Abstract
OBJECTIVE
Transcriptional peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α) plays a key role in mitochondrial biogenesis and energy metabolism and is suggested to be involved in the exercise-induced increase in mitochondrial content. PGC-1α activity is regulated by posttranslational modifications, among them acetylation or phosphorylation. Accordingly, the deacetylase SIRT1 and the kinase AMPK increase PGC-1α activity.
RESEARCH DESIGN AND METHODS
We tested whether chronic treadmill exercise or a single exercise session modifies PGC-1α activation and mitochondrial biogenesis differentially in obese ob/ob mice with dysregulated adiponectin/leptin-mediated AMPK activation compared with C57BL/6J wild-type mice.
RESULTS
Exercise training (12 weeks) induced adiponectin and lowered plasma insulin and glucose, suggesting improved insulin sensitivity in wild-type mice. It enhanced mitochondrial biogenesis in red gastrocnemius muscle, as indicated by increased mRNA expression of transcriptional regulators and primary mitochondrial transcripts, increased mtDNA content, and citrate synthase activity. Parallel to this, we observed AMPK activation, PGC-1α deacetylation, and SIRT1 induction in trained wild-type mice. Although none of these exercise-induced changes were detected in ob/ob mice, comparable effects on mitochondrial respiration were observed. A single exercise session resulted in comparable changes in wild-type mice. These changes remained detectable 6 h after the exercise session but had disappeared after 24 h. Treatment of C2C12 myoblasts with leptin or adiponectin resulted in increased AMPK phosphorylation and PGC-1α deacetylation.
CONCLUSIONS
Chronic exercise induces mitochondrial biogenesis in wild-type mice, which may require intact AMPK activation by adipocytokines and involve SIRT1-dependent PGC-1α deacetylation. Trained ob/ob mice appear to have partially adapted to reduced mitochondrial biogenesis by AMPK/SIRT1/PGC-1α–independent mechanisms without mtDNA replication.
Several studies have reported alterations in mitochondrial morphology, reductions in mitochondrial mass, impaired oxidative capacity, and altered expression of peroxisome proliferator–activated receptor-γ coactivator-1α (PGC-1α) (1,2) in the skeletal muscle of insulin-resistant individuals. This impairment in mitochondrial function results in a decrease in glucose and fatty acid oxidation and an increase in intramuscular triglycerides and insulin resistance (3). Emerging evidence suggests that adaptations to heavy lipid load depend on the coordinated actions of transcriptional regulators such as the peroxisome proliferator–activated receptors (PPARs) and PGC-1α (4). Chronic exercise (5) enhances muscular mitochondrial biogenesis and performance, favoring tighter coupling between β-oxidation and trichloroacetic acid cycle, and may concomitantly improve insulin sensitivity. PGC-1α was suggested to play a key role in coordinating metabolic flux and mitochondrial biogenesis (6,7).
Besides an increase in size and number of mitochondria, chronic exercise results in the induction of mitochondrial transporters, enzymes involved in β-oxidation of fatty acids, or enzymes of the mitochondrial respiratory chain in the muscle. Mitochondrial biogenesis requires the coordination of the nuclear and mitochondrial genome. Transcription factors involved in this process include PGC-1α, the nuclear respiratory factors (NRF-1/NRF-2), and the mitochondrial transcription factor A (Tfam), which are downstream of PGC-1α (6). PGC-1α is preferentially expressed in muscles enriched in slow-twitch type I fibers and drives the formation of slow-twitch fibers (8). Exercise was shown to result in an increased PGC-1α expression (9). The induction of PGC-1α expression and the concomitant increase in mitochondrial biogenesis in muscle involves the activation of calcium/calmodulin-dependent protein kinase (CaMK), p38 mitogen-activated protein kinase (9,10), or AMP-dependent protein kinase (AMPK) (11). AMPK phosphorylates PGC-1α at Thr177 and Ser538, which is required for the PGC-1α–dependent induction of the PGC-1α promoter and the mitochondrial biogenic response (12). However, the extent of endurance training may determine whether the CaMK or the AMPK pathway is primarily activated (13).
PGC-1α activity is influenced by various posttranslational modifications. AMP-activated protein kinase (12), p38 MAPK (9,10), and protein kinase B (Akt) (14) phosphorylate PGC-1α, modifying its stability and activity (rev. in 15). Besides these phosphorylation sites, PGC-1α contains multiple distinct acetylation sites. PGC-1α deacetylation has been demonstrated to occur via SIRT1 in vitro as well as in vivo during fasting (7,16) and can be mimicked by resveratrol (17). Furthermore, an exercise-induced increase in SIRT1 activity has been described in both heart and adipose tissue (18). Recently, a reduced PGC-1α acetylation was described after a single session of exercise (19). In addition, the mechanisms underlying the interaction between PGC-1α deacetylation by SIRT1 and PGC-1α phosphorylation by AMPK have been investigated in detail (19).
However, the effects of chronic exercise on AMPK-induced and SIRT1-mediated deacetylation of PGC-1α have not been investigated so far. Exercise is associated with an intermittent increase in muscular AMP content during the replenishing of cellular ATP and can thus result in the activation of AMPK by a repetitive increase in intracellular AMP-to-ATP ratio (20). The posttranslational modifications of PGC-1α induced by fasting (7,16,21), high-fat diet (21), or physical activity (19) may contribute to changes in mitochondrial function, energy metabolism, and insulin sensitivity. Because of the major role of PGC-1α in the control of both energy metabolism and insulin sensitivity, it is seen as a candidate factor in the etiology of type 2 diabetes and a drug target for its therapy.
Leptin (22) and adiponectin (23,24) have both been shown to activate AMPK and may thus promote mitochondrial biogenesis. Adiponectin directly improves mitochondrial function, increases the number of mitochondria, and exerts antidiabetic effects (24). Leptin deficiency on the other hand is associated with impaired mitochondria (25). Furthermore, ob/ob mice with premature obesity show lower levels of circulating adiponectin (26) and reduced muscle AMPK phosphorylation (27) compared with wild-type mice.
Therefore, the purpose of the present study was to investigate the influence of impaired adipokine-related AMPK activation on exercise-mediated effects on mitochondrial function and biogenesis in the skeletal muscle of ob/ob mice with early-onset obesity in comparison to wild-type mice. In addition, we tried to define the influence of physical activity on PGC-1α acetylation as a putatively important determinant of mitochondrial biogenesis.
RESEARCH DESIGN AND METHODS
Animals and exercise protocol.
Mice heterozygous for the obese spontaneous mutation (Lepob commonly referred to as ob) on a C57BL/6J background were purchased from Jackson Laboratories. Animals were housed in an environmentally controlled laboratory with a 12-h/12-h light–dark cycle with two to four mice in each cage. Age-matched male ob/ob mice and the corresponding littermates (wild-type mice on a C57BL/6J background) were randomly assigned to the sedentary control group (C, n = 6 per group) or the training group (T, n = 6 per group). Treadmill exercise training on an Exer 3/6 treadmill (equipped with a stimulus assembly; Columbus Instruments) started at age 10–12 weeks. We tested critical speed in both mouse strains at the age of 10–12 weeks in a pilot experiment. Critical speed during involuntary treadmill exercise performance in C57BL/6J mice, as used here in the present study, has been shown to be low compared with other commonly used mouse strains (28,29). Although voluntary exercise avoids some of the stressful factors related to forced training, we used treadmill exercise, since ob/ob mice are very resistant to spontaneous running. Running speed was lowest in the ob/ob mice and was therefore adjusted in both mouse strains according to the critical speed determined for the ob/ob mice. The highest speed tolerated by the ob/ob mice was 15 m/min. This maximum speed was therefore used in the wild-type mice as well. Pilot experiments also showed that exhaustion in both mouse strains occurred after ∼60–70 min at this speed. Two weeks before the experimental period, the treadmill speed was gradually increased from 5 to 15 m/min and 10% incline. During the following 12 weeks, a 10-min warming up at 10 m/min was followed by 60 min exercise at a speed of 15 m/min and a 10-min cool down at a speed of 10 m/min (5 days weekly). Concurrently, matched untrained mice maintained familiarity with the treadmill (5 m/min, 0 degrees incline, 15-min duration, three times/week).
For measurement of fasting serum parameters 24 h after the last exercise, food was withdrawn for 12 h before death by cervical dislocation. Muscles were removed 24 h after the last exercise session, dissected, and stored in liquid nitrogen if not otherwise stated. Blood was drawn by aortic puncture at that time.
To investigate whether the changes observed after chronic exercise can be mimicked by a single bout of exercise, we performed additional experiments in a subset of C57BL/6J and ob/ob mice. Animals were acclimatized to the treadmill before the running test. For the habituation, mice were run at 10 m/min for 15 min on the day before the test. For the actual test, the speed was gradually increased from 5 to 15 m/min and a 10% incline. Mice were run until exhaustion, which was assumed when mice received more than five shocks in a 2-min interval. Average running distance was 1,050 ± 69 m in C57BL/6J mice and 989 ± 93m in ob/ob mice. Mice were either killed 6 or 24 h after the exercise test (n = 6 per group). Nonexercising C57BL/6J and ob/ob mice served as the respective control groups (n = 6 per group). The protocols were approved by the Animal Care and Use Committee of the Martin Luther University Halle-Wittenberg.
Tissue sampling.
Mouse gastrocnemius muscle is a heterogeneous tissue (30). To avoid this problem, we carefully dissected the gastrocnemius muscle before analyses by respirometric measurements or before freezing in small aliquots in liquid nitrogen (for gene expression analyses as well as enzymatic measurements) and used only the deep red portion. Although fiber composition of the deep red portion of gastrocnemius muscle was not evaluated in the present study, it can be distinguished macroscopically from the white portion. There are data on gastrocnemius fiber composition from rats available in the literature (31), showing a clear difference between the deep-red and the superficial-white portion of the muscle.
RNA and DNA extraction.
Total RNA was isolated from frozen tissue (deep-red portion of the gastrocnemius muscle) by guanidine thiocyanate/cesium chloride centrifugation. Integrity and quality of the RNA was confirmed by agarose gel electrophoresis, and the concentration was determined by measuring ultraviolet absorption. DNA isolation was performed with the Puregene DNA isolation kit (Biozym).
Real-time PCR.
Reverse transcription of RNA samples (500 ng total RNA) was carried out for 30 min at 42°C using the SuperScript III First-Strand cDNA Synthesis Kit (Invitrogen). Real-time PCR (primer sequences, Table 1) and data analysis were performed using the Mx3000P Multiplex Quantitative PCR System (Stratagene) as described previously (32). 18S rRNA expression was not affected by any of the interventions and was therefore used as a housekeeping gene. All data of mRNA are given as relative units of 18S rRNA concentrations (18S rRNA control kit, Yakima Yellow-Eclipse Dark Quencher; Eurogentec). The relative copy number of mtDNA per diploid nuclear genome was measured as described previously (33) using a fragment of mtDNA and a fragment of β-globin.
TABLE 1.
Primer sequences
| GenBank accession number | Forward primer | Reverse primer | |
|---|---|---|---|
| COX III | AY999076 | CGTGAAGGAACCTACCAAGG | ATTCCTGTTGGAGGTCAGCA |
| Cytochrome b | AY999076 | CCCTAGCAATCGTTCACCTC | TCTGGGTCTCCTAGTATGTCTGG |
| β-Globin | NM_008220 | TGGGTAATCCCAAGGTGAAG | TTCTCAGGATCCACATGCAG |
| Mitofusin-2 | NM_133201 | GGGGCCTACATCCAAGAGA | AAAAAGCCACCTTCATGTGC |
| ND5 | AY999076 | ACCAGCATTCCAGTCCTCAC | ATGGGTGTAATGCGGTGAAT |
| NRF-1 | BC005410 | GCACCTTTGGAGAATGTGGT | GGGTCATTTTGTCCACAGAGA |
| NRF-2 | U20532 | CCAGCTACTCCCAGGTTGC | CCTGATGAGGGGCAGTGA |
| PGC-1α | NM_00894 | AAACTTGCTAGCGGTCCTCA | TGGCTGGTGCCAGTAAGAG |
| PEPCK | NM_011044 | AGTGCCCATCCCCAAAACT | CACCACATAGGGCGAGTCTG |
| SIRT1 | NM_019812 | AAAGGAATTGGTTCATTTATCAGAG | TTGTGGTTTTTCTTCCACACA |
| Tfam | NM_009360 | CCTTCGATTTTCCACAGAACA | GCTCACAGCTTCTTTGTATGCTT |
| CDS | NM_173370 | TGTTCCCATATCAAGCGTCA | GGCTCACACTCTGTCACGAA |
| PGPS | NM_133757 | CGACCTCAAGGTCTCCATTC | GTTTGCACCACTCAGGATGA |
| CLS | NM_025646 | ATCAGCTTTGGGAAGTGCTC | ACCTTGCTGATGAATGTTGGT |
| MLCL AT | NM_001081071 | CCCAAGAACCACTGGCTTTA | TTTCTTCCCACCTTCTGTGG |
| Tafazzin | NM_181516 | CTGGGGGATCCTAAAACTCC | AGCGCAGGAACTCAGAACTC |
Western blotting and immunoprecipitation.
Protein samples from frozen tissue (deep-red portion of the gastrocnemius muscle) were prepared as described previously (32). A total of 50 μg protein was separated by SDS-PAGE gel and immunoblotted with antibodies directed against SIRT1 (1:2,000; Upstate), PGC-1α (1:1,000; Santa Cruz), phospho-AMPK (Thr172, 1:1,000) and phospho-acetyl CoA carboxylase (Ser79, 1:1,000; both Cell Signaling Technology), and GAPDH (1:3,000; Abcam). After incubation with peroxidase-conjugated secondary antibody (1:10,000), blots were subjected to the enhanced chemiluminescent detection method (Amersham) and exposed to a film. PGC-1α protein expression was calculated from both bands observed on Western blots.
To obtain protein extracts from tissue samples for immunoprecipitation, 50 mg frozen tissue was rapidly homogenized in 250 μl cold lysis buffer (25 mmol/l Tris HCl, pH 7.9, 5 mmol/l MgCl2, 10% glycerol, 100 mmol/l KCl, 1% NP40, 0.3 mmol/l dithiothreitol, protease/phosphatase inhibitor cocktail, 10 mmol/l nicotinamide, and 1 μmol/l Trichostatin A (TSA) with a Precellys 24 Homogenizer (Peqlab), sonicated, and separated by centrifugation. A total of 1,000 μg protein from muscle samples were used for immunoprecipitation. Extracts were diluted with two volumes PBS and incubated with anti–PGC-1α antibody (1 μg antibody/250 μg total protein; Santa Cruz) rotating at 4°C overnight. Immunoprecipitates were then further incubated with protein A/G agarose (Santa Cruz) at 4°C. The beads were collected by centrifugation and washed with PBS. After the final wash, the samples were dissolved in 2 × SDS sample buffer and boiled. The immunoprecipitates were then separated by SDS-PAGE and immunoblotted using an acetyl-lysine antibody (1:1,000; Cell Signaling Technology) and anti–PGC-1α antibody, respectively.
Preparation of skinned fibers and respirometric measurements.
Immediately before oxygraphic measurements, the muscular fibers (deep-red portion of the gastrocnemius muscle) were permeabilized 30 min with saponin. After removal of saponin and adenine nucleotides, the measurements were performed on an OROBOROS oxygraph at 30°C in incubation medium (75 mmol/l mannitol, 25 mmol/l sucrose, 100 mmol/l KCl, 10 mmol/l KH2PO4, 0.5 mmol/l EDTA, 5 mmol/l MgCl2, 20 mmol/l Tris-HCl, and 1 mg/ml BSA) using different substrates: 10 mmol/l pyruvate + 2 mmol/l malate and 10 mmol/l succinate + 5 μmol/l rotenone. The weight-specific oxygen consumption was calculated as the time derivative of the oxygen concentration (DATGRAPH Analysis software, OROBOROS).
Determination of enzyme activities.
Small pieces of frozen tissue (deep-red portion of the gastrocnemius muscle) were homogenized in a solution containing 50 mmol/l Tris buffer (pH 7.5), 100 mmol/l potassium chloride, 5 mmol/l MgCl2, and 1 mmol/l EDTA using a glass/glass homogenizer. Enzyme activities were referenced to the activity of the mitochondrial marker enzyme citrate synthase. The activity of complex I, complex I + III, complex II + III, complex III, complex II, and complex IV were performed as described previously (34) using a Cary 50 spectrophotometer (Varian GmbH, Darmstadt, Germany).
Serum analyses.
Serum adiponectin, leptin, tumor necrosis factor (TNF)-α, and insulin were measured by using commercial enzyme-linked immunosorbent assays (Mouse/Rat Adiponectin ELISA kit, B-Bridge International; Mouse/Rat Leptin ELISA kit, BioVendor; Mouse TNF-α ELISA kit, Raybiotech; Mouse Insulin ELISA, Mercodia). Serum values for glucose were determined with a Glucose Assay Kit (BioCat). Triglycerides were measured using a commercial kit (GPO Trinder; Sigma). Blood lactate concentration was determined three times in all mice: at the beginning of the chronic exercise, after 6 weeks of exercise, and at the end of the last session. Blood was collected from the tip of the tail for this purpose immediately after the end of the session, and lactate was measured with an enzymatic Lactate Assay Kit (BioCat).
Cell culture.
C2C12 myoblasts were cultured in Dulbecco's modified Eagle medium supplemented with 10% FBS, 2 mmol/l l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all PAA) in 5% CO2 at 37°C in a humidified chamber. Cells were treated with 25, 50, 100, or 200 ng/ml of mouse recombinant leptin (BioVendor) or 500 ng/ml of mouse recombinant adiponectin (BioVendor) for 24 h.
Plasmid construction and transfection.
The cDNA of PGC-1α (GenBank accession number NM_013261) was amplified by PfuUltra II Fusion HS DNA Polymerase (Stratagene). PCR products were subcloned into pcDNA3.1 (Invitrogen). Site-directed mutations at Thr177 and Ser538 in PGC-1α were introduced by a PCR-based strategy with the QuikChange site-directed mutagenesis kit (Stratagene). Plasmid DNA preparation was performed with the Midi Plasmid Kit (Qiagen). One day before transfection, C2C12 myoblasts were seeded at a density of 5 × 105 cells per well on a six-well dish. DNA transfection was carried out by liposome-mediated transfection using 2 μg plasmid (pcDNA3.1, pcDNA3.1 PGC-1α, pcDNA3.1 PGC1α ΔThr177/ΔSer538) and Lipofectamine 2000 (Invitrogen) as the transfection reagent according to the manufacturer's instructions. The next day, cell culture medium was changed, and cells were left untreated or stimulated with adiponectin (BioVendor) for 24 h. C2C12 myoblasts transfected with the empty plasmid (pcDNA3.1) were treated accordingly and served as an internal control for the effects of adiponectin on AMPK activation and PGC-1α deacetylation. Cells were harvested 48 h after transfection.
Statistical analysis.
All values are expressed as mean ± SEM. Statistical analysis of differences observed between numeric parameters of all groups was performed by one-way ANOVA using an all pair-wise multiple comparison procedure (Tukey test). Statistical significance was accepted at the level of P < 0.05.
RESULTS
Systemic effects of chronic exercise.
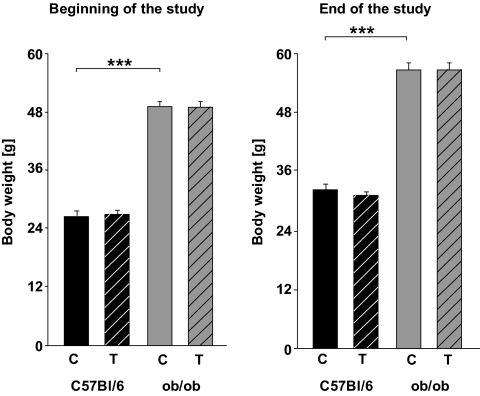
Throughout the study, the body weight was higher in ob/ob mice than in C57BL/6J wild-type mice (Fig. 1). The chronic exercise did not result in less weight gain in any of the trained groups (Fig. 1). Furthermore, chronic exercise did not result in significant changes in epididymal fat pads (C57BL/6: 320 mg ± 26; ob/ob: 1,120 mg ± 189, P < 0.001).
FIG. 1.
Body weight before and after 3 months of chronic exercise. Black columns: C57BL/6J wild-type mice (12 controls [C], 12 trained animals [T]); gray columns: ob/ob mice (6 controls, 6 trained animals). Results obtained from the trained mice are depicted with hatched columns. All data are mean ± SEM. ***P < 0.001.
After the first exercise session, blood lactate in both strains showed an increase compared with the respective control mice (C57BL/6J: 3.69 ± 0.48 vs. 5.15 ± 0.43, P < 0.05; ob/ob: 3.86 ± 0.64 vs. 6.16 ± 0.52, P < 0.01). After 6 weeks of chronic exercise, blood lactate concentration in the trained C57BL/6J mice was no longer different from the respective control mice (not shown). Blood lactate in trained ob/ob mice had decreased but remained higher than in the untrained ob/ob mice (3.84 ± 0.35 vs. 5.06 ± 0.73, P < 0.05). After 12 weeks of chronic exercise, blood lactate levels were comparable between the groups (Table 2) and did not reveal a statistically significant difference compared with the respective untrained mice.
TABLE 2.
Serum/blood parameters at the end of the study
| C57BL/6J |
ob/ob |
|||
|---|---|---|---|---|
| Control | Training | Control | Training | |
| Glucose (mmol/l) | 6.72 ± 0.54 | 4.52 ± 0.62* | 15.26 ± 0.98‡ | 12.23 ± 0.95* |
| Insulin (ng/ml) | 1.55 ± 0.39 | 0.51 ± 0.15* | 10.24 ± 0.65‡ | 10.26 ± 0.42 |
| Leptin (pg/ml) | 1,427.6 ± 242.1 | 328.8 ± 52.5* | 43.6 ± 9.6§ | 36.3 ± 8.9 |
| Adiponectin (μg/ml) | 17.8 ± 0.8 | 34.4 ± 1.7* | 8.1 ± 0.5† | 8.7 ± 1.3 |
| Triglycerides (mg/dl) | 79.7 ± 14.4 | 61.2 ± 9.5 | 177.5 ± 18.7† | 156.9 ± 24.4 |
| Lactate (mmol) | 3.79 ± 0.41 | 4.52 ± 0.30 | 3.81 ± 0.78 | 4.92 ± 0.22 |
| TNF-α (pg/ml) | 23.6 ± 1.4 | 24.9 ± 0.9 | 86.2 ± 2.5‡ | 79.2 ± 2.1 |
*P < 0.05 vs. respective control;
†P < 0.05,
‡P < 0.01,
§P < 0.001 vs. C57BL/6J controls.
Glucose, insulin, and leptin serum levels demonstrated a significant reduction together with a strong induction of serum adiponectin in wild-type mice in response to chronic exercise (Table 2), indicating a change in insulin sensitivity in these animals. None of the serum parameters except for minor changes in serum glucose was altered in trained compared with untrained ob/ob mice (Table 2), pointing to the major impact of the deficient leptin on whole-body metabolism. Furthermore, ob/ob mice had higher serum TNF levels than wild-type mice, and these were not altered after chronic exercise (Table 2).
Influence of chronic exercise on skeletal muscle mitochondrial biogenesis.
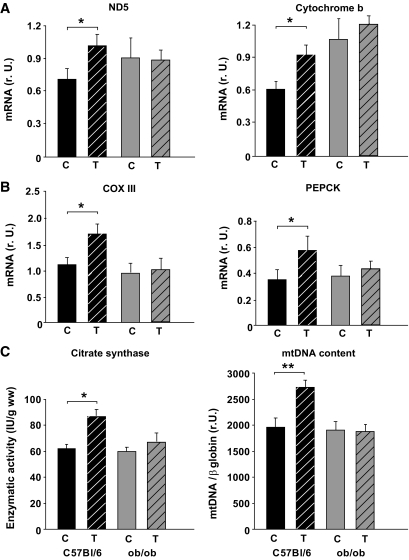
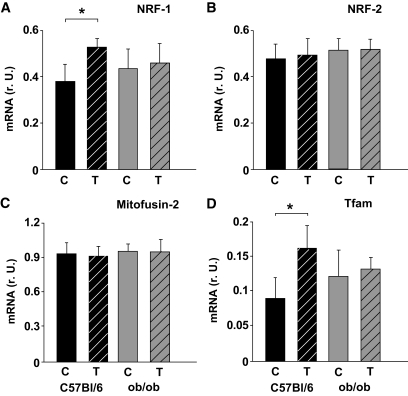
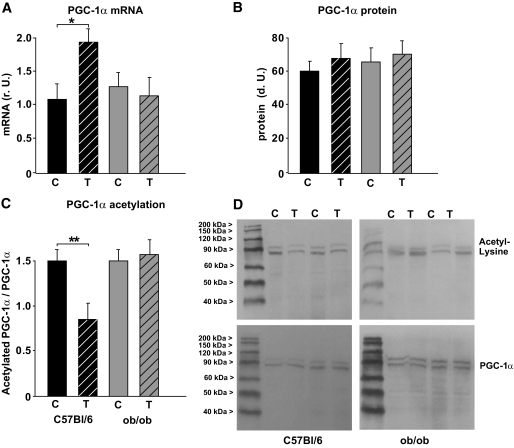
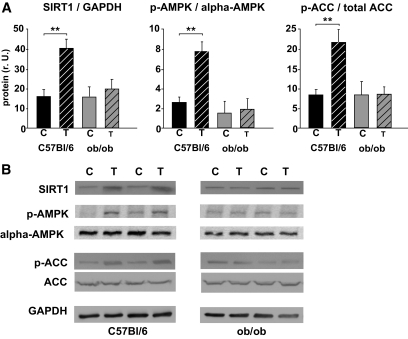
The mRNA expression of ND5 (complex I), cytochrome b (CYTB, complex III), COX III (complex IV), and the PGC-1α–dependent gluconeogenic gene PEPCK (phosphoenolpyruvate carboxykinase) was higher in wild-type mice but not in ob/ob mice after 3 months of chronic exercise (Fig. 2A and B). Furthermore, exercise increased citrate synthase activity in wild-type mice (Fig. 2C), suggesting a higher mitochondrial density. This was not observed in ob/ob mice in response to chronic exercise (Fig. 2C). Accordingly, no significant change in mtDNA content was observed in the ob/ob mice (Fig. 2C), whereas an ∼40% increase occurred in the wild-type mice (Fig. 2C). Similarly, we observed a mild induction of NRF-1 (Fig. 3A) and Tfam mRNA (Fig. 3D) in wild-type mice but not in ob/ob mice. NRF-2 mRNA (Fig. 3B) was not altered in any of the two groups. Mitofusin-2 (MFN2) mRNA, which may be involved in PGC-1α–independent mechanisms during mitochondrial turnover and assembly (mitochondrial fusion and fission), was not different among the groups or in response to chronic exercise (Fig. 3C). The expression of PGC-1α mRNA was doubled only in wild-type mice, whereas PGC-1α protein was not significantly altered in any of the groups (Fig. 4A and B). Acetylation status of PGC-1α was analyzed, since it has a major impact on PGC-1α activity/stability and was shown to be altered in response to low nutrient conditions (7,16). As shown in Fig. 4C and D, PGC-1α acetylation was significantly reduced in trained wild-type mice, whereas ob/ob mice showed no difference between the two treatment groups (Fig. 4C and D). Accordingly, the deacetylase SIRT1, which specifically deacetylates PGC-1α in vivo and in vitro, was induced in these mice but not in ob/ob mice (Fig. 5) after chronic exercise.
FIG. 2.
Markers of mitochondrial biogenesis after chronic exercise. A: Real-time PCR analyses of primary mitochondrial transcripts ND5 (complex I) and CYTB (complex III). B: Real-time PCR analyses of primary mitochondrial transcript cytochrome oxidase III (COX III; complex IV) and the gluconeogenic phosphoenolpyruvate carboxykinase (PEPCK) in skeletal muscle samples. All data are normalized per 18S rRNA. C: Citrate synthase enzyme activity (IU/g wet weight) and relative copy number of mtDNA per diploid nuclear genome in the deep-red portion of the gastrocnemius muscle. Numbers of animals are as described in Fig. 1. Data are mean ± SEM. *P < 0.05; **P < 0.01. C, controls; r.U., relative units; T, trained mice.
FIG. 3.
mRNA expression of transcriptional activators and coactivators of mitochondrial biogenesis after chronic exercise. Results are from real-time PCR analyses in skeletal muscle samples. A: Nuclear respiratory factor 1 (NRF-1). B: NRF-2. C: Mitofusin-2 (MFN2). D: Mitochondrial transcription factor A (Tfam). All data are normalized per 18S rRNA. Numbers of animals are as described in Fig. 1. Data are means ± SEM. *P < 0.05. C, controls; r.U., relative units; T, trained mice.
FIG. 4.
Changes in PGC-1α expression and acetylation after chronic exercise. A: Real-time PCR analyses of PGC-1α mRNA expression in the skeletal muscle of C57BL/6J wild-type mice (black columns) and ob/ob mice (gray columns). All data are normalized per 18S rRNA. B: Densitometry of protein data. Homogenates of skeletal muscle were probed with an antibody detecting PGC-1α (90 kDa). Blots were also probed with GAPDH as a loading control. C: Densitometry of immunoprecipitation (IP) experiments performed on skeletal muscle lysates, using PGC-1α for precipitation and an antibody directed against acetyl-lysine or PGC-1α for detection. Data are given as acetylated PGC-1α per total PGC-1α in these samples. All data are means ± SEM. *P < 0.05; **P < 0.01. C, controls; r.U., relative units; T, trained mice. D: Representative full-size blots of IP experiments.
FIG. 5.
SIRT1, phospho-AMPK, and phospho-ACC protein expression after chronic exercise. A: Densitometry of protein data. Homogenates of skeletal muscle probed with an antibody detecting SIRT1 (110 kDa), phospho-AMPK (62 kDa, Thr172), AMPK (62kDa), phospho-ACC (280 kDa, Ser79), ACC (280 kDa), and GAPDH (42 kDa) as a loading control. Data are means ± SEM. **P < 0.01. C, control animals; r.U., relative units; T, trained animals. B: Representative Western blots.
Besides PGC-1α deacetylation at various lysines by SIRT1, phosphorylation of PGC-1α by AMPK at residues Thr177 and Ser538 was suggested to result in a more active protein (15). Both mechanisms have been described to occur in response to exercise and contribute to training-induced changes in mitochondrial biogenesis (11,19). Indeed, chronic exercise resulted in AMPK activation and increased phosphorylation of acetyl-CoA carboxylase in wild-type mice (Fig. 5). Phosphorylation of AMPK or ACC was, however, not altered in ob/ob mice after exercise (Fig. 5). Even when muscles were harvested and analyzed shortly after the last training session (30 min, 2 h) to identify short-lived effects, no change in AMPK activation or PGC-1α expression/acetylation was observed in the ob/ob mice (not shown). The protein expression of α-AMPK (Fig. 5), α-1 or α-2 AMPK (not shown), was not altered by treadmill exercise in any of the groups. A single session of exhaustive exercise was sufficient to reduce PGC-1α acetylation, increase AMPK phosphorylation, and increase ND5 and CYTB mRNA expression in wild-type mice, if animals were killed 6 h after the end of exercise (supplementary Figure 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0331/DC1). When animals were killed 24 h after exercise, changes induced by this single exercise session were no longer detectable (supplementary Figure 2).
Influence of chronic exercise on skeletal muscle mitochondrial function.
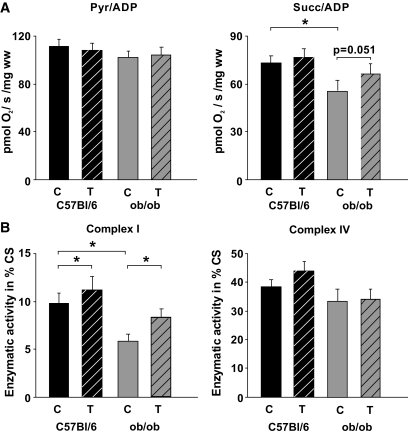
Pyruvate- and succinate-dependent respiration was lower in untrained ob/ob animals than in C57BL/6J mice, but 3 months of chronic exercise resulted in increased succinate-dependent respiration in these mice (Fig. 6A). A typical feature of an impaired respiratory chain function is the reduction in the activity of complex I and IV (35), both containing proteins transcribed from the mitochondrial DNA. Here we show that the skeletal muscle of obese animals demonstrated a significant reduction in complex I activity, and 3 months of exercise training resulted in a higher activity in C57BL/6J and ob/ob mice (Fig. 6B). The complex I activity in trained ob/ob mice, however, was only mildly increased and significantly lower than in untrained wild-type mice (P < 0.05). Complex IV (Fig. 6B) and the other complexes (not shown) of the respiratory chain demonstrated only minor changes related to obesity or physical activity. Because PGC-1α was shown to be of particular importance in slow-twitch fibers (8), microrespirometry and enzymatic measurements were performed on soleus muscle as well, which is also predominantly composed of slow type I fibers and yielded comparable results (not shown).
FIG. 6.
Mitochondrial function after chronic exercise. A: Active rates of respiration (state 3) were measured in saponin-skinned fibers in the presence of 5 mmol/l ADP and either 10 mmol/l pyruvate (Pyr) + 2 mmol/l malate or 10 mmol/l succinate (Succ) + 5 μmol/l rotenone. Rates of respiration are given as pmol O2/second/mg wet weight. B: Effects on enzymatic activity of the respiratory chain complexes. Citrate synthase (CS) normalized complex I activity (rotenone-sensitive NADH:CoQ1 oxidoreductase) is higher in the skeletal muscle of all trained mice (hatched columns) compared with the respective controls. CS normalized activity of complex IV is similar among the groups (numbers of animals as described in Fig. 1). Data are means ± SEM. *P < 0.05. C, controls; T, trained mice.
The mRNA expression of enzymes involved in primary cardiolipin synthesis (CDS, PGPS, CLS) was not different between the groups (supplementary Figure 3). To attain its high levels of linoleic acid, newly synthesized cardiolipin must initially undergo remodeling by monolysocardiolipin acyltransferase and tafazzin. Whereas the former enzyme was not altered, we observed a mild increase in tafazzin mRNA in the skeletal muscle of ob/ob mice after 3 months of exercise training (supplementary Figure 3).
Effects of leptin and adiponectin on PGC-1α acetylation in vitro.
Leptin treatment in C2C12 myoblasts resulted in a dose-dependent increase in AMPK phosphorylation and PGC-1α deacetylation as well as a concomitant induction of adiponectin protein expression (supplementary Figure 4A). Similarly, adiponectin treatment of C2C12 cells also resulted in AMPK phosphorylation and PGC-1α deacetylation (supplementary Figure 4B). The observed effects of adiponectin were abolished in C2C12 myoblasts overexpressing a mutant PGC-1α lacking the two AMPK phosphorylation sites compared with cells overexpressing wild-type PGC-1α or cells expressing the empty plasmid.
DISCUSSION
The present study demonstrates that enhanced mitochondrial biogenesis (Fig. 2) induced by chronic exercise is paralleled by PGC-1α deacetylation (Fig. 4), SIRT1 protein induction, and increased phosphorylation of AMPK and ACC (Fig. 5) in wild-type mice. Although none of these exercise-induced changes were detected in ob/ob mice, comparable effects on mitochondrial respiration (Fig. 6) were observed.
All mice were trained at a speed of 15 m/min, which is a moderate speed compared with some other studies described in the literature. Critical speed was tested in ob/ob mice and used accordingly in wild-type mice. Exhaustion in both mouse strains occurred after ∼60–70 min at this speed. As deducted from the time course of blood lactate levels in the two mouse strains, the process of adaptation to chronic exercise differs between the C57BL/6J and the ob/ob mice. Future studies are needed to elucidate the mechanisms underlying these differences. Measurements of maximal oxygen uptake or heart rate under exercise conditions may yield more conclusive data (36) but require additional equipment, which was not available for the present study.
Three months of exercise resulted in enhanced mitochondrial biogenesis and respiration in wild-type mice, together with PGC-1α deacetylation (Fig. 4), AMPK phosphorylation, and SIRT1 protein induction (Fig. 5), which suggests that these may play a role for the mitochondrial biogenic response. No change in any mitochondrial biogenic parameter including citrate synthase occurred in the ob/ob mice with training, despite the fact that they ran at a rate close to their maximum. A single session of exercise had comparable effects on PGC-1α, AMPK, SIRT1, and the mRNA expression of primary mitochondrial transcripts (supplementary Fig. 1). This suggests the involvement of fast-acting mechanisms. Long-term maintenance, however, appears to require chronic exercise or repetitive stimuli (supplementary Fig. 2). Although PGC-1α mRNA was increased in wild-type mice, we observed only minor changes in PGC-1α protein (Fig. 4). Other studies have shown exercise-induced increases in PGC-1α mRNA (37) and protein (38) in skeletal muscle. However, while our mice performed moderate chronic exercise for 3 months, these studies (37,38) used rats that were trained for 5–7 days. Furthermore, a similar study performed in mice (8 weeks voluntary wheel running) demonstrated that no change in PGC-1α protein was evident in plantaris and soleus muscle, although changes in mitochondrial gene expression were observed (39).
To our knowledge, this is the first study showing that chronic exercise has similar effects on SIRT1 protein expression and PGC-1α acetylation as previously shown by fasting (7,16) or a single exercise session (19). SIRT1-mediated lysine deacetylation can be mimicked by resveratrol resulting in increased aerobic capacity and expression of oxphos genes, together with decreased PGC-1α acetylation (17). Gerhart-Hines et al. (16) showed that SIRT1 deacetylation of PGC-1α is required for activation of mitochondrial fatty acid oxidation genes as well as oxphos genes. The present study suggests that SIRT1 is involved in exercise-induced changes in mitochondrial gene expression, biogenesis, and respiration in wild-type mice (Fig. 5). The ratio of the mitochondrial redox pair NADH/NAD is ∼80–100 under muscle resting conditions, but during intense exercise, falls to 2–3 (35,40). Because SIRT1 is activated by NAD (41), the repeated decrease in the NADH/NAD ratio during chronic exercise may stimulate SIRT1, as observed here in mouse muscular SIRT1 protein expression (Fig. 5) or in the heart of old rats (18). However, the mechanisms leading to the lack of a SIRT1 response in ob/ob mice require further investigation.
Despite a similar degree of chronic exercise, the ob/ob mice did not respond to the stimulatory effects of exercise training (12 weeks) in any of the parameters of mitochondrial biogenesis (Figs. 2 and 3) or indicators of insulin sensitivity (Table 2). This suggests that the disturbed leptin signaling contributes to the lack of mitochondrial biogenesis via AMPK/SIRT1/PGC-1α. Similarly, it was shown that leptin is necessary for basal and cold-stimulated PGC-1α expression in brown adipose tissue in ob/ob mice (42). Chronic exercise resulted in a significant increase in AMPK phosphorylation in wild-type mice (Fig. 5). Increased phosphorylation of AMPK was shown to occur after a single session of treadmill running (19,43) and after chronic exercise in mouse skeletal muscle (44). However, others have shown no differences between trained or nontrained gastrocnemius muscles in rats (45). Furthermore, the stimulatory effects of an acute session of exercise on α-2 AMPK activity were lower in trained compared with untrained rats (46). The reason for these discrepancies in AMPK phosphorylation is not clear at this time but species differences may exist. Ob/ob mice have significantly higher serum TNF-α levels than wild-type mice (Table 2). This may have contributed to the reduction in AMPK signaling as shown by others (27). Plasma adiponectin levels in ob/ob mice are low (Table 2), and leptin treatment increases these plasma levels markedly (26). The lack of AMPK activation in ob/ob mice (Fig. 5) correlated with plasma adiponectin (Table 2) in these mice (R2 = 0.67, P < 0.05). Accordingly, leptin deficiency is associated with impaired mitochondria (25) and reduced muscle AMPK phosphorylation (27).
Leptin (22) and adiponectin (23) stimulate AMPK and may thus influence mitochondrial biogenesis via increased phosphorylation of PGC-1α (12). Although chronic leptin treatment in rodents was shown to increase AMPK expression and activation (47), others did not detect significant changes in the expression of mitochondrial genes in response to leptin treatment compared with pair-fed mice (48). Furthermore, whereas leptin-mediated activation of AMPK was demonstrated in H9C2 cardiomyoblasts (49), others have reported that leptin attenuates AMPK activation induced by globular or full-length adiponectin in L6 myotubes (50). Thus, whereas adiponectin has consistently been shown to activate AMPK, the effects of leptin are more obscure and may depend on additional not-yet-identified coactivators. Our in vitro experiments in C2C12 myoblasts demonstrated an increased AMPK phosphorylation and reduced PGC-1α acetylation after leptin as well as adiponectin treatment (supplementary Fig. 4A). Leptin treatment also resulted in the induction of adiponectin protein expression. Thus, the effects of leptin on AMPK and PGC-1α might be mediated via adiponectin. The observed direct effects of adiponectin were abolished in C2C12 myoblasts overexpressing mutant PGC-1α lacking the two AMPK phosphorylation sites (supplementary Fig. 4B). This suggests that direct phosphorylation of PGC-1α by AMPK is required for adipocytokine-induced PGC-1α deacetylation. The mechanisms underlying the concomitant effect of adiponectin on PGC-1α deacetylation and AMPK activation as well as the increased expression of adiponectin in C2C12 myoblasts after leptin treatment will require further investigations. The interaction between PGC-1α deacetylation by SIRT1 and the AICAR-induced PGC-1α phosphorylation have been investigated recently by others (19). An adiponectin-mediated mechanism can also be envisioned in wild-type mice, which demonstrate a strong increase in adiponectin plasma levels after chronic exercise (Table 2). Leptin and adiponectin plasma levels in ob/ob mice on the other hand remain low in sedentary as well as in trained obese mice (Table 2). Accordingly, it was demonstrated recently that mice with muscle-specific disruption of AdipoR1 show reduced adiponectin-mediated activation of AMPK and SIRT1 together with reduced PGC-1α expression and mitochondrial content (51).
The experimental design of our study does not allow a conclusion concerning a superior role of AMPK or SIRT1, since we found similar changes in both, putatively promoting mitochondrial biogenesis. However, others have recently shown that AMPK enhances SIRT1 activity by increasing cellular NAD+ levels, resulting in the deacetylation of various SIRT1 targets (19). The ob/ob mice have a major deficit in both pathways, which may have contributed to the complete lack of a mitochondrial biogenic response. Data from DNA microarrays have shown that PGC-1α target genes are coordinately downregulated in diabetic humans (2,52). Although basal expression of PGC-1α target genes was not impaired, the severely obese ob/ob mice did not respond to the stimulatory effects of chronic exercise with increased mRNA expression of PGC-1α, Tfam (Figs. 3 and 4), or PGC-1α target genes (Fig. 2).
The obese ob/ob mice showed a mild increase in succinate-dependent respiration and complex I activity (Fig. 6), although no signs of mitochondrial biogenesis or changes of the AMPK/SIRT1/PGC-1α signaling pathway were detected. However, the complex I activity was lower in trained ob/ob mice than in untrained wild-type mice (Fig. 6). Complex I catalytic activity critically depends on the surrounding phospholipid environment, in particular on cardiolipin (53), which is the main functional phospholipid of the mitochondrial inner membrane. Cardiolipin content falls in response to decreased muscle performance, and this fall precedes the loss of enzyme activity during decreases in mitochondrial content (54). A recent study (33) showed that exercise combined with weight loss resulted in increased oxidative enzyme activity in skeletal muscle without a significant increase in mitochondrial biogenesis, as concluded from mtDNA content. The authors suggest that the increase in mitochondrial cardiolipin facilitates the formation of mitochondrial supercomplexes with enhanced efficiency of electron transport (33). A similar mechanism can be envisioned in the severely obese ob/ob mice, which showed a moderate increase in complex I activity (Fig. 6) but no change in the expression of primary mitochondrial transcripts (Fig. 2) or transcription factors involved in mitochondrial biogenesis (Fig. 3). Changes in cardiolipin content or composition may underlie the reduced complex I activity in the ob/ob mice. It has been shown in the heart of ob/ob mice that cardiolipin composition is altered and contributes to the mitochondrial dysfunction in diabetic cardiomyopathy (55). However, mitochondrial cardiolipin content or composition was not measured in the present study, since it required the use of freshly isolated mitochondria. After primary cardiolipin synthesis, the tafazzin-mediated cardiolipin remodeling results in a specific set of acyl chains. The mildly increased mRNA expression of tafazzin in trained ob/ob mice (supplementary Fig. 3) suggests an increased cardiolipin remodeling. Future studies are needed to elucidate whether the increased tafazzin expression results in altered cardiolipin composition and increased complex I activity in the ob/ob mice after chronic exercise.
In summary, chronic exercise induces mitochondrial biogenesis together with changes in indicators of insulin sensitivity and enhanced mitochondrial respiration in wild-type mice, which requires AMPK activation and may involve SIRT1-dependent PGC-1α deacetylation. Ob/ob mice show no signs of increased mitochondrial biogenesis after treadmill exercise but similar changes in mitochondrial function as observed in wild-type mice. Thus, ob/ob mice appear to have partially adapted to reduced mitochondrial biogenesis by AMPK/SIRT1/PGC-1α–independent mechanisms without mtDNA replication, putatively involving changes in cardiolipin content or composition.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft (RO 2328/2-1), Deutsche Stiftung für Herzforschung (F/05/05), Doktor Robert Pfleger-Stiftung, and the Wilhelm-Roux program of the Medical Faculty Halle (FKZ: 14/42, FKZ: 14/47, FKZ: 14/07), which is funded by the German “Bundesministerium für Bildung und Forschung.”
No potential conflicts of interest relevant to this article were reported.
L.L., R.P., R.L, A.-C.A., and Y.C. researched data. B.N. researched data and contributed to discussion. S.R. researched data and wrote/reviewed/edited the manuscript.
The authors appreciate the technical assistance of R. Gall, B. Heinze, and R. Busath (Institute of Pathophysiology, Martin Luther University Halle-Wittenberg).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kelley DE, He J, Menshikova EV, Ritov VB: Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 2.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003;100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravussin E, Klimes I, Sebokova E, Howard BV: Lipids and insulin resistance: what we've learned at the Fourth International Smolenice Symposium. Ann N Y Acad Sci 2002;967:576–580 [DOI] [PubMed] [Google Scholar]
- 4.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM: Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 2005;280:33588–33598 [DOI] [PubMed] [Google Scholar]
- 5.Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA: Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med 2003;33:783–793 [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98:115–124 [DOI] [PubMed] [Google Scholar]
- 7.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P: Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005;434:113–118 [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM: Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002;418:797–801 [DOI] [PubMed] [Google Scholar]
- 9.Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO: Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 2007;282:18793–18799 [DOI] [PubMed] [Google Scholar]
- 10.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM: Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 2001;8:971–982 [DOI] [PubMed] [Google Scholar]
- 11.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO: Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 2000;88:2219–2226 [DOI] [PubMed] [Google Scholar]
- 12.Jager S, Handschin C, St-Pierre J, Spiegelman BM: AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 2007;104:12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojuka EO: Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc 2004;63:275–278 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Monks B, Ge Q, Birnbaum MJ: Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 2007;447:1012–1016 [DOI] [PubMed] [Google Scholar]
- 15.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P: Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett 2008;582:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P: Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 2007;26:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J: Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–1122 [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A: Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res 2008;11:139–150 [DOI] [PubMed] [Google Scholar]
- 19.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J: AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW: Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol 2002;92:2475–2482 [DOI] [PubMed] [Google Scholar]
- 21.Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, Schoonjans K, Puigserver P, O'Malley BW, Auwerx J: The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc Natl Acad Sci U S A 2008;105:17187–17192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB: Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002;415:339–343 [DOI] [PubMed] [Google Scholar]
- 23.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB: Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A 2002;99:16309–16313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, Ravussin E, Smith SR: Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 2006;4:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED: Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 2005;112:2686–2695 [DOI] [PubMed] [Google Scholar]
- 26.Delporte ML, El Mkadem SA, Quisquater M, Brichard SM: Leptin treatment markedly increased plasma adiponectin but barely decreased plasma resistin of ob/ob mice. Am J Physiol Endocrinol Metab 2004;287:E446–E453 [DOI] [PubMed] [Google Scholar]
- 27.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE: Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 2006;4:465–474 [DOI] [PubMed] [Google Scholar]
- 28.Billat VL, Mouisel E, Roblot N, Melki J: Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol 2005;98:1258–1263 [DOI] [PubMed] [Google Scholar]
- 29.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA: Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol 2002;92:2245–2255 [DOI] [PubMed] [Google Scholar]
- 30.Burkholder TJ, Fingado B, Baron S, Lieber RL: Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 1994;221:177–190 [DOI] [PubMed] [Google Scholar]
- 31.Staron RS, Kraemer WJ, Hikida RS, Fry AC, Murray JD, Campos GE: Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol 1999;111:117–123 [DOI] [PubMed] [Google Scholar]
- 32.Rohrbach S, Gruenler S, Teschner M, Holtz J: The thioredoxin system in aging muscle: key role of mitochondrial thioredoxin reductase in the protective effects of caloric restriction? Am J Physiol Regul Integr Comp Physiol 2006;291:R927–R935 [DOI] [PubMed] [Google Scholar]
- 33.Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE: Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol 2007;103:21–27 [DOI] [PubMed] [Google Scholar]
- 34.Gellerich FN, Deschauer M, Chen Y, Muller T, Neudecker S, Zierz S: Mitochondrial respiratory rates and activities of respiratory chain complexes correlate linearly with heteroplasmy of deleted mtDNA without threshold and independently of deletion size. Biochim Biophys Acta 2002;1556:41–52 [DOI] [PubMed] [Google Scholar]
- 35.Boveris A, Navarro A: Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med 2008;44:224–229 [DOI] [PubMed] [Google Scholar]
- 36.Hoydal MA, Wisloff U, Kemi OJ, Ellingsen O: Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 2007;14:753–760 [DOI] [PubMed] [Google Scholar]
- 37.Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T: cDNA cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 2000;274:350–354 [DOI] [PubMed] [Google Scholar]
- 38.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO: Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 2002;16:1879–1886 [DOI] [PubMed] [Google Scholar]
- 39.Ikeda S, Kawamoto H, Kasaoka K, Hitomi Y, Kizaki T, Sankai Y, Ohno H, Haga S, Takemasa T: Muscle type-specific response of PGC-1 alpha and oxidative enzymes during voluntary wheel running in mouse skeletal muscle. Acta Physiol (Oxf) 2006;188:217–223 [DOI] [PubMed] [Google Scholar]
- 40.Korzeniewski B: Regulation of oxidative phosphorylation in different muscles and various experimental conditions. Biochem J 2003;375:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V: Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell 2003;12:51–62 [DOI] [PubMed] [Google Scholar]
- 42.Kakuma T, Wang ZW, Pan W, Unger RH, Zhou YT: Role of leptin in peroxisome proliferator-activated receptor gamma coactivator-1 expression. Endocrinology 2000;141:4576–4582 [DOI] [PubMed] [Google Scholar]
- 43.Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H: Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J 2005;19:1146–1148 [DOI] [PubMed] [Google Scholar]
- 44.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM: AMPK and PPARdelta agonists are exercise mimetics. Cell 2008;134:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurst D, Taylor EB, Cline TD, Greenwood LJ, Compton CL, Lamb JD, Winder WW: AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance-trained rats. Am J Physiol Endocrinol Metab 2005;289:E710–E715 [DOI] [PubMed] [Google Scholar]
- 46.Durante PE, Mustard KJ, Park SH, Winder WW, Hardie DG: Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am J Physiol Endocrinol Metab 2002;283:E178–E186 [DOI] [PubMed] [Google Scholar]
- 47.Steinberg GR, Rush JW, Dyck DJ: AMPK expression and phosphorylation are increased in rodent muscle after chronic leptin treatment. Am J Physiol Endocrinol Metab 2003;284:E648–E654 [DOI] [PubMed] [Google Scholar]
- 48.Alon T, Friedman JM, Socci ND: Cytokine-induced patterns of gene expression in skeletal muscle tissue. J Biol Chem 2003;278:32324–32334 [DOI] [PubMed] [Google Scholar]
- 49.Shin EJ, Schram K, Zheng XL, Sweeney G: Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol 2009;221:490–497 [DOI] [PubMed] [Google Scholar]
- 50.Fang X, Fetros J, Dadson KE, Xu A, Sweeney G: Leptin prevents the metabolic effects of adiponectin in L6 myotubes. Diabetologia 2009;52:2190–2200 [DOI] [PubMed] [Google Scholar]
- 51.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T: Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010;464:1313–1319 [DOI] [PubMed] [Google Scholar]
- 52.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC: PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 [DOI] [PubMed] [Google Scholar]
- 53.Ohtsuka T, Nishijima M, Suzuki K, Akamatsu Y: Mitochondrial dysfunction of a cultured Chinese hamster ovary cell mutant deficient in cardiolipin. J Biol Chem 1993;268:22914–22919 [PubMed] [Google Scholar]
- 54.Wicks KL, Hood DA: Mitochondrial adaptations in denervated muscle: relationship to muscle performance. Am J Physiol 1991;260:C841–C850 [DOI] [PubMed] [Google Scholar]
- 55.Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW: Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry 2007;46:6417–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.