Abstract
OBJECTIVE
TRPM2 is a Ca2+-permeable nonselective cation channel activated by adenosine dinucleotides. We previously demonstrated that TRPM2 is activated by coapplication of heat and intracellular cyclic adenosine 5′-diphosphoribose, which has been suggested to be involved in intracellular Ca2+ increase in immunocytes and pancreatic β-cells. To clarify the involvement of TRPM2 in insulin secretion, we analyzed TRPM2 knockout (TRPM2-KO) mice.
RESEARCH DESIGN AND METHODS
Oral and intraperitoneal glucose tolerance tests (OGTT and IPGTT) were performed in TRPM2-KO and wild-type mice. We also measured cytosolic free Ca2+ in single pancreatic cells using fura-2 microfluorometry and insulin secretion from pancreatic islets.
RESULTS
Basal blood glucose levels were higher in TRPM2-KO mice than in wild-type mice without any difference in plasma insulin levels. The OGTT and IPGTT demonstrated that blood glucose levels in TRPM2-KO mice were higher than those in wild-type mice, which was associated with an impairment in insulin secretion. In isolated β-cells, smaller intracellular Ca2+ increase was observed in response to high concentrations of glucose and incretin hormone in TRPM2-KO cells than in wild-type cells. Moreover, insulin secretion from the islets of TRPM2-KO mice in response to glucose and incretin hormone treatment was impaired, whereas the response to tolbutamide, an ATP-sensitive potassium channel inhibitor, was not different between the two groups.
CONCLUSIONS
These results indicate that TRPM2 is involved in insulin secretion stimulated by glucose and that further potentiated by incretins. Thus, TRPM2 may be a new target for diabetes therapy.
Under physiological conditions, blood glucose levels are kept in a narrow range despite periods of food intake and fasting. Insulin secretion from pancreatic β-cells is the only efficient means to decrease blood glucose concentration. Accordingly, insulin secretion is strictly controlled by glucose, hormones, and autonomic nervous system activity. Glucose is the principal stimulator of insulin secretion from pancreatic β-cells. The primary and best-characterized pathway involved in glucose-stimulated insulin secretion is the ATP-sensitive potassium channel (KATP channel)-dependent pathway. ATP closes KATP channels, causing depolarization, resulting in Ca2+ influx from the extracellular space via l-type voltage-gated Ca2+ channels followed by exocytosis. Glucose-stimulated insulin secretion is potentiated by incretins such as glucagon-like peptide-1 (GLP-1).
Transient receptor potential melastatin 2 (TRPM2, previously named TRPC7 or LTRPC2) is a Ca2+-permeable nonselective cation channel, expressed predominantly in brain and also detected in bone marrow, spleen, heart, liver, lung, and immunocytes (1–3). This channel is activated by nicotinamide adenine dinucleotide (NAD), adenosine 5′-diphosphoribose (ADPR), and hydrogen peroxide (H2O2). Previously, we reported that TRPM2 is expressed in mouse pancreatic β-cells and is dramatically activated at body temperature by treatment with intracellular cyclic ADPR (cADPR) (4). Although some reports have shown that TRPM2 is involved in H2O2-mediated apoptosis in insulin-secreting cell lines (5,6), the physiological significance of TRPM2 in pancreas, especially at an in vivo level, has not been well characterized.
To clarify the involvement of TRPM2 in insulin secretion, we analyzed TRPM2 knockout (TRPM2-KO) mice. We found that lack of TRPM2 impairs insulin secretion not only stimulated by glucose but also potentiated by incretins.
RESEARCH DESIGN AND METHODS
Animals.
The study was conducted in 9- to 12-week-old male and female C57BL/6Cr wild-type mice and TRPM2-KO mice (7). TRPM2-KO mice were backcrossed onto the C57BL/6Cr strain over eight generations. The animals were maintained on 12 h–12 h artificial light–dark cycles at 23 ± 1°C and allowed free access to food and water. The in vivo experiments were performed between December and April. All procedures involving the care and use of animals were approved by the National Institute for Physiological Sciences and carried out in accordance with the National Institutes of Health Guidelines for the care and use of laboratory animals (NIH publication no. 85-23; revised 1985).
Metabolic and biochemical measurements.
Measurement of food and water intake and body weight, and collection of blood samples were carried out every week. Blood glucose levels were measured using a glucose monitor (G sensor, GLUCOCARD DIA meter, Arkray, Kyoto, Japan), and plasma insulin, glucagon, and somatostatin levels were measured using ELISAs (Morinaga Institute of Biological Science, Inc., Yokohama, Japan; WAKO Pure Chemical Industries, Ltd., Osaka, Japan; and Phoenix Pharmaceuticals, Inc., CA, USA, respectively). Core body temperature and locomotor activity were monitored using transmitter devices (Mini Mitter Company, OR, USA) surgically implanted in the peritoneal cavity under anesthesia (50 mg/kg sodium pentobarbital, Dainippon Sumitomo Pharma Co., Ltd., Osaka, Japan). After surgery, each animal was housed alone in a recording cage and allowed to recover from surgery for >1 week. Data were recorded by placing each cage containing an animal implanted with a radiotransmitter on a receiver plate (ER-4000, Mini Mitter Company). A Vital View data acquisition system (Mini Mitter Company) was used for data collection and analysis.
Isolation of pancreatic islets.
Islets of Langerhans were isolated from mouse pancreas by collagenase digestion as described previously (8) with minor modifications. Animals were anesthetized with sodium pentobarbital (80 mg/kg, i.p., Dainippon Sumitomo Pharma Co., Ltd.), followed by injection of 1.05 mg/ml collagenase (Sigma-Aldrich, St. Louis, MO, USA) into the common bile duct. The collagenase was dissolved in 5 mmol/l Ca2+-containing HEPES-added Krebs-Ringer bicarbonate buffer solution (HKRB, 129 mmol/l NaCl, 5 mmol/l NaHCO3, 4.7 mmol/l KCl, 1.2 mmol/l KH2PO4, 2 mmol/l CaCl2, 1.2 mmol/l MgSO4, 10 mmol/l HEPES, 0.1% BSA, pH 7.4). The pancreas was removed by dissection and incubated at 37°C for 20 min. The islets were then collected and used either for insulin release experiments or dispersed into single cells in Ca2+-free HKRB.
Immunocytochemistry.
Single cells were plated sparsely on coverslips and cultured for 12–16 h at 37°C in an atmosphere of 5% CO2 and 95% air in Eagle's minimal essential medium containing 5.6 mmol/l glucose supplemented with 10% FBS, 100 μg/ml streptomycin, and 100 units/ml penicillin. Samples were fixed for 10 min at room temperature in ethanol, followed by washing (3 × 5 min) with PBS. Samples were then washed (3 × 5 min) in PBS supplemented with 0.1% Tween-20 (PBST) and blocked with PBST containing 3% BSA for 1 h at room temperature. Cells were incubated with primary antibodies (rabbit anti-TRPM2 antibody at 1:100, generous gift from Dr. Y. Mori, and guinea pig anti-insulin antibody at 1:700, Dako Japan, Tokyo, Japan) dissolved in PBST containing 3% BSA and then washed (3 × 10 min) with PBST for 1.5 h at room temperature. This was followed by incubation with secondary antibodies (Alexa Rb488 and Alexa GpCy3, Molecular Probes, Invitrogen Corp., CA, USA) for 1 h at room temperature. Images were then obtained with a fluorescence microscope (Olympus, Tokyo, Japan).
Glucose tolerance test.
Oral and intraperitoneal glucose tolerance tests (OGTT and IPGTT) were performed after 16–17-h fasting. Glucose (1 g/kg body wt, Otsuka Pharmaceutical, Tokyo, Japan) was administered orally or injected intraperitoneally. Blood glucose levels were measured before (0 min) and 15, 30, 45, 60, and 120 min after administration or injection. Blood samples were taken from the tail vein and blood glucose levels were measured using a glucose monitor. Plasma insulin levels were determined by ELISA. Area under the curve (AUC) of insulin secretion was calculated after subtraction of basal insulin levels (at time zero).
Insulin tolerance test.
An insulin tolerance test was performed after 4-h fasting. Porcine insulin (1 unit/kg body wt, Sigma-Aldrich) was injected into the intraperitoneal space and blood glucose levels were measured with a glucose monitor before (0 min) and 15, 30, 45, and 60 min after injection of insulin.
Measurement of cytosolic-free Ca2+ in single mouse β-cells.
Single cells were sparsely plated on coverslips and maintained in short-term culture for up to 2 days at 37°C in an atmosphere of 5% CO2 and 95% air in Eagle's minimal essential medium containing 5.6 mmol/l glucose supplemented with 10% FBS, 100 μg/ml streptomycin, and 100 units/ml penicillin. Single β-cells on coverslips were mounted in an open chamber and superfused in HKRB. Cytosolic-free Ca2+ ([Ca2+]i) in single β-cells was measured by dual-wavelength fura-2 (Molecular Probes, Invitrogen Corp.) microfluorometry with excitation at 340/380 nm and emission at 510 nm. The ratio image was calculated and acquired using an imaging processing system (IP-Lab, Scanalytic Inc., VA, USA). Cells showing an increase in the ratio of >0.3 from basal values in response to heat stimulation were defined as heat-sensitive cells. All glucose- and tolbutamide-positive cells were confirmed to be β-cells. AUC for changes in [Ca2+]i was calculated after subtraction of basal [Ca2+]i.
Measurement of insulin release in mouse islets.
Islets were collected in RPMI-1640 medium (WAKO Pure Chemical Industries, Ltd.) containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin, incubated in that medium for 3 h and then preincubated in Krebs-Ringer buffer (KRB, 129 mmol/l NaCl, 5 mmol/l NaHCO3, 5.2 mmol/l KCl, 1.3 mmol/l KH2PO4, 2.7 mmol/l CaCl2, 1.3 mmol/l MgSO4, 0.2% BSA, pH 7.4) containing 3.3 mmol/l glucose for 30 min at 37°C. The solution was then replaced with fresh KRB buffer containing glucose (3.3, 8.3, 11.2, or 16.7 mmol/l, Sigma-Aldrich) and islets were incubated for 10, 15, or 60 min at 37°C. In some experiments, GLP-1 (10 or 30 nmol/l, Sigma-Aldrich) or exendin-4 (Ex-4, 10 or 30 nmol/l, Sigma-Aldrich) was present throughout the incubation with 8.3 mmol/l glucose. In some experiments, tolbutamide (30 or 100 μmol/l, Sigma-Aldrich) was present throughout the incubation with basal glucose (3.3 mmol/l). To examine the contribution of TRPM2 to KATP channel-independent glucose-stimulated insulin release, islets were incubated at 37°C for 60 min in KRB containing glucose (3.3 or 16.7 mmol/l), 250 μmol/l diazoxide (Sigma-Aldrich), and 30 mmol/l K+. Insulin content in the supernatant was measured by ELISA. Second-phase insulin secretion (from 10 to 60 min) was calculated by subtracting first-phase secretion (from 0 to 10 min) from the total secretion.
Electrophysiology.
Whole-cell patch-clamp recordings were performed 1 day after plating single pancreatic cells on coverslips. The standard bath solution contained (mM) 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, pH 7.4 (adjusted with NaOH). The pipette solution contained (mM) 140 CsCl or KCl, 5 EGTA, and 10 HEPES, pH 7.4 (adjusted with CsOH or KOH). Data from whole-cell voltage-clamp or current-clamp recordings were sampled at 10 kHz and filtered at 5 kHz for analysis (Axon 200B amplifier with pCLAMP software, Axon Instruments, CA, USA). Membrane potential was clamped at −60 mV in the voltage-clamp recordings. Heat stimulation was applied by increasing the bath temperature with a preheated solution through the inline heater (1°C/s, with a maximum of 45°C).
Statistical analysis.
Data are expressed as means ± SEM. Statistical analysis was performed using Student t test, two-factor repeated measures ANOVA, followed by Dunnett post hoc test, or ANOVA followed by the Bonferroni-type multiple t test. P values < 0.05 were considered significant.
RESULTS
Characterization of the TRPM2-KO mouse.
In cells isolated from pancreatic islets of wild-type mice, TRPM2 immunoreactive cells were also shown to be positive for insulin as described in a previous report (4) (Fig. 1A). In contrast, no TRPM2-like immunoreactivity was seen in TRPM2-KO cells (Fig. 1B). Heat stimulation increased cytosolic Ca2+ ([Ca2+]i) in wild-type cells (45% heat-sensitive cells, 25/56 cells) (Fig. 1C), whereas the majority of TRPM2-KO cells (92%, 48/52 cells) failed to respond to heat stimulation (Fig. 1D).
FIG. 1.
Relationship between TRPM2 expression and heat response in isolated pancreatic cells. A and B: Immunocytochemistry of cultured single pancreatic cells from wild-type (WT) (A) and TRPM2-KO (B) mice. Green, TRPM2; red, insulin. Scale bars: 200 μm. C and D: Quantification of [Ca2+]i changes in individual cells isolated from WT (C) and TRPM2-KO (D) mice displaying heat-evoked [Ca2+]i changes. Ordinates, fura-2 ratio (upper) and temperature (lower); abscissa, time. (A high-quality digital representation of this figure is available in the online issue.)
Table 1 depicts a comprehensive evaluation of the metabolic parameters of 12 week-old TRPM2-KO mice. Body weight, food intake, and water intake were similar in wild-type and TRPM2-KO mice. Under ad libitum feeding conditions, blood glucose levels were significantly higher in TRPM2-KO mice. Although plasma insulin levels measured between 10:00 and 11:00 a.m. tended to be lower in TRPM2-KO mice, this difference was not statistically significant. Plasma glucagon and somatostatin levels did not differ between wild-type and TRPM2-KO mice. The data from 9- to 11-week-old mice were similar to those from 12-week-old mice (data not shown). Core body temperature and locomotor activity also did not differ between wild-type and TRPM2-KO mice (Table 1).
TABLE 1.
Characterization of TRPM2-KO mice
| Body weight (g) | Food intake (kcal/day) | Water intake (mL/day) | Blood glucose (mg/dl) | Plasma insulin (ng/ml) | Plasma glucagon (pg/ml) | Plasma somatostatin (ng/ml) | Body temperature (°C) |
Activity (counts/20 min) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dark | Light | Dark | Light | ||||||||
| WT | 25.92 ± 0.51 | 10.78 ± 0.50 | 3.79 ± 0.11 | 103.2 ± 4.6 | 0.682 ± 0.101 | 322.5 ± 55.3 | 7.18 ± 0.73 | 37.46 ± 0.24 | 36.27 ± 0.15 | 480.0 ± 130.2 | 159.4 ± 20.1 |
| TRPM2-KO | 25.87 ± 0.56 | 10.70 ± 0.35 | 3.89 ± 0.17 | 118.5 ± 4.0* | 0.520 ± 0.113 | 321.1 ± 61.7 | 6.70 ± 1.14 | 37.42 ± 0.17 | 36.06 ± 0.13 | 369.3 ± 53.5 | 133.5 ± 20.8 |
Each value represents the mean ± SEM of 4–9 mice. Statistical significance was assessed using Student t test.
*P < 0.05 for WT versus TRPM2-KO mice.
Glucose and insulin tolerance tests.
To assess glucose metabolism and insulin secretion, OGTT and IPGTT were performed in fasted (16–17 h) male wild-type and TRPM2-KO mice. Basal fasting blood glucose and plasma insulin levels were similar between the two groups of mice. In the OGTT, TRPM2-KO mice exhibited significantly higher blood glucose levels (Fig. 2A) with significantly lower plasma insulin levels (Fig. 2C) 15 min after glucose administration. In the IPGTT, TRPM2-KO mice also exhibited significantly higher blood glucose levels (Fig. 2B) associated with significantly lower plasma insulin levels (Fig. 2D) after glucose administration. Lower plasma insulin levels in TRPM2-KO mice were also detected by another analysis with integrated insulin secretion expressed as the AUC (Fig. 2C and 2D). Impaired glucose tolerance with lower insulin secretion was also observed in female TRPM2-KO mice (supplementary Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0276/DC1). Measurement of blood glucose levels during the insulin tolerance test showed only minor differences between wild-type and TRPM2-KO mice (supplementary Fig. 2, available in an online appendix).
FIG. 2.
Glucose tolerance in WT and TRPM2-KO mice. Oral (A, C) and intraperitoneal (B, D) glucose tolerance tests (OGTT and IPGTT, respectively) were performed in male TRPM2-KO and WT mice fasted for 16–17 h. A 20% glucose solution (100 μl/10 g BW) was administered at time zero. A and B: Ordinates, blood glucose concentrations; abscissa, time after injection of glucose. C and D: Ordinates, plasma insulin concentrations; abscissa, time after injection of glucose. Each point represents the mean ± SEM of 9–12 mice. White and black symbols indicate data from WT and TRPM2-KO, respectively. Insets in C and D: Calculation of the AUC for the data presented in (C) and (D). White and black bars indicate data from WT and TRPM2-KO, respectively. Statistical significance was assessed using Student t test or repeated-measures ANOVA followed by Dunnett post hoc test. *P < 0.05, **P < 0.01 for WT versus TRPM2-KO mice.
Ca2+ imaging.
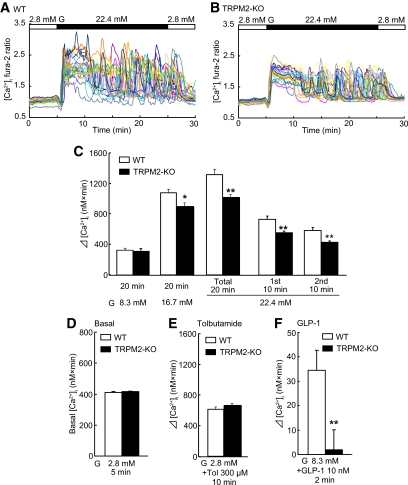
[Ca2+]i is considered to be the primary regulator of insulin secretion in pancreatic β-cells. To determine whether TRPM2 is involved in control of [Ca2+]i, fura-2 microfluorometry was used to measure [Ca2+]i in pancreatic β-cells isolated from wild-type and TRPM2-KO mice. Figure 3A and B shows representative changes in [Ca2+]i after application of increasing concentrations of glucose from 2.8 to 22.4 mmol/l. The increase in [Ca2+]i tended to be lower in pancreatic β-cells from TRPM2-KO mice than in those from wild-type mice during the first-phase. Moreover, the peak [Ca2+]i increases in TRPM2-deficient β-cells appeared to be lower than in wild-type cells, although Ca2+ oscillations were also observed in TRPM2-KO mice. We then analyzed the integrated [Ca2+]i increase in 20 min in each cell, expressed as the AUC. As shown in Fig. 3C, high glucose-stimulated (16.7 and 22.4 mmol/l) increases in [Ca2+]i were significantly blunted in β-cells from TRPM2-KO mice, whereas basal (2.8 mmol/l glucose) [Ca2+]i and moderate glucose-stimulated (8.3 mmol/l) increases in [Ca2+]i did not differ between the two groups of mice (Fig. 3C and D). In addition, analysis of first- and second-phase AUC revealed that [Ca2+]i response to 22.4 mmol/l glucose was significantly reduced in β-cells from TRPM2-KO mice in both phases (Fig. 3C). In contrast, the [Ca2+]i response to incubation with 300 μmol/l tolbutamide for 10 min, which causes membrane depolarization by inhibiting KATP channels, was not different in pancreatic β-cells from TRPM2-KO mice under basal glucose (2.8 mmol/l) conditions (Fig. 3E). We also measured [Ca2+]i increases potentiated by GLP-1 (10 nmol/l) with 8.3 mmol/l glucose for 2 min. Increases in [Ca2+]i were significantly reduced in β-cells from TRPM2-KO mice (Fig. 3F).
FIG. 3.
Measurement of [Ca2+]i in β-cells isolated from WT and TRPM2-KO mice. A and B: Quantification of [Ca2+]i changes in individual cells from WT (A) and TRPM2-KO (B) mice in response to 22.4 mmol/l glucose (G). Ordinates, fura-2 ratios; abscissa, time. C: Quantification of [Ca2+]i changes in response to 8.3–22.4 mmol/l G for 20 min. Total [Ca2+]i changes in response to 22.4 mmol/l G in 20 min were separated into values from the first 10 min and those from the second 10 min (values for 2.8 mmol/l G alone were subtracted). D: Quantification of basal [Ca2+]i in 2.8 mmol/l G. E: Quantification of [Ca2+]i changes in response to 300 μmol/l tolbutamide with 2.8 mmol/l G (values for 2.8 mmol/l G alone were subtracted). F: Quantification of [Ca2+]i changes in response to 10 nmol/l GLP-1 with 8.3 mmol/l G (values for 8.3 mmol/l G alone were subtracted). Ordinates, AUC of [Ca2+]i. Each bar represents the mean + SEM. White and black bars indicate data from WT and TRPM2-KO, respectively. Statistical significance was assessed using Student t test. *P < 0.05, **P < 0.01 for WT versus TRPM2-KO mice.
In vitro insulin secretion test.
To determine whether β-cells isolated from TRPM2-KO mice exhibited impaired insulin secretion, an insulin secretion test was carried out using islets obtained from TRPM2-KO mice. As shown in Fig. 4A, basal and moderate glucose-stimulated (3.3 and 8.3 mmol/l) insulin secretion did not differ in islets obtained from wild-type and TRPM2-KO mice. However, high glucose-stimulated (11.2 and 16.7 mmol/l) insulin secretion was significantly impaired in islets from TRPM2-KO mice. Next, to examine whether the impairment in glucose-stimulated insulin secretion observed in TRPM2-KO islets was time-dependent, we measured insulin secretion for 60 min, separating the values from the first 10 min (first phase) and the second 50 min (second phase). Insulin secretion stimulated by a high concentration of glucose (16.7 mmol/l) was significantly reduced in islets from TRPM2-KO mice in both the first and second phases (Fig. 4B). However, insulin secretion induced by either 30 or 100 μmol/l tolbutamide with basal glucose (3.3 mmol/l) was not different between wild-type and TRPM2-KO mice (Fig. 4C). We previously reported that TRPM2 may be involved in the potentiation of glucose-stimulated insulin secretion by Ex-4, a glucagon-like peptide analog (4). In this study, we examined insulin secretion induced by GLP-1 and Ex-4 and found that GLP-1 (10 and 30 nmol/l) and Ex-4 (10 and 30 nmol/l) significantly potentiated insulin secretion in wild-type islets exposed to 8.3 mmol/l glucose, whereas this potentiation was markedly reduced in islets from TRPM2-KO mice (Fig. 4D). To determine to what extent TRPM2 is involved in KATP channel-independent glucose-stimulated insulin release, we measured insulin release after stimulation with a high concentration of glucose (16.7 mmol/l) with diazoxide (250 μmol/l) and 30 mmol/l K+, as shown in another report (9), conditions that would be expected to completely inactivate KATP channel-mediated pathways. Under these conditions, glucose-stimulated insulin secretion was not observed in islets obtained from TRPM2-KO mice (Fig. 5).
FIG. 4.
Measurement of insulin release from islets isolated from WT and TRPM2-KO mice. A: Glucose-stimulated insulin secretion. Ten islets were incubated in medium containing 3.3, 8.3, 11.2, or 16.7 mmol/l glucose (G) for 15 min at 37°C. Ordinates, insulin content in the supernatant. Each bar represents the mean + SEM of 6–8 tubes. White and black bars indicate data from WT and TRPM2-KO, respectively. Statistical significance was assessed using ANOVA followed by the Bonferroni-type multiple t test. *P < 0.05 for WT versus TRPM2-KO mice. #P < 0.05, ##P < 0.01 versus 3.3 mmol/l G. B: Glucose-stimulated insulin secretion in the first and second phases. Ten islets were incubated in medium containing 3.3 or 16.7 mmol/l G for 10 or 60 min at 37°C. Ordinates, insulin content in the supernatant. Each bar represents the mean + SEM of 6–9 tubes. White and black bars indicate data from WT and TRPM2-KO, respectively. Statistical significance was assessed using ANOVA followed by the Bonferroni-type multiple t test. *P < 0.05 for WT versus TRPM2-KO mice. #P < 0.05, ##P < 0.01 versus 3.3 mmol/l G. C: Effects of tolbutamide on 3.3 mmol/l G-stimulated insulin release. Ten islets were incubated in the presence of 30 or 100 μmol/l tolbutamide (Tol) for 15 min at 37°C. Ordinates, insulin content in the supernatant. Each bar represents the mean + SEM of 6–8 tubes. White and black bars indicate data from WT and TRPM2-KO, respectively. Statistical significance was assessed using ANOVA followed by the Bonferroni-type multiple t test. #P < 0.05, ##P < 0.01 versus 3.3 mmol/l G. D: Effects of GLP-1 and Ex-4 on 8.3 mmol/l G-stimulated insulin secretion. Ten islets were incubated in the presence of GLP-1 (10 or 30 nmol/l) or Ex-4 (10 or 30 nmol/l) for 15 min at 37°C. Ordinates, insulin content in the supernatant. Each bar represents the mean + SEM of 6–8 tubes. White and black bars indicate data from WT and TRPM2-KO, respectively. Statistical significance was assessed using ANOVA followed by the Bonferroni-type multiple t test. **P < 0.01 for WT versus TRPM2-KO mice. #P < 0.05, ##P < 0.01 versus 8.3 mmol/l G.
FIG. 5.
Measurement of insulin release from islets evoked by glucose, diazoxide, and high K+. Insulin secretion from 10 islets treated with a mixture of glucose (G), diazoxide (Dia) (250 μmol/l), and K+ (30 mmol/l) at 37°C for 60 min was measured. Ordinates, insulin content in the supernatant. Each bar represents the mean + SEM of 7–9 tubes. White and black bars indicate data from WT and TRPM2-KO islets, respectively. Statistical significance was assessed using ANOVA followed by the Bonferroni-type multiple t test. **P < 0.01 for WT versus TRPM2-KO mice. #P < 0.05 versus 3.3 mmol/l G.
DISCUSSION
In this study, we confirmed the expression of TRPM2 at both the protein and functional levels in mouse pancreatic β-cells, confirming previous reports (4,6,10), and also confirmed the deletion of TRPM2 in TRPM2-KO mice (Fig. 1). To date, many TRP channels (TRPC1, TRPC4, TRPC6, TRPV2, TRPV4, TRPV5, TRPM3, TRPM4, TRPM5, and TRPM7) have been shown to be expressed in pancreatic β-cells (11–14), with TRPV2, TRPM3, TRPM4, and TRPM5 reported to be involved in insulin secretion (13,15–18). Some of these TRP channels (TRPV2, TRPV4, TRPM4, and TRPM5) exhibit thermosensitivity. In this study, a temperature increase up to 42–43°C increased intracellular Ca2+ levels, an effect which was nearly abolished in pancreatic β-cells obtained from TRPM2-KO mice (Fig. 1C and D). This suggests that the functional expression of thermosensitive TRP channels other than TRPM2, except for TRPV2 (activated over 52°C), may be modest. Alternatively, TRPM2 might function in concert with other thermosensitive TRP channels in the pancreas.
In vivo experiments in TRPM2-KO mice showed that insulin secretion during an OGTT was significantly reduced in these mice 15 min after glucose administration (Fig. 2C). This early phase of insulin secretion is believed to involve the action of incretin hormones released from gut endocrine cells after absorption of nutrients (19). This reduction in insulin secretion during the OGTT is in agreement with the finding that incretin-potentiated [Ca2+]i increases were nearly abolished in β-cells from TRPM2-KO mice (Fig. 3F) and that incretin-potentiated insulin secretion was markedly impaired in islets from TRPM2-KO mice (Fig. 4D). These findings support the notion that TRPM2 is involved in incretin-potentiated insulin secretion. In the IPGTT, where the incretin-mediated component is thought not to predominate, TRPM2-KO mice showed impaired glucose tolerance with a lower insulin response (Fig. 2B and D). This impairment is similar to what was observed in the OGTT and is consistent with the reduction in glucose-stimulated [Ca2+]i increases and insulin secretion in TRPM2-KO β-cells and islets (Figs. 3C and 4A). These data indicate that TRPM2 is involved in insulin secretion stimulated by glucose and that further promoted by incretins. Insulin sensitivity was also found to be slightly impaired in TRPM2-KO mice (supplementary Fig. 2), suggesting that insulin resistance may also be partially involved in the impaired glucose tolerance observed in TRPM2-KO mice.
An increase in intracellular ATP concentration is one of the most important events in glucose-stimulated insulin secretion. In this study, the effects of tolbutamide, a KATP channel inhibitor, were not changed in TRPM2-KO mice (Figs. 3E and 4C), suggesting that the capacity for insulin secretion is intact in β-cells from TRPM2-KO mice. The results of our in vitro experiments suggest that impairment of glucose-stimulated insulin secretion in TRPM2-KO islets is caused by reduced increases in [Ca2+]i (Figs. 3C and 4A). We previously reported that cADPR activates TRPM2 at body temperature (4), and we observed heat-evoked inward currents in the presence of 100 μmol/l cADPR in the pipette in isolated pancreatic cells from wild-type but not TRPM2-KO mice (supplementary Fig. 3, available in an online appendix). Although the physiological significance of cADPR in β-cell function is still controversial (20), cADPR, synthesized from NAD by CD38, has been reported to be a second messenger that increases intracellular Ca2+ not only in lymphocytes (21) but also in pancreatic β-cells (22), and to be involved in insulin secretion. Given the fact that NAD also activates TRPM2, NAD and its metabolites may cooperatively activate TRPM2 at body temperature.
[Ca2+]i increases resulting from high concentrations of glucose were impaired in TRPM2-KO β-cells, suggesting that Ca2+ influx through TRPM2 can modulate [Ca2+]i levels. However, glucose-stimulated insulin secretion evoked under conditions of glucose, diazoxide, and high K+ was lost in TRPM2-KO islets, but changes in [Ca2+]i resulting from stimulation by glucose, diazoxide, and high K+ were not different in β-cells from wild-type and TRPM2-KO mice (Fig. 5 and supplementary Fig. 4, available in an online appendix). These results suggest that the TRPM2-mediated insulin secretion is KATP channel-independent to some extent and that it involves a pathway not related to changes in [Ca2+]i. The possible existence of a pathway not involving Ca2+ influx could explain the fact that glucose-stimulated insulin secretion from islets (Fig. 4A) was impaired to a greater extent than [Ca2+]i changes in isolated β-cells (Fig. 3C) from TRPM2-KO mice. Gene expression of signaling molecules for glucose-stimulated exocytotic machinery may also be changed by ablation of TRPM2. More experiments are necessary to clarify the detailed mechanism of TRPM2-mediated insulin secretion.
As shown in Figs. 3F and 4D, the responses to GLP-1 and Ex-4 in pancreatic β-cells and islets, respectively, were markedly reduced in TRPM2-KO mice. We have previously shown that potentiation of insulin secretion by forskolin, an adenylate cyclase activator, is mediated by TRPM2 activation. Furthermore, TRPM2 currents induced by cADPR at an elevated temperature were potentiated by forskolin, and this potentiation was blocked by H-89, a cAMP-dependent protein kinase (PKA) inhibitor (4). GLP-1 is known to cause a rise in intracellular Ca2+ concentration (23), and GLP-1 receptor is a G-protein coupled receptor coupled to Gs and involved in insulin secretion through PKA-dependent and -independent mechanisms (24). Thus, we hypothesize that TRPM2 potentiation by incretin may occur via the cAMP-PKA pathway, possibly through phosphorylation of TRPM2 by PKA. Moreover, it has been reported that TRPM2 is activated by intracellular Ca2+ through binding of Ca2+/calmodulin to the NH2 terminus of TRPM2 (25). In addition, the receptors for vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide are also G-protein coupled receptors coupled to Gs (26) and may modulate TRPM2 function. On the other hand, TRPM2 activation could evoke l-type voltage-gated Ca2+ channel-mediated Ca2+ influx to increase insulin secretion because activation of TRPM2 causes influx of Na+ as well as Ca2+, leading to the depolarization of pancreatic β-cells, followed by activation of the voltage-gated Ca2+ channel. Indeed, we observed generation of action potentials upon heating in the isolated pancreatic cells from wild-type mice (supplementary Fig. 5, available in an online appendix). Collectively, these findings support the notions that TRPM2 activity can be modulated in several ways and that TRPM2 activation can cause intracellular Ca2+ increase in different ways, which may, in turn, regulate insulin secretion.
In conclusion, endogenous TRPM2 in pancreatic β-cells plays a key role in insulin secretion induced by glucose and incretins. This indicates that TRPM2 is a sensor not only for signals from the gut but also for glucose metabolism and that TRPM2 integrates this information to control insulin secretion by modulating intracellular Ca2+ levels and by another mechanism not involving changes in intracellular Ca2+ levels. Pharmacological activation of TRPM2 channels may therefore be useful for enhancing insulin secretion and may potentially lead to the development of effective drugs for the treatment of diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants to M.T. and Y.Mi. from the Ministry of Education, Culture, Sports, Science and Technology in Japan, and to M.T. from Hayama Center for Advanced Studies Projects. No potential conflicts of interest relevant to this article were reported.
K.U. performed the experiments and wrote the manuscript. K.D. performed the experiments and contributed to discussion of the results. B.D. performed the experiments. H.I., T.S., Y.Mo., T.Y., and Y.Mi. contributed to discussion of the results. M.T. wrote the manuscript.
We thank N. Fukuta and A. Fukuda at the National Institute for Physiological Sciences for technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 28.
REFERENCES
- 1.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM: ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001;411:595–599 [DOI] [PubMed] [Google Scholar]
- 2.Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N: Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 1998;54:124–131 [DOI] [PubMed] [Google Scholar]
- 3.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y: LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 2002;9:163–173 [DOI] [PubMed] [Google Scholar]
- 4.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M: TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 2006;25:1804–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R: TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal 2009;2:ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bari MR, Akbar S, Eweida M, Kühn FJ, Gustafsson AJ, Lückhoff A, Islam MS: H2O2-induced Ca2+ influx and its inhibition by N-(p-amylcinnamoyl) anthranilic acid in the beta-cells: involvement of TRPM2 channels. J Cell Mol Med 2009;13:3260–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y: TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 2008;14:738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton R, Peters M, McShane P, Gray DW, Morris PJ: Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation 1986;42:689–691 [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Henquin JC: The K+-ATP channel-independent pathway of regulation of insulin secretion by glucose: in search of the underlying mechanism. Diabetes 1998;47:1713–1721 [DOI] [PubMed] [Google Scholar]
- 10.Qian F, Huang P, Ma L, Kuznetsov A, Tamarina N, Philipson LH: TRP genes: candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes 2002;51(Suppl. 1):S183–S189 [DOI] [PubMed] [Google Scholar]
- 11.Nilius B, Voets T: A TRP channel-steroid marriage. Nat Cell Biol 2008;10:1383–1384 [DOI] [PubMed] [Google Scholar]
- 12.Hiriart M, Aguilar-Bryan L: Channel regulation of glucose sensing in the pancreatic beta-cell. Am J Physiol Endocrinol Metab 2008;295:E1298–E1306 [DOI] [PubMed] [Google Scholar]
- 13.Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I: Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. Diabetes 2009;58:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casas S, Novials A, Reimann F, Gomis R, Gribble FM: Calcium elevation in mouse pancreatic beta cells evoked by extracellular human islet amyloid polypeptide involves activation of the mechanosensitive ion channel TRPV4. Diabetologia 2008;51:2252–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Düfer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J: Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 2008;10:1421–1430 [DOI] [PubMed] [Google Scholar]
- 16.Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R: TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium 2007;41:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R: Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci U S A 2010;107:5208–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D: TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch 2010;460:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B: Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 2004;113:635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HC: Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol 2001;41:317–345 [DOI] [PubMed] [Google Scholar]
- 21.Guse AH, da Silva CP, Berg I, Skapenko AL, Weber K, Heyer P, Hohenegger M, Ashamu GA, Schulze-Koops H, Potter BV, Mayr GW: Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature 1999;398:70–73 [DOI] [PubMed] [Google Scholar]
- 22.Takasawa S, Nata K, Yonekura H, Okamoto H: Cyclic ADP-ribose in insulin secretion from pancreatic beta cells. Science 1993;259:370–373 [DOI] [PubMed] [Google Scholar]
- 23.Yada T, Itoh K, Nakata M: Glucagon-like peptide-1-(7–36)amide and a rise in cyclic adenosine 3′,5′-monophosphate increase cytosolic free Ca2+ in rat pancreatic beta-cells by enhancing Ca2+ channel activity. Endocrinology 1993;133:1685–1692 [DOI] [PubMed] [Google Scholar]
- 24.Doyle ME, Egan JM: Glucagon-like peptide-1. Recent Prog Horm Res 2001;56:377–399 [DOI] [PubMed] [Google Scholar]
- 25.Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ, Miller BA: Regulation of the transient receptor potential channel TRPM2 by the Ca2+ sensor calmodulin. J Biol Chem 2006;281:9076–9085 [DOI] [PubMed] [Google Scholar]
- 26.Winzell MS, Ahrén B: Role of VIP and PACAP in islet function. Peptides 2007;28:1805–1813 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.