Type 1 diabetes—or, more accurately, type 1A diabetes—is thought to arise from selective immunologically mediated destruction of the insulin-producing β-cells in the pancreatic islets of Langerhans with consequent insulin deficiency (1). This occurs in individuals in whom genetic susceptibility outweighs genetic protection and is probably initiated by environmental factors not yet clearly defined. The disease arises via a cellular-mediated immune process, presumably a specific reaction to one or more β-cell proteins (autoantigens). There is consequent progressive impairment of β-cell function and apparent decline in β-cell mass. A secondary humoral immune response is characterized by the appearance of autoantibodies that serve as markers of the immune damage to β-cells. This insidious type 1 diabetes disease process generally evolves over a variable period of years (Fig. 1). The decline in β-cell function—and presumably in mass—is evidenced metabolically by loss of first-phase insulin response to an intravenous glucose challenge and later by the appearance of impairment in glycemic regulation, which is manifested as dysglycemia either as impaired glucose tolerance, impaired fasting glucose, or “indeterminate” glucose levels (values >200 mg/dl [11.1 mmol/l] at 30, 60, or 90 min during an oral glucose tolerance test). Ultimately, the clinical syndrome of type 1 diabetes becomes evident when the majority of β-cell function has been lost and presumably most β-cells have been destroyed; at this juncture, frank hyperglycemia supervenes. Although that broad sequence can be articulated, there are still gaps in many of the details. Further understanding of the nature of the disease process will facilitate the design of intervention strategies aimed at abrogating β-cell destruction and ultimately at prophylaxis of type 1 diabetes.
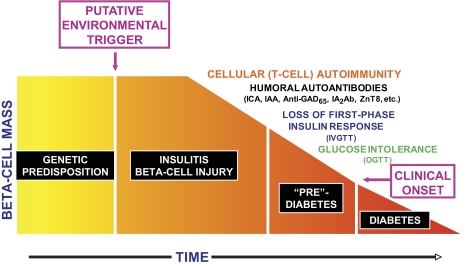
FIG. 1.
Progression of the type 1 diabetes disease process. This is a cellular autoimmune process occurring in individuals with a genetic predisposition to the disease, presumably triggered by some environmental factor. Humoral antibodies indicate that the disease process is underway, and there is then progressive impairment of β-cell function manifested by progressive deterioration of glucose metabolism. The time frame is variable, so the x-axis is dimensionless. IAA, insulin autoantibody; ICA, islet cell antibody; IVGTT, intravenous glucose tolerance test; OGTT, oral glucose tolerance test.
It should be evident from the above sequence of events that if type 1 diabetes is to be conquered, it is necessary 1) to stop immune destruction of β-cells, 2) to replace or regenerate β-cells, and 3) to preserve β-cell function and mass. In regards to all three of these, much has been accomplished in animal models of type 1 diabetes. Yet in human beings, success has been elusive. That is not to say that progress has not been made, for indeed it has. In this Perspectives in Diabetes article, written in honor of the 40th anniversary of the Juvenile Diabetes Research Foundation (JDRF), we review the progress that has been made, indicate the challenges that have confronted investigators in these efforts, and propose a vision for how such research efforts might unfold in the future. We also note that several important consortia are addressing various aspects of this sequence, and these are listed in Table 1. These consortia have been supported by several institutes of the National Institutes of Health (NIH), JDRF, and the American Diabetes Association (ADA).
TABLE 1.
Consortia studying type 1 diabetes
| Consortium | Full name of consortium | Consortium activities | Consortium website |
|---|---|---|---|
| T1DGC | Type 1 Diabetes Genetics Consortium | T1DGC was established with the primary goal of organizing international efforts to identify genes that determine an individual's risk of type 1 diabetes (2). | www.t1dgc.org |
| TEDDY | The Environmental Determinants of Diabetes in the Young | The primary objective(s) of TEDDY is the identification of infectious agents, dietary factors, or other environmental exposures that are associated with increased risk of autoimmunity and type 1 diabetes (3). | teddy.epi.usf.edu |
| nPOD | Network for Pancreatic Organ donors with Diabetes | The mission of nPOD is to characterize pancreata and related tissues from organ donors with type 1 diabetes or who are islet autoantibody positive and utilize the tissues to address key immunological, histological, viral, and metabolic questions related to how type 1 diabetes develops (4). | www.jdrfnpod.org |
| SEARCH | Search for Diabetes in Youth | SEARCH identifies cases of diabetes in children/youth <20 years of age in six geographically dispersed populations that encompass the ethnic diversity of the United States (5). | www.searchfordiabetes.org |
| TrialNet | Type 1 Diabetes TrialNet | TrialNet is an international network conducting studies that will improve the understanding of type 1 diabetes disease development and test interventions to interdict the type 1 diabetes disease process, particularly strategies for type 1 diabetes prevention (6). | www.diabetestrialnet.org |
| ITN | Immune Tolerance Network | ITN is an international consortium dedicated to the clinical evaluation of novel tolerogenic approaches for the treatment of autoimmune diseases (including type 1 diabetes), asthma, and allergic diseases, and the prevention of graft rejection (7). | www.immunetolerance.org |
| TRIGR | Trial to Reduce IDDM in the Genetically at Risk | TRIGR is testing whether weaning to a casein hydrolysate formula during the first 6–8 months of life—in place of cow's milk-based formula—reduces the incidence of autoimmunity and type 1 diabetes in genetically susceptible newborn infants (8). | trigr.epi.usf.edu/ |
| ICRs | Islet Cell Resource Centers | ICRs' goals are: 1) to provide high-quality islets for use in transplantation; 2) to optimize characterization of quality and effectiveness of islets transplanted into patients; and 3) to provide islets for basic science studies (9). | icr.coh.org/ |
| CITR | Collaborative Islet Transplant Registry | CITR is expediting progress and promoting safety in islet transplantation through the collection, analysis, and communication of comprehensive and current data on all such transplants performed in North America (10). | www.citregistry.org |
| CIT | Clinical Islet Transplantation Consortium | This consortium is implementing a program of clinical studies, accompanied by mechanistic studies, in islet transplantation with or without accompanying kidney transplantation, for the treatment of type 1 diabetes (11). | www.isletstudy.org |
| DirecNet | Diabetes Research in Children Network | DirecNet is investigating the potential use of glucose monitoring technology and its impact on the management of type 1 diabetes in children (12). | public.direc.net/ |
| EDIC | Epidemiology of Diabetes Interventions and Complications | EDIC is studying the clinical course and risk factors associated with long-term complications of type 1 diabetes in the Diabetes Control and Complications Trial (DCCT) cohort (13). | www.bsc.gwu.edu/bsc/studies/edic.html |
Stopping immune destruction of β-cells.
Much investigation has been directed at interrupting the type 1 diabetes disease process both during the stage of evolution of the disease and at the time of disease onset.
The goal of intervention before clinical disease onset is to arrest the immune destruction and thus delay or prevent clinical disease. To effectively accomplish this requires identification of individuals at risk of type 1 diabetes (14). Therefore, a significant amount of attention has been given to identify potential risk markers and to quantify risk projection—with considerable success among relatives of individuals with type 1 diabetes (15,16) and some success based on the screening of newborns for genetic markers (17). Such prediction of the development of type 1 diabetes is based on risk assessment, which is accomplished using genetic, immunologic, and metabolic parameters. Yet, the majority of individuals who present with type 1 diabetes do not have a known relative who had the disease, and newborn screening programs for genetic risk markers are not yet universal. If there were an available clinical prevention strategy with demonstrated effectiveness, the case could be made that such newborn screening be mandatory. At some point, that clearly will be the case. This approach would identify most (over 95%) of those destined to develop type 1 diabetes, but it would also identify a larger number of people who will not develop the disease. This would facilitate the introduction of interventions designed to prevent autoimmunity as opposed to interventions in individuals with autoimmune markers in whom the goal is to prevent clinical type 1 diabetes. Yet, because of the fact that many newborns identified as having genetic risk will not ultimately develop type 1 diabetes, any intervention imposed at such time must be very safe.
The ability to predict type 1 diabetes on the basis of immunologic, genetic, and metabolic markers has led to several large studies designed to determine whether type 1 diabetes can be prevented by intervening in individuals with identified autoimmunity (Fig. 2). Unfortunately, to date, such studies have been without clear success (18). In part, this can be attributed to the selection of interventions that impose minimal risk to the subjects participating in the trials. However, it also may be that once autoimmunity is established, the immune process progressively expands, involving more and more components of the immune system and directed at an increasing number of islet autoantigens. The consequence is that therapies targeted at one specific component of the immune system, or at one specific autoantigen, may be inadequate to arrest the type 1 diabetes disease process. Nonetheless, any therapeutic strategy that has proven safe in new-onset type 1 diabetes, even if only with minor or transient efficacy, may be worth considering for prevention. We recognize that safety must be paramount for any study involving individuals who do not yet have disease. On the other hand, one must be cautious to not equate unpleasant but transient side effects, such as those associated with an infusion, as an obstacle for consideration of agents that are devoid of serious sustained adverse effects.
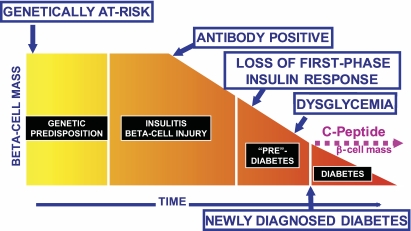
FIG. 2.
Potential time points for intervention to alter the type 1 diabetes disease process. Intervention may be attempted in the genetically at-risk to try to abrogate autoimmunity, in those with antibodies signifying that the disease process is underway, or in those with varying degrees of metabolic abnormalities, including at the time of clinical onset of type 1 diabetes.
Studies of immune intervention begun at or shortly after diagnosis of clinical type 1 diabetes have the advantage that research subjects have an unambiguous diagnosis, but they have the disadvantage that such individuals have fewer β-cells to preserve. The goal of such studies is the preservation of residual β-cell function, thus allowing for easier glycemic management while also decreasing the likelihood of both hypoglycemia and long-term complications. β-Cell function is assessed by measuring C-peptide response to a provocative stimulus (19). A whole variety of interventions have been explored at this stage (20). Some interventions have shown potential benefit but were limited by toxicity or by the benefit being of limited magnitude and/or short duration of benefit. A number of agents showed promising preliminary effects but were not studied further. Others have failed to show efficacy. Some have shown mixed results. Others have only been evaluated in pilot studies that were too small to draw firm conclusions. Yet some approaches have shown promise in recent-onset type 1 diabetes, including two different anti-CD3 monoclonal antibodies (teplizumab [21] and otelixizumab [22]), the anti-CD20 monoclonal antibody rituximab (23), a GAD vaccine (24), and a somewhat extreme approach involving profound immunoablation with cyclophosphamide and anti-thymocyte globulin followed by rescue with autologous bone marrow transplantation to prevent the theoretical risk of aplastic anemia that could, in theory, develop after the cytoreductive-induction treatment (25). However, this risk should be virtually absent since the induction is not fully cytoablative. Additional strategies continue to be explored including cellular approaches such as the use of immature autologous dendritic cells (26).
Yet careful examination even of the approaches that have shown promise in recent-onset type 1 diabetes reveals that the effects seen are transient in nature (Fig. 3). As noted above, this could be due to progressive expansion of the immune process to involve more of the immune system and/or expansion of the number of islet autoantigens involved. One insight into the complicated and aggressive nature of the autoimmune response in type 1 diabetes is the observation that in individuals who have received combined kidney and pancreas transplants, there is often recurrent autoimmunity in the face of significant immunosuppression and absent the signs of organ rejection (27). This suggests that the immune pathways leading to autoimmunity are different than those leading to allorejection, and it implies that different approaches may be needed if both are to be controlled. Yet in organ transplantation, combinations of multiple immunologic modulators or suppressants are routinely used. Moreover, in animal models of type 1 diabetes, some of the best successes are seen with combination therapies. Thus it may very well be that combination therapy is needed if we are to successfully abrogate the immune processes leading to β-cell destruction (28). In Fig. 4, one example of a theoretical approach to combination therapy for interdicting the type 1 diabetes disease process is schematically depicted. This potential approach involves the use of a number of agents, including 1) an anti-inflammatory agent, such as an anti–interleukin-1β or an anti-tumor necrosis factor, since considerable evidence exists that pancreatic islets are engulfed by an inflammatory response in both type 1 and type 2 diabetes (29); 2) transient use of a potent immunomodulator such as an anti-CD3 (21,22), or an anti-CD20 monoclonal antibody (23), or a co-stimulation blocker; 3) provision of an antigen-specific therapy such as GAD vaccine (24), and/or oral insulin (30), or another antigen-specific approach, perhaps continued indefinitely; 4) enhancement of the protective immune response, perhaps by stimulation with granulocyte colony stimulation factor (31) or the infusion of regulatory T-lymphocytes (T-regs) (32); and 5) the addition of agents that may enhance β-cell function and potentially stimulate repair, regeneration, or neogenesis of β-cells, such as glucagon-like peptide 1 (33), exenatide (34), islet neogenesis–associated peptide, or human proislet peptide-2B (35). Such a combination approach involves many challenges, including projecting the timing and duration of use of each of the components, concerns over additive toxicities, regulatory hurdles, and ethical considerations. Nonetheless, similar combination approaches have been required both for the prevention of organ rejection and for the treatment of many malignancies. Even in type 1 diabetes, the longest term beneficial effect has been seen with an aggressive combination approach, as noted above (25). Nonetheless, the acceptable cost-to-benefit ratio in type 1 diabetes currently is different from that in organ rejection and malignancy; thus, more caution may be warranted.
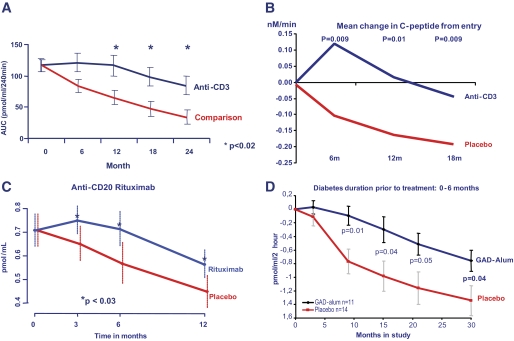
FIG. 3.
Progressive decline of β-cell function (measured by C-peptide) is evident even in studies that are successful. These include intervention with the anti-CD3 monoclonal antibodies teplizumab (A) (21) and otelixizumab (B) (22), the anti-CD20 monoclonal antibody rituximab (C) (23), and a GAD vaccine with aluminum (Alum) adjuvant (D) (24). AUC, area under the curve.
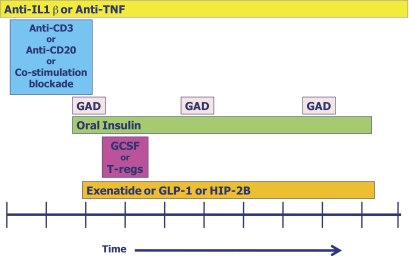
FIG. 4.
Potential scheme for combination therapy to interdict the type 1 diabetes disease process. Such a combination might include an anti-inflammatory therapy (e.g., anti–interleukin-1β [anti-IL1β] or anti-tumor necrosis factor [anti-TNF]), an immunomodulatory therapy (e.g., anti-CD3, anti-CD20, or co-stimulatory blockade), followed by initiation of antigen-specific therapy (e.g., GAD and/or oral insulin), with stimulation of protective immunity (e.g., with granulocyte colony stimulation factor [GCSF]) or provision of protective immunity by infusion of T-regulatory (T-reg) cells, and with stimulation of β-cells (e.g., with glucagon-like peptide 1 [GLP-1], exenatide, or human proislet peptide-2B [HIP-2B]). The time frame needs to be determined, so the x-axis is dimensionless.
It is interesting that intervention studies, particularly those that show beneficial effects, have challenged prevailing views regarding the pathogenesis of type 1 diabetes. An example is the finding that B-lymphocyte depletion with rituximab results in slowing of the decline of β-cell function (23). This suggests that β-cell destruction might also depend on B-lymphocyte antigen capture and presentation, which is associated with determinant spreading. If that is the case, early intervention with a B-lymphocyte–depleting strategy may delay the evolution of type 1 diabetes.
Replacement or regeneration of β-cells.
Pancreatic transplantation, usually in the context of renal transplantation with the obligatory immunosuppression needed for the latter, has been applied successfully in patients with type 1 diabetes for over two decades. Indeed, when patients with type 1 diabetes develop end-stage renal disease, the most effective intervention strategy is combined kidney and pancreas or islet transplantation. Isolated islet cell transplantation has proven to be an effective treatment modality for patients with type 1 diabetes who suffer from hypoglycemia unawareness resulting in frequent emergency room visits (10). Both approaches to β-cell replacement result in dramatic improvements in prevailing glycemia, yet both are limited by the need for immune system alteration to prevent both allorejection and recurrent autoimmunity, as well as the limited availability of tissue.
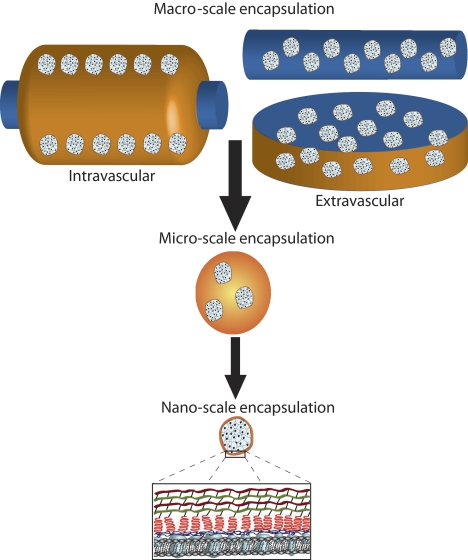
The immunologic problems partly may be addressed in an identical manner to that discussed above. In addition, it may be possible to protect cells used for β-cell replacement by immunoisolation (Fig. 5) (36,37). To that end, various encapsulation methods have been attempted for many years. More recently, progress has been made with nanoencapsulation using conformal approaches to fabricate polymer capsules. Biomaterial scaffolds have also been used to provide mechanical stability and three-dimensional distribution of β-cells to reduce stress and improve nutrient distribution (38). Implantable biohybrid devices can be used both to protect cells and to provide localized delivery of immunomodulatory drugs, thereby limiting systemic toxicity (39).
FIG. 5.
The scale of various approaches to immunoisolation (37). Macro-scale encapsulation devices include intravascular, which are perfused with blood, or extravascular devices. Micro-scale devices are typically microcapsules (as illustrated). Nano-scale encapsulation commonly employs the coating of the islet spheroid with polymeric layers, such as conformal coating.
The availability of an adequate source of cells for β-cell replacement has been a vexing problem, but one for which there are a host of potentially innovative solutions (40). Clearly, cadaveric organ donation cannot meet the potential needs, especially since in the U.S. in 2009 there were but 8,021 organ donors with only 1,739 pancreata collected. Although distal pancreatectomy for either pancreas or islet transplantation has been done, this is not likely a robust source of tissue. Xenotransplantation with porcine islets has been successful in nonhuman primates (41,42) and has been attempted in human beings. A variety of stem cells—embryonic, mesenchymal, bone marrow, cord blood, adipocyte-derived, and others—have been explored. There has been progressive improvement in this field, first with insulin-expressing cells being created, then with insulin-producing cells being developed, and finally with the generation of glucose-responsive insulin-secreting cells (43). Another approach to modifying cells is by protein transduction domain strategies, whereby several proteins can be delivered simultaneously to targeted intracellular compartments with their presence in the medium only as long as required and with maximum transfection efficiency (44). Although much work still remains, there has been substantial progress in the generation of “β-cells” from stem cells, such that these cells demonstrate insulin gene expression, insulin synthesis and processing, insulin packaging and storage in granules, glucose sensing, and appropriate release of insulin and C-peptide.
Alternatively, other types of existing adult cells may be reprogrammed or transdifferentiated into β-cells. In animal systems, functional insulin-producing cells have been generated from pancreatic α-cells (45), pancreatic ductal cells (46), pancreatic acinar cells (47), and from human liver cells (48). A recent study generated pluripotent stem cells from fibroblasts of patients with type 1 diabetes (49). In theory, one could envision obtaining such tissue (such as liver) by biopsy, manipulating that tissue in the laboratory to convert it to insulin-producing cells, and then reinfusing them as an autotransplant back into the individual from whom the tissue was obtained.
An alternative to external differentiation in vitro is to stimulate neogenesis or regeneration of insulin-secreting tissue in vivo. As noted above, agents that may enhance β-cell function and potentially stimulate repair, regeneration, or neogenesis of β-cells include glucagon-like peptide 1 (33), exenatide (34), islet neogenesis-associated peptide, and human proislet peptide-2B (35). They clearly have demonstrated the ability to do so in rodents and/or in vitro, although the potential for regeneration or neogenesis of β-cells in human beings is still unclear. It should also be noted that, in conjunction with adequate immunomodulation, exenatide has been used in individuals receiving a second islet transplant (50) with remarkable success in preserving posttransplant islet function and maintaining insulin independence, especially if the drug is administered also in the peritransplant period and not just when islet function is beginning to decline at variable times following transplantation. Yet some investigators have raised concern that the improved β-cell function seen with exenatide in type 2 diabetes is not sustained after discontinuation of exenatide, and they have assumed that this is indicative of the lack of an effect on islet mass. Although islet function serves as a surrogate of islet mass in the absence of an independent measure of islet mass, it may be that continued exposure to the drug is required for improved islet function even after mass has been expanded. Thus the interpretation of such experiments is complex, and they cannot be used to conclude that these agents do not expand islet cell mass in human beings. In addition, the benefit of exenatide could go well beyond an insulinotrophic, β-cell–enhancing effect. This is because an antiapoptotic, anti-inflammatory, and immunomodulatory effect has been associated with exenatide treatment (51).
Preserving β-cell function and mass.
A successful approach to the prevention or cure of type 1 diabetes requires that β-cell function and mass be preserved. This is true both for native β-cells, regenerated β-cells, and for any cells used to replace β-cells. From the discussion above, there are at least three components to preserving β-cell function and mass. These include: 1) abrogating or controlling the immune response, both in terms of autoimmunity and allorejection (if a nonidentical source of β-cells is used); 2) protecting cells used for β-cell replacement by immunoisolation; and 3) using pharmacologic agents to enhance β-cell function and potentially stimulate repair, regeneration, or neogenesis of β-cells. The appropriate manner of use of such strategies remains to be determined. For example, it may be that it is necessary to arrest autoimmunity and induce immune regulation or immune tolerance prior to replacing or regenerating β-cells. Alternatively, it may be possible to apply such strategies simultaneously, such as combined islet cell and hematopoietic stem cell transplantation to induce chimerism and graft tolerance (52).
Conclusions.
Although considerable work remains to be accomplished, the potential to prevent and to cure type 1 diabetes is clearly within reach. The clinical trials necessary to demonstrate this must be well designed, adequately powered, carefully controlled, and vigilantly conducted. Depending on the modality being tested and the population being used for such trials, sample sizes likely will require a collaborative, cooperative, multicenter approach. The diabetes community of scientists, clinical trialists, patients, families, funding agencies, and regulatory agencies must work together in a cooperative and collegial manner if we are to be successful in our efforts to prevent and cure type 1 diabetes. Approaches that are more aggressive than those used in the past, including combination approaches and novel interventions, will likely be needed. Despite current impediments, the progress in recent years has been greater than in preceding decades, and together, we are uniquely poised to address existing challenges and conquer type 1 diabetes.
ACKNOWLEDGMENTS
J.S.S. is chairman of Type 1 Diabetes TrialNet, supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Child Health and Human Development, and National Institute of Allergy and Infectious Diseases (NIAID). C.R. is chairman of the Clinical Islet Transplant Consortium, supported by NIDDK and NIAID. J.S.S. and C.R. are both supported by the Diabetes Research Institute Foundation.
J.S.S. notes potential conflicts of interest as a member of the board of directors of Amylin Pharmaceuticals, the manufacturer of exenatide, and as chairman of the Type 1 Diabetes Advisory Board of sanofi-aventis, who have licensed human proislet peptide-2B. No other potential conflicts of interest relevant to this article were reported.
This Perspectives in Diabetes article is written in honor of the 40th anniversary of the Juvenile Diabetes Research Foundation (JDRF). An interesting side note is that at 15 years of age, J.S.S. was a newspaper delivery carrier for the Philadelphia Bulletin and among the customers on his delivery route was Lee Ducat, the founder of JDRF.
REFERENCES
- 1.Eisenbarth GS: Banting Lecture 2009: an unfinished journey: molecular pathogenesis to prevention of type 1 diabetes. Diabetes 2010;59:759–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rich SS, Akolkar B, Concannon P, Erlich H, Hilner JE, Julier C, Morahan G, Nerup J, Nierras C, Pociot F, Todd JA: Overview of the Type I Diabetes Genetics Consortium. Genes Immun 2009;10(Suppl. 1):S1–S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 4.Gianani R, Campbell-Thompson M, Sarkar SA, Wasserfall C, Pugliese A, Solis JM, Kent SC, Hering BJ, West E, Steck A, Bonner-Weir S, Atkinson MA, Coppieters K, von Herrath M, Eisenbarth GS: Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia 2010;53:690–698 [DOI] [PubMed] [Google Scholar]
- 5.The SEARCH Writing Group SEARCH for Diabetes in Youth: a multi-center study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 6.Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, Spain L: Type 1 Diabetes TrialNet Study Group Type 1 Diabetes TrialNet—an international collaborative clinical trials network. Ann N Y Acad Sci 2008;1150:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rotrosen D, Matthews JB, Bluestone JA: The immune tolerance network: a new paradigm for developing to1erance-inducing therapies. J Allergy Clin Immunol 2002;110:17–23 [DOI] [PubMed] [Google Scholar]
- 8.TRIGR Study Group Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR). Pediatr Diabetes 2007;8:117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knazek RA: The human pancreatic islet cell resource consortium. Diabetes Technol Ther 2002;4:551–552 [DOI] [PubMed] [Google Scholar]
- 10.Alejandro R, Barton FB, Hering BJ, Wease S: Collaborative Islet Transplant Registry Investigators 2008 update from the Collaborative Islet Transplant Registry. Transplantation 2008;86:1783–1788 [DOI] [PubMed] [Google Scholar]
- 11.Mineo D, Pileggi A, Alejandro R, Ricordi C: Point: steady progress and current challenges in clinical islet transplantation. Diabetes Care 2009;32:1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruedy KJ, Beck RW, Xing D, Kollman C: Diabetes Research in Children Network: availability of protocol data sets. J Diabetes Sci Technol 2007;1:738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Epidemiology of Diabetes Interventions and Complications (EDIC): design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler AG, Nepom GT: Prediction and pathogenesis in type 1 diabetes. Immunity 2010;32:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, Yu L, Palmer JP, Schatz D, Eisenbarth G: Diabetes Prevention Trial-Type 1 Study Group Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Mahon J, Greenbaum CJ, Cowie CC, Skyler JS: Diabetes Prevention Trial-Type 1 Study Group Incident dysglycemia and progression to type 1 diabetes among participants in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knip M, Korhonen S, Kulmala P, Veijola R, Reunanen A, Raitakari OT, Viikari J, Akerblom HK: Prediction of type 1 diabetes in the general population. Diabetes Care 2010;33:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skyler JS: Type 1 Diabetes TrialNet Study Group Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci 2008;1150:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, Battelino T, Haastert B, Ludvigsson J, Pozzilli P, Lachin JM, Kolb H: Type 1 Diabetes Trial Net Research Group, European C-Peptide Trial Study Group Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rewers M, Gottlieb P: Immunotherapy for the prevention and treatment of type 1 diabetes: human trials and a look into the future. Diabetes Care 2009;32:1769–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA: Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 22.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 23.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS: Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R: GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 25.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simões BP, Martinez EZ, Foss MC, Burt RK, Voltarelli JC: C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–1579 [DOI] [PubMed] [Google Scholar]
- 26.Giannoukakis N, Phillips B, Trucco M: Toward a cure for type 1 diabetes mellitus: diabetes-suppressive dendritic cells and beyond. Pediatr Diabetes 2008;9(3 Pt 2):4–13 [DOI] [PubMed] [Google Scholar]
- 27.Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, Diamantopoulos S, Standifer N, Geubtner K, Falk BA, Ichii H, Takahashi H, Snowhite I, Chen Z, Mendez A, Chen L, Sageshima J, Ruiz P, Ciancio G, Ricordi C, Reijonen H, Nepom GT, Burke GW, 3rd, Pugliese A: Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes 2010;59:947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews JB, Staeva TP, Bernstein PL, Peakman M, von Herrath M: ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol 2010;160:176–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandrup-Poulsen T, Pickersgill L, Donath MY: Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol 2010;6:158–166 [DOI] [PubMed] [Google Scholar]
- 30.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E: Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 31.Parker MJ, Xue S, Alexander JJ, Wasserfall CH, Campbell-Thompson ML, Battaglia M, Gregori S, Mathews CE, Song S, Troutt M, Eisenbeis S, Williams J, Schatz DA, Haller MJ, Atkinson MA: Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009;58:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brusko T, Bluestone J: Clinical application of regulatory T cells for treatment of type 1 diabetes and transplantation. Eur J Immunol 2008;38:931–934 [DOI] [PubMed] [Google Scholar]
- 33.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A: Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 2008;57:3281–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC: Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 2007;148:5136–5144 [DOI] [PubMed] [Google Scholar]
- 35.Levetan CS, Upham LV, Deng S, Laury-Kleintop L, Kery V, Nolan R, Quinlan J, Torres C, El-Hajj RJ: Discovery of a human peptide sequence signaling islet neogenesis. Endocr Pract 2008;14:1075–1083 [DOI] [PubMed] [Google Scholar]
- 36.Hall KK, Gattas-Asfura KM, Stabler CL: Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via Staudinger ligation. Acta Biomater. 21July2010[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giraldo JA, Weaver JD, Stabler CL: Tissue engineering approaches to enhancing clinical islet transplantation through tissue engineering strategies. J Diabetes Sci Technol 2010;4:1238–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman DM, O'Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS: Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant 2009;9:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fort A, Fort N, Ricordi C, Stabler CL: Biohybrid devices and encapsulation technologies for engineering a bioartificial pancreas. Cell Transplant 2008;17:997–1003 [DOI] [PubMed] [Google Scholar]
- 40.Halban PA, German MS, Kahn SE, Weir GC: Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab 2010;95:1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hering BJ, Walawalkar N: Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol 2009;21:81–86 [DOI] [PubMed] [Google Scholar]
- 42.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, Murase N, Hara H, Ball S, Loveland BE, Ayares D, Lakkis FG, Cooper DK, Trucco M: Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant 2009;9:2716–2726 [DOI] [PubMed] [Google Scholar]
- 43.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE: Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 44.Dominguez-Bendala J, Pastori RL, Ricordi C, Inverardi L: Protein transduction: a novel approach to induce in vitro pancreatic differentiation. Cell Transplant 2006;15(Suppl. 1):S85–S90 [DOI] [PubMed] [Google Scholar]
- 45.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL: Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Pagola A, Sisino G, Allende G, Dominguez-Bendala J, Gianani R, Reijonen H, Nepom GT, Ricordi C, Ruiz P, Sageshima J, Ciancio G, Burke GW, Pugliese A: Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 2008;51:1803–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA: In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S, Ben-Dor L, Polak-Charcon S, Karasik A, Shimon I, Mor E, Ferber S: Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A 2005;102:7964–7969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA: Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A 2009;106:15768–15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, Mendez AJ, Selvaggi G, Ricordi C, Alejandro R: Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation 2008;86:1658–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buss JL, Rajab A, Diakoff E, Wang J, Miller K, Osei K: Exenatide pre-treatment improved islet graft function compared to treatment post-transplant only (Abstract). Diabetes 2010;59(Suppl. 1):A38 [Google Scholar]
- 52.Mineo D, Ricordi C, Xu X, Pileggi A, Garcia-Morales R, Khan A, Baidal DA, Han D, Monroy K, Miller J, Pugliese A, Froud T, Inverardi L, Kenyon NS, Alejandro R: Combined islet and hematopoietic stem cell allotransplantation: a clinical pilot trial to induce chimerism and graft tolerance. Am J Transplant 2008;8:1262–1274 [DOI] [PubMed] [Google Scholar]