Abstract
OBJECTIVE
Animal studies indicate a prominent role of brain insulin signaling in the regulation of peripheral energy metabolism. We determined the effect of intranasal insulin, which directly targets the brain, on glucose metabolism and energy expenditure in humans.
RESEARCH DESIGN AND METHODS
In a double-blind, placebo-controlled, balanced within-subject comparison, 19 healthy normal-weight men (18–26 years old) were intranasally administered 160 IU human insulin after an overnight fast. Energy expenditure assessed via indirect calorimetry and blood concentrations of glucose, insulin, C-peptide, and free fatty acids (FFAs) were measured before and after insulin administration and the subsequent consumption of a high-calorie liquid meal of 900 kcal.
RESULTS
Intranasal insulin, compared with placebo, increased postprandial energy expenditure, i.e., diet-induced thermogenesis, and decreased postprandial concentrations of circulating insulin and C-peptide, whereas postprandial plasma glucose concentrations did not differ from placebo values. Intranasal insulin also induced a transient decrease in prandial serum FFA levels.
CONCLUSIONS
Enhancing brain insulin signaling by means of intranasal insulin administration enhances the acute thermoregulatory and glucoregulatory response to food intake, suggesting that central nervous insulin contributes to the control of whole-body energy homeostasis in humans.
Animal studies have yielded evidence that the regulation of whole-body energy flux critically depends on intact brain insulin signaling (1,2). Most recent findings have shown that the hypothalamic administration of insulin increases brown adipose tissue thermogenesis by direct inhibitory effects on warm-sensitive neurons (3). Moreover, studies in rodents have demonstrated that in addition to its direct inhibitory effect on hepatic gluconeogenesis, insulin acts in the hypothalamus to decrease glucose production in the liver (4,5), thus establishing an insulin-driven brain-liver axis that controls systemic glucose homeostasis. We examined whether insulin acting in the human brain exerts comparable effects on energy homeostasis by administering intranasal insulin that bypasses the blood-brain barrier and reaches the brain compartment along the olfactory nerve (6,7), modulating central nervous functions in the absence of relevant peripheral effects (8). Notably, intranasal insulin reduces food intake (9) and body fat content (10) in healthy men, indicating that following intranasal administration, the hormone accesses neuronal networks relevant for energy homeostasis. Against this background, in the present study we assessed the effects of intranasal insulin on the glucoregulatory and thermoregulatory response to food intake in humans.
RESEARCH DESIGN AND METHODS
Nineteen healthy men (mean ± SEM age 23.2 ± 0.6 years and BMI 23.5 ± 0.3 kg/m2) who were free of medication participated in the experiments. They gave written informed consent to the study, which conformed to the Declaration of Helsinki and was approved by the local ethics committee.
Experimental protocol.
Each subject participated in two conditions (insulin and placebo) spaced apart by at least 4 weeks. The order of conditions was balanced across subjects. Body weight and body composition (BIA 2000-M; Data Input, Frankfurt, Germany) did not differ between conditions.
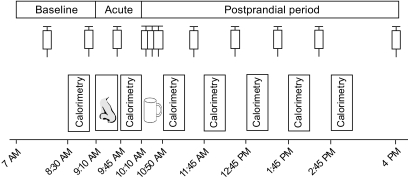
After a 12-h fast, experimental sessions started at 7:00 a.m. with baseline assessments of energy expenditure and blood parameters (Fig. 1). Throughout the experiment, subjects rested in bed in a supine position in a quiet room of constant temperature (23°C). At 9:10 a.m., sixteen 0.1-ml puffs (eight per nostril) of insulin and placebo, respectively, were intranasally administered in 2-min intervals, amounting to a total dose of 1.6 ml insulin (160 IU Insulin Actrapid; Novo Nordisk, Mainz, Germany) or vehicle (HOE 31 dilution buffer; Aventis Pharma, Bad Soden, Germany). Insulin and placebo were administered using precision air pumps (Aero Pump, Hochheim, Germany) that fill the nostrils and the nasal cavity with aerosol, thus enabling the solution to effectively target the olfactory epithelium. The dose of intranasal insulin used here has previously been shown to be functionally effective in healthy humans (9,11). Following postinsulin administration measurements (see below), subjects consumed 600 ml of a standard liquid meal (Fresubin energy drink, Fresenius Kabi, Bad Homburg, Germany) at a dose of 20 ml/min (totaling 900 kcal, 33.6 g protein, 34.8 g fat, and 112.8 g carbohydrate) from 10:15–10:45:00 a.m., and subsequently assessments were continued until 4:00 p.m.
FIG. 1.
Experimental schedule. Nineteen healthy subjects who had fasted overnight spent the experimental day sitting in bed in a supine position. Measurements of energy expenditure by 30-min periods of indirect calorimetry were performed during baseline (8:30–9:00 a.m.), immediately after intranasal insulin administration (9:45–10:15:00 a.m., 1.6 ml [160 IU] insulin and placebo, respectively; nose symbol), and five times following the standardized consumption of a predefined liquid meal of 900 kcal (cup symbol). Blood samplings for the determination of plasma glucose, serum insulin, C-peptide, and free fatty acids concentrations are indicated by syringe symbols.
Assessments.
Energy expenditure (expressed as kcal/min) was measured via indirect calorimetry using a ventilated-hood system (Deltatrac II, MBM-200 Metabolic Monitor; Datex-Engström Deutschland, Achim, Germany). Before each use, the device was calibrated with Quick Cale calibration gas to 5% Co2 and 95% O2. Calorimetric measurements took place from 8:30 to 9:00 a.m. (baseline), from 9:45 to 10:15:00 a.m. (to assess effects of intranasal insulin alone), and five times between 10:45 and 3:15:00 p.m., i.e., after liquid food intake (Fig. 1). The rise in energy expenditure between the fasting state (baseline measurement from 8:45 to 9:15:00 a.m.) and the postprandial state (mean energy expenditure from 10:45 to 3:15:00 p.m.) reflects diet-induced thermogenesis, i.e., the energy that is emitted as heat during food metabolization and thus does not contribute to the production of ATP. Postprandial measurements were separated by 30-min breaks during which the ventilation hood was not worn but the subjects remained in bed.
For the assessment of plasma glucose levels and serum concentrations of insulin, C-peptide, and free fatty acids (FFAs), blood was sampled twice during baseline (8:00 and 9:00 a.m.), immediately after intranasal insulin administration (9:40 a.m.), every 10 min during liquid food intake (10:20–10:40 a.m.), and at 60-min intervals thereafter (11:30 a.m.–2:30 p.m.) with a final sample taken at 4:00 p.m. (Fig. 1). Plasma glucose levels were measured in fluoride plasma (hexokinase method, Aeroset; Abbott Diagnostics, North Chicago, IL). Serum concentrations of insulin and C-peptide were measured by an Immulite analyzer (Siemens Medical Solutions Diagnostics, Los Angeles, CA). FFA concentrations were measured by enzymatic assays as previously described (12).
Statistical analysis.
Data are presented as means ± SEM. Statistical analyses were based on ANOVA including the repeated-measures factors condition and time (referring to the immediate posttreatment and postprandial periods). Postprandial glucose and hormone concentrations (10:20 a.m.–4:00 p.m.) were expressed as areas under the curve (AUCs) calculated according to the trapezoidal rule. Post hoc two-sided t tests were used for single time point comparisons. A P value <0.05 was considered significant.
RESULTS
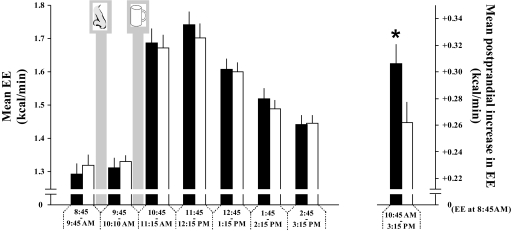
Resting metabolic rates were comparable between conditions during baseline and immediately after insulin administration (P > 0.19 for all comparisons) (Fig. 2). However, the increase in metabolic rate following liquid food intake was on average ∼17% greater in the insulin than in the placebo condition (P < 0.05 for the insulin/placebo main effect; Fig. 2), indicating an increase in postprandial energy expenditure, i.e., diet-induced thermogenesis, due to intranasal insulin.
FIG. 2.
Intranasal insulin enhances postprandial energy expenditure. Following baseline assessment of energy expenditure (EE) (expressed per kcal/min), acute effects of intranasal administration (nose symbol) of insulin (160 IU) (■), and placebo (□), respectively, on energy expenditure were frequently measured before and after ingestion of liquid food (900 kcal; cup symbol) for a total of 6.5 h (left panel). The rise in energy expenditure between baseline (8:30–9:45:00 a.m.) and the postprandial state (10:45:00 a.m.–3:15:00 p.m.) reflects the energy emitted mainly as heat during food metabolization (diet-induced thermogenesis [DIT]) (right panel). Data are means ± SEM; N = 19. *P < 0.05.
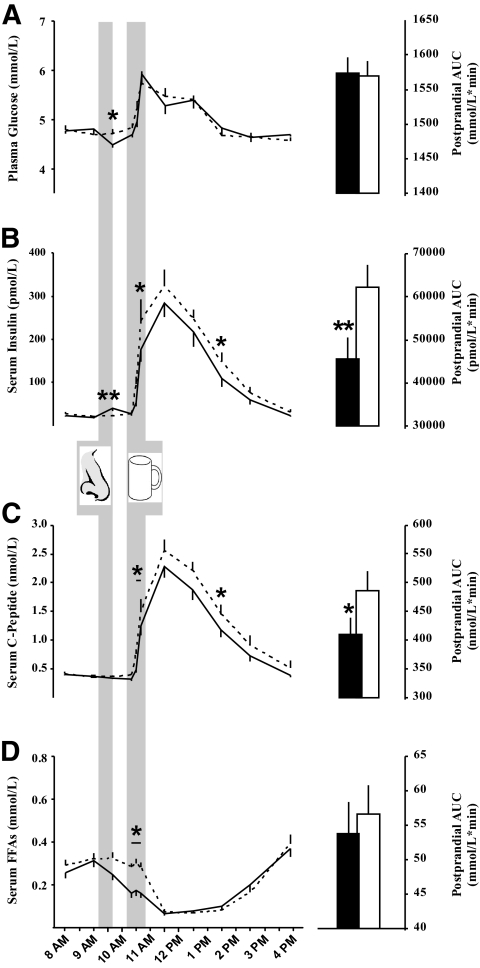
Plasma glucose, hormonal, and FFA concentrations did not differ between conditions during baseline (P > 0.25). Immediately after intranasal insulin administration, i.e., at 9:40 a.m., a slight and transient decrease in fasting plasma glucose was detected (insulin vs. placebo 4.5 ± 0.1 vs. 4.7 ± 0.1 mmol/l; P < 0.02 [insulin/placebo main effect]), but the subsequent postprandial increase in glucose concentrations did not differ between conditions (AUC, 10:20 a.m.–4:00 p.m., insulin vs. placebo 1,574 ± 23 vs. 1,570 ± 20 mmol/l/min; P > 0.90) (Fig. 3A). In parallel with the slight postinsulin administration drop in plasma glucose, a small increase in serum insulin (insulin vs. placebo 40.1 ± 6.3 vs. 22.6 ± 3.0 pmol/l; P < 0.01) but not in C-peptide concentrations (0.34 ± 0.02 vs. 0.37 ± 0.03 nmol/l; P > 0.31) emerged. Following liquid food intake, the postprandial increase in both insulin and C-peptide concentrations was reduced by intranasal insulin in comparison with placebo (AUC, 10:20 a.m.–4:00 p.m., serum insulin 45,521 ± 5,052 vs. 62,315 ± 4,973 pmol/l/min; serum C-peptide 409 ± 30 vs. 487 ± 33 nmol/l/min; P < 0.001 and P < 0.02, respectively) (Fig. 3B and C). Serum FFA concentrations showed a transient decrease during food intake in the insulin compared with the placebo condition (P < 0.01 for condition × time) but did not differ between conditions during the postprandial period (AUC, 10:20 a.m.–4:00 p.m., insulin vs. placebo 53.7 ± 4.6 vs. 56.6 ± 4.2 mmol/l/min; P < 0.60) (Fig. 3D).
FIG. 3.
Intranasal insulin lowers postprandial serum insulin levels. Concentrations of plasma glucose (A), serum insulin (B), serum C-peptide (C), and serum free fatty acids (D) before and after acute intranasal administration (nose symbol) of intranasal insulin (160 IU; solid lines and black bars) and placebo (dashed lines and white bars) followed by the standardized ingestion of 900 kcal of liquid food (cup symbol). Postprandial levels (10:20 a.m.–4:00 p.m.) were also expressed as AUCs (right panels). All values are presented as means ± SEM. N = 19. *P < 0.05; **P < 0.01.
Supplementary analyses revealed that the immediate effects of intranasal insulin on preprandial glucose and insulin levels as well as the prandial decrease in FFA levels were statistically unrelated to the treatment-induced increase in postprandial thermogenesis and the suppression in postprandial serum insulin concentrations (Pearson's correlations; P > 0.23 for all coefficients).
DISCUSSION
We demonstrated in humans that acutely enhancing brain insulin signaling by intranasal administration of the hormone increases postprandial thermogenesis. The parallel treatment-induced reduction in postprandial serum insulin concentrations while plasma glucose levels were comparable between conditions indicates that following intranasal insulin administration to the brain, lower circulating levels of the hormone are sufficient to dispose of meal-related increases in plasma glucose. In line with findings in animals (4,5,13), our results support the notion that brain insulin signaling in humans is involved in the control of whole-body energy homeostasis.
In keeping with previous experiments (9,11), intranasal administration of 160 IU insulin induced a transient and mild increase in serum insulin concentrations accompanied by a slight drop in prefood intake plasma glucose that clearly remained within the euglycemic range. Due to the relatively high dose administered here compared with that in previous studies (6,8), a small ratio of the hormone may have entered the circulation via the nasal mucosa. However, the transient nature and limited size of these immediate effects argues against an involvement of systemic uptake of intranasal insulin in its impact on postprandial thermogenesis and glucose metabolism. This conclusion is corroborated by the fact that immediate and postprandial effects were not statistically related.
The balanced regulation of nutrient intake and energy expenditure relies on the hypothalamus as a major integrator of nutritional and hormonal signals from the body periphery, including glucose and insulin (1). Direct injections of insulin into the preoptic area of the hypothalamus induce a dose-dependent increase in core body temperature due to stimulation of brown adipose tissue thermogenesis that is assumed to be mediated by inhibitory insulinergic action on warm-sensitive hypothalamic neurons (3). In our experiments, intranasal administration of the hormone to the brain did not affect resting energy expenditure but evoked a distinct increase in postprandial thermogenesis. Increased postprandial energy expenditure due to enhanced brain insulin signaling adds to the reduction in food intake previously observed after intranasal administration of the hormone (9), suggesting that the catabolic impact of central nervous insulin (10,14) is mediated not only by anorexigenic but also by thermogenic effects of the hormone. Still, further studies on this issue are needed and should include measurements of body temperature, brown adipose tissue activity, and relevant vital signs like heart rate and blood pressure to elucidate the effect of brain insulin signaling on energy expenditure in humans.
A most remarkable finding of our study is the intranasal insulin–induced reduction in postprandial serum insulin concentrations while the food intake-induced rise in plasma glucose remained unaffected, suggesting that intranasal insulin improves postprandial insulin sensitivity. A regulatory effect of central nervous insulin on hepatic glucose metabolism has been indicated by animal studies showing that a selective decrease in hypothalamic insulin receptors reduces hepatic insulin sensitivity and results in marked increases in hepatic glucose production in the presence of plasma insulin concentrations equaling those of control animals (15). Fittingly, insulin hyperpolarizes glucose-responsive hypothalamic neurons by opening ATP-sensitive K+ channels (16) which triggers a decrease in hepatic glucose production that is mediated by vagal efferences (5,15). This pattern suggests that enhancing brain insulin signaling by intranasal administration of the hormone may act on glucose homeostasis in the body periphery by supporting hepatic insulin action. Nevertheless, given that postprandial liver glucose production accounts for approximately one-fifth to one-half of fasting values (17), improved insulin-dependent metabolization of ingested glucose may also have contributed to the intranasal insulin-induced decrease in postprandial serum insulin levels. Such an effect could basically be supported by the observed decrease in prandial FFA levels due to intranasal insulin inasmuch as FFAs are known to impair insulin-stimulated muscle uptake of glucose (18). However, FFA effects on peripheral insulin-stimulated glucose uptake slowly develop over some hours (19), which, in conjunction with the lack of a significant correlation between the decreases in prandial FFA and postprandial insulin concentrations, makes this view unlikely. Furthermore, a contribution of enhanced noninsulin-mediated glucose disposal, i.e., glucose effectiveness, to our effects cannot be ruled out.
Although the present results suggest that insulin administration to the human brain enhances the efficiency of the glucoregulatory brain-liver axis in response to nutrient intake, our observations should be corroborated in future studies that rely on more refined measurements of insulin sensitivity, e.g., euglycemic hyperinsulinemic clamps. It is also noteworthy that most recent animal data hint at divergent effects of hypothalamic insulinergic signaling on peripheral glucose homeostasis and energy expenditure depending on the involvement of agouti-related protein or proopiomelanocortin neuronal pathways (13). In this regard, general enhancements in brain insulin signaling as performed in our study do not permit differentiations. Taken together, our findings indicate that intranasal insulin acutely increases postprandial thermogenesis and improves the glucoregulatory response to food intake, suggesting that boosting brain insulin signaling in humans enhances the body's ability to cope with calorie consumption (20,21). Against the background of studies indicating that obesity and peripheral insulin resistance are associated with reduced central nervous insulin sensitivity (22–24), enhancing brain insulin signaling may emerge as a useful approach in the therapeutic management of disorders hallmarked by disturbed glucose homeostasis (25).
ACKNOWLEDGMENTS
This study was supported by Deutsche Forschungsgemeinschaft (KFO126/B5), Bonn, Germany, and Tore Nilsons Foundation for Medical Research, Stockholm, Sweden. The funding sources had no input in the design and conduct of this study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Aero Pump, Hochheim, Germany, generously provided us with precision nasal air pumps. No other potential conflicts of interest relevant to this article were reported.
C.B. designed the study, analyzed the data, contributed to writing the manuscript, and collected data or performed experiments for the study. S.B. enrolled patients and collected data or performed experiments for the study. H.B.S. contributed to writing the manuscript. H.L. contributed to writing the manuscript. B.S. contributed to writing the manuscript. J.B. contributed to writing the manuscript. M.H. designed the study, analyzed the data, and contributed to writing the manuscript. All authors had full access to all of the data and take responsibility for the integrity and accuracy of the data analysis.
We thank I. von Lützau, M. Grohs, and H. Ruf (Department of Neuroendocrinology, University of Lübeck, Lübeck, Germany) for their expert and invaluable laboratory work.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW: Central nervous system control of food intake and body weight. Nature 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 2.Porte D, Jr, Baskin DG, Schwartz MW: Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes 2005;54:1264–1276 [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, Holmberg KH, Klein I, Klaus J, Gomez LF, Kolb H, Secrest J, Jochems J, Myashiro K, Buckley P, Hadcock JR, Eberwine J, Conti B, Bartfai T: Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes 2010;59:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obici S, Zhang BB, Karkanias G, Rossetti L: Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 5.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, guilar-Bryan L, Rossetti L: Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005;434:1026–1031 [DOI] [PubMed] [Google Scholar]
- 6.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL: Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5:514–516 [DOI] [PubMed] [Google Scholar]
- 7.Thorne RG, Emory CR, Ala TA, Frey WH: Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res 1995;692:278–282 [DOI] [PubMed] [Google Scholar]
- 8.Hallschmid M, Schultes B, Marshall L, Molle M, Kern W, Bredthauer J, Fehm HL, Born J: Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes 2004;53:2202–2208 [DOI] [PubMed] [Google Scholar]
- 9.Benedict C, Kern W, Schultes B, Born J, Hallschmid M: Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 2008;93:1339–1344 [DOI] [PubMed] [Google Scholar]
- 10.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W: Intranasal insulin reduces body fat in men but not in women. Diabetes 2004;53:3024–3029 [DOI] [PubMed] [Google Scholar]
- 11.Krug R, Benedict C, Born J, Hallschmid M: Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab. 18August2010[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Shimizu S, Tani Y, Yamada H, Tabata M, Murachi T: Enzymatic determination of serum-free fatty acids: a colorimetric method. Anal Biochem 1980;107:193–198 [DOI] [PubMed] [Google Scholar]
- 13.Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D: Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 2010;59:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC: Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 2003;52:682–687 [DOI] [PubMed] [Google Scholar]
- 15.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L: Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 2002;5:566–572 [DOI] [PubMed] [Google Scholar]
- 16.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford M: Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci Aug 2000;3:757–758 [DOI] [PubMed] [Google Scholar]
- 17.Roden M, Bernroider E: Hepatic glucose metabolism in humans: its role in health and disease. Best Pract Res Clin Endocrinol Metab 2003;17:365–383 [DOI] [PubMed] [Google Scholar]
- 18.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI: Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996;97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden G: Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997;46:3–10 [PubMed] [Google Scholar]
- 20.Woods SC: The eating paradox: how we tolerate food. Psychol Rev 1991;98:488–505 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MW, Porte D, Jr: Diabetes, obesity, and the brain. Science 2005;307:375–379 [DOI] [PubMed] [Google Scholar]
- 22.Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klosel B, Lutzenberger W, Birbaumer N, Haring HU, Fritsche A: The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci U S A 2006;103:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallschmid M, Benedict C, Schultes B, Born J, Kern W: Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282 [DOI] [PubMed] [Google Scholar]
- 24.Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA: Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 2006;55:2986–2992 [DOI] [PubMed] [Google Scholar]
- 25.Hallschmid M, Schultes B: Central nervous insulin resistance: a promising target in the treatment of metabolic and cognitive disorders? Diabetologia 2009;52:2264–2269 [DOI] [PubMed] [Google Scholar]