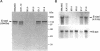Abstract
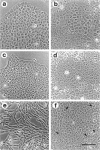
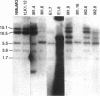
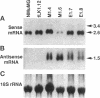
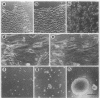
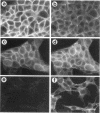
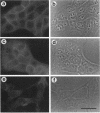
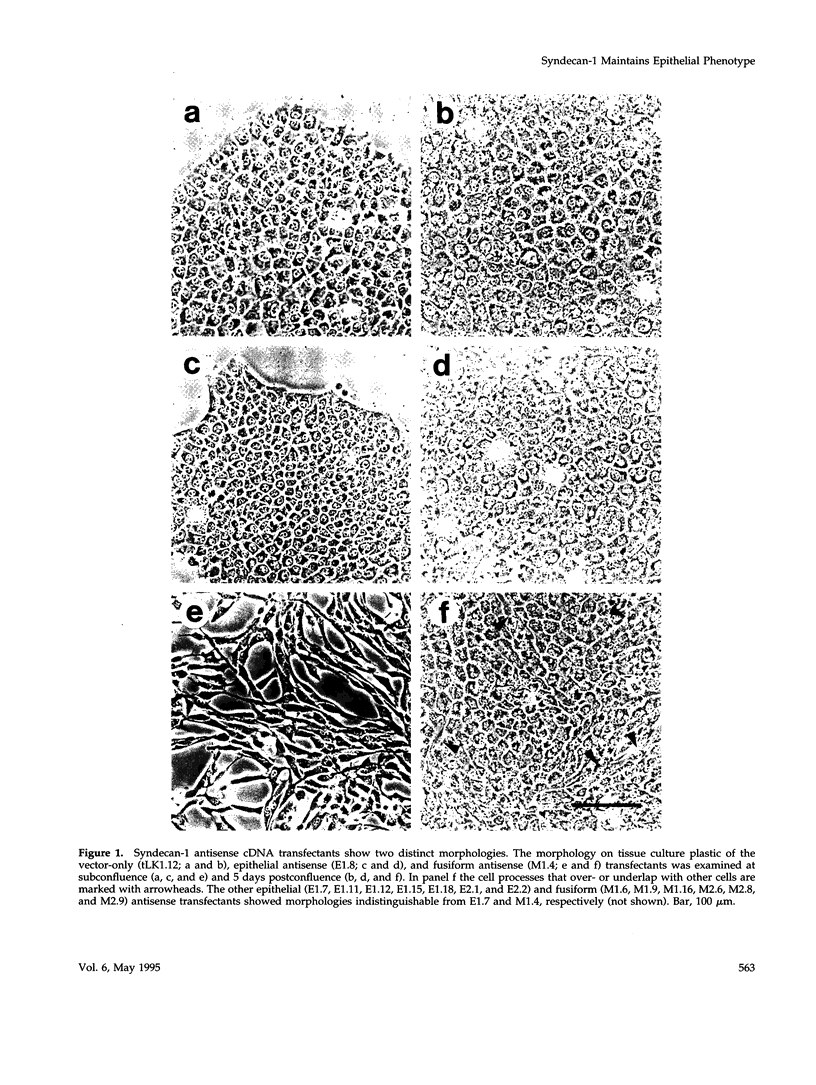
Simple epithelial cells are polygonal in shape, polarized in an apical-basal orientation, and organized into closely adherent sheets, characteristics that result from a variety of cellular specializations and adhesive proteins. These characteristics are lost when the epithelia transform during embryogenesis into mesenchymal cells or after neoplasia into invasive carcinoma cells. Of the syndecan family of transmembrane heparan sulfate proteoglycans, simple epithelia produce predominantly syndecan-1, which is found at basolateral surfaces and within adhesive junctions. To elucidate the function of this syndecan-1, normal murine mammary gland epithelia were made deficient in syndecan-1 by transfection with an expression vector containing the syndecan-1 cDNA in the antisense configuration. Several independently derived clones of stable transfectants contained the antisense cDNA in their genome and expressed the antisense transcript. These grew either as epithelial islands of closely adherent polygonal cells, identical to both the parental cells and the vector-only control transfectants, or as individual elongated fusiform cells that invaded and migrated within collagen gels, like mesenchymal cells, but were anchorage-independent for growth. The clones that retained epithelial characteristics were moderately deficient in cell surface syndecan-1 (greater than 48% of control levels) but did not differ from control cells in expression of beta 1-integrins and E-cadherin, or in F-actin organization. However, the clones of fusiform cells were severely deficient in cell surface syndecan-1 (less than 12% of control levels) and showed rearranged beta 1-integrins, markedly reduced E-cadherin expression, and disorganized F-actin filaments, but retained mammary epithelial markers. Therefore, depleting epithelia of cell surface syndecan-1 alters cell morphology and organization, the arrangement and expression of adhesion molecules, and anchorage-dependent growth controls. Thus, cell surface syndecan-1 is required to maintain the normal phenotype of simple epithelia.
Full text
PDF



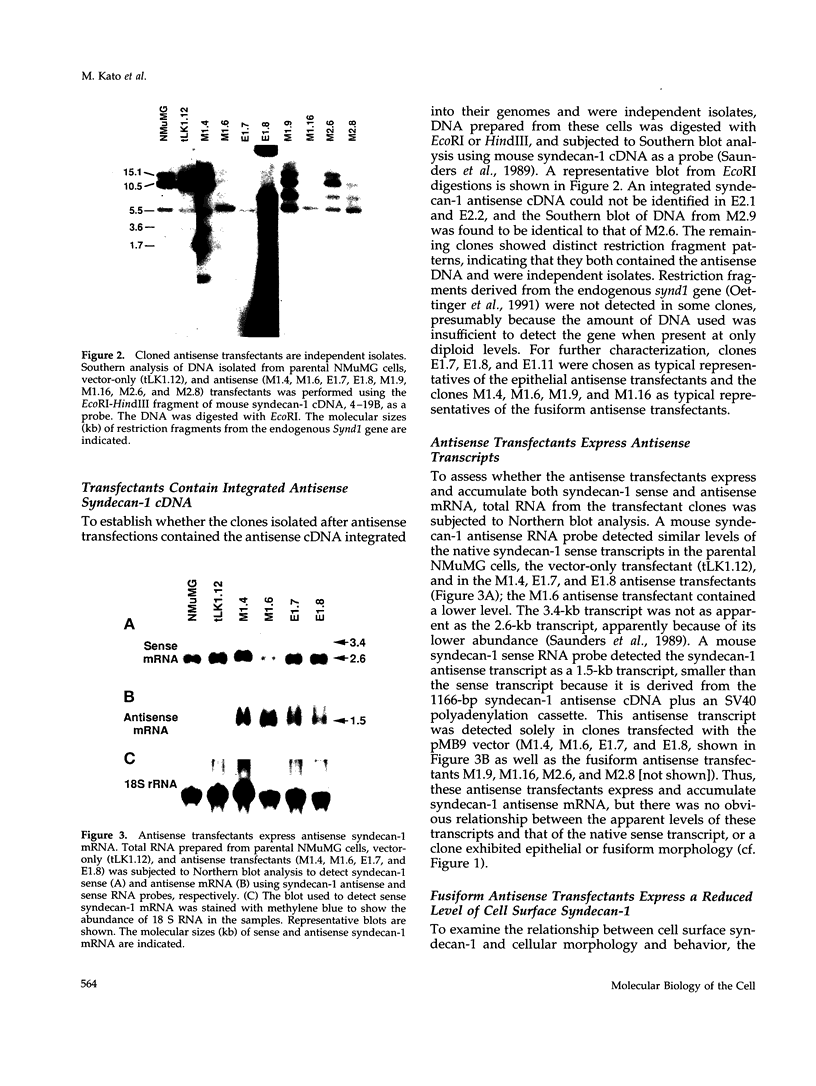
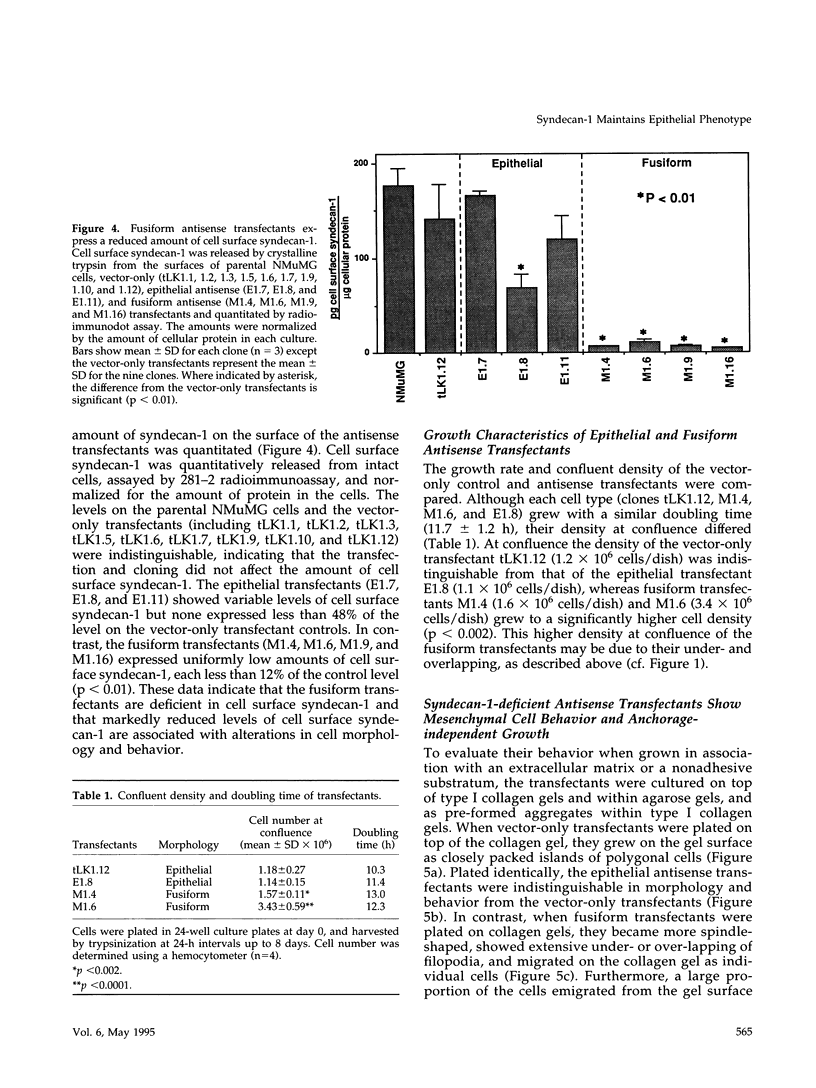
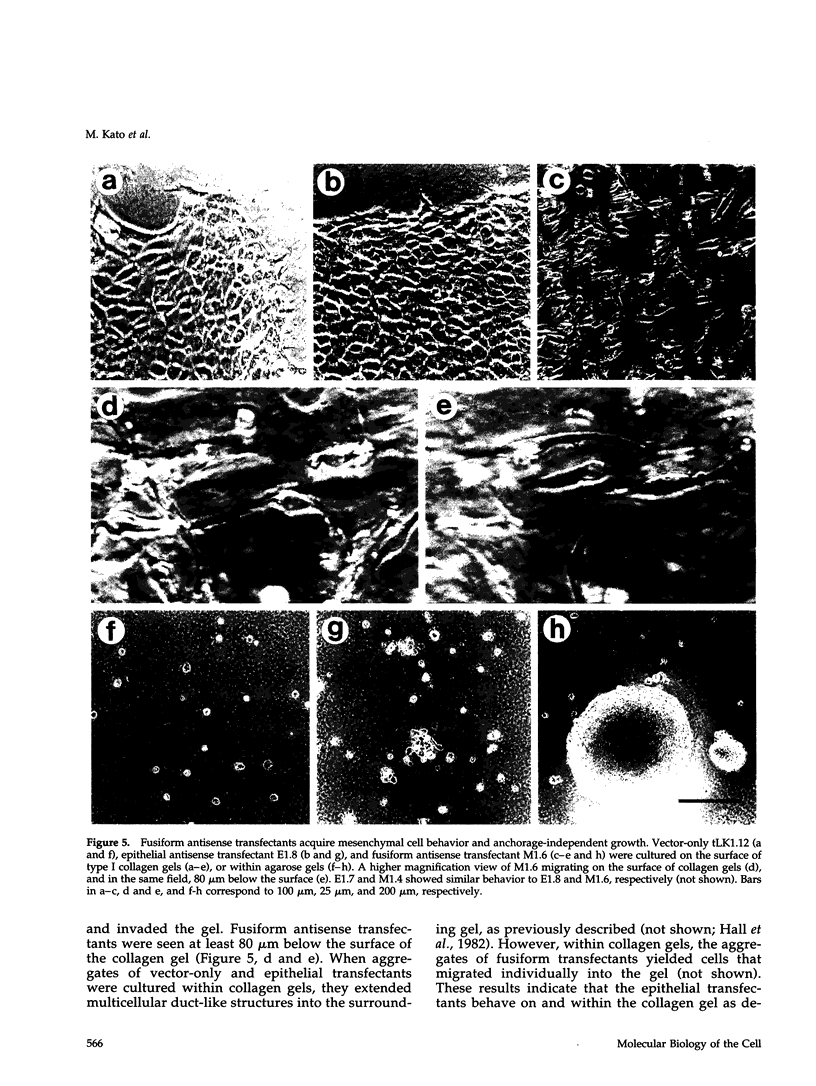

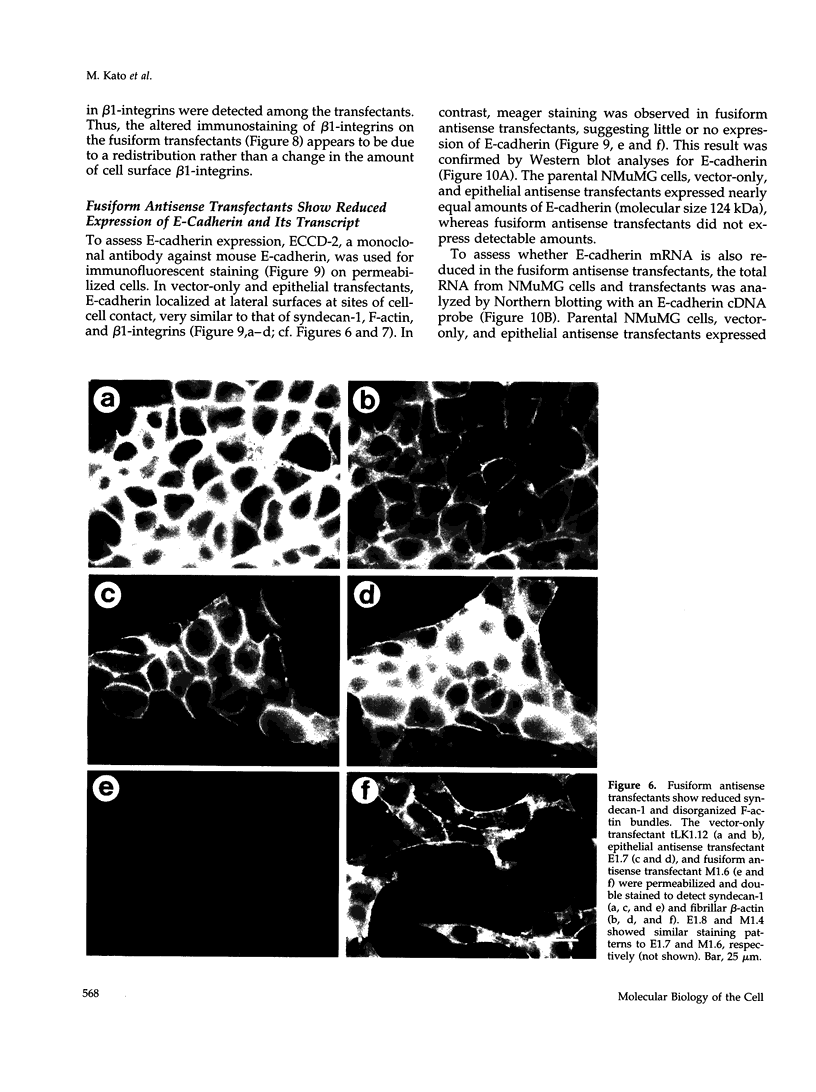
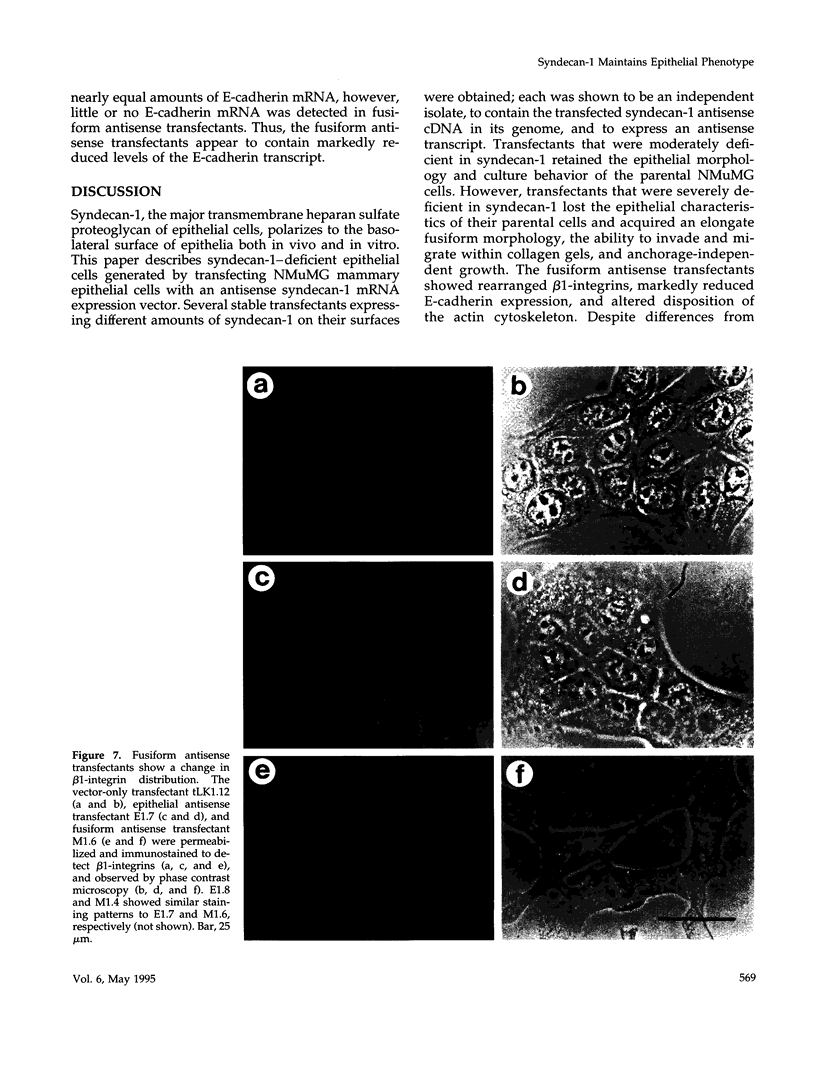

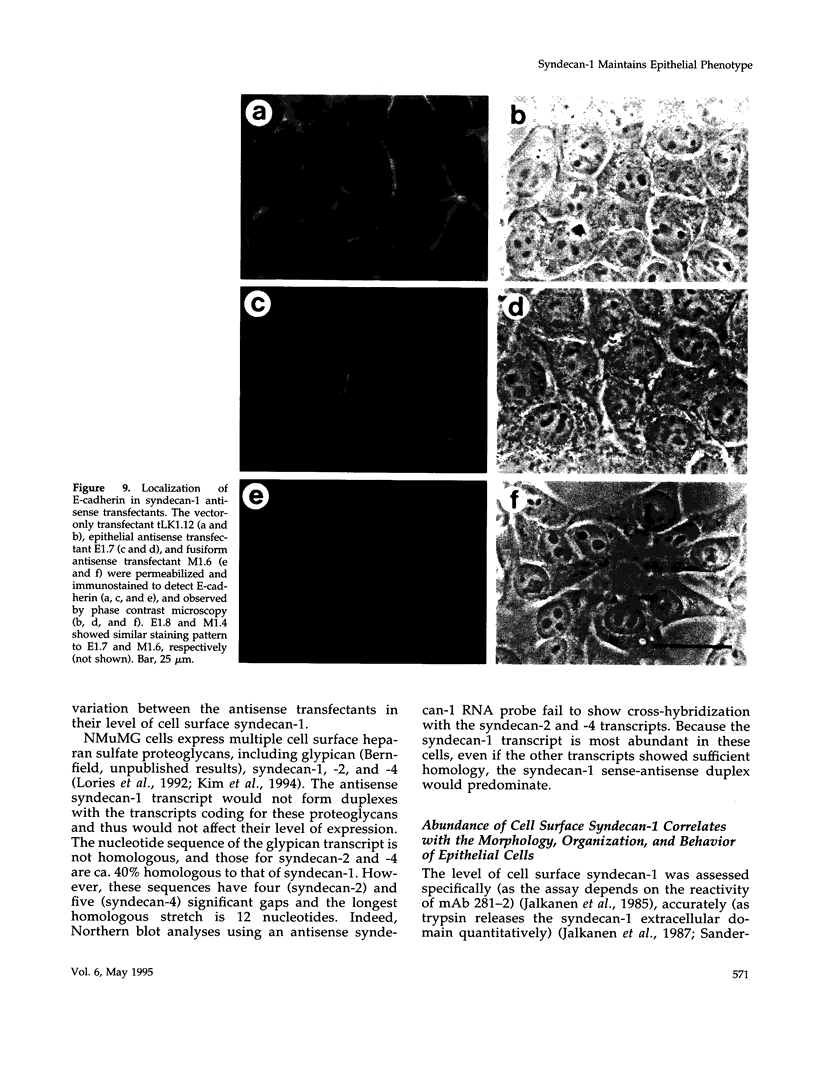
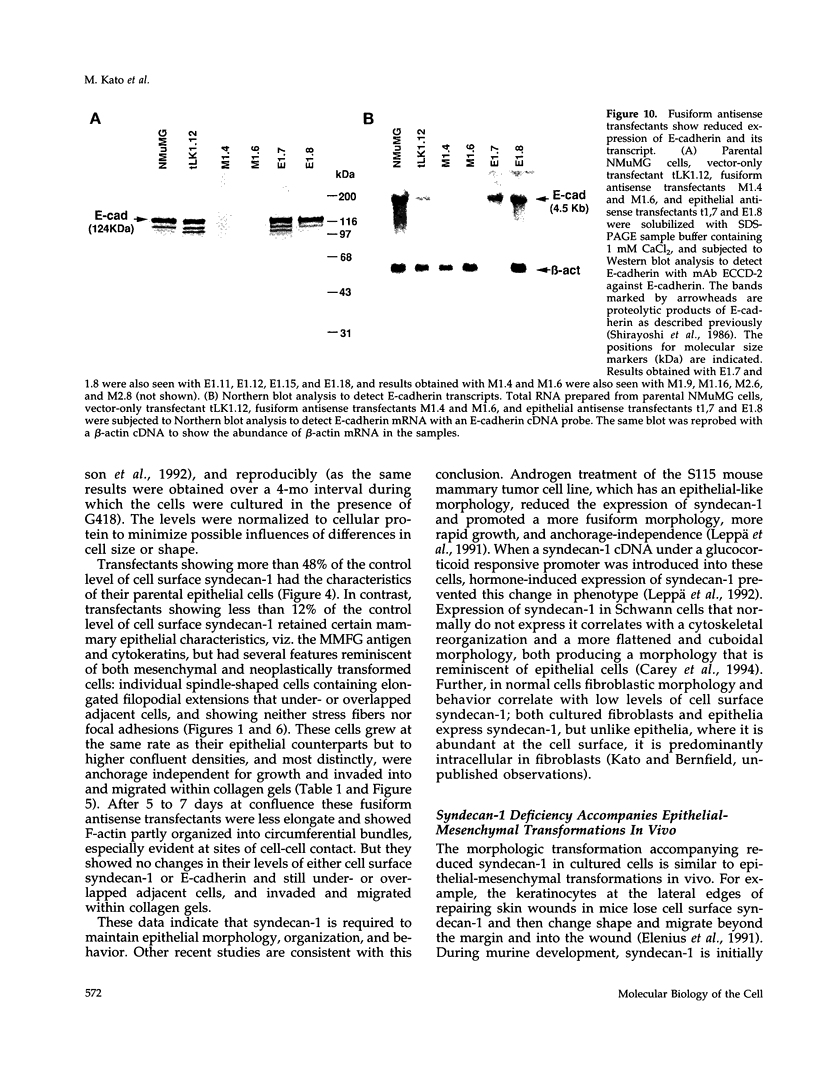




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amini S., DeSeau V., Reddy S., Shalloway D., Bolen J. B. Regulation of pp60c-src synthesis by inducible RNA complementary to c-src mRNA in polyomavirus-transformed rat cells. Mol Cell Biol. 1986 Jul;6(7):2305–2316. doi: 10.1128/mcb.6.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Mareel M. M., Van Roy F. M., Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989 Jun;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Banerjee S. D., Koda J. E., Rapraeger A. C. Remodelling of the basement membrane: morphogenesis and maturation. Ciba Found Symp. 1984;108:179–196. doi: 10.1002/9780470720899.ch12. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Hooper K. C. Possible regulation of FGF activity by syndecan, an integral membrane heparan sulfate proteoglycan. Ann N Y Acad Sci. 1991;638:182–194. doi: 10.1111/j.1749-6632.1991.tb49029.x. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Birchmeier W., Weidner K. M., Behrens J. Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J Cell Sci Suppl. 1993;17:159–164. doi: 10.1242/jcs.1993.supplement_17.23. [DOI] [PubMed] [Google Scholar]
- Burdsal C. A., Damsky C. H., Pedersen R. A. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993 Jul;118(3):829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Stahl R. C., Cizmeci-Smith G., Asundi V. K. Syndecan-1 expressed in Schwann cells causes morphological transformation and cytoskeletal reorganization and associates with actin during cell spreading. J Cell Biol. 1994 Jan;124(1-2):161–170. doi: 10.1083/jcb.124.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani R. L., Peterson J. A. Characterization of differentiation antigens of the mouse mammary epithelial cell (MME antigens) carried on the mouse milk fat globule. Cell Differ. 1978 Dec;7(6):355–366. doi: 10.1016/0045-6039(78)90036-2. [DOI] [PubMed] [Google Scholar]
- D'Souza B., Berdichevsky F., Kyprianou N., Taylor-Papadimitriou J. Collagen-induced morphogenesis and expression of the alpha 2-integrin subunit is inhibited in c-erbB2-transfected human mammary epithelial cells. Oncogene. 1993 Jul;8(7):1797–1806. [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Van der Schueren B., Bernfield M. Basal lamina formation by normal and transformed mouse mammary epithelial cells duplicated in vitro. J Natl Cancer Inst. 1981 Sep;67(3):719–728. [PubMed] [Google Scholar]
- Ekblom P., Nordling S., Saxén L. Inhibition of kidney tubule induction by charged polymers. Cell Differ. 1978 Dec;7(6):345–353. doi: 10.1016/0045-6039(78)90035-0. [DOI] [PubMed] [Google Scholar]
- Elenius K., Mättä A., Salmivirta M., Jalkanen M. Growth factors induce 3T3 cells to express bFGF-binding syndecan. J Biol Chem. 1992 Mar 25;267(9):6435–6441. [PubMed] [Google Scholar]
- Elenius K., Vainio S., Laato M., Salmivirta M., Thesleff I., Jalkanen M. Induced expression of syndecan in healing wounds. J Cell Biol. 1991 Aug;114(3):585–595. doi: 10.1083/jcb.114.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estival A., Louvel D., Couderc B., Prats H., Hollande E., Vaysse N., Clémente F. Morphological and biological modifications induced in a rat pancreatic acinar cancer cell line (AR4-2J) by unscheduled expression of basic fibroblast growth factors. Cancer Res. 1993 Mar 1;53(5):1182–1187. [PubMed] [Google Scholar]
- Fitchett J. E., Hay E. D. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989 Feb;131(2):455–474. doi: 10.1016/s0012-1606(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Gallo R. L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M., Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E., Gray J., Stoker M., Perryman M., Furlong R. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5844–5848. doi: 10.1073/pnas.86.15.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson C., Bell K. S., Insel P. A. Inhibition of expression of protein kinase C alpha by antisense cDNA inhibits phorbol ester-mediated arachidonate release. J Biol Chem. 1993 Jun 5;268(16):11946–11950. [PubMed] [Google Scholar]
- Greenburg G., Hay E. D. Cytodifferentiation and tissue phenotype change during transformation of embryonic lens epithelium to mesenchyme-like cells in vitro. Dev Biol. 1986 Jun;115(2):363–379. doi: 10.1016/0012-1606(86)90256-3. [DOI] [PubMed] [Google Scholar]
- Greenburg G., Hay E. D. Cytoskeleton and thyroglobulin expression change during transformation of thyroid epithelium to mesenchyme-like cells. Development. 1988 Mar;102(3):605–622. doi: 10.1242/dev.102.3.605. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M., McCrea P. D. Catenins as mediators of the cytoplasmic functions of cadherins. J Cell Sci Suppl. 1993;17:155–158. doi: 10.1242/jcs.1993.supplement_17.22. [DOI] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H. G., Farson D. A., Bissell M. J. Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Hayashi M., Jalkanen M., Firestone J. H., Trelstad R. L., Bernfield M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J Histochem Cytochem. 1987 Oct;35(10):1079–1088. doi: 10.1177/35.10.2957423. [DOI] [PubMed] [Google Scholar]
- Hirai Y., Nose A., Kobayashi S., Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. I. Lung epithelial morphogenesis. Development. 1989 Feb;105(2):263–270. doi: 10.1242/dev.105.2.263. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Marcantonio E. E., Stepp M. A., Urry L. A., Yee G. H. Integrin heterodimer and receptor complexity in avian and mammalian cells. J Cell Biol. 1989 Jul;109(1):409–420. doi: 10.1083/jcb.109.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inki P., Larjava H., Haapasalmi K., Miettinen H. M., Grenman R., Jalkanen M. Expression of syndecan-1 is induced by differentiation and suppressed by malignant transformation of human keratinocytes. Eur J Cell Biol. 1994 Feb;63(1):43–51. [PubMed] [Google Scholar]
- Jalkanen M., Nguyen H., Rapraeger A., Kurn N., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells: localization on the cell surface with a monoclonal antibody. J Cell Biol. 1985 Sep;101(3):976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Rapraeger A., Saunders S., Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987 Dec;105(6 Pt 2):3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jue S. F., Bradley R. S., Rudnicki J. A., Varmus H. E., Brown A. M. The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol. 1992 Jan;12(1):321–328. doi: 10.1128/mcb.12.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Stephans J. C., Crawford K., Okino K., Barr P. J. Ligand-affinity cloning and structure of a cell surface heparan sulfate proteoglycan that binds basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6985–6989. doi: 10.1073/pnas.87.18.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. W., Goldberger O. A., Gallo R. L., Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994 Jul;5(7):797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda J. E., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Basal extracellular proteoglycan binds specifically to native type I collagen fibrils. J Biol Chem. 1984 Oct 10;259(19):11763–11770. [PubMed] [Google Scholar]
- Koda J. E., Rapraeger A., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985 Jul 5;260(13):8157–8162. [PubMed] [Google Scholar]
- Kokenyesi R., Bernfield M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem. 1994 Apr 22;269(16):12304–12309. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppä S., Härkönen P., Jalkanen M. Steroid-induced epithelial-fibroblastic conversion associated with syndecan suppression in S115 mouse mammary tumor cells. Cell Regul. 1991 Jan;2(1):1–11. doi: 10.1091/mbc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppä S., Mali M., Miettinen H. M., Jalkanen M. Syndecan expression regulates cell morphology and growth of mouse mammary epithelial tumor cells. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):932–936. doi: 10.1073/pnas.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Differential expression of cell surface heparan sulfate proteoglycans in human mammary epithelial cells and lung fibroblasts. J Biol Chem. 1992 Jan 15;267(2):1116–1122. [PubMed] [Google Scholar]
- McAlmon K. R. Matrix receptors and development. Semin Perinatol. 1992 Apr;16(2):90–96. [PubMed] [Google Scholar]
- McNeill H., Ozawa M., Kemler R., Nelson W. J. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990 Jul 27;62(2):309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Oettinger H. F., Streeter H., Lose E., Copeland N. G., Gilbert D. J., Justice M. J., Jenkins N. A., Mohandas T., Bernfield M. Chromosome mapping of the murine syndecan gene. Genomics. 1991 Oct;11(2):334–338. doi: 10.1016/0888-7543(91)90140-a. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Trescony P., Lindman B., Oegema T. R. Heparin and heparan sulfate delimit nephron formation in fetal metanephric kidneys. Dev Biol. 1990 Jun;139(2):338–348. doi: 10.1016/0012-1606(90)90303-z. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann E., Schwarz H., Deiner E. M., Leitner I., Eilers M., Berger J., Busslinger M., Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992 Dec 24;71(7):1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- Salmivirta M., Elenius K., Vainio S., Hofer U., Chiquet-Ehrismann R., Thesleff I., Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J Biol Chem. 1991 Apr 25;266(12):7733–7739. [PubMed] [Google Scholar]
- Sanderson R. D., Hinkes M. T., Bernfield M. Syndecan-1, a cell-surface proteoglycan, changes in size and abundance when keratinocytes stratify. J Invest Dermatol. 1992 Oct;99(4):390–396. doi: 10.1111/1523-1747.ep12616103. [DOI] [PubMed] [Google Scholar]
- Saunders S., Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988 Feb;106(2):423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989 Apr;108(4):1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Nose A., Iwasaki K., Takeichi M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct Funct. 1986 Sep;11(3):245–252. doi: 10.1247/csf.11.245. [DOI] [PubMed] [Google Scholar]
- Solursh M., Reiter R. S., Jensen K. L., Kato M., Bernfield M. Transient expression of a cell surface heparan sulfate proteoglycan (syndecan) during limb development. Dev Biol. 1990 Jul;140(1):83–92. doi: 10.1016/0012-1606(90)90055-n. [DOI] [PubMed] [Google Scholar]
- Sun X., Mosher D. F., Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989 Feb 15;264(5):2885–2889. [PubMed] [Google Scholar]
- Sutherland A. E., Sanderson R. D., Mayes M., Seibert M., Calarco P. G., Bernfield M., Damsky C. H. Expression of syndecan, a putative low affinity fibroblast growth factor receptor, in the early mouse embryo. Development. 1991 Sep;113(1):339–351. doi: 10.1242/dev.113.1.339. [DOI] [PubMed] [Google Scholar]
- Symington B. E., Takada Y., Carter W. G. Interaction of integrins alpha 3 beta 1 and alpha 2 beta 1: potential role in keratinocyte intercellular adhesion. J Cell Biol. 1993 Jan;120(2):523–535. doi: 10.1083/jcb.120.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Thesleff I., Jalkanen M., Vainio S., Bernfield M. Cell surface proteoglycan expression correlates with epithelial-mesenchymal interaction during tooth morphogenesis. Dev Biol. 1988 Oct;129(2):565–572. doi: 10.1016/0012-1606(88)90401-0. [DOI] [PubMed] [Google Scholar]
- Trautman M. S., Kimelman J., Bernfield M. Developmental expression of syndecan, an integral membrane proteoglycan, correlates with cell differentiation. Development. 1991 Jan;111(1):213–220. doi: 10.1242/dev.111.1.213. [DOI] [PubMed] [Google Scholar]
- Vallés A. M., Tucker G. C., Thiery J. P., Boyer B. Alternative patterns of mitogenesis and cell scattering induced by acidic FGF as a function of cell density in a rat bladder carcinoma cell line. Cell Regul. 1990 Dec;1(13):975–988. doi: 10.1091/mbc.1.13.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet L., Jr, Mareel M., Fiers W., van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991 Jul 12;66(1):107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Weidner K. M., Behrens J., Vandekerckhove J., Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990 Nov;111(5 Pt 1):2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz H. M., Hinck L., Cannon P., Navre M., Ringold G. M. Reduced expression of AP27 protein, the product of a growth factor-repressible gene, is associated with diminished adipocyte differentiation. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1065–1069. doi: 10.1073/pnas.89.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida-Noro C., Suzuki N., Takeichi M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol. 1984 Jan;101(1):19–27. doi: 10.1016/0012-1606(84)90112-x. [DOI] [PubMed] [Google Scholar]
- Zuk A., Matlin K. S., Hay E. D. Type I collagen gel induces Madin-Darby canine kidney cells to become fusiform in shape and lose apical-basal polarity. J Cell Biol. 1989 Mar;108(3):903–919. doi: 10.1083/jcb.108.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]