Abstract
OBJECTIVE
Hypothalamic nutrient sensing regulates glucose production, but the neuronal circuits involved remain largely unknown. Recent studies underscore the importance of N-methyl-d-aspartate (NMDA) receptors in the dorsal vagal complex in glucose regulation. These studies raise the possibility that hypothalamic nutrient sensing activates a forebrain-hindbrain NMDA-dependent circuit to regulate glucose production.
RESEARCH DESIGN AND METHODS
We implanted bilateral catheters targeting the mediobasal hypothalamus (MBH) (forebrain) and dorsal vagal complex (DVC) (hindbrain) and performed intravenous catheterizations to the same rat for infusion and sampling purposes. This model enabled concurrent selective activation of MBH nutrient sensing by either MBH delivery of lactate or an adenovirus expressing the dominant negative form of AMPK (Ad-DN AMPK α2 [D157A]) and inhibition of DVC NMDA receptors by either DVC delivery of NMDA receptor blocker MK-801 or an adenovirus expressing the shRNA of NR1 subunit of NMDA receptors (Ad-shRNA NR1). Tracer-dilution methodology and the pancreatic euglycemic clamp technique were performed to assess changes in glucose kinetics in the same conscious, unrestrained rat in vivo.
RESULTS
MBH lactate or Ad-DN AMPK with DVC saline increased glucose infusion required to maintain euglycemia due to an inhibition of glucose production during the clamps. However, DVC MK-801 negated the ability of MBH lactate or Ad-DN AMPK to increase glucose infusion or lower glucose production. Molecular knockdown of DVC NR1 of NMDA receptor via Ad-shRNA NR1 injection also negated MBH Ad-DN AMPK to lower glucose production.
CONCLUSIONS
Molecular and pharmacological inhibition of DVC NMDA receptors negated hypothalamic nutrient sensing mechanisms activated by lactate metabolism or AMPK inhibition to lower glucose production. Thus, DVC NMDA receptor is required for hypothalamic nutrient sensing to lower glucose production and that hypothalamic nutrient sensing activates a forebrain-hindbrain circuit to lower glucose production.
Hypothalamic nutrient and hormonal sensing regulate glucose and lipid homeostasis (1–7). Although much effort has been put in by laboratories to elucidate the neuronal circuits involved in glucose regulation, an experimental challenge remains in assessing whether extrahypothalamic regions are involved in relaying the hypothalamic signal(s) to the liver to regulate glucose production.
The dorsal vagal complex (DVC) within the hindbrain processes peripheral signals to regulate homeostasis (8–11). N-methyl-d-aspartate (NMDA) receptor–mediated neurotransmission in the DVC has recently been shown to be sufficient (12) and necessary for gut nutrient sensing (13,14) to regulate glucose production. Although one study reports that hypothalamic lipid sensing elucidates DVC neuronal activation in association with an inhibition of glucose production (15), the necessity of the DVC neuronal activation and the neuronal population involved in hypothalamic regulation of glucose production remain unknown.
We here attempted to elucidate in conscious, unrestrained rodents whether direct activation of hypothalamic nutrient sensing by either an enhancement of hypothalamic lactate metabolism (16,17) or a molecular knockdown of hypothalamic AMP-activated protein kinase (AMPK) (18) triggers a forebrain-hindbrain NMDA–dependent axis to lower glucose production. To address this, we inhibited NMDA receptor–mediated neuronal transmission in the DVC hindbrain in the same rats whose nutrient sensing in the forebrain hypothalamus was activated and examined whether glucose regulation was affected accordingly.
RESEARCH DESIGN AND METHODS
All study protocols were approved by the Institutional Animal Care and Use Committee of the University Health Network. Eight-week-old male SD rats were used and were housed in individual cages and maintained on a standard light-dark cycle with access to standard rat chow and water ad libitum. Rats were stereotaxically implanted with indwelling bilateral catheters into both the mediobasal hypothalamus (MBH) (3.1 mm posterior of bregma, 0.4 mm lateral from midline, and 9.6 mm below skull surface) (19) and dorsal vagal complex (DVC) (0.0 mm on occipital crest, 0.4 mm lateral to midline, and 7.9 mm below skull surface) (12). After 1 week of recovery, rats underwent intravenous catheterization where the internal jugular vein and carotid artery were catheterized for infusion and sampling.
MBH/DVC infusion and pancreatic-euglycemic clamp.
Four days post intravenous catherization, animals whose food intake and body weight had recovered back to within 10% of baseline underwent the clamp studies. Rats were restricted to ∼55 kcal of food the night before the experiment to ensure the same nutritional status during the clamps, which lasted 210 min. At t = 0 min, MBH/DVC infusions were initiated and maintained throughout the clamps at 0.33 μl/h. The groups wereas follows: MBH saline + DVC saline, MBH saline + DVC MK-801 (0.06 ng/min), MBH lactate (5 mmol/l) + DVC saline, and MBH lactate (5 mmol/l) + DVC MK-801 (0.06 ng/min, with 2 h preinfusion starting at t = − 120 min). A primed continuous infusion of [3-3H] glucose (40 μCi bolus, 0.4 μCi/min; Perkin Elmer) was initiated at 0 min and maintained throughout. A pancreatic (basal insulin)-euglycemic clamp was started at t = 90 min until 210 min. Somatostatin (3 μg/kg/min) was infused along with insulin (0.8 mU/kg/min) to replace insulin back to basal (supplemental Table S1, available in the online appendix [http://diabetes.diabetesjournals.org/cgi/content/full/db10-0994/DC1rsqb]). A 25% glucose solution was started and periodically adjusted to maintain plasma glucose levels (Table S1). Samples for the determination of [3-3H] glucose specific activity and insulin levels were obtained at 10-min intervals. At the end, the rats were anesthetized and 3 μl diluted bromophenol blue (BPB) was injected through each side of the MBH catheter to ensure the correct placement of the catheter upon dissection of an MBH wedge that contains the entire mediolateral and dorsoventral extent of the arcuate nuclei while minimizing ventromedial nucleus tissue. In parallel, the DVC was sampled by injecting 3 μl (volume found to be restricted locally to the DVC) of BPB via the DVC cannulae and cutting coronally across the medulla. Upon verifying that the location of the BPB staining in the coronal sections was anatomically representative of the DVC, DVC tissues were obtained by dissecting the BPB-stained regions. To a different set of rats, we also performed tracer infusion to verify cannuale location. Radioactive glucose tracer [3-3H] was administrated via the bilateral cannulae into the MBH or DVC at the same infusion rate (0.33 μl/h) and duration (210 min) as the clamps. MBH, DVC, and cortical tissue samples were obtained and homogenized for counting.
MBH/DVC adenoviral infection and pancreatic-euglycemic clamp.
Immediately poststereotaxic surgery, rats were injected with 3 μl adenovirus per side of cannulae into the MBH and/or DVC as previously described (12,18,20). The adenoviral infected groups were as follows: MBH GFP + DVC saline, MBH GFP + DVC MK-801, MBH dominant negative (DN) AMPK + DVC saline, MBH DN AMPK + DVC MK-801, MBH GFP + DVC mismatch control (mm), MBH GFP + DVC shRNA NR1, MBH DN AMPK + DVC mm, and MBH DN AMPK + DVC shRNA NR1. We had previously validated the specificity of brain injections of these adenoviruses (12,18). Green fluorescent protein (GFP) was localized in the MBH with 40% colocalized with AgRP-positive neurons and another ∼40% colocalized with proopiomelanocortin-positive neurons in MBH Ad-GFP–injected rats (18). Furthermore, MBH Ad-DN AMPK injection decreased hypothalamic AMPK activity by ∼50% (18). DVC injection of Ad-shRNA NR1 decreased NR1 protein levels in the DVC but not in the MBH wedges (12). This was confirmed by immunohistochemistry showing that NR1 expression within the DVC was reduced in rats injected with DVC shRNA-NR1 (12). Four days post intravenous catherization, animals with MBH GFP + DVC null and MBH AMPK + DVC null were infused with either DVC saline or MK-801 from t = 0 min to 210 min at 0.33 μl/h. Initially, MK-801 was given at 0.03 ng/min because it was previously shown that DVC MK-801 negated the gut lipid-sensing mechanism to lower glucose production (13,14). However, when this dose of MK-801 was infused in DN AMPK-infected rats, it only partially blocked the glucose production–lowering effect (glucose production suppression −42.5%). Given this, MK-801 dose was doubled (0.06 ng/min in 210 min; 12 ng) and was found to fully abolish the GP-lowering effect of DN AMPK (Fig. 2D and E), whereas no effect on cumulative food intake was detected (Fig. 4C). Hence, this dose of DVC MK-801 was used for all studies.
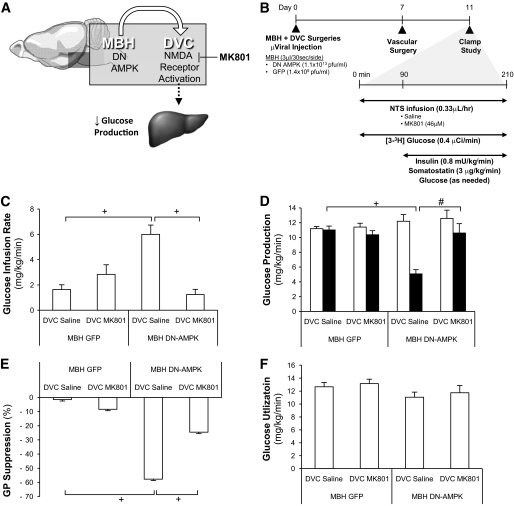
FIG. 2.
DVC MK-801 abolished the glucose production–lowering effect induced by hypothalamic expression of DN AMPK. A: Schematic representation of working hypothesis that infusion of NMDA antagonist MK-801 in the DVC negates the glucose production–lowering effect of MBH DN AMPK. B: Schematic representation of experimental design. MBH and DVC stereotaxic surgery was performed on male Sprague-Dawley rats (∼240–280 g). Virus-injected animals were administered 3 μl GFP or DN AMPK–expressing adenovirus per side of cannulae immediately postsurgery. After 1 week of recovery (day 7), vascular surgery (i.e., intravenous catherization) was performed. Rats were given 5 recovery days until clamp studies (day 11), upon which DVC infusion of saline or MK-801 was given to MBH virus–injected rodents. DVC administration of MK-801 in adenoviral-DN AMPK–injected rodents prevented the expected increase in glucose infusion rate (C) and lowering in glucose production (GP) (D) found to be elicited by DN AMPK. □, basal glucose production; ■, clamp glucose production. E: Suppression of glucose production (GP) during the clamp period was expressed as percent decrease from basal glucose production. F: Glucose utilization was comparable in all groups: MBH GFP + DVC saline (n = 5), MBH GFP + DVC MK-801 (n = 6), MBH DN AMPK + DVC saline (n = 6), and MBH DN AMPK + DVC MK-801 (n = 5). #P < 0.01; +P < 0.001.
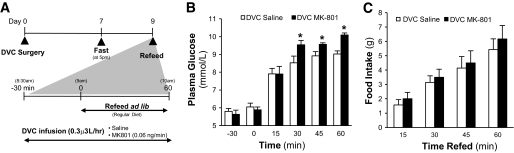
FIG. 4.
DVC MK-801 administration disrupts glucose homeostasis during refeeding. A: Schematic representation of experimental design. DVC stereotaxic surgery was performed on male SD rats (∼240–280 g). After 1 week of recovery, animals were subjected to a 40-h fast (from 5:00 p.m. on day 7 until 9:00 a.m. on day 9). Thirty minutes before the completion of the 40-h fast (i.e., time − 30 min of refeeding protocol), rats were infused with DVC-saline or –MK-801. Rats were refed on regular chow ad libitum at time 0 min, when food intake and blood glucose levels were monitored for 1 h (time 15, 30, 45, and 60 min). At the fasting state (time −30 and 0 min), rats receiving DVC saline or MK-801 have comparable plasma glucose levels representative of fasting animals. B: During 1 h of refeeding, DVC MK-801–infused rats have significantly higher plasma glucose levels compared with DVC-saline–infused rats at 30, 45, and 60 min after refeeding. C: Cumulative food intake was comparable in both groups during 1 h refeeding. DVC saline, n = 7; DVC MK-801, n = 6. *P < 0.001.
Fasting-refeeding experiment.
Seven days poststereotaxic surgery (DVC cannulae), animals whose food intake and body weight had recovered back to baseline underwent the fasting-refeeding studies. Rats were fasted for 40 h. Thirty minutes before completion of the fast, rats began a DVC infusion of either saline or MK-801, and upon completion of the fast rats were fed ad libitum on regular chow diet. Blood glucose levels and food intake were measured.
Biochemical analysis.
Plasma glucose concentrations were measured by the glucose oxidase method (Glucose Analyzer GM9; Analox Instruments, Lunenbertg, MA). Plasma insulin levels were determined by radioimmunoassay (RIA) (Linco Research, St. Charles, MO).
Statistical analysis.
Statistical analysis was done by two-way ANOVA to compare across the groups, followed by a Tukey post hoc test to compare between groups. Statistical analysis was accepted as significant with a P value of <0.05. Data are presented as means ± SE.
RESULTS
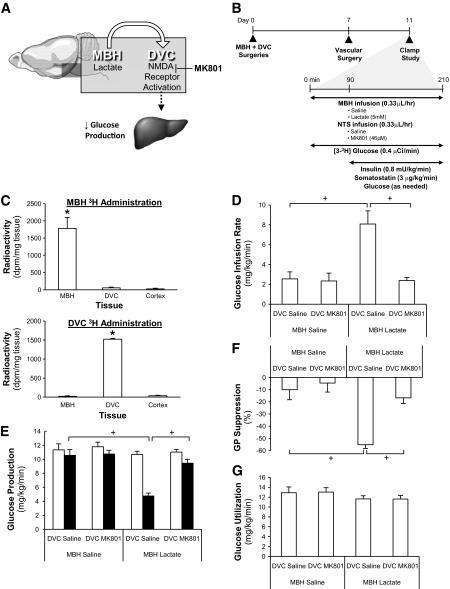
We first developed a model that received bilateral catheters into both the MBH and DVC (Fig. 1A and B). On day 7, [3-3H] glucose was infused at the same rate as hypothalamic lactate or DVC MK-801 either into the MBH or DVC (Fig. 1A and B). MBH [3-3H] glucose selectively increased radioactive counts in the MBH but not in the DVC of the same rats (Fig. 1C). DVC [3-3H] glucose increased radioactive counts in the DVC but not MBH (Fig. 1C). Counts were not detected in cortex samples (Fig. 1C). Thus, a rat model is developed that enabled concurrent delivery of substances of interest directly into the MBH and DVC, but with no direct infusate contact between the two brain regions, and examination of neuronal communication between the MBH and the DVC in glucose regulation in vivo.
FIG. 1.
DVC MK-801 abolished the glucose production–lowering effect induced by hypothalamic lactate infusion. A: Schematic representation of working hypothesis. Infusion of NMDA antagonist MK-801 in the DVC negates the glucose production–lowering effect of MBH lactate. B: Schematic representation of experimental design. MBH and DVC stereotaxic surgery was performed on male Sprague-Dawley rats (∼240–280 g). After 1 week of recovery (day 7), vascular surgery (i.e., intravenous catherization) was performed. Rats were given 5 recovery days until clamp studies (day 11), upon which DVC infusion of saline or MK-801 was given to MBH lactate-infusing animals. To verify the anatomical placement of cannulae and to confirm that infusion was localized, radioactive tracer administered in the MBH or DVC via the bilateral cannulae was found to be contained within the respective tracer-infused tissues (C). DVC administration of MK-801 in lactate-infused animals prevented the expected increase in glucose infusion rate (D) and lowering in glucose production (E) found to be elicited by MBH lactate. □, basal glucose production; ■, clamp glucose production. F: suppression of glucose production (GP) during the clamp period was expressed as percent decrease from basal glucose production. G: glucose utilization was comparable in all groups: MBH saline + DVC saline (n = 5), MBH saline + DVC MK-801 (n = 5), MBH lactate + DVC saline (n = 5), and MBH lactate + DVC MK-801 (n = 6). *P < 0.01; +P < 0.001.
Pharmacological inhibition of DVC NMDA receptors abolishes hypothalamic lactate metabolism to lower glucose production.
We inhibited DVC NMDA receptors via DVC delivery of MK-801 at a concentration that blocked DVC NMDA receptor agonist to lower glucose production (12). We infused DVC MK-801 and lactate into MBH (Fig. 1A and B). During the clamps, MBH lactate + DVC saline increased glucose infusion (Fig. 1D) and decreased glucose production (Fig. 1E and F) compared with MBH/DVC saline in the presence of similar plasma insulin levels (Table S1) and glucose uptake (Fig. 1G). In contrast, DVC MK-801 abolished the ability of MBH lactate to increase glucose infusion (Fig. 1D) and decrease glucose production (Fig. 1E and F) in the presence of similar plasma insulin levels (Table S1). These data suggest that DVC NMDA receptors mediate hypothalamic lactate metabolism to lower glucose production.
Pharmacological inhibition of DVC NMDA receptors abolishes hypothalamic AMPK inhibition to lower glucose production.
To alternatively activate hypothalamic nutrient sensing and evaluate the necessity of DVC NMDA receptors, DVC MK-801 was infused to rats that had hypothalamic expression of the dominant negative form of AMPK via MBH injection of adenovirus expressing the dominant negative form of AMPK (Ad-DN AMPK α2 [D157A] [21]) during the clamps (Fig. 2A and B). In the presence of similar plasma insulin levels (Table S2), MBH DN-AMPK rats with DVC saline had higher glucose infusion (Fig. 2C) and lower glucose production (Fig. 2D and E) compared with MBH GFP rats with DVC saline. This is consistent with our recent findings indicating that MBH DN-AMPK lowers glucose production independent of changes in food intake and body weight (18) and strengthens the claim that DVC surgery itself does not affect glucose regulation. DVC MK-801 negated the increase in glucose infusion (Fig. 2C) and decrease in glucose production (Fig. 2D and E) in MBH DN-AMPK rats. Glucose uptake was comparable in all groups (Fig. 2F). Thus, DVC NMDA receptors are required for hypothalamic AMPK inhibition to lower glucose production.
Molecular inhibition of DVC NMDA receptors abolishes hypothalamic AMPK inhibition to lower glucose production.
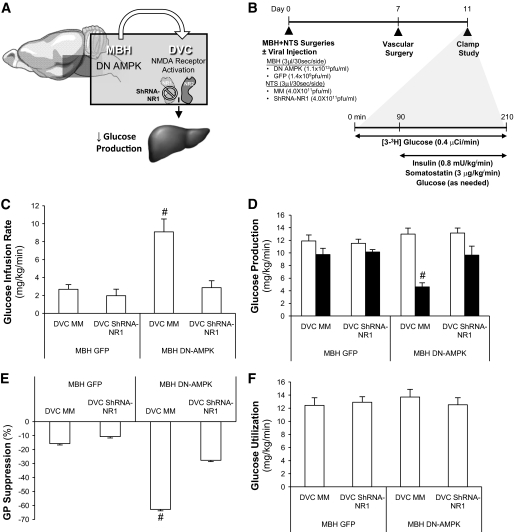
The NMDA receptor is composed of NR1 and NR2 subunits (22). We recently constructed an adenovirus expressing the shRNA of NR1 (Ad-shRNA NR1) and injected this Ad-shRNA NR1 into the DVC of rats to knock down DVC NR1 (12). Here, we injected the same Ad-shRNA NR1 into the DVC to inhibit DVC NMDA receptor–mediated neurotransmission and tested whether hypothalamic nutrient sensing activated by MBH Ad-DN AMPK is impaired (Fig. 3A and B). MBH DN AMPK/DVC Ad-mm rats required higher glucose infusion compared with MBH GFP/DVC mm rats to maintain euglycemia (Fig. 3C) due to an inhibition of glucose production (Fig. 3D and E) rather than changes in glucose uptake (Fig. 3F) or plasma insulin levels (Table S3). MBH DN AMPK/DVC shRNA-NR1 rats did not have this elevated glucose infusion (Fig. 3C) or a drop in glucose production (Fig. 3D and E) compared with the corresponding control in the presence of similar plasma insulin levels (Table S3). These studies demonstrate that DVC NR1 subunit of the NMDA receptor is required for hypothalamic AMPK inhibition to lower glucose production.
FIG. 3.
Molecular knockdown of DVC NR1 negated the glucose production–lowering effect of hypothalamic expression of AMPK-DN. A: Schematic representation of working hypothesis that molecular knockdown of the NR1 subunit of the NMDA receptor in the DVC negates the glucose production–lowering effect of MBH DN AMPK. B: Schematic representation of experimental design. MBH and DVC stereotaxic surgeries were performed on male Sprague-Dawley rats (∼240–280 g). Animals were injected with 3 μl/side of cannulae of adenovirus into the MBH (GFP or DN AMPK) and into the DVC (MM or shRNA-NR1) immediately postsurgery. After 1 week of recovery (day 7), vascular surgery (i.e., intravenous catherization) was performed. Rats were given 5 recovery days until clamp studies (day 11). Animals with simultaneous disruption of both MBH AMPK and DVC NR1 did not exhibit the expected increase in glucose infusion rate (C) or lowering in glucose production (D) of DN AMPK animals. □, basal glucose production; ■, clamp glucose production. E: Suppression of glucose production (GP) during the clamp period was expressed as percent decrease from basal glucose production. F: Glucose utilization was comparable in all groups. MBH GFP+ DVC MM (n = 5), MBH GFP + DVC NR1 (n = 5), MBH DN AMPK + DVC MM (n = 6), and MBH DN AMPK + DVC NR1 (n = 5). #P < 0.01.
DVC NMDA receptor blockage disrupts glucose homeostasis during refeeding.
We addressed the physiological relevance of the ability of DVC NMDA receptor to integrate nutrient sensing to regulate glucose homeostasis using a fasting-refeeding nonclamp protocol. When fasted rodents are subjected to refeeding, the rise in plasma glucose levels is restrained as a result of an inhibition of gluconeogenesis (23). We reasoned that if the nutrient sensing–related signal(s) activated by refeeding is integrated at the DVC NMDA receptors to regulate glucose homeostasis (as suggested by the above hypothalamic as well as the recent gut [13,14] nutrient sensing studies), direct inhibition of DVC NMDA receptors during refeeding should disrupt glucose homeostasis. We first subjected our rats to a fast after 1 week of DVC surgery (Fig. 4A and B). Plasma glucose levels (Fig. 4B) and cumulative food intake (Fig. 4C) rose, respectively, after refeeding for the DVC saline rats. We next infused DVC MK-801 (at the same dose that negated the ability of hypothalamic lactate metabolism [Fig. 1E] and DN AMPK [Fig. 2D] to lower glucose production) and upon refeeding, plasma glucose levels rose significantly higher than DVC saline rats (Fig. 4B) in the presence of similar cumulative food intake (Fig. 4C). Thus, our data indicate that DVC NMDA receptors integrate nutrient sensing to regulate glucose homeostasis.
DISCUSSION
We demonstrated that hypothalamic nutrient sensing triggers a forebrain-hindbrain NMDA–dependent neuronal axis to lower glucose production. The cross-talk between the hypothalamus and the DVC in glucose regulation, however, remains to be clarified. For example, it is crucial to assess whether the descending projection from the hypothalamus to the DVC is direct or indirect through other nuclei such as the paraventricular hypothalamus (PVN) because a recent study reports that hypothalamic leucine sensing activates DVC neurons via PVN oxytocin neurons to lower food intake (24). The necessity of a forebrain-hindbrain axis in glucose regulation by hypothalamic hormonal action also warrants future investigations because hepatic vagus is required for hypothalamic leptin to increase hepatic insulin sensitivity as well (2).
Our findings imply that DVC neurons expressing NMDA receptors are required specifically for the hypothalamic nutrient sensing to lower glucose production. Inhibition of DVC NMDA receptors, however, also negate lipid sensing in the intestine to lower glucose production (13,14) and food intake (9,10), while the activation of hypothalamic nutrient sensing is absence. These studies suggest that DVC NMDA receptor–expressing neurons integrate signal(s) derived from nutrient sensing to regulate glucose and energy homeostasis but do not exclude the involvement of other neuronal systems because a recent study reports that serotonergic neurons in the brainstem mediates leptin action to regulate food intake (25). Lastly, in addition to the need to clarify the neuronal systems in extrahypothalamic regions that mediate hypothalamic nutrient sensing to lower glucose production, it is equally important to test for a glucose regulatory role of nutrient sensing in MBH-proximate regions such as the dorsomedial and lateral hypothalamic area so that advancement in this field is made in a better defined context.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a research grant to T.K.T.L. from the Canadian Institute of Health Research (MOP-86554). C.K.L.L. was supported by a Canadian Institute of Health Research graduate award. M.C. was supported by an Ontario Graduate Scholarship and a graduate studentship from the Banting and Best Diabetes Centre at the University of Toronto. G.A.R. is supported by a Programme Grant from the Wellcome Trust, by the Medical Research Council (U.K.), and by the European Union (FP7 “IMIDIA”).
No potential conflicts of interest relevant to this article were reported.
C.K.L.L. conducted and designed experiments, performed data analyses, and wrote the manuscript. M.C. assisted in experiments. G.A.R. supplied the adenovirus expressing the dominant negative form of AMPK or GFP control. T.K.T.L. supervised the project, designed experiments, and reviewed and edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Caspi L, Wang PY, Lam TK: A balance of lipid-sensing mechanisms in the brain and liver. Cell Metab 2007;6:99–104 [DOI] [PubMed] [Google Scholar]
- 2.German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ: Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology 2009;150:4502–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC: Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest 2008;118:2132–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC: Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007;5:438–449 [DOI] [PubMed] [Google Scholar]
- 5.Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L: Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med 2007;13:171–180 [DOI] [PubMed] [Google Scholar]
- 6.Lam TK: Neuronal regulation of homeostasis by nutrient sensing. Nat Med 2010;16:392–395 [DOI] [PubMed] [Google Scholar]
- 7.van den Hoek AM, Voshol PJ, Karnekamp BN, Buijs RM, Romijn JA, Havekes LM, Pijl H: Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes 2004;53:2529–2534 [DOI] [PubMed] [Google Scholar]
- 8.Bourque CW: Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 2008;9:519–531 [DOI] [PubMed] [Google Scholar]
- 9.Coll AP, Farooqi IS, O'Rahilly S: The hormonal control of food intake. Cell 2007;129:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings DE, Overduin J: Gastrointestinal regulation of food intake. J Clin Invest 2007;117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW: Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 2005;115:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam CK, Chari M, Su BB, Cheung GW, Kokorovic A, Yang CS, Wang PY, Lai TY, Lam TK: Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem 2010;285:21913–21921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK: Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab 2009;10:99–109 [DOI] [PubMed] [Google Scholar]
- 14.Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK: Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 2008;452:1012–1016 [DOI] [PubMed] [Google Scholar]
- 15.Pocai A, Obici S, Schwartz GJ, Rossetti L: A brain-liver circuit regulates glucose homeostasis. Cell Metab 2005;1:53–61 [DOI] [PubMed] [Google Scholar]
- 16.Chari M, Lam CK, Wang PY, Lam TK: Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes 2008;57:836–840 [DOI] [PubMed] [Google Scholar]
- 17.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L: Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 2005;309:943–947 [DOI] [PubMed] [Google Scholar]
- 18.Yang CS, Lam CK, Chari M, Cheung GW, Kokorovic A, Gao S, Leclerc I, Rutter GA, Lam TK: Hypothalamic AMP-activiated protein kinase regulates glucose production. Diabetes 2010;59:2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross R, Wang PY, Chari M, Lam CK, Caspi L, Ono H, Muse ED, Li X, Gutierrez-Juarez R, Light PE, Schwartz GJ, Rossetti L, Lam TK: Hypothalamic protein kinase C regulates glucose production. Diabetes 2008;57:2061–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He W, Lam TK, Obici S, Rossetti L: Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 2006;9:227–233 [DOI] [PubMed] [Google Scholar]
- 21.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA: Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 2003;371:761–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBain CJ, Mayer ML: N-methyl-D-aspartic acid receptor structure and function. Physiol Rev 1994;74:723–760 [DOI] [PubMed] [Google Scholar]
- 23.Duran-Sandoval D, Cariou B, Percevault F, Hennuyer N, Grefhorst A, van Dijk TH, Gonzalez FJ, Fruchart JC, Kuipers F, Staels B: The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem. 2005;280:29971–29979 [DOI] [PubMed] [Google Scholar]
- 24.Blouet C, Jo YH, Li X, Schwartz GJ: Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 2009;29:8302–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G: A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 2009;138:976–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.