Stim1 [71]. Using these approaches including gene transfection and silencing of GM2/GD2 synthase and exogenous GD1a exposure, it also has been found that GD1a suppresses MMP-9 expression level in FBJ-S1 osteosarcoma cells [71-72]. Additionally, GD1a (50 μM, 12 hr) and GM1 suppresses TNF-α expression. Deletion of glycosphingolipid by inhibition of GCS with D-PDMP (12.5 μM, six days) increases TNF-α expression in FBJ cell variants by protein kinase N1 (Pkn1) that is a serine/threonine kinase, like PKC, and mediates cellular response to stress [73]. In contrast to GD1a, GM3 upregulates TNF-α expression via PI3K, Rictor/mTOR, Akt and also the Rho GDP-dissociation inhibitor 2 (Arhgdib) in mouse melanoma B16 cells [74-75]. GM3 (25 μM, 24 hr) induces the levels of mRNA and protein in mouse B16, B11 and CAH-3 melanoma cell lines; silencing of St3gal 5 gene, an enzyme responsible for GM3 synthesis represses TNF-α expression. Furthermore, inhibition of PI3K with LY294002 (20 μM) or silencing of Akt suppresses TNF-α expression in B16 cells [74]. Monosialyl-Gb5 (MSGb5), one of the globo-series of GSLs is also known as SSEA-4 (stage-specific embryonic antigen-4) and is a GSL found in GSL-enriched microdomains (GEMs) that are maximally expressed in human renal cell carcinomas and correlated with metastasis [76-77]. It has been found that clustering MSGb5 by ET-18-OMe (1-o-octadecyl-2-O-methyl-glycerophosphocholine) (15 μg/ml, 30 min) in GEMs increases the expressions of MMP-2 and MMP-9, and decreases the expression of integrin α1 and integrin β1 in human MCF-7 breast cancer cell variants [78]. Inhibition of cSrc kinase with PP1 (4-amino-1-tert-butyl-3-1’-naphthyl)pyrazolo[3,4-d]pyrimidine) suggests that the inductive effects of MSGb5 on the gene expression are dependent on cSrc signaling [78-79].
It can be concluded that S1P and GSLs are major molecules to promote cancer metastasis. S1P upregulates MMP-2, uPA, and ZNF580 expression promoting cancer cell invasion, migration and angiogenesis. Among GSLs, GD1a upregulates caveolin-1 and Stim1 expression, meanwhile MSGb5 induces MMP-2 and MMP-9 expression contributing to metastasis.
2.4. Sphingolipids regulate genes associated with cancer stem cells
Sphingolipids play crucial roles in determining stem cell fate including self-renewal, proliferation and differentiation; and in particular, sphingolipids can be developed as therapeutic agents to eliminate cancer stem cells [80-82]. Cell surface GSLs, globopentaosylceramide (Gb5) and MSGb5 are known SSEA-3 and SSEA-4 (stage specific embryonic antigen-3, -4), as markers on human ES cells [81, 83-84]. SSEA-3 and Globo H are markers for a subpopulation of CSC in breast cancer patients [85]. Breast cancer stem cells with CD55 are highly resistance to ceramide or serum-deprivation induced apoptosis, and exposure to ceramide (nano-liposomal C6-ceramide 3 μM) prevents premature human ES cell differentiation and maintains pluripotent stem cell populations in vitro [86-87]. Addition of serum (10% fetal bovine serum, 24 hr) or inhibition of STAT3 phosphorylation with WP1193 (5 μM, 24 hr) significantly decreases the numbers of human GCS11 (CD133+) glioblastoma CSC cells, accompanied with decreased glucosylceramide synthase [88]. Deoxycholate promotes the survival of mouse breast CSC cells (CD44+/Flk-1+) by reducing ceramide levels [89]. A novel ceramide analogue S18 (N-oleoyl serinol) that has ceramide bioactivity can selectively induce apoptosis in PAR-4+ embryonic stem cells [90]. In cell and animal models, S18 exposure (80 μM, 72 hr) enriches the embryonic stem cells that have low levels of PAR-4 and Oct-4, and are able to undergo neural differentiation [91]. Together, these suggest that ceramide glycosylation is one of the mechanisms that maintain CSC cells in their de-differentiated state.
S1P mediates proliferation and multipotency of adult stem cells including mesoangiblast, bone marrow stem cells and adipose tissues-derived stem cells [88, 92]. S1P stimulates the functional capacity of endothelial progenitor cells, and augments neovascularization in hindlimb ischemia, since S1P or its synthetic analog FTY720 activates CXCR4-dependent signaling pathway via the S1P3 receptor [93]. S1P generated from osteoclasts recruits osteoblasts and promotes their differentiation to bone formation via the S1P1 receptor and induced expression of Wnt10b and BMP6; since a S1P1 receptor antagonist, VPC 23019 (1 μM, 7 days) decreases the differentiating effects of S1P on osteoblasts [94]. S1P exposure (20 μM, 5-9 hr) upregulates the expression of BAX, BID, cadherins and integrins, meanwhile it down-regulates LEFTY1, Oct-4, and FGF4 expression and pluripotency. In this way, S1p determines the fate of human Shef 4 embryonic stem cells [95]. Sphingolipid extracts (C18) from placenta restore the expression of microphthalmia-associated transcription factor (Mift) that is reduced in deactivated melanocyte stem cells, and then promote hair pigmentation from melanocyte stem via p38 stress-signaling [96-97]. Neuronal and gial cells in the central nervous system are generated from common neural precursor cells, neuroepithelial cells during development. Inhibition of ceramide glycosylation by using GCS inhibitor, D-PDMP depletes GD3, a major ganglioside in neuroepithelial cells and represses FGF-2 induced proliferation via the Ras-MAPK pathway [98]. Decreased GM1 and GD3 via silencing of GCS decrease the expression levels of microtubule-associated protein 2 (MAP-2) and glial fibrillary acidic protein (GFAP) in mouse embryonic stem cells [99].
In addition to Gb5 and MS-Gb5 that act as the stage specific markers of human ES cells, GM1 and GD3 mediate MAP-2 and GFAP expression in mouse ES cells. S1P mediates LEFTY1, Oct-4 and FGF4 expression promoting osteoblasts to differentiation. It is clear that ceramide glycosylation is one important mechanism modulating the self-renewal and pluripotency of stem cells. Ceramide and S1P can significantly mediate c-myc, c-fos, c-jun and hTERT expression (Fig. 2). It should be interested to observe how sphingolipids mediate oncogene and SC regulator expression synergistically to determine the formation and pluripotency of CSC. It may find novel approaches based on disruption of ceramide glycosylation or sphingosine phosphorylation to target CSC.
2.5. Sphingolipids regulate genes associated with drug resistance
Drug resistance of cancer cells is the outcome of multiple-gene interactions in cancer cells under the action of antineoplastic agents or irradiation [100-101]. In addition to affecting signal transduction and membrane transportation, sphingolipids can modulate the expression as well as function of drug-resistant genes including Bcl-2, p53 and ABC transporters to alter cell responses to treatments. Disruption of S1P lyase that irreversibly degrades S1P in knockout mice demonstrates S1P is a cause of drug resistance and tumorigenesis through induction of Bcl2/Bcl-xl expression [102]. Recently, we and other groups have characterized the role of ceramide glycosylation in drug resistance [61, 63, 103-104]. Ceramide upregulates GCS expression that confers cell resistance to anticancer drugs [61, 104]; furthermore, we have found that globo-series GSL (Gb3, Gb5) upregulates MDR1 gene expression via cSrc and β-catenin pathways [103] (Fig. 2). The mixed-backbone oligonucleotide against GCS (MBO-asGCS) effectively represses MDR1 and GCS expression and effectively sensitizes drug resistance in several different cancer cell lines and tumor-bearing mice [103, 105-106].
It is clear that S1P and globo-series GSL can upregulate drug-resistant genes including Bcl-2 and MDR1. Ceramide is a nature substrate of GCS and it does feedback upregulate GCS expression (Fig. 2). In this manner, the enhanced GCS and the upregulated MDR1 cause drug resistance via eliminating ceramide-induced apoptosis and drug efflux. Ceramide-induced cell death is an effective way for anticancer drugs to kill cancer cells. Understanding the details showing how ceramide induce GCS in cancer cell may find any specific approach to prevent and reverse drug resistance.
3. Mechanisms of gene regulation by sphingolipids
Understanding how sphingolipids mediate gene expression requires elucidation of the mechanisms by which these lipids act. Currently, based on available literature, sphingolipids impose their actions by two mechanisms: lipid-lipid interactions, whereby the candidate bioactive sphingolipid affects membrane structure and/or the interaction of membrane proteins with the membrane bilayer [107-108]; or lipid-protein interactions, whereby changes in sphingolipids modulate the functions of target proteins that interact specifically with the candidate bioactive lipid [1]. Bioactive sphingolipids which are regulated by various extracellular signals, themselves mediate expression of other genes. Based on available evidence, we have briefly generalized that sphingolipids mediate the expression of genes three mechanisms, as follows.
3.1. Modulation of protein kinases and their signal cascades alters transcription factor actions in gene transcription
The important research from the Kahari and Ogretmen groups have elucidated how the cellular lipid, ceramide regulates gene expression of MMP-1 [68] and hTERT [19, 41-42]. Ceramide upregulates MMP-1 expression by transactivation of its promoter at the AP-1 cis-element and this depends on the activation of three distinct MAPKs including ERK1/2, SAPK/JNK and p38 [68]. This cascade also has been demonstrated in ceramide-induced COX-2, in which MAPKs activate COX-2 promoter in CRE site [26]. Cellular ceramide (C18-Cer) synthesized by CerS1 represses the hTERT promoter activity by decreasing Sp1 and Sp3 deacetylation by HDAC1 to diminish recruitment of RNA polymerase II to hTERT promoter [41-42]. The repressive effects of ceramide on hTERT are also dependent on JNK activation [109]. Ceramide suppresses PKC activity (PKCθ, PKCα) and NF-κB activation to repress IL-2 expression [110]. Ceramide activates GCS promoter by Sp1 [61] to upregulate GCS expression and represses GST transactivation via HNF1 degradation [38]. Ceramide may mediate the expressions of Txnip, p21 and other genes in the same way, but these are not fully understood yet.
Ganglio-series GM3 induces PTEN expression by AP-2α binding to the PTEN promoter [45]. Our group has recently proved that Globo-series GSLs upregulate MDR1 via activation of cSrc signaling and TCF4/β-catenin recruitment on MDR1 promoter [103]. As summarized in Fig. 3, sphingolipid activates cellular protein kinases (MAPK, PKC) and their signaling cascades, and phosphorylation modulates actions of transcription factors and activates or represses gene expression at the transcription level. In contrast to other sphingolipids, increase or decrease of GSLs will alter lipid-lipid interactions or lipid-protein interactions and mediate protein kinases (cSrc kinases) in GEMs of the plasma membrane. As showed in Fig. 3, doxorubicin increases ceramide generation via the de novo synthesis pathway and transactivates GCS expression via Sp1 transcription factor; enhanced globo-series GSLs (Gb3, Gb5) activates cSrc kinases, increases nuclear β-catenin by diminishing its degradation after phosphorylation and transactivates MDR1 expression. In this way, sphingolipids (ceramide, globo-series) upregulates GCS and MDR1 expressions in response to anticancer drugs and confer cell resistance by preventing ceramide-induced apoptosis and MDR1 drug-efflux [61, 103]. Interestingly, MBO-asGCS that silences GCS in the nanomole range reverses cell resistance by suppression of MDR1 and GCS [103, 106].
Fig. 3.
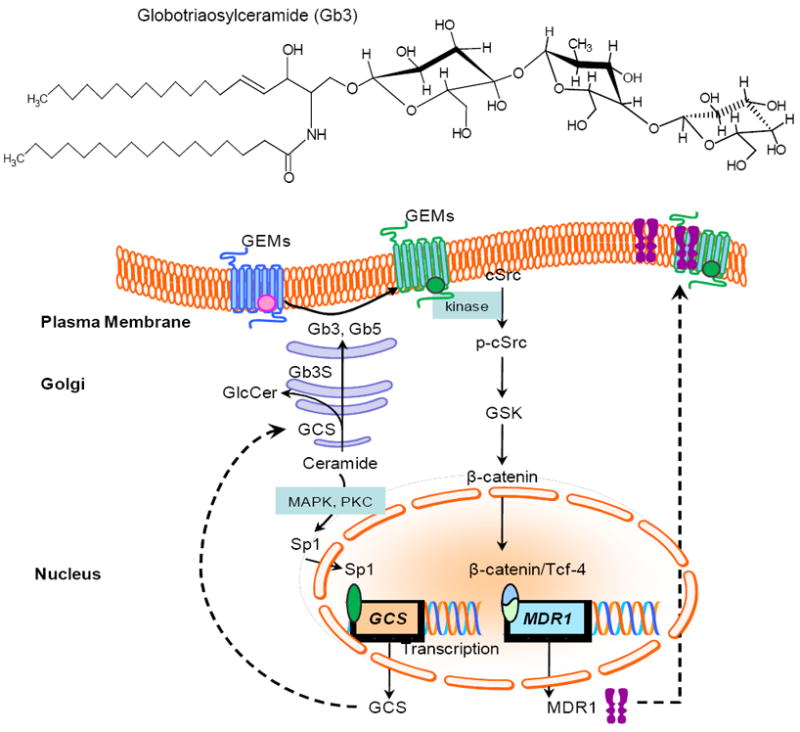
Ceramide and globo-series GSLs upregulate GCS and MDR1 leading cell resistant to anticancer drugs via protein kinase cascades and recruitment of transcription factors. Ceramide generated by de novo synthesis in response to stress transactivates GCS expression possibly by the MAPK or PKC cascades and the Sp1 transcription factor; globo-series GSLs (Gb3, Gb5) interact with lipids/protein on GEMs and activate cSrc-GSK cascade, consequently increase recruitment of β-catenin/Tcf-4 to upregulate MDR1. Mitogen-activated protein kinase; GEMs, GSL-enriched microdomains; GSK, glycogen synthase kinase-3.
3.2. Intracellular sphingolipids mediate gene expression via protein dephosphorylation and posttranscriptional processing
Chalfant et al have demonstrated that ceramide regulates the expressions of Bcl-x and caspase-9 isoforms in RNA splicing after transcription in human A549 lung [57-59]. As illustrated in Fig. 4, after generation, cellular ceramide activates PP1 (also named ceramide-activated protein phosphatase, CAPP) and dephosphorylates SRp30a (also named ASF/SF2), a SR protein that directs alternative splicing of pre-mRNA. SRp30a binds to the splicing sites of Bcl-x pre-mRNA or caspase-9 pre-mRNA with other proteins of spliceosome and selectively generates Bcl-x (S) or caspase-9. Ceramide thus inhibits Bcl-x (L) and caspase-9b splice variants that restrain apoptosis and increases Bcl-x (S) and caspase-9 variants which are pro-apoptotic [58]. Ceramide generated by the TNF-α-activated sphingomyelin cycle down-regulates c-myc expression through inducing a block to transcription elongation of the c-myc transcript in Exon II, without affecting transcription through the first exon [39]. This effect can be directed by PP1 [39]. Ceramide and subsequent PP1 activation also mediates alternative splicing of TRAIL and caspase-2 to generate TRAIL-β and 2S [111].
Fig. 4.
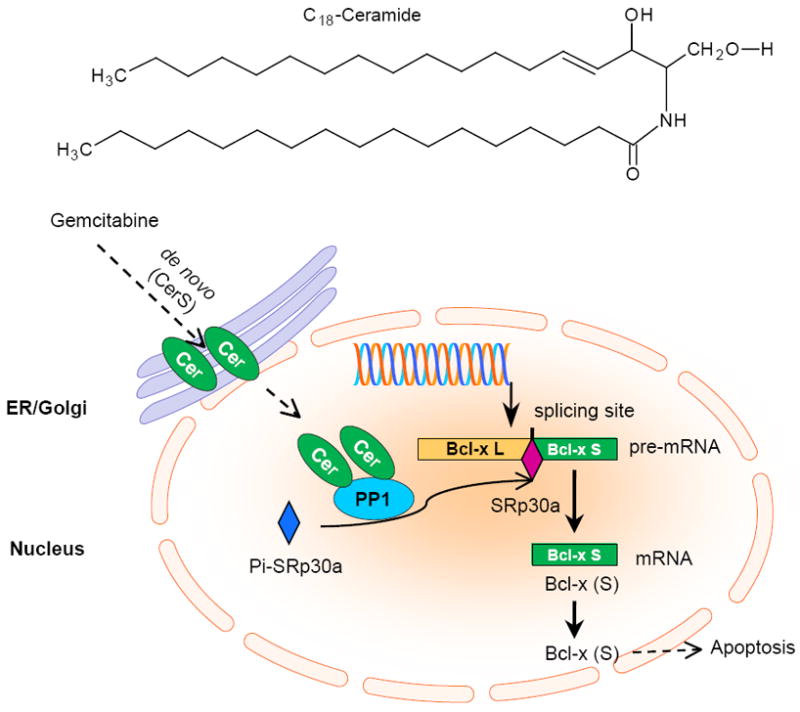
Cellular ceramide upregulates apoptotic Bcl-x or caspase-9 expression via activation of PP1 and RNA splicing. Ceramide generated in the de novo synthesis pathway responding to gemcitabine activates nuclear PP1 and increases the amounts of non-phosphorylated SRp30a that binds to the splicing sites of pre-mRNA of Bcl-x or caspase-9. These will increase the expression of Bcl-x (L) or the caspase-9b isoform that is the pro-apoptosis. PP1, protein phosphatase 1.
3.3. Nuclear sphingolipids modulate histone acetylation and upregulate gene transcription
Recently, the Spiegel group has demonstrated that nuclear S1P inhibits HDAC1/HDAC2 activities, increases histone H3 acetylation and releases the repressor complex from the promoter region to upregulate p21 and c-fos transcriptions [36-37, 69]. It provides a perfect model showing how phosphorsphingolipids closely mediate gene expression in the nucleus (Fig. 5). It is very interesting that SphK2 is predominately located with histone H3 in mononucleosomes of MCF-7 cells and generates S1P. S1P binds to HDAC1/HDAC2 to increase acetylation of lysine 9 of histone H3 (H3-K9) [37]. Sequentially, the H3 repressor complex is released from the promoter and transactivates the expression of p21 or c-fos [36-37, 69]. It is still not clear whether S1P also mediates other genes through this mechanism. C18-Ceramide synthesized by CerS1 results in repression of the hTERT promoter via deacetylation of Sp3 by HDAC1 [41]. C2-Ceramide down-regulates MMP-2 is associated with histone H3 acetylation [112].
Fig. 5.
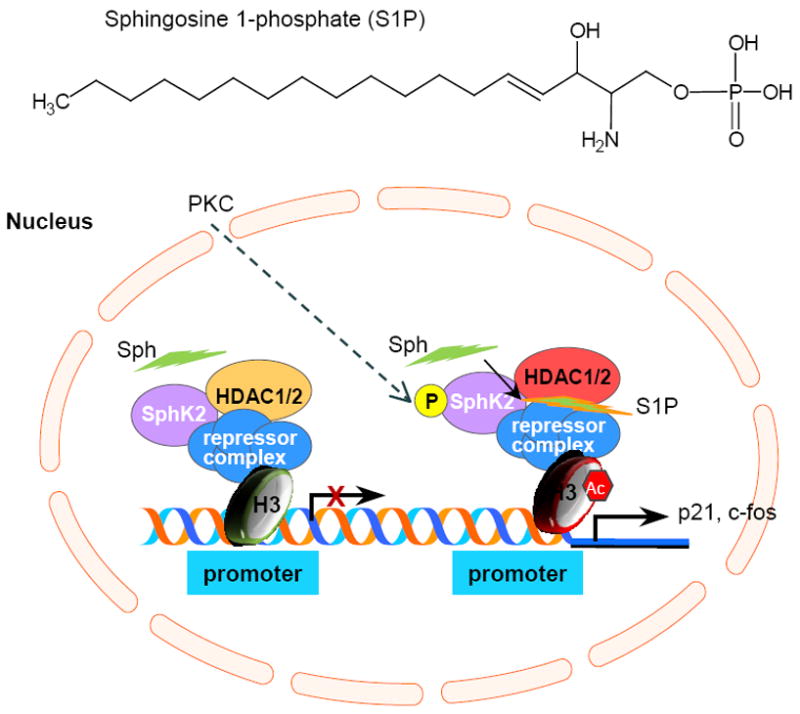
Nuclear sphingosine 1-phosphate upregulates p21 or c-fos expression via histone H3 acetylation and release of repressor complex. Nuclear SphK2 that is located with HDA1 and HDA2 produces S1P in response to PKC activation. S1P bound to HDAC1 or HDAC2 and prevents their deacetylation on histone H3 and increases the release of repressor complex from promoter region to express p21 or c-fos. SphK2, sphingosine kinase 2; Sph, sphingosine; AC, acetyl; HDAC1, histone deacetylase 1; HDAC2, histone deacetylase.
4. Concluding remarks and future directions
Bioactive lipids have promises to occupy centre-stage in cell biological research in the twenty-first century [1]. More than ten diverse species of sphingolipids mediate expressions of genes at the levels of transcriptional and posttranscriptional processing, besides altering cellular signaling to affect cell functions. As selected effectors to strengthen sphingolipid impacts, more than 50 genes that affect cell proliferation, apoptosis, metastasis, cancer stem cells and drug resistance have been identified in diverse cancer cells and animal models. Based on current evidence, it would be inaccurately to state which sphingolipids are pro- or anti-cancer compounds. However, glycosphingolipids (glucosylceramide, GM3, Gb3, Gb5, Globo H) and S1P are more likely to favor tumorigenesis and cancer progression, while ceramide as well as C1P often display anticancer effects, after they mediate particular genes. We have put forward three mechanisms to explain how sphingolipids mediate genes in cancer. These tentative models are based on available literature, but needs to be confirmed by further experimentations.
It is noticed that each type of sphingolipid (excepting sphingosine) has several distinctive molecular species that share similar chemical properties, but differ in length of their fatty acid chains (Fig. 1). Diverse ceramides (C16-24) are synthesized by six enzymes (CerS1-6) in different cellular compartments, cells and tissues, and each of these ceramides has its own effects on cells and in disorders [8, 34]. C18-Ceramide generated by CerS1, but not C16-ceramide by CerS5 or CerS6, represses hTERT promoter via deacytylation of Sp3 [113]. In addition to identification of particular signaling with gene silencing and profiling, and the molecular species of lipids with modern analytical techniques, characterization of enzymes that prefer particular carbon-chains in sphingolipid metabolism and application of cell-permeable bioactive ceramides (C16-C24) to investigate how they mediate genes are required. Sphingolipid transfer proteins (CERT, ceramide transfer protein; FAPP2, four-phosphate adaptor protein 2) that are regulated by PI4P mediate the ER-to-Golgi trafficking of ceramide and the cis- to trans-Golgi to ER trafficking of glucosylceramide [114-115]. CERT and FAPP2 may also be involved in mediating the effects of ceramide and glycosphingolipids on gene expression. Other sphingolipid-associated proteins that mediate the nuclear location of sphingolipids, like HDAC1/HDAC2 [37] may also play a regulatory role in gene expression.
On reviewing this sprouting area of research, we can say with confidence that sphingolipid-mediated gene expression play crucial roles in cancer biological research. By this epigenetic manner, the profound effects of bioactive sphingolipids formed in responding to oncogene activation, DNA damage and chemotherapy become relatively specific and consolidated, as exampled in ceramide induced drug resistance via the GCS and MDR1 (Fig. 3). Clearly, more study in this field will allow us understand insights of gene regulation, including potential mechanism via lipid-DNA interaction. These should offer novel and exciting strategies to tackle cancer effectively.
Acknowledgments
Current work in Y.Y.L. laboratory is supported by United State Public Health Service/NIH grant P20 RR16456 from the NCRR, and the Department Deference Breast Cancer Research Program DAMD17-01-1-0536.
Abbreviations
- C1P
ceramide 1-phosphate
- CAPP
ceramide-activated protein phosphatase
- CerK
ceramide kinase
- CerS
ceramide synthase
- CERT
ceramide transfer protein
- COX-2
cyclooxygenase-2
- CPPase
ceramide phosphate phosphatase
- CSC
cancer stem cell
- ES
embryo stem cell
- FAPP2
four-phosphate adaptor protein 2
- Gb3
globotriaosylceramide
- Gb5
globopentosylceramide
- GCase
glucosylceramide β-glucosidase
- GCS
glucosylceramide synthase
- GEM
GSL-enriched microdomain
- GSL
glycosphingolipid
- HDAC
histone deacetylase
- hTERT
human telomerase reverse transcriptase
- MAPK
mitogen-activated protein kinase
- MDR1
multidrug resistance gene 1
- MMP
matrix metalloproteinase
- MSGb5
monosialyl globopentosylceramide
- PKC
protein kinase C
- S1P
sphingosine 1-phosphate
- SMase
sphingomyelinase
- siRNA
small interfering RNA
- SphK
sphingosine kinase
- SPPase
sphingosine phosphate phosphatase
- SSEA
stage specific embryonic antigen
- TNF-α
tumor necrosis factor α
- uPA
urokinase plasminogen activator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Goni FM, Alonso A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta. 2006;1758:1902–21. doi: 10.1016/j.bbamem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–82. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson KA. On the character and functions of sphingolipids. Acta Biochim Pol. 1998;45:429–38. [PubMed] [Google Scholar]
- 5.Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. J Lipid Res. 2009;50(Suppl):S440–45. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolesnick RN, Haimovitz-Friedman A, Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem Cell Biol. 1994;72:471–74. doi: 10.1139/o94-063. [DOI] [PubMed] [Google Scholar]
- 7.Rabionet M, van der Spoel AC, Chuang CC, von Tumpling-Radosta B, Litjens M, Bouwmeester D, et al. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J Biol Chem. 2008;283:13357–69. doi: 10.1074/jbc.M800870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–05. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 9.Merrill AH, Jr, Stokes TH, Momin A, Park H, Portz BJ, Kelly S, et al. Sphingolipidomics: a valuable tool for understanding the roles of sphingolipids in biology and disease. J Lipid Res. 2009;50(Suppl):S97–102. doi: 10.1194/jlr.R800073-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–80. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett. 2006;580:5467–76. doi: 10.1016/j.febslet.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Ogretmen B, Hannun YA. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resist Updat. 2001;4:368–77. doi: 10.1054/drup.2001.0225. [DOI] [PubMed] [Google Scholar]
- 13.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 14.Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism--a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001;93:347–57. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- 15.Ruckhaberle E, Karn T, Rody A, Hanker L, Gatje R, Metzler D, et al. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J Cancer Res Clin Oncol. 2009;135:1005–13. doi: 10.1007/s00432-008-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Haefen C, Wieder T, Gillissen B, Starck L, Graupner V, Dorken B, et al. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21:4009–19. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- 17.Modrak DE, Gold DV, Goldenberg DM. Sphingolipid targets in cancer therapy. Mol Cancer Ther. 2006;5:200–08. doi: 10.1158/1535-7163.MCT-05-0420. [DOI] [PubMed] [Google Scholar]
- 18.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 19.Ogretmen B, Kraveka JM, Schady D, Usta J, Hannun YA, Obeid LM. Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line. J Biol Chem. 2001;276:32506–14. doi: 10.1074/jbc.M101350200. [DOI] [PubMed] [Google Scholar]
- 20.Ogretmen B, McCauley MD, Safa AR. Molecular mechanisms of loss of beta 2-microglobulin expression in drug-resistant breast cancer sublines and its involvement in drug resistance. Biochemistry. 1998;37:11679–91. doi: 10.1021/bi980573c. [DOI] [PubMed] [Google Scholar]
- 21.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, et al. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002;277:12960–69. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 22.Ogretmen B, Safa AR. Down-regulation of apoptosis-related bcl-2 but not bcl-xL or bax proteins in multidrug-resistant MCF-7/Adr human breast cancer cells. Int J Cancer. 1996;67:608–14. doi: 10.1002/(SICI)1097-0215(19960904)67:5<608::AID-IJC3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Ogretmen B, Safa AR. Expression of the mutated p53 tumor suppressor protein and its molecular and biochemical characterization in multidrug resistant MCF-7/Adr human breast cancer cells. Oncogene. 1997;14:499–506. doi: 10.1038/sj.onc.1200855. [DOI] [PubMed] [Google Scholar]
- 24.Ogretmen B, Schady D, Usta J, Wood R, Kraveka JM, Luberto C, et al. Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells. J Biol Chem. 2001;276:24901–10. doi: 10.1074/jbc.M100314200. [DOI] [PubMed] [Google Scholar]
- 25.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–18. [PubMed] [Google Scholar]
- 26.Subbaramaiah K, Chung WJ, Dannenberg AJ. Ceramide regulates the transcription of cyclooxygenase-2. Evidence for involvement of extracellular signal-regulated kinase/c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:32943–49. doi: 10.1074/jbc.273.49.32943. [DOI] [PubMed] [Google Scholar]
- 27.Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gomez-Munoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal. 2008;20:726–36. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Gangoiti P, Granado MH, Arana L, Ouro A, Gomez-Munoz A. Activation of protein kinase C-alpha is essential for stimulation of cell proliferation by ceramide 1-phosphate. FEBS Lett. 2010;584:517–24. doi: 10.1016/j.febslet.2009.11.086. [DOI] [PubMed] [Google Scholar]
- 29.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–21. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 30.Ohama T, Okada M, Murata T, Brautigan DL, Hori M, Ozaki H. Sphingosine-1-phosphate enhances IL-1{beta}-induced COX-2 expression in mouse intestinal subepithelial myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2008;295:G766–75. doi: 10.1152/ajpgi.90423.2008. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh HL, Wu CB, Sun CC, Liao CH, Lau YT, Yang CM. Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J Cell Physiol. 2006;207:757–66. doi: 10.1002/jcp.20621. [DOI] [PubMed] [Google Scholar]
- 32.Phillips DC, Hunt JT, Moneypenny CG, Maclean KH, McKenzie PP, Harris LC, et al. Ceramide-induced G2 arrest in rhabdomyosarcoma (RMS) cells requires p21Cip1/Waf1 induction and is prevented by MDM2 overexpression. Cell Death Differ. 2007;14:1780–91. doi: 10.1038/sj.cdd.4402198. [DOI] [PubMed] [Google Scholar]
- 33.Kim WH, Kang KH, Kim MY, Choi KH. Induction of p53-independent p21 during ceramide-induced G1 arrest in human hepatocarcinoma cells. Biochem Cell Biol. 2000;78:127–35. [PubMed] [Google Scholar]
- 34.Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J Biol Chem. 2010;285:10911–23. doi: 10.1074/jbc.M109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang KH, Kim WH, Choi KH. p21 promotes ceramide-induced apoptosis and antagonizes the antideath effect of Bcl-2 in human hepatocarcinoma cells. Exp Cell Res. 1999;253:403–12. doi: 10.1006/excr.1999.4644. [DOI] [PubMed] [Google Scholar]
- 36.Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–74. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 37.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–57. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park IN, Cho IJ, Kim SG. Ceramide negatively regulates glutathione S-transferase gene transactivation via repression of hepatic nuclear factor-1 that is degraded by the ubiquitin proteasome system. Mol Pharmacol. 2004;65:1475–84. doi: 10.1124/mol.65.6.1475. [DOI] [PubMed] [Google Scholar]
- 39.Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM, Hannun YA. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem. 1994;269:19605–09. [PubMed] [Google Scholar]
- 40.Sultan I, Senkal CE, Ponnusamy S, Bielawski J, Szulc Z, Bielawska A, et al. Regulation of the sphingosine-recycling pathway for ceramide generation by oxidative stress, and its role in controlling c-Myc/Max function. Biochem J. 2006;393:513–21. doi: 10.1042/BJ20051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007;21:3386–97. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- 42.Wooten LG, Ogretmen B. Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J Biol Chem. 2005;280:28867–76. doi: 10.1074/jbc.M413444200. [DOI] [PubMed] [Google Scholar]
- 43.Sonoda Y, Hada N, Kaneda T, Suzuki T, Ohshio T, Takeda T, et al. A synthetic glycosphingolipid-induced antiproliferative effect in melanoma cells is associated with suppression of FAK, Akt, and Erk activation. Biol Pharm Bull. 2008;31:1279–83. doi: 10.1248/bpb.31.1279. [DOI] [PubMed] [Google Scholar]
- 44.Choi HJ, Chung TW, Kang SK, Lee YC, Ko JH, Kim JG, et al. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression--transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology. 2006;16:573–83. doi: 10.1093/glycob/cwj105. [DOI] [PubMed] [Google Scholar]
- 45.Choi HJ, Chung TW, Kim SJ, Cho SY, Lee YS, Lee YC, et al. The AP-2alpha transcription factor is required for the ganglioside GM3-stimulated transcriptional regulation of a PTEN gene. Glycobiology. 2008;18:395–407. doi: 10.1093/glycob/cwn016. [DOI] [PubMed] [Google Scholar]
- 46.Li MH, Sanchez T, Pappalardo A, Lynch KR, Hla T, Ferrer F. Induction of antiproliferative connective tissue growth factor expression in Wilms’ tumor cells by sphingosine-1-phosphate receptor 2. Mol Cancer Res. 2008;6:1649–56. doi: 10.1158/1541-7786.MCR-07-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 48.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–23. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An S, Zheng Y, Bleu T. Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem. 2000;275:288–96. doi: 10.1074/jbc.275.1.288. [DOI] [PubMed] [Google Scholar]
- 50.Hsieh HL, Sun CC, Wu CB, Wu CY, Tung WH, Wang HH, et al. Sphingosine 1-phosphate induces EGFR expression via Akt/NF-kappaB and ERK/AP-1 pathways in rat vascular smooth muscle cells. J Cell Biochem. 2008;103:1732–46. doi: 10.1002/jcb.21563. [DOI] [PubMed] [Google Scholar]
- 51.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–58. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 52.Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G. Mitochondrial membrane permeabilization during the apoptotic process. Ann N Y Acad Sci. 1999;887:18–30. doi: 10.1111/j.1749-6632.1999.tb07919.x. [DOI] [PubMed] [Google Scholar]
- 53.Phillips DC, Martin S, Doyle BT, Houghton JA. Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer Res. 2007;67:756–64. doi: 10.1158/0008-5472.CAN-06-2374. [DOI] [PubMed] [Google Scholar]
- 54.Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–74. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- 55.Carracedo A, Gironella M, Lorente M, Garcia S, Guzman M, Velasco G, et al. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006;66:6748–55. doi: 10.1158/0008-5472.CAN-06-0169. [DOI] [PubMed] [Google Scholar]
- 56.Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9:301–12. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Massiello A, Roesser JR, Chalfant CE. SAP155 Binds to ceramide-responsive RNA cis-element 1 and regulates the alternative 5’ splice site selection of Bcl-x pre-mRNA. FASEB J. 2006;20:1680–82. doi: 10.1096/fj.05-5021fje. [DOI] [PubMed] [Google Scholar]
- 58.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–95. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 59.Massiello A, Chalfant CE. SRp30a (ASF/SF2) regulates the alternative splicing of caspase-9 pre-mRNA and is required for ceramide-responsiveness. J Lipid Res. 2006;47:892–97. doi: 10.1194/jlr.C600003-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Gomez-Munoz A, Kong JY, Parhar K, Wang SW, Gangoiti P, Gonzalez M, et al. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett. 2005;579:3744–50. doi: 10.1016/j.febslet.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 61.Liu YY, Yu JY, Yin D, Patwardhan GA, Gupta V, Hirabayashi Y, et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 2008;22:2541–51. doi: 10.1096/fj.07-092981. [DOI] [PubMed] [Google Scholar]
- 62.Liu YY, Han TY, Giuliano AE, Ichikawa S, Hirabayashi Y, Cabot MC. Glycosylation of ceramide potentiates cellular resistance to tumor necrosis factor-alpha-induced apoptosis. Exp Cell Res. 1999;252:464–70. doi: 10.1006/excr.1999.4649. [DOI] [PubMed] [Google Scholar]
- 63.Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719–30. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- 64.Bharti AC, Singh SM. Inhibition of macrophage nitric oxide production by gangliosides derived from a spontaneous T cell lymphoma: the involved mechanisms. Nitric Oxide. 2003;8:75–82. doi: 10.1016/s1089-8603(02)00145-3. [DOI] [PubMed] [Google Scholar]
- 65.Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- 66.Young N, Pearl DK, Van Brocklyn JR. Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol Cancer Res. 2009;7:23–32. doi: 10.1158/1541-7786.MCR-08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu WT, Chen CN, Lin CI, Chen JH, Lee H. Lysophospholipids enhance matrix metalloproteinase-2 expression in human endothelial cells. Endocrinology. 2005;146:3387–400. doi: 10.1210/en.2004-1654. [DOI] [PubMed] [Google Scholar]
- 68.Reunanen N, Westermarck J, Hakkinen L, Holmstrom TH, Elo I, Eriksson JE, et al. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J Biol Chem. 1998;273:5137–45. doi: 10.1074/jbc.273.9.5137. [DOI] [PubMed] [Google Scholar]
- 69.Spiegel S, English D, Milstien S. Sphingosine 1-phosphate signaling: providing cells with a sense of direction. Trends Cell Biol. 2002;12:236–42. doi: 10.1016/s0962-8924(02)02277-8. [DOI] [PubMed] [Google Scholar]
- 70.Sun HY, Wei SP, Xu RC, Xu PX, Zhang WC. Sphingosine-1-phosphate induces human endothelial VEGF and MMP-2 production via transcription factor ZNF580: novel insights into angiogenesis. Biochem Biophys Res Commun. 2010;395:361–66. doi: 10.1016/j.bbrc.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 71.Wang L, Takaku S, Wang P, Hu D, Hyuga S, Sato T, et al. Ganglioside GD1a regulation of caveolin-1 and Stim1 expression in mouse FBJ cells: augmented expression of caveolin-1 and Stim1 in cells with increased GD1a content. Glycoconj J. 2006;23:303–15. doi: 10.1007/s10719-006-5742-3. [DOI] [PubMed] [Google Scholar]
- 72.Hu D, Man Z, Wang P, Tan X, Wang X, Takaku S, et al. Ganglioside GD1a negatively regulates matrix metalloproteinase-9 expression in mouse FBJ cell lines at the transcriptional level. Connect Tissue Res. 2007;48:198–205. doi: 10.1080/03008200701458731. [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Wang Y, Sato T, Yamagata S, Yamagata T. Ganglioside GD1a suppresses TNFalpha expression via Pkn1 at the transcriptional level in mouse osteosarcoma-derived FBJ cells. Biochem Biophys Res Commun. 2008;371:230–35. doi: 10.1016/j.bbrc.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 74.Wang P, Wu P, Zhang J, Sato T, Yamagata S, Yamagata T. Positive regulation of tumor necrosis factor-alpha by ganglioside GM3 through Akt in mouse melanoma B16 cells. Biochem Biophys Res Commun. 2007;356:438–43. doi: 10.1016/j.bbrc.2007.02.152. [DOI] [PubMed] [Google Scholar]
- 75.Wang P, Yang X, Wu P, Zhang J, Sato T, Yamagata S, et al. GM3 signals regulating TNF-alpha expression are mediated by Rictor and Arhgdib in mouse melanoma B16 cells. Oncology. 2007;73:430–38. doi: 10.1159/000136801. [DOI] [PubMed] [Google Scholar]
- 76.Saito S, Orikasa S, Ohyama C, Satoh M, Fukushi Y. Changes in glycolipids in human renal-cell carcinoma and their clinical significance. Int J Cancer. 1991;49:329–34. doi: 10.1002/ijc.2910490303. [DOI] [PubMed] [Google Scholar]
- 77.Kannagi R, Cochran NA, Ishigami F, Hakomori S, Andrews PW, Knowles BB, et al. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–61. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Slambrouck S, Steelant WF. Clustering of monosialyl-Gb5 initiates downstream signalling events leading to invasion of MCF-7 breast cancer cells. Biochem J. 2007;401:689–99. doi: 10.1042/BJ20060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steelant WF, Kawakami Y, Ito A, Handa K, Bruyneel EA, Mareel M, et al. Monosialyl-Gb5 organized with cSrc and FAK in GEM of human breast carcinoma MCF-7 cells defines their invasive properties. FEBS Lett. 2002;531:93–98. doi: 10.1016/s0014-5793(02)03484-1. [DOI] [PubMed] [Google Scholar]
- 80.Yu RK, Suzuki Y, Yanagisawa M. Membrane glycolipids in stem cells. FEBS Lett. 2010;584:1694–99. doi: 10.1016/j.febslet.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bieberich E. Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: review and hypothesis. Glycoconj J. 2004;21:315–27. doi: 10.1023/B:GLYC.0000046274.35732.47. [DOI] [PubMed] [Google Scholar]
- 82.Hakomori SI. Structure and function of glycosphingolipids and sphingolipids: recollections and future trends. Biochim Biophys Acta. 2008;1780:325–46. doi: 10.1016/j.bbagen.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stewart MH, Bosse M, Chadwick K, Menendez P, Bendall SC, Bhatia M. Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat Methods. 2006;3:807–15. doi: 10.1038/nmeth939. [DOI] [PubMed] [Google Scholar]
- 84.Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713–20. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- 85.Chang WW, Lee CH, Lee P, Lin J, Hsu CW, Hung JT, et al. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc Natl Acad Sci U S A. 2008;105:11667–72. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu JX, Morii E, Liu Y, Nakamichi N, Ikeda J, Kimura H, et al. High tolerance to apoptotic stimuli induced by serum depletion and ceramide in side-population cells: high expression of CD55 as a novel character for side-population. Exp Cell Res. 2007;313:1877–85. doi: 10.1016/j.yexcr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 87.Salli U, Fox TE, Carkaci-Salli N, Sharma A, Robertson GP, Kester M, et al. Propagation of undifferentiated human embryonic stem cells with nano-liposomal ceramide. Stem Cells Dev. 2009;18:55–65. doi: 10.1089/scd.2007.0271. [DOI] [PubMed] [Google Scholar]
- 88.He X, H’Ng SC, Leong DT, Hutmacher DW, Melendez AJ. Sphingosine-1-phosphate mediates proliferation maintaining the multipotency of human adult bone marrow and adipose tissue-derived stem cells. J Mol Cell Biol. 2010;2:199–208. doi: 10.1093/jmcb/mjq011. [DOI] [PubMed] [Google Scholar]
- 89.Krishnamurthy K, Wang G, Rokhfeld D, Bieberich E. Deoxycholate promotes survival of breast cancer cells by reducing the level of pro-apoptotic ceramide. Breast Cancer Res. 2008;10:R106. doi: 10.1186/bcr2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG. Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol. 2003;162:469–79. doi: 10.1083/jcb.200212067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167:723–34. doi: 10.1083/jcb.200405144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Donati C, Cencetti F, Nincheri P, Bernacchioni C, Brunelli S, Clementi E, et al. Sphingosine 1-phosphate mediates proliferation and survival of mesoangioblasts. Stem Cells. 2007;25:1713–19. doi: 10.1634/stemcells.2006-0725. [DOI] [PubMed] [Google Scholar]
- 93.Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–82. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 94.Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2008;105:20764–69. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Avery K, Avery S, Shepherd J, Heath PR, Moore H. Sphingosine-1-phosphate mediates transcriptional regulation of key targets associated with survival, proliferation, and pluripotency in human embryonic stem cells. Stem Cells Dev. 2008;17:1195–205. doi: 10.1089/scd.2008.0063. [DOI] [PubMed] [Google Scholar]
- 96.Saha B, Singh SK, Sarkar C, Bera R, Ratha J, Tobin DJ, et al. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 2006;19:595–605. doi: 10.1111/j.1600-0749.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 97.Saha B, Singh SK, Mallick S, Bera R, Datta PK, Mandal M, et al. Sphingolipid-mediated restoration of Mitf expression and repigmentation in vivo in a mouse model of hair graying. Pigment Cell Melanoma Res. 2009;22:205–18. doi: 10.1111/j.1755-148X.2009.00548.x. [DOI] [PubMed] [Google Scholar]
- 98.Yanagisawa M, Nakamura K, Taga T. Glycosphingolipid synthesis inhibitor represses cytokine-induced activation of the Ras-MAPK pathway in embryonic neural precursor cells. J Biochem. 2005;138:285–91. doi: 10.1093/jb/mvi129. [DOI] [PubMed] [Google Scholar]
- 99.Jung JU, Ko K, Lee DH, Chang KT, Choo YK. The roles of glycosphingolipids in the proliferation and neural differentiation of mouse embryonic stem cells. Exp Mol Med. 2009;41:935–45. doi: 10.3858/emm.2009.41.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perez-Tomas R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859–76. doi: 10.2174/092986706777585077. [DOI] [PubMed] [Google Scholar]
- 101.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 102.Colie S, Van Veldhoven PP, Kedjouar B, Bedia C, Albinet V, Sorli SC, et al. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 2009;69:9346–53. doi: 10.1158/0008-5472.CAN-09-2198. [DOI] [PubMed] [Google Scholar]
- 103.Liu YY, Gupta V, Patwardhan GA, Bhinge K, Zhao Y, Bao J, et al. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol Cancer. 2010;9:145. doi: 10.1186/1476-4598-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu YY, Han TY, Giuliano AE, Cabot MC. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem. 1999;274:1140–46. doi: 10.1074/jbc.274.2.1140. [DOI] [PubMed] [Google Scholar]
- 105.Patwardhan G, Gupta V, Huang J, Gu X, Liu YY. Direct assessment of P-glycoprotein efflux to determine tumor response to chemotherapy. Biochem Pharmacol. 2010;80:72–79. doi: 10.1016/j.bcp.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patwardhan GA, Zhang QJ, Yin D, Gupta V, Bao J, Senkal CE, et al. A new mixed-backbone oligonucleotide against glucosylceramide synthase sensitizes multidrug-resistant tumors to apoptosis. PLoS One. 2009;4:e6938. doi: 10.1371/journal.pone.0006938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Meer G, Hoetzl S. Sphingolipid topology and the dynamic organization and function of membrane proteins. FEBS Lett. 2010;584:1800–05. doi: 10.1016/j.febslet.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 108.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 109.Beyne-Rauzy O, Prade-Houdellier N, Demur C, Recher C, Ayel J, Laurent G, et al. Tumor necrosis factor-alpha inhibits hTERT gene expression in human myeloid normal and leukemic cells. Blood. 2005;106:3200–05. doi: 10.1182/blood-2005-04-1386. [DOI] [PubMed] [Google Scholar]
- 110.Abboushi N, El-Hed A, El-Assaad W, Kozhaya L, El-Sabban ME, Bazarbachi A, et al. Ceramide inhibits IL-2 production by preventing protein kinase C-dependent NF-kappaB activation: possible role in protein kinase Ctheta regulation. J Immunol. 2004;173:3193–200. doi: 10.4049/jimmunol.173.5.3193. [DOI] [PubMed] [Google Scholar]
- 111.Kamachi M, Aramaki T, Tanimura S, Ichinose K, Fujikawa K, Iwamoto N, et al. Activation of protein phosphatase causes alternative splicing of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): potential effect on immune surveillance. Biochem Biophys Res Commun. 2007;360:280–85. doi: 10.1016/j.bbrc.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 112.Debret R, Brassart-Pasco S, Lorin J, Martoriati A, Deshorgue A, Maquart FX, et al. Ceramide inhibition of MMP-2 expression and human cancer bronchial cell invasiveness involve decreased histone acetylation. Biochim Biophys Acta. 2008;1783:1718–27. doi: 10.1016/j.bbamcr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 113.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 115.Yamaji T, Kumagai K, Tomishige N, Hanada K. Two sphingolipid transfer proteins, CERT and FAPP2: their roles in sphingolipid metabolism. IUBMB Life. 2008;60:511–18. doi: 10.1002/iub.83. [DOI] [PubMed] [Google Scholar]


