Abstract
Wiskott-Aldrich Syndrome Protein (WASP) is a hematopoietic cell-specific regulator of Arp2/3-dependent actin polymerization. Despite the presence of the highly homologous N-WASP (neural-WASP), macrophages from WAS patients are devoid of podosomes, adhesion structures in cells of the monocytic lineage capable of matrix degradation via matrix metalloproteases (MMPs), suggesting that WASP and N-WASP play unique roles in macrophages. To determine whether N-WASP also plays a unique role in macrophage function, N-WASP expression was reduced using silencing RNA in a sub-line of RAW 264.7 macrophages (RAW/LR5). Similar to reduction in WASP levels, cells with reduced N-WASP levels were rounder and less polarized. Interestingly, podosomes still formed when N-WASP was reduced but they were unable to perform matrix degradation. This defect was rescued by re-expression of N-WASP, but not by over-expression of WASP, indicating that these proteins play distinct roles in podosome function. Additionally, reducing N-WASP levels mistargets the metalloprotease MT1-MMP and it no longer localizes to podosomes. However, N-WASP was only found to co-localize with MT1-MMP positive vesicles at podosomes, suggesting that N-WASP may play a role on the targeting or fusion of MMP containing vesicles to podosomes in macrophage-like cells.
Keywords: WASP, N-WASP, podosomes, macrophages, matrix metalloproteases, matrix degradation, vesicle trafficking
Introduction
Macrophages play an important role in the immune response through phagocytosis of foreign pathogens and subsequent antigen presentation. However, macrophages may also destroy non-infected cells when they are recruited to sites of inflammation. In tumors, they may speed the progression of metastasis and their recruitment into breast tumors has been correlated with a poor prognosis (reviewed in (Pixley and Stanley 2004)).
Wiskott-Aldrich Syndrome and X-linked thrombocytopenia are X-linked diseases caused by mutations in the gene encoding WASP and are characterized by immune system malfunctions including thrombocytopenia, eczema, recurring infections, and autoimmune disorders (reviewed in Thrasher and Burns (Thrasher and Burns)). However, while WASP is only found in hematopoetic cells, the ubiquitously expressed WASP homolog N-WASP is also present in these cells, albeit at low levels (Isaac et al. 2010; Snapper et al. 2001; Stamm et al. 2005; Suzuki et al. 2002), suggesting that N-WASP cannot compensate for the reduced WASP protein levels typically seen in WAS patients or in the WASP knockout mouse.
WASP is important in Arp2/3 mediated actin polymerization and, among other things, regulates the formation of podosomes, dynamic adhesion structures that are localized to the ventral surface of cells that mediate matrix degradation by localization of various enzymes including matrix metalloproteases (MMPs) (Linder 2007; Yamaguchi et al. 2006). It has also been shown that podosomes are absent in macrophages and DCs derived from WAS patients, with disorganized clusters of F-actin lacking the typical vinculin ring (Burns et al. 2001; Linder et al. 1999). Podosome-like structures are also present in non-hematopoietic cells such as endothelial cells, vascular smooth muscle cells, Src-transformed cells and in certain aggressive cancer cells where they are often called invadopodia. These structures have distinct organization and they contain N-WASP in the F-actin core instead of WASP (reviewed in (Linder 2007)).
N-WASP has 50% overall sequence homology with WASP, with conserved domain organization, including a WH1 domain that binds WASP interacting protein (WIP), a proline-rich region that binds SH3 proteins, a GBD domain that binds Cdc42-GTP, and a basic sequence that binds PIP2 (Miki et al. 1996). At the C terminus, both proteins have a VCA domain, which binds actin via the V domain and the Arp2/3 complex via the CA region but N-WASP has an additional V domain. In vitro data using the purified VVCA domain of N-WASP showed that this domain alone was sufficient for actin polymerization and its activity was higher than the isolated VCA domain of WASP (Zalevsky et al. 2001). This suggests that N-WASP is a better inducer of actin polymerization due to its additional V domain. Additionally, WASP and N-WASP are differentially activated by SH3 proteins in vitro where Grb2 and Nck1 are more potent activators of N-WASP than WASP (Tomasevic et al. 2007), consistent with potentially different cellular functions. Also, studies using pathogenic microorganisms suggest WASP family proteins may not compensate for each other completely. For example, Shigella outer membrane protein VirG binds to N-WASP and not to WASP and the defect in Shigella motility in the absence of N-WASP cannot be restored by ectopic expression of WASP (Snapper et al. 2001; Suzuki et al. 2002). However, WASP does compensate for N-WASP in cytoplasmic vaccinia virus motility (Snapper et al. 2001) and Mycobacterium marinum can exploit either WASP or N-WASP interchangeably (Stamm et al. 2005). These studies suggest that WASP and N-WASP may serve redundant or non-redundant functions depending on the cellular context.
Due to the differences between WASP and N-WASP in their activation and cellular processes, it is speculated that they may play unique, nonredundant roles. While many studies have focused on WASP, the function of N-WASP in macrophages has not been clearly elucidated and is the subject of this short communication.
Material and Methods
Cells, Constructs, antibodies and other reagents
All cells were maintained at 37°C in a 5% CO2 incubator. Lac Repressor expressing murine monocyte/macrophage RAW 264.7 cell line (RAW/LR5) (Cox et al. 1997) were grown in RPMI medium (Mediatech Inc, Manassas, VA) containing 10% new born calf serum (Cambrex, Walkersville, MD), 100 U/ml penicillin and 100 ug/ml streptomycin (Sigma, Saint Louis, MO). RAW/LR5 cells were transfected using Fugene HD (Roche, Indianapolis, IN) with bovine Wild-N-WASP and N-WASPΔV in pSRα (Miki et al. 1998), GFP-N-WASP (Yamaguchi et al. 2002), myc-tagged WT human WASP construct in pEFBos. GFP or mCherry tagged MT1-MMP was a generous gift from Jose Bravo-Cordero (Bravo-Cordero et al. 2007). RNA-mediated interference of WASP and N-WASP was performed according to Park and Cox (Park and Cox 2009). Chicken anti-N-WASP (Clone AE920) (Isaac et al. 2010) was generated based on a sequence indicated in (Miki et al. 1996). The following commercial antibodies were also used: monoclonal mouse anti-β-actin (Clone AC-15) and anti-vinculin (hVin1; Sigma), monoclonal mouse anti-MT1-MMP (Clone Ab-4; Calbiochem), mouse anti-GFP (Roche), rabbit anti-WASP (H250; Santa Cruz). Alexa Fluor 647-, 568-, and 488-conjugated secondary antibodies or phalloidin were from Molecular Probes, Eugene, OR).
Immunofluorescence microscopy
Cells were plated on either 12 mm glass coverslips or 35 mm uncoated glass bottom microwell dishes (MatTek Corporation, Ashland, MA) and were allowed to adhere overnight. Cells were fixed for 7 minutes in 3.7% formaldehyde in BWD buffer (20 mM HEPES, 125 mM NaCl, 5 mM KCl, 5mM glucose, 1mM KH2PO4, 10 mM NaHCO3, 1mM CaCl2, 1mM MgCl2, pH 7.4), permeabilized for 5 minutes in 0.2% Triton X-100 (in BWD), and blocked in 10% milk in TBS (137 mM NaCl, 24.7 mM Tris, pH 7.4). For MT1-MMP staining, LR5 cells were simultaneously fixed and permeabilized with saponin to preserve membrane integrity, as described (Eddy et al., 2000). Cells were incubated with the primary antibodies of interest in TBS and then with fluorophore-conjugated secondary antibodies. Finally, coverslips were mounted in antifade mounting medium (50% glycerol, 1X PBS, 100mM n-Propyl gallate (Sigma)) for widefield or in PBS for TIRF microscopy. Images were taken by either TIRF or epifluorescence on an Olympus IX71 microscope (Olympus, X150/1.45 NA, oil objective) coupled to a Sensicam cooled CCD camera.
Morphological parameters were analyzed as follows: a cell displaying migratory morphology was defined by an unambiguous leading and trailing edge (Wheeler et al. 2006). The elongation index was determined using Image J (http://rsb.info.nih.gov/ij/) by tracing the cells and measuring the ratio of the major axis over the minor axis of the fit ellipse. At least 60 cells per experiment were analyzed for each determination. Podosomes were identified as F-actin punctate structures surrounded by a vinculin ring. F-actin core intensity was obtained by identifying the area inside the vinculin ring from F-actin and vinculin merged image and circling the corresponding area on F-actin alone image for intensity measurement. At least 100 podosomes per cell type were analyzed for each experiment.
Matrix degradation assay
Degradation of fluorescently-labeled fibronectin (FN) by RAW/LR5 cells was determined by a protocol detailed in (Dovas et al. 2009). Briefly, cells were plated on either Alexa488 or Alexa568 fluorescently labeled fibronectin for 16 hours and areas of loss of fluorescence were measured as degradation area per cell. For rescue experiments shWASp or shN-WASP cells, were transfected with the indicated constructs followed by plating on Alex568 labeled fibronectin as described above. Transfected cells were identified by either GFP labeling or staining for Myc. At least 50 cells per experiment were analyzed.
Western blotting
Cells were lysed for 10 minutes in ice-cold lysis buffer (1% Triton X-100, 25 mM Tris, 137 mM NaCl, 2 mM EDTA, 1 mM sodium orthovanadate, 1 mM benzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin, pH 7.4). Clarified whole-cell lysates were mixed with 5X Laemmli buffer and boiled. Samples were run on an 8% SDS-PAGE gel, transferred onto PVDF membranes (Immobilon-P, Millipore), and blotted with appropriate antibodies. Blots were exposed using Kodak IS440 machine and bands were quantified using Kodak ID 3.6 (Kodak Imaging Systems).
Data analysis
Results were considered statistically different when analysis using a Student t-test resulted in differences between two means with a p value of less than 0.05. All graphs indicate mean values and error bars signify standard error of the mean.
Results and Discussion
N-WASP is required for macrophage polarization
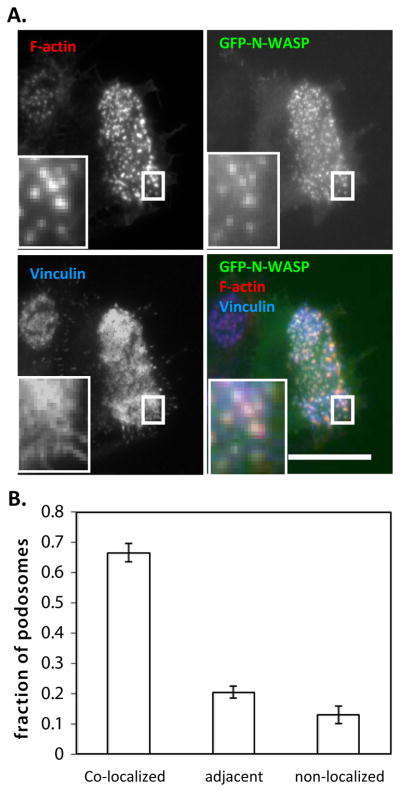
It has been suggested in the field that WASP and N-WASP have non-redundant functions given the defect associated with the absence of WASP despite the presence of N-WASP (Calle et al. 2006). To determine if N-WASP has a distinct function from WASP, we initially determined N-WASP localization in a macrophage cell line (RAW/LR5) transiently transfected with GFP-N-WASP (Fig. 1). While WASP has been consistently localized to podosomes in human and mouse macrophages, dendritic cells and osteoclasts (Calle et al. 2004a; Calle et al. 2004b; Linder et al. 1999), N-WASP was observed in both podosomes (Fig. 1A, inset) and other cellular puncta (arrows; Fig. 1A). These puncta did not display distinct podosome characteristics, namely, an F-actin core surrounded by a vinculin ring, and are dispersed throughout the cytoplasm consistent with the vesicular localization of N-WASP observed in dendritic cells and in breast carcinoma cells (Calle et al. 2006; Parsons et al. 2005). It should be noted that while N-WASP was not present in dendritic cell podosomes, GFP-N-WASP significantly localized to macrophage podosomes (Fig. 1B). The localization of N-WASP to F-actin rich adherent structures was also confirmed using TIRF microscopy (Supplemental Fig. 1). This result may be due differences in cell types, dendritic versus macrophages, or to the over-expression of N-WASP. To minimize the latter possibility only cells that expressed low levels of GFP-N-WASP were analyzed. Attempts to localize endogenous N-WASP were unsuccessful since all of the antibodies available against N-WASP cross-reacted with WASP. However, the presence of N-WASP in cellular puncta suggests that it may perform unique functions in macrophages.
Figure 1. GFP-N-WASP localizes to podosomes.
RAW/LR5 cells transiently transfected with GFP-N-WASP were fixed and stained with F-actin (red) and vinculin (blue) (to indicate podosomes). Epifluorescence images are shown in (A) and the inset contains a magnified image of the boxed region (Bar, 20 μm). (B) The colocalization of N-WASP with podosomes was analyzed using ImageJ and the fraction of podosomes where GFP-N-WASP was co-localized, adjacent to podosomes, or non-localized was determined. Error bars represent SEM. (n=17 cells).
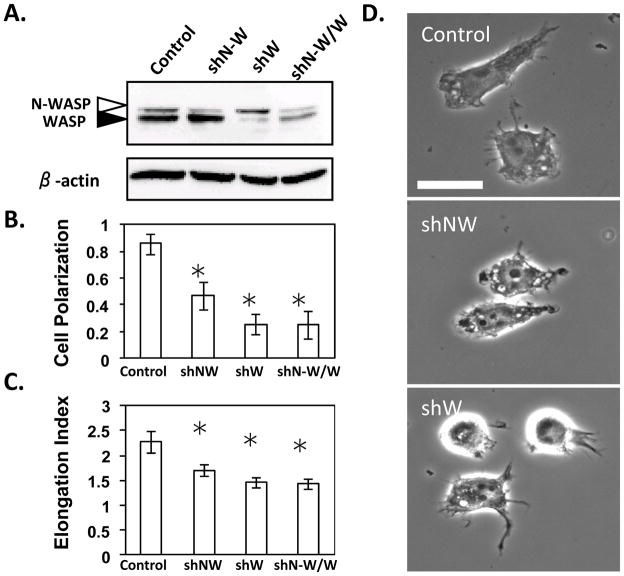
To determine the specific role of N-WASP in macrophages, N-WASP levels were reduced using silencing RNA alone or in combination with silencing RNA against WASP. Reduction of N-WASP using short hairpin RNA (shRNA) resulted in no stable, viable cells, suggesting that N-WASP is an essential protein. In mice, unlike WAS−/−, N-WASP−/− is embryonic lethal (Snapper et al. 2001). Therefore, a transient approach to reducing N-WASP was used by selecting in puromycin media for two days to remove non-infected cells. At this point N-WASP levels were reduced by approximately 80% and yet the cells were still viable as determined by trypan blue exclusion (data not shown). Under the same conditions WASP levels were reduced by more than 85% (Fig. 2A). Consistent with other reports using monocytes and macrophages from WAS patients (Badolato et al. 1998; Zicha et al. 1998), shWASP cells appeared to be more round and fewer cells showed a clear head to tail migratory phenotype than control cells. Reduced levels of N-WASP also decreased cell elongation and migratory morphology. Consistent with a role for N-WASP in the generation of a migratory morphology others have shown that N-WASP is required for carcinoma cells to extend polarized protrusions towards a chemoattractant (Desmarais et al. 2009) and epithelial cell chemotaxis (Yamaguchi et al. 2002). While reduction of both N-WASP and WASP did not enhance the effect on cell polarity and elongation greater than that caused by the reduction of WASP alone (Fig. 2B and C), this was most likely because the combined shRNA treatment did not reduce the levels of each as effectively as use of single shRNA (Fig. 2A). The obeserved effects on cell morphology may be caused by the effects of WASP and N-WASP in lamellipodial and filopodial extension through their interaction with the Arp2/3 complex in macrophages (Hufner et al. 2002). In addition, this data also suggest that WASP and N-WASP may have partially overlapping functions in macrophage morphology.
Figure 2. Cells with reduced N-WASP are less polar and elongated.
RAW/LR5 cells were transfected with control shRNA (Control), shN-WASP (shN-W), shWASP (shW), or both plasmids (shN-W/W) and selected for 2 days in puromycin. (A) Representative western blot is shown of whole cell lysates probed using an antibody that recognizes both WASP and N-WASP. The open arrow represents N-WASP and the closed arrow represents WASP. The fraction of cells with a polarized or migratory phenotype (B) or the elongation index (C) was determined as described in the Materials and Methods. Error bars represent SEM. * p<0.05, n ≥ 3.
Interestingly, it was observed that reduction of either WASP or N-WASP led to a 3 and 1.5-fold increase in N-WASP or WASP expression, respectively (data not shown) suggesting the possibility that the cells were attempting to compensate for the loss of the expression of one WASP family member by enhancing the expression of remaining member. In a recent study we have found that WASP is expressed at fifteen times the level of N-WASP in primary macrophages, dendritic cells and RAW/LR5 macrophages and forced over-expression of N-WASP in RAW/LR5 cells with reduced levels of WASP restored the morphology defects observed in WASP deficient cells (Isaac et al. 2010).
N-WASP deficient macrophages produce podosomes, but they are non-functional
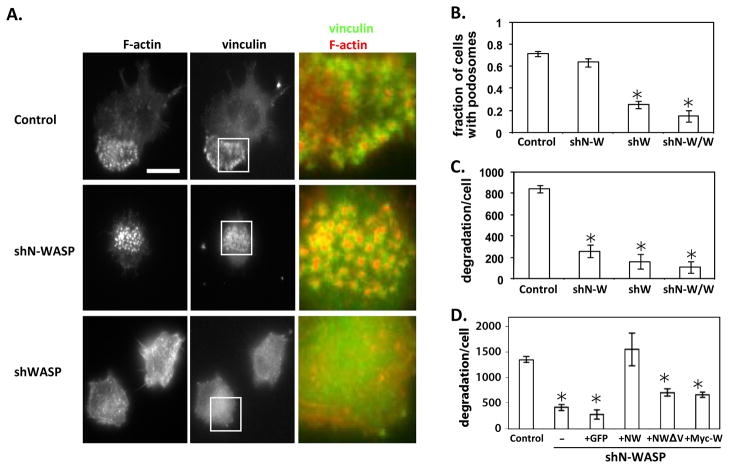
Even though cells with reduced N-WASP demonstrated clear morphological defects and GFP-N-WASP was localized to podosomes, cells with reduced N-WASP levels still contained podosomes (Fig. 3A). There was no significant difference in the number of cells containing podosomes but there was a slight decrease in the number of podosomes per cell (Fig. 3B and data not shown). To determine whether the podosomes present in cells with reduced N-WASP level are still functional, their ability to degrade a fibronectin substrate was analyzed. Consistent with previous results (Dovas et al. 2009), cells with reduced WASP levels displayed reduced podosomes and degradation. Unlike WASP, N-WASP deficient cells still contain podosomes, but they are unable to degrade matrix as efficiently as control cells (Fig. 3C). Subsequent rescue experiments found that expression of GFP-tagged N-WASP, that was not recognized by the targeting siRNA, was able to restore the ability of shN-WASP cells to degrade matrix (Fig. 3D). Surprisingly however, expression of myc–WASP was unable to restore the degradation defect following N-WASP reduction (Fig. 3D). Myc tagged WASP was fully capable of restoring both podosome formation and function in shWASP cells (data not shown and (Dovas et al. 2009)). This data indicated that the role of N-WASP in matrix degradation was unique and was non-redundant with WASP.
Figure 3. N-WASP deficient cells produce podosomes, but are unable to degrade a fibronectin matrix.
(A) Representative images of RAW/LR5 cells transfected with either control shRNA, shN-WASP, shWASP and stained for F-actin and vinculin (to identify podosomes) are shown. Image on right is a merged imaged of a 10 μm square shown in the inset. (Bar, 10 μm). (B-D) Cells transfected with control shRNA(Control), shN-WASP (shN-W), shWASP (shW), or both plasmids (shN-W/W) cells were plated on coverslips coated with fluorescently labeled fibronectin for 16 hrs and (B) the fraction of cells with podosomes or (C) the degradation area per cell were determined as described in Material and Methods. (D) Degradation area per cell was determined for control and shN-WASP cells or those rescued with GFP alone (GFP), GFP-N-WASP (NW), mutated N-WASP (NWΔV) or Myc-WASP (Myc-W). Error bars represent SEM. * p<0.05, n ≥ 3.
To determine whether actin polymerization induced by N-WASP was required for degradation, shN-WASP cells were transfected with a mutant version of N-WASP containing a deletion in the V domain that results in decreased actin polymerization (Miki and Takenawa 1998). Unlike wild-type N-WASP, this mutant (NW-ΔV) was unable to fully restore matrix degradation but a partial rescue of degradation was observed (Fig. 3D), suggesting that other functions of N-WASP that are independent of actin polymerization may be involved. However, the ΔV mutant used is a deletion of only one of the V domains and still retains a partial ability to polymerize actin in vitro and this may be the reason for the partial recue observed (Co et al. 2007). In summary, the role of N-WASP in podosomes may only partially be associated with its role on the induction of actin polymerization and another function of N-WASP in podosomes may exist.
Several studies have implicated N-WASP in the trafficking of vesicles. For example, N-WASP has been shown to localize to endosomal and lysosomal vesicles in Xenopus oocyte extracts (Taunton et al. 2000). Additionally, actin-based vesicle movement induced by overexpression of phosphatidylinositol 5-kinase in fibroblasts was dependent on N-WASP (Rozelle et al. 2000). Expression of N-WASP truncation mutants in N-WASP defective cells indicated that the WH1 and polyproline, but not the GBD and basic, domains were important in recruitment to the vesicle surface potentially by recruiting SH3-SH2 adaptor proteins, Nck and Grb2, and WIP (Benesch et al. 2002). The physiological relevance of N-WASP recruitment for endocytosis was demonstrated by a decrease in EGF internalization in N-WASP deficient fibroblasts (Benesch et al. 2005; Innocenti et al. 2005). N-WASP remains associated with actin filaments barbed ends after nucleation via the V domains and is involved in actin “comet tail” motility by linking the vesicle membrane to actin filaments in vitro (Co et al. 2007).
N-WASP is required to localize MMPs to podosomes
It has been shown in the literature that specific MMPs are localized at podosomes. In endothelial cells, MT1-MMP and MMP 9 have degradation activity beneath podosomes (Varon et al. 2006)(reviewed in (Poincloux et al. 2009). Additionally, the polarized exocytosis of MT1-MMP–containing vesicles has been demonstrated in invading breast cancer cells (Bravo-Cordero et al. 2007). Based on the above, it is possible that N-WASP may play a role in MMP trafficking. In addition to the delivery of MMPs to invadopodia and podosomes, it has been suggested that active MT1-MMP is localized to invadopodia by means of the recycling pathway (Itoh and Seiki 2004; Remacle et al. 2005; Wang et al. 2004). N-WASP activity was observed in vesicles and in particular recycling vesicles, using FRET to determine the interaction between Cdc42 and N-WASP in breast carcinoma cells (Parsons et al. 2005).
To explore the role of N-WASP on matrix degradation through MMP targeting to podosomes, colocalization experiments were performed with mCherry MT1-MMP. MT1-MMP demonstrated a punctate distribution with partial overlap with GFP-N-WASP both by mCherry MT1-MMP and by staining for endogenous MT1-MMP (Fig. 4A and data not shown). However, when examined further it was determined that MT1-MMP colocalized with GFP-N-WASP predominantly at F-actin rich puncta that appeared to be podosomes. MT1-MMP localization to podosomes was confirmed using vinculin as an additional marker for podosomes (Fig. 4B). Next, the effect of N-WASP on MT1-MMP localization to podosomes was analyzed. MT1-MMP localization to podosomes was inhibited by silencing of N-WASP (Fig. 5A and 5B). Additionally, MT1-MMP localization was altered in cells that overexpressed the ΔV N-WASP mutant that has been demonstrated to inhibit endogenous N-WASP activity (Miki and Takenawa 1998) (Fig. 5B). This was not simply due to over-expression since overexpression of wild-type GFP-N-WASP did not significantly alter MT1-MMP localization. In either case, there was reduced MT1-MMP at podosomes (Fig. 5B), explaining the reduced matrix proteolysis observed (Fig. 3D). These studies suggest that N-WASP may play a role in the recruitment of MMPs to podosomes.
Figure 4. N-WASP co-localizes with MT1-MMP at podosomes.
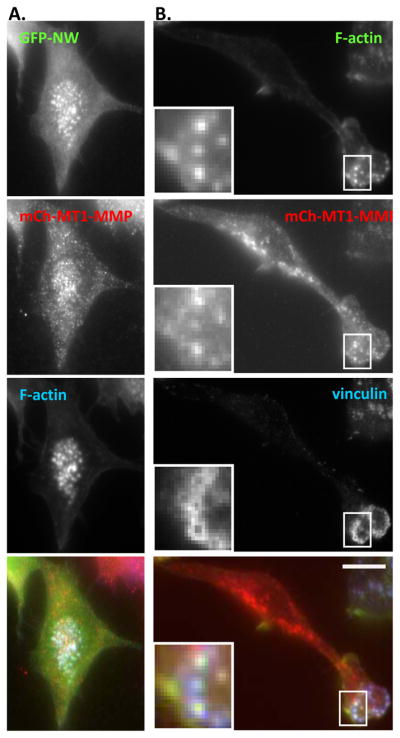
RAW/LR5 cells transiently tranfected with (A) mCherry MT1-MMP and GFP-N-WASP and (B) mCherry-MT1-MMP. Cells were fixed and stained with vinculin and F-actin as indicated and representative epifluorescence images are shown. (Bar, 10 μm)
Figure 5. N-WASP is required for MT1-MMP localization to podosomes.
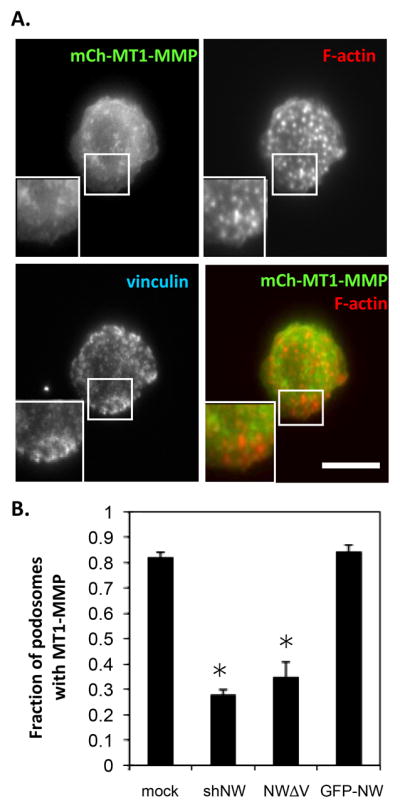
(A) mCherryMT1-MMP expressing shN-WASP cells were fixed and stained with vinculin and F-actin and representative epifluorescence images are shown. (Bar, 10 μm) (B) The fraction of mCherry MT1-MMP containing podosomes was analyzed using ImageJ for control, shN-WASP cells (shNW), mutated N-WASP (NWΔV) or GFP-N-WASP (GFP-NW). Error bars represent SEM. * p<0.05, n ≥ 3.
In support of a role of N-WASP in mediating MMP recruitment, others have demonstrated a role for N-WASP in vesicle trafficking (Benesch et al. 2002; Co et al. 2007; Kovacs et al. 2006; Taunton et al. 2000). Therefore, studies were performed using immunofluorescence microscopy to determine the colocalisation of N-WASP with several vesicle markers, such as Rab 5, 8, 8b and 11 as well as the golgi marker TGN38. None of these markers appeared to colocalize with N-WASP (data not shown). However, it is possible that N-WASP is only transiently associated with vesicles and further studies using live cell imaging would be needed. This in conjunction with the association of N-WASP with MT1-MMP only at sites of podosomes supports the hypothesis that N-WASP is required for vesicle fusion during exocytosis.
Several recent studies have shown a role for the exocyst in MMP secretion in carcinoma cells. Cdc42 is required to recruit IQGAP, another actin binding protein to the exocyst [(Sakurai-Yageta et al. 2008) and the exocyst binds to Arp2/3 and both of these interactions are required for invadopodia function (Liu et al. 2009). Cdc42 activated N-WASP has been shown to stimulate secretion in neuroendocrine cells. N-WASP mutants with a VVCA deletion were not recruited to the cell periphery and exocytosis of growth hormone in PC12 cells was impaired. However, the VVCA or GBD domains alone were not sufficient for exocytosis suggesting that other domains of N-WASP are also required for exocytosis (Gasman et al. 2004). It is interesting to speculate that N-WASP dependent actin polymerization is required for MMP containing vesicles to target and/or fuse at the podosome similar to the role of actin polymerization in neuroendocrine cells (reviewed in (Malacombe et al. 2006)).
Alternatively, it is possible that N-WASP may play a role in podosome maturation. It has been shown by others that MMPs are only targeted to mature invadopodia (Artym et al. 2006) and only the static podosomes of macrophages degrade matrix (Kopp et al. 2006). While the fraction of cells containing podosomes is unaltered in cells with reduced N-WASP levels, it should be noted that these cells do have fewer podosomes per cell (17.8±1.1 versus 27.8±0.8 for control cells). The reduced number of podosomes in shN-WASP cells is not due to decreased podosome formation since podosomes reform with the same kinetics as control cells when recovering from cytochalasin D treatment (data not shown). Future studies are needed to determine whether N-WASP regulates podosome lifetime.
Conclusions
Thus, we find that in the RAW264.7 macrophage-like cell-line that WASP is necessary for podosome formation and N-WASP is required for podosome dependent degradation of ECM indicating that these proteins serve non-redundant roles in podosome function. Unlike invadopodia, matrix degrading structures in carcinoma cells, N-WASP is not necessary for podosome formation since podosomes are still present in shN-WASP cells. Collectively these data indicate that WASP is responsible for podosome formation and N-WASP is only required for matrix degradation, while N-WASP serves two functions in invadopodia; 1) actin polymerization required for invadodopodia formation and 2) maturation (Oser et al. 2009).
We speculate that N-WASP regulation of podosome degradation occurs via directed MT1-MMP transport. Consistent with other reports (Linder 2007), we find that MT1-MMP is localized to podosomes and importantly this localization is altered by N-WASP silencing. However, the precise role of N-WASP in the localization of MT1-MMP to podosomes is not clear. N-WASP may be involved in transport of nascent MT1-MMP to podosomes or it acts in the recycling of MT1-MMP vesicles to the cell surface via an intermediate recycling compartment. Further work will be needed to investigate the spatial involvement of N-WASP on MT1-MMP trafficking.
Supplementary Material
RAW/LR5 cells transiently transfected with GFP-N-WASP were fixed and stained with F-actin (red) (to indicate podosomes). TIRF images are shown and the inset contains a magnified image of the boxed region. Arrows indicate examples of non-podosomal puncta (Bar, 10 μm)
Acknowledgments
This work was funded by an NIH (GM07828 to DC). TIRF imaging was performed at the Gruss-Lipper Biophotonics Center.
ABBREVIATIONS LIST
- WASP
Wiskott-Aldrich Syndrome Protein
- N-WASP
neural-WASP
- MMP
matrix metalloproteinase
- siRNA
small-interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66(6):3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Badolato R, Sozzani S, Malacarne F, Bresciani S, Fiorini M, Borsatti A, Albertini A, Mantovani A, Ugazio AG, Notarangelo LD. Monocytes from Wiskott-Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1998;161(2):1026–33. [PubMed] [Google Scholar]
- Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem. 2002;277(40):37771–6. doi: 10.1074/jbc.M204145200. [DOI] [PubMed] [Google Scholar]
- Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118(Pt 14):3103–15. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26(6):1499–510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98(4):1142–9. doi: 10.1182/blood.v98.4.1142. [DOI] [PubMed] [Google Scholar]
- Calle Y, Burns S, Thrasher AJ, Jones GE. The leukocyte podosome. Eur J Cell Biol. 2006;85(3–4):151–7. doi: 10.1016/j.ejcb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Calle Y, Chou HC, Thrasher AJ, Jones GE. Wiskott-Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J Pathol. 2004a;204(4):460–9. doi: 10.1002/path.1651. [DOI] [PubMed] [Google Scholar]
- Calle Y, Jones GE, Jagger C, Fuller K, Blundell MP, Chow J, Chambers T, Thrasher AJ. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood. 2004b;103(9):3552–61. doi: 10.1182/blood-2003-04-1259. [DOI] [PubMed] [Google Scholar]
- Co C, Wong DT, Gierke S, Chang V, Taunton J. Mechanism of actin network attachment to moving membranes: barbed end capture by N-WASP WH2 domains. Cell. 2007;128(5):901–13. doi: 10.1016/j.cell.2006.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. j Exp Med. 1997;186(9):1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton. 2009;66(6):303–16. doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovas A, Gevrey JC, Grossi A, Park H, Abou-Kheir W, Cox D. Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J Cell Sci. 2009;122(Pt 21):3873–82. doi: 10.1242/jcs.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Malacombe M, Way M, Bader MF. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol Biol Cell. 2004;15(2):520–31. doi: 10.1091/mbc.E03-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufner K, Schell B, Aepfelbacher M, Linder S. The acidic regions of WASp and N-WASP can synergize with CDC42Hs and Rac1 to induce filopodia and lamellipodia. FEBS Lett. 2002;514(2–3):168–74. doi: 10.1016/s0014-5793(02)02358-x. [DOI] [PubMed] [Google Scholar]
- Innocenti M, Gerboth S, Rottner K, Lai FP, Hertzog M, Stradal TE, Frittoli E, Didry D, Polo S, Disanza A, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol. 2005;7(10):969–76. doi: 10.1038/ncb1304. [DOI] [PubMed] [Google Scholar]
- Isaac BM, Ishihara D, Nusblat LM, Gevrey J-C, Dovas A, Condeelis J, Cox D. N-WASP has the Ability to Compensate for the Loss of WASP in Macrophage Podosome Formation and Function. Exp Cell Res. 2010 doi: 10.1016/j.yexcr.2010.06.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Seiki M. MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem Sci. 2004;29(6):285–9. doi: 10.1016/j.tibs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kopp P, Lammers R, Aepfelbacher M, Woehlke G, Rudel T, Machuy N, Steffen W, Linder S. The kinesin KIF1C and microtubule plus ends regulate podosome dynamics in macrophages. Mol Biol Cell. 2006;17(6):2811–23. doi: 10.1091/mbc.E05-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Makar RS, Gertler FB. Tuba stimulates intracellular N-WASP-dependent actin assembly. J Cell Sci. 2006;119(Pt 13):2715–26. doi: 10.1242/jcs.03005. [DOI] [PubMed] [Google Scholar]
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17(3):107–17. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96(17):9648–53. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue P, Artym VV, Mueller SC, Guo W. The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol Biol Cell. 2009;20(16):3763–71. doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacombe M, Bader MF, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochim Biophys Acta. 2006;1763(11):1175–83. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15(19):5326–35. [PMC free article] [PubMed] [Google Scholar]
- Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391(6662):93–6. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- Miki H, Takenawa T. Direct binding of the verprolin-homology domain in N-WASP to actin is essential for cytoskeletal reorganization. Biochem Biophys Res Commun. 1998;243(1):73–8. doi: 10.1006/bbrc.1997.8064. [DOI] [PubMed] [Google Scholar]
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186(4):571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Cox D. Cdc42 regulates Fc gamma receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol Biol Cell. 2009;20(21):4500–8. doi: 10.1091/mbc.E09-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M, Monypenny J, Ameer-Beg SM, Millard TH, Machesky LM, Peter M, Keppler MD, Schiavo G, Watson R, Chernoff J, et al. Spatially distinct binding of Cdc42 to PAK1 and N-WASP in breast carcinoma cells. Mol Cell Biol. 2005;25(5):1680–95. doi: 10.1128/MCB.25.5.1680-1695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14(11):628–38. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122(Pt 17):3015–24. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- Remacle AG, Rozanov DV, Baciu PC, Chekanov AV, Golubkov VS, Strongin AY. The transmembrane domain is essential for the microtubular trafficking of membrane type-1 matrix metalloproteinase (MT1-MMP) J Cell Sci. 2005;118(Pt 21):4975–84. doi: 10.1242/jcs.02610. [DOI] [PubMed] [Google Scholar]
- Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10(6):311–20. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D’Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181(6):985–98. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper SB, Takeshima F, Anton I, Liu CH, Thomas SM, Nguyen D, Dudley D, Fraser H, Purich D, Lopez-Ilasaca M, et al. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol. 2001;3(10):897–904. doi: 10.1038/ncb1001-897. [DOI] [PubMed] [Google Scholar]
- Stamm LM, Pak MA, Morisaki JH, Snapper SB, Rottner K, Lommel S, Brown EJ. Role of the WASP family proteins for Mycobacterium marinum actin tail formation. Proc Natl Acad Sci U S A. 2005;102(41):14837–42. doi: 10.1073/pnas.0504663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Mimuro H, Suetsugu S, Miki H, Takenawa T, Sasakawa C. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell Microbiol. 2002;4(4):223–33. doi: 10.1046/j.1462-5822.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol. 2000;148(3):519–30. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 10(3):182–92. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- Tomasevic N, Jia Z, Russell A, Fujii T, Hartman JJ, Clancy S, Wang M, Beraud C, Wood KW, Sakowicz R. Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck, and PI(4,5)P2. Biochemistry. 2007;46(11):3494–502. doi: 10.1021/bi062152y. [DOI] [PubMed] [Google Scholar]
- Varon C, Tatin F, Moreau V, Van Obberghen-Schilling E, Fernandez-Sauze S, Reuzeau E, Kramer I, Genot E. Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol. 2006;26(9):3582–94. doi: 10.1128/MCB.26.9.3582-3594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma D, Keski-Oja J, Pei D. Co-recycling of MT1-MMP and MT3-MMP through the trans-Golgi network. Identification of DKV582 as a recycling signal. J Biol Chem. 2004;279(10):9331–6. doi: 10.1074/jbc.M312369200. [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Wells CM, Smith SD, Vega FM, Henderson RB, Tybulewicz VL, Ridley AJ. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J Cell Sci. 2006;119(Pt 13):2749–57. doi: 10.1242/jcs.03024. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Miki H, Takenawa T. Neural Wiskott-Aldrich syndrome protein is involved in hepatocyte growth factor-induced migration, invasion, and tubulogenesis of epithelial cells. Cancer Res. 2002;62(9):2503–9. [PubMed] [Google Scholar]
- Yamaguchi H, Pixley F, Condeelis J. Invadopodia and podosomes in tumor invasion. Eur J Cell Biol. 2006;85(3–4):213–8. doi: 10.1016/j.ejcb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Zalevsky J, Lempert L, Kranitz H, Mullins RD. Different WASP family proteins stimulate different Arp2/3 complex-dependent actin-nucleating activities. Curr Biol. 2001;11(24):1903–13. doi: 10.1016/s0960-9822(01)00603-0. [DOI] [PubMed] [Google Scholar]
- Zicha D, Allen WE, Brickell PM, Kinnon C, Dunn GA, Jones GE, Thrasher AJ. Chemotaxis of macrophages is abolished in the Wiskott-Aldrich syndrome. Br J Haematol. 1998;101(4):659–665. doi: 10.1046/j.1365-2141.1998.00767.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RAW/LR5 cells transiently transfected with GFP-N-WASP were fixed and stained with F-actin (red) (to indicate podosomes). TIRF images are shown and the inset contains a magnified image of the boxed region. Arrows indicate examples of non-podosomal puncta (Bar, 10 μm)