Abstract
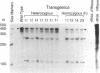
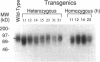
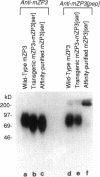
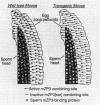
To initiate fertilization in mice, free-swimming sperm bind to mZP3, an approximately 83-kDa glycoprotein present in the ovulated egg zona pellucida (ZP). mZP3 is located periodically along the filaments that constitute the ZP. Sperm recognize and bind to specific oligosaccharides linked to one or more of five Ser residues clustered in the carboxy-terminal one-third of the mZP3 polypeptide. When all five Ser residues are converted to nonhydroxy amino acids by site-directed mutagenesis of the mZP3 gene, an inactive form of mZP3, called mZP3[ser], is secreted by embryonal carcinoma cells stably transfected with the mutated gene. Here, seven independent transgenic mouse lines were established that harbor the mutated mZP3 gene. In all lines, the mutant gene is expressed by growing oocytes and mZP3[ser] is synthesized, secreted, and incorporated into the ZP. Purified mZP3[ser] prepared from ovaries of transgenic mice, like mZP3[ser] from transfected embryonal carcinoma cells, is inactive in sperm binding assays in vitro. On the other hand, the presence of mZP3[ser] in the ZP does not significantly affect either the binding of sperm to ovulated eggs in vitro or the reproduction of the mice, i.e., the transgenic mice are fertile, breed at normal intervals, and produce litters of normal sizes. These results indicate that the number of functional sperm receptors in the ZP can be reduced by more than 50% without adversely affecting fertilization of eggs in vivo.
Full text
PDF
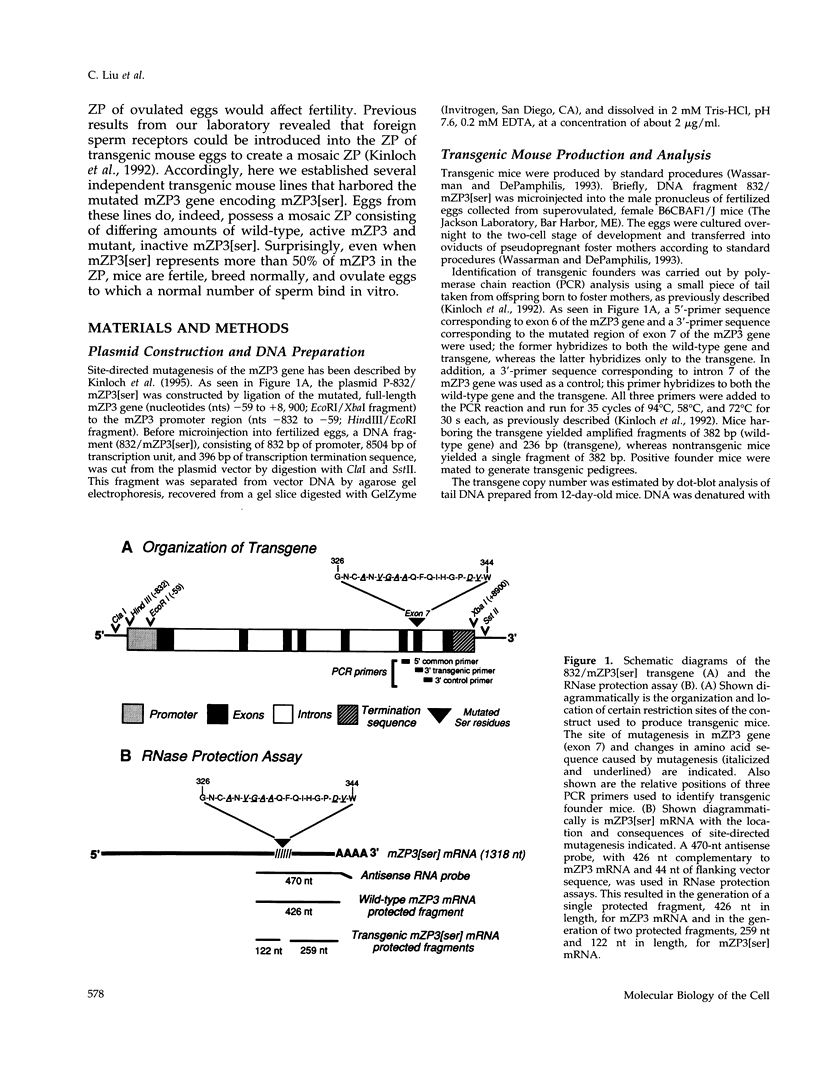

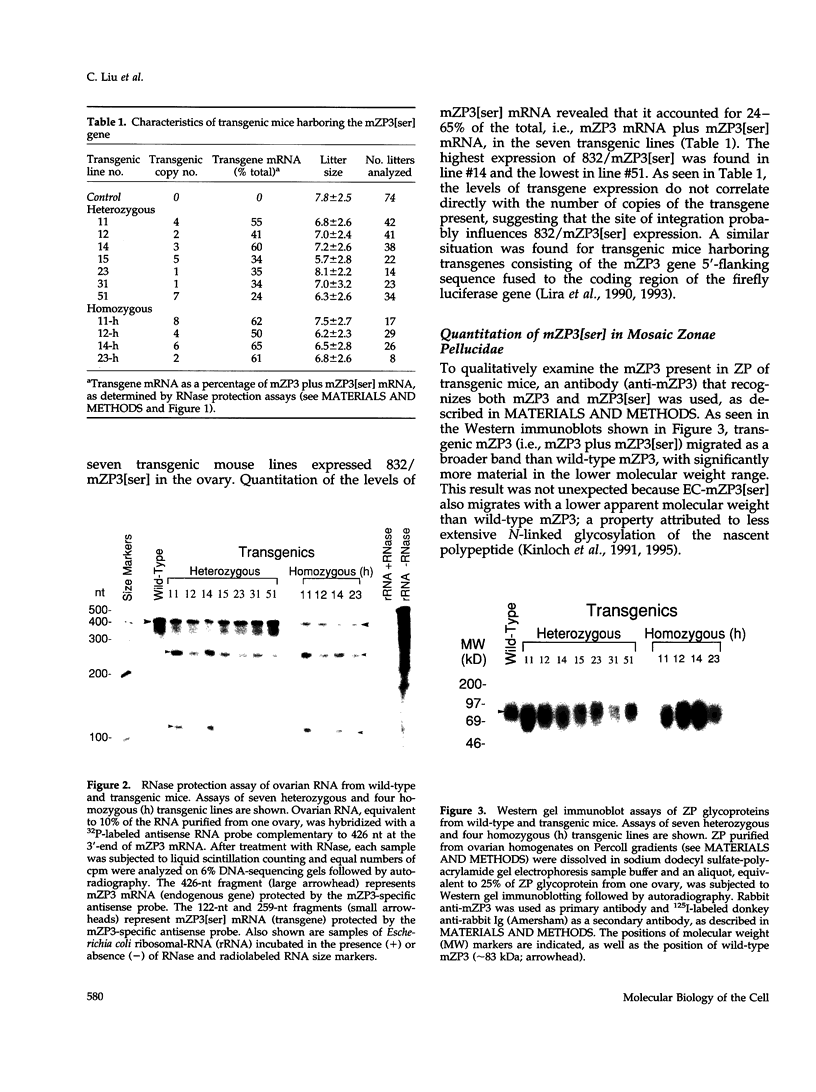
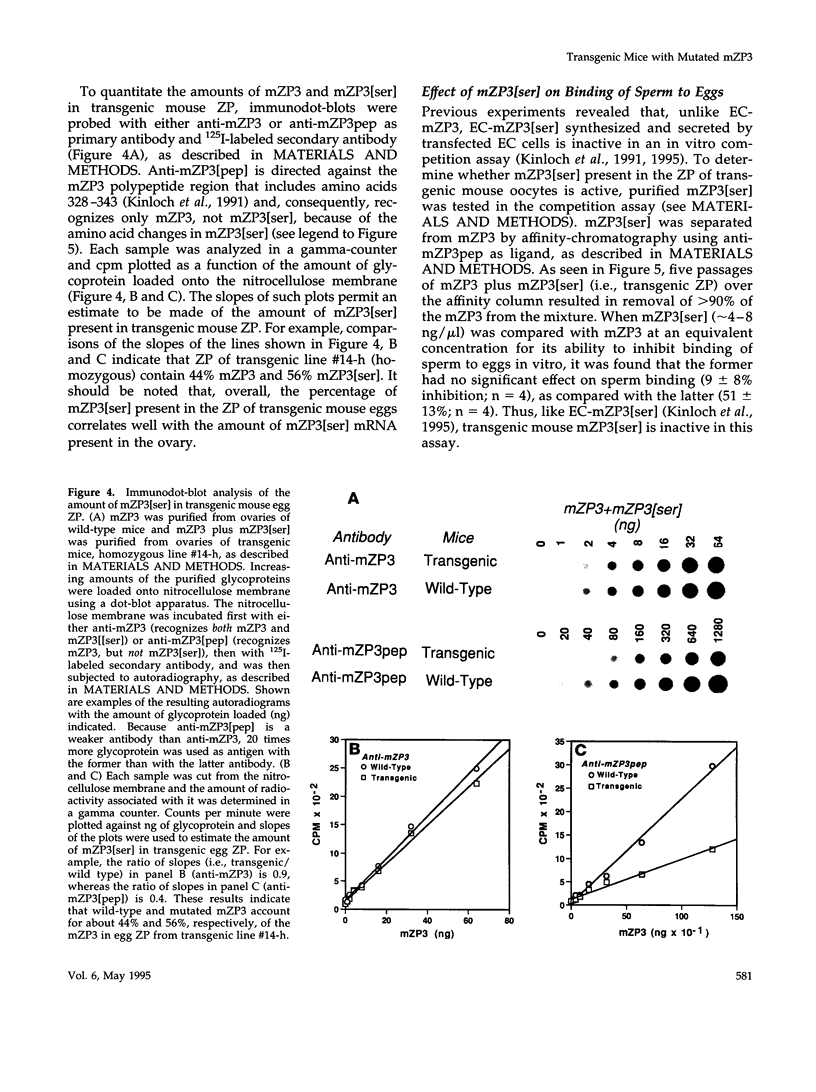

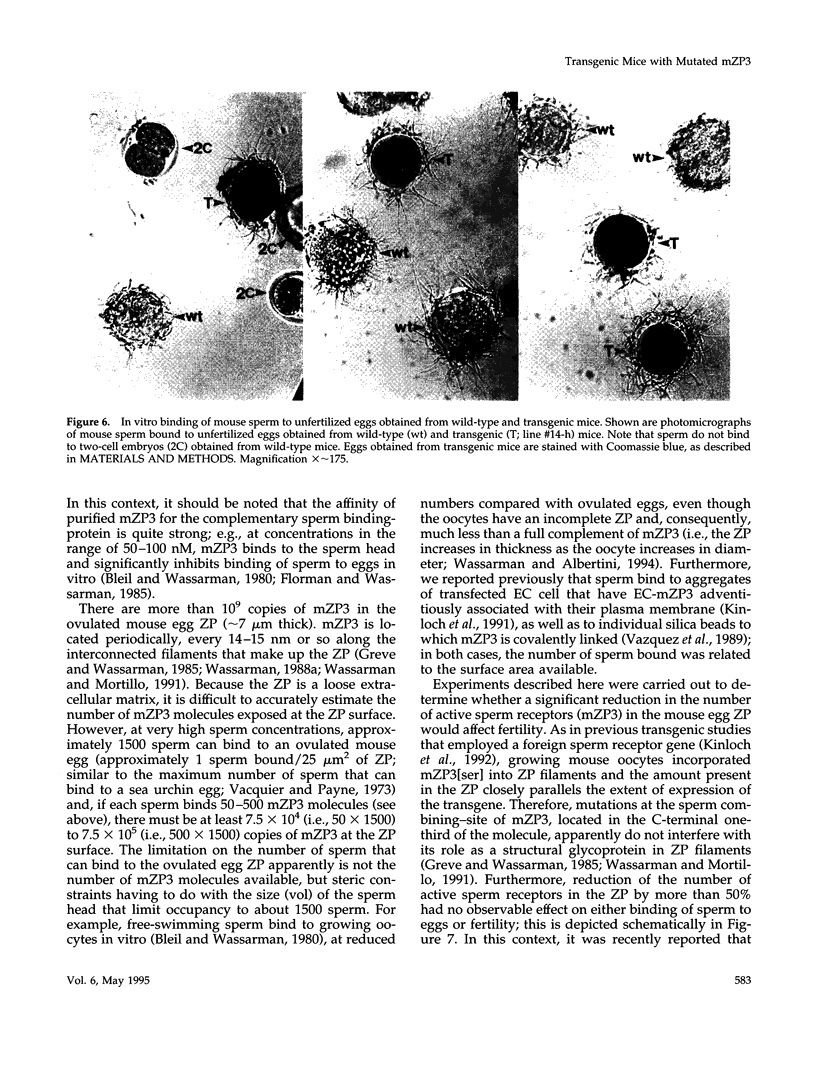

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz J. M., Cardullo R. A. On the number and rate of formation of sperm-zona bonds in the mouse. Gamete Res. 1989 Sep;24(1):1–8. doi: 10.1002/mrd.1120240103. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Greve J. M., Wassarman P. M. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Dev Biol. 1988 Aug;128(2):376–385. doi: 10.1016/0012-1606(88)90299-0. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Autoradiographic visualization of the mouse egg's sperm receptor bound to sperm. J Cell Biol. 1986 Apr;102(4):1363–1371. doi: 10.1083/jcb.102.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 1980 Jul;20(3):873–882. doi: 10.1016/0092-8674(80)90334-7. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983 Feb;95(2):317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Wassarman P. M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985 May;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Wassarman P. M. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol. 1985 Jan 20;181(2):253–264. doi: 10.1016/0022-2836(85)90089-0. [DOI] [PubMed] [Google Scholar]
- Kinloch R. A., Mortillo S., Stewart C. L., Wassarman P. M. Embryonal carcinoma cells transfected with ZP3 genes differentially glycosylate similar polypeptides and secrete active mouse sperm receptor. J Cell Biol. 1991 Nov;115(3):655–664. doi: 10.1083/jcb.115.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch R. A., Mortillo S., Wassarman P. M. Transgenic mouse eggs with functional hamster sperm receptors in their zona pellucida. Development. 1992 Aug;115(4):937–946. doi: 10.1242/dev.115.4.937. [DOI] [PubMed] [Google Scholar]
- Kinloch R. A., Sakai Y., Wassarman P. M. Mapping the mouse ZP3 combining site for sperm by exon swapping and site-directed mutagenesis. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):263–267. doi: 10.1073/pnas.92.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch R. A., Wassarman P. M. Profile of a mammalian sperm receptor gene. New Biol. 1989 Dec;1(3):232–238. [PubMed] [Google Scholar]
- Lira S. A., Kinloch R. A., Mortillo S., Wassarman P. M. An upstream region of the mouse ZP3 gene directs expression of firefly luciferase specifically to growing oocytes in transgenic mice. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7215–7219. doi: 10.1073/pnas.87.18.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira S. A., Schickler M., Wassarman P. M. cis-acting DNA elements involved in oocyte-specific expression of mouse sperm receptor gene mZP3 are located close to the gene's transcription start site. Mol Reprod Dev. 1993 Dec;36(4):494–499. doi: 10.1002/mrd.1080360414. [DOI] [PubMed] [Google Scholar]
- Melton D. W. Gene targeting in the mouse. Bioessays. 1994 Sep;16(9):633–638. doi: 10.1002/bies.950160907. [DOI] [PubMed] [Google Scholar]
- Moller C. C., Bleil J. D., Kinloch R. A., Wassarman P. M. Structural and functional relationships between mouse and hamster zona pellucida glycoproteins. Dev Biol. 1990 Feb;137(2):276–286. doi: 10.1016/0012-1606(90)90254-g. [DOI] [PubMed] [Google Scholar]
- Mortillo S., Wassarman P. M. Differential binding of gold-labeled zona pellucida glycoproteins mZP2 and mZP3 to mouse sperm membrane compartments. Development. 1991 Sep;113(1):141–149. doi: 10.1242/dev.113.1.141. [DOI] [PubMed] [Google Scholar]
- Rosiere T. K., Wassarman P. M. Identification of a region of mouse zona pellucida glycoprotein mZP3 that possesses sperm receptor activity. Dev Biol. 1992 Dec;154(2):309–317. doi: 10.1016/0012-1606(92)90070-w. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Payne J. E. Methods for quantitating sea urchin sperm-egg binding. Exp Cell Res. 1973 Nov;82(1):227–235. doi: 10.1016/0014-4827(73)90265-6. [DOI] [PubMed] [Google Scholar]
- Vazquez M. H., Phillips D. M., Wassarman P. M. Interaction of mouse sperm with purified sperm receptors covalently linked to silica beads. J Cell Sci. 1989 Apr;92(Pt 4):713–722. doi: 10.1242/jcs.92.4.713. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Bleil J. D., Florman H. M., Greve J. M., Roller R. J., Salzmann G. S., Samuels F. G. The mouse egg's receptor for sperm: what is it and how does it work? Cold Spring Harb Symp Quant Biol. 1985;50:11–19. doi: 10.1101/sqb.1985.050.01.004. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Early events in mammalian fertilization. Annu Rev Cell Biol. 1987;3:109–142. doi: 10.1146/annurev.cb.03.110187.000545. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Fertilization in mammals. Sci Am. 1988 Dec;259(6):78–84. doi: 10.1038/scientificamerican1288-78. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Litscher E. S. Sperm--egg recognition mechanisms in mammals. Curr Top Dev Biol. 1995;30:1–19. doi: 10.1016/s0070-2153(08)60562-1. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Mortillo S. Structure of the mouse egg extracellular coat, the zona pellucida. Int Rev Cytol. 1991;130:85–110. doi: 10.1016/s0074-7696(08)61502-8. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Profile of a mammalian sperm receptor. Development. 1990 Jan;108(1):1–17. doi: 10.1242/dev.108.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. The biology and chemistry of fertilization. Science. 1987 Jan 30;235(4788):553–560. doi: 10.1126/science.3027891. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- Youakim A., Hathaway H. J., Miller D. J., Gong X., Shur B. D. Overexpressing sperm surface beta 1,4-galactosyltransferase in transgenic mice affects multiple aspects of sperm-egg interactions. J Cell Biol. 1994 Sep;126(6):1573–1583. doi: 10.1083/jcb.126.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]