Abstract
OBJECTIVE
Metformin has been well characterized in vitro as a substrate of liver-expressed organic cation transporters (OCTs). We investigated the gene expression and protein levels of OCT-1 and OCT-2 in adipose tissue and during adipogenesis and evaluated their possible role in metformin action on adipocytes.
RESEARCH DESIGN AND METHODS
OCT1 and OCT2 gene expressions were analyzed in 118 adipose tissue samples (57 visceral and 61 subcutaneous depots) and during human preadipocyte differentiation. To test the possible role of OCT1 mediating the response of adipocytes to metformin, cotreatments with cimetidine (OCT blocker, 0.5 and 5 mmol/l) and metformin were made on human preadipocytes and subcutaneous adipose tissue (SAT).
RESULTS
OCT1 gene was expressed in both subcutaneous and visceral adipose tissue. In both fat depots, OCT1 gene expression and protein levels were significantly increased in obese subjects. OCT1 gene expression in isolated preadipocytes significantly increased during differentiation in parallel to adipogenic genes. Metformin (5 mmol/l) decreased the expression of lipogenic genes and lipid droplets accumulation while increasing AMP-activated protein kinase (AMPK) activation, preventing differentiation of human preadipocytes. Cotreatment with cimetidine restored adipogenesis. Furthermore, metformin decreased IL-6 and MCP-1 gene expression in comparison with differentiated adipocytes. Metformin (0.1 and 1 mmol/l) decreased adipogenic and inflammatory genes in SAT. OCT2 gene expression was not detected in adipose tissue and was very small in isolated preadipocytes, disappearing during adipogenesis.
CONCLUSIONS
OCT1 gene expression and protein levels are detectable in adipose tissue. Increased OCT1 gene expression in adipose tissue of obese subjects might contribute to increased metformin action in these subjects.
Metformin (dimethylbiguanidine) is the most widely used drug for the treatment of type 2 diabetes (1,2). This insulin-sensitizing agent has well known beneficial effects not only on glycemic control, but also on the cardiovascular system. In the Diabetes Prevention Program, treatment with metformin reduced the incidence of type 2 diabetes by 31%. Interestingly, metformin was less effective in persons with a lower baseline BMI than in those with obesity (3). The reason for this observation is unknown. Shikata et al. (4) also found that BMI was a strong predictor of metformin effects: the higher the BMI, the higher the response.
Metformin has been well characterized in vitro as a substrate of organic cation transporters (OCTs) (5–9). Members of the OCT family play essential roles in the handling of cationic drugs and endogenously synthesized organic cations. Human solute carrier family 22 (organic cation transporter), member 1 (OCT1) is expressed primarily in the liver, localized in the basolateral membrane of hepatocytes, mediating the hepatic uptake of several cationic drugs (metformin, as well as cimetidine, desipramine, midazolam, citalopram, or clonidine). OCT1 has been reported to be necessary for metabolic activities of metformin in liver cell lines (10). In fact, different polymorphisms in the OCT1 gene have been associated with metformin action (3).
To our knowledge, OCT1-dependent metformin activity on other cells has not been previously studied. In addition to the liver-specific OCT1, its paralog, human solute carrier family 22 (organic cation transporter), member 2 (OCT2) is a transporter expressed in abundance in the kidney (7,8).
The most prominent feature of obesity is increased fat mass. Despite the important observed effects of metformin in obesity, there is relatively scarce information about in vitro models. Metformin effects have been evaluated in the murine 3T3-L1 cell line, in which an inhibition of adipogenesis was found (11–13). To our knowledge, the effects of this drug on adipogenesis has not been tested in human preadipocytes despite the potentially important mechanistic effects. However, the effects of metformin (1 mmol/l, during 24 h) increasing glucose intake in subcutaneous and visceral human adipocytes have been reported (14).
We first observed that metformin inhibited the differentiation of human adipocytes, decreasing the expression of different lipogenic genes. For this reason, we hypothesized that OCT1 was mediating these effects. In fact, the inhibition of this transporter using cimetidine reversed the blunted differentiation induced by metformin. Finally, we evaluated the potential in vivo importance of these observations by studying the expression of OCT1 in human adipose tissue.
RESEARCH DESIGN AND METHODS
Differentiation of human subcutaneous preadipocytes.
Isolated subcutaneous preadipocytes from lean (BMI <25) and obese (BMI >30) subjects (Zen-Bio, Research Triangle Park, NC) were plated on T-75 cell culture flasks and cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM)/Nutrient Mix F-12 medium (1:1, v/v) supplemented with 10U/ml P/S, FBS 10%, HEPES 1% and glutamine 1% (all from GIBCO, Invitrogen S.A, Barcelona, Spain). One week later, the isolated and expanded human subcutaneous preadipocytes were cultured (∼40.000 cells/cm2) in 12-well plates with preadipocytes medium (Zen-Bio) composed of DMEM/Nutrient Mix F-12 medium (1:1, v/v), HEPES, FBS, penicillin and streptomycin in a humidified 37°C incubator with 5% CO2. Twenty-four hours after plating, cells were checked for complete confluence (day 0) and differentiation was induced using differentiation medium (DM, Zen-Bio) composed of preadipocytes medium, human insulin, dexamethasone, isobutylmethylxanthine and peroxisome proliferator–activated receptor (PPAR)γ agonists (rosiglitazone). After 7 days (day 7), DM was replaced with fresh adipocyte medium (AM, Zen-Bio) composed of DMEM/Nutrient Mix F-12 medium (1:1, v/v), HEPES, FBS, biotin, panthothenate, human insulin, dexamethasone, penicillin, streptomycin and amphotericin. Negative control (nondifferentiated cell) was performed with preadipocyte medium during all differentiation process. Fourteen days after the initiation of differentiation, cells appeared rounded with large lipid droplets apparent in the cytoplasm. Cells were then considered mature adipocytes, harvested, and stored at −80°C for RNA extraction to study OCT1 and OCT2 gene expression levels after human adipocyte differentiation. Nondifferentiated control was performed using proliferation medium along 14 days. Metformin (Sigma, Barcelona, Spain) (5 mmol/l), cimetidine (Sigma, Barcelona, Spain) (0.5 mmol/l and 5 mmol/l) and metformin and cimetidine coincubations were performed with differentiation medium along 14 days. The experiment was performed in triplicate for each sample. The differentiation was monitored with the fatty acid synthase (FASN, Hs00188012_m1, Applied Biosystems, Madrid, Spain), adiponectin (Adipoq, Hs00605917_m1, Applied Biosystems), acetyl-CoA carboxylase α (ACC1, Hs00167385_m1, Applied Biosystems), peroxisome proliferator-activated receptor γ (PPARγ, Hs00234592_m1, Applied Biosystems), fatty acid binding protein 4(FABP4, HS00609791_m1, Applied Biosystems), interleukin 6 (IL6, Hs00985639_m1, Applied Biosystems), and monocyte chemoattractant protein-1 (MCP1, Hs00234140_m1, Applied Biosystems) expression.
Ex vivo experiments using subcutaneous adipose tissue explants.
Subcutaneous adipose tissue was obtained from six obese subjects undergoing open abdominal surgery (gastrointestinal bypass) under anesthesia after an overnight fast. The mean age was 46 ± 6.4 years (range, 39–58 years) and the BMI 44.9 ± 12.4 kg/m2. Medical histories, physical examinations, electrocardiogram, and blood screening showed that all patients were in good health. None of the subjects had a history of hepatic or renal disorders. The study had the approval of the Ethics Committee and all patients gave informed written consent.
Samples of subcutaneous adipose tissue were immediately transported to the laboratory (5–10 min). The handling of tissue was carried out under strict aseptic conditions. The tissue was cut with scissors into small pieces (5–10 mg), and incubated in buffer plus albumin (3 ml/g of tissue) for ∼5–30 min. After incubation, the tissue explants were centrifuged for 30 s at 400 g. Then 100 mg of minced tissue was placed into 1-ml M199 (Gibco, Invitrogen) containing 10% FBS (Hyclone, Thermo Fisher Scientific), 100 unit/ml penicillin (Gibco, Invitrogen), 100 μg/ml streptomycin (Gibco, Invitrogen), and incubated for 48 h in suspension culture under aseptic conditions (15). Control treatment (M199) and metformin (Sigma, Barcelona, Spain) (0.1 and 1 mmol/l) were compared. After 48 h, all samples were immediately flash-frozen in liquid nitrogen before being stored at −80°C. To evaluate cell integrity, lactate dehydrogenase activity released from damaged cells was analyzed by Cytotoxicity Detection Kit (lactate dehydrogenase [LDH]; Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions in all treatments. OCT1, ACC1, FASN, Adipoq, PPARγ, IL-6, and MCP-1 relative gene expression were analyzed using TaqMan technology suitable for relative genetic expression quantification (described below).
Human adipose tissue samples.
A group of 118 adipose tissue samples (57 visceral and 61 subcutaneous depots), from participants with a BMI within 20 and 68 kg/m2, who were recruited at the Endocrinology Service of the Hospital Universitari Dr. Josep Trueta (Girona, Spain), were analyzed. All subjects were of Caucasian origin and reported that their body weight had been stable for at least 3 months before the study. Liver and renal diseases were specifically excluded by biochemical workup. All subjects gave written informed consent after the purpose of the study was explained to them.
Adipose tissue samples were obtained from subcutaneous and visceral depots during elective surgical procedures (cholecystectomy, abdominal hernia surgery, and gastric by-pass surgery). All samples were washed, fragmented, and immediately flash-frozen in liquid nitrogen before stored at −80°C.
To perform the isolation of adipocyte and stromo-vascular fraction, tissues were washed three to four times with PBS and suspended in an equal volume of PBS supplemented with 1% bovine serum and 0.1% collagenase type I prewarmed to 37°C. The tissue was placed in a shaking water bath at 37°C with continuous agitation for 60 min, and centrifuged for 5 min at 300 to 500g at room temperature. The supernatant, containing mature adipocytes, was recollected. The pellet was identified as the SVF cell. The adipose tissue fractionation was performed from eight subcutaneous fat depots.
RNA expression.
To study gene expressions, RNA was prepared from these samples using RNeasy Lipid Tissue Mini Kit (Qiagen,Izasa SA, Barcelona, Spain). The integrity of each RNA sample was checked by Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA). Total RNA was quantified by means of spectrophotometer (GeneQuant, GE Health Care, Piscataway NJ) reverse transcribed to cDNA using a High Capacity cDNA Archive Kit (Applied Biosystems) according to the manufacturer's protocol.
Gene expression was assessed by real time PCR using an ABI Prism 7,000 Sequence Detection System (Applied Biosystems, Madrid, Spain), using TaqMan technology suitable for relative genetic expression quantification.
The commercially available and prevalidated TaqMan primer/probe sets used were as follows: endogenous control PPIA (4333763, cyclophilin A, Applied Biosystems, Madrid, Spain) and target gene human organic cation transporter 1 and human organic cation transporter 2 (OCT1, Hs00427552_m1; and OCT2, Hs00161893_m1, Applied Biosystems). The RT-PCR TaqMan reaction was performed in a final volume of 25 μl. The cycle program consisted of an initial denaturing of 10 min at 95°C, and then 40 cycles of 15-s denaturizing phase at 95°C and 1 min annealing and extension phase at 60°C. A threshold cycle (Ct value) was obtained for each amplification curve, and a ΔCt value was first calculated by subtracting the Ct value for human Cyclophilin A (PPIA) RNA from the Ct value for each sample. Fold changes compared with the endogenous control were then determined by calculating 2−ΔCt, so gene expression results are expressed as expression ratios relative to PPIA gene expression according to manufacturers' guidelines.
Western blot analysis.
Adipose tissue lysates (from eight subcutaneous fat depots) were washed in ice-cold PBS followed by homogenization assay using RIPA lysis buffer (Millipore, Madrid, Spain) supplemented with a protease inhibitor cocktail (Sigma-Aldrich, Madrid, Spain) at 4°C for 30 min. Cellular debris were eliminated by centrifugation of the diluted samples at 14,000 g for 30 min (4°C). Protein concentration was determined using a Lowry assay. RIPA protein extracts (50 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes by conventional procedures. Membranes were immunoblotted with OCT1, FASN, and β-actin antibodies (Santa Cruz Biotechnology, CA). Anti-rabbit IgG and anti-mouse IgG coupled to horseradish peroxidase was used as a secondary antibody. Horseradish peroxidase activity was detected by chemiluminescence, and quantification of protein expression was performed using scion image software.
AMPK and acetyl-CoA carboxylase-α activity.
AMP-activated protein kinase (AMPK) activity was determined measuring pThr172AMPK by ELISA (KHO0651, Invitrogen, Barcelona, Spain). pThr172AMPK is directly associated with AMPK activity. According to the manufacturer, the analytical sensitivity of this assay is <1 unit/ml of AMPKα [pT172]. This was determined by adding two SDs to the mean O.D. obtained when the zero standard was assayed 30 times. The average recovery was 90% The specificity of this assay for phosphorylated AMPKα (pT172) was confirmed by peptide competition. Intra- and interassay coefficients of variation for all these determinations were between 5 and 10%.
Acetyl-CoA carboxylase-α (ACC) activity was calculated measuring pSer79ACC1 and ACC1 (total) by ELISA (KHO1061 and KHO1071, respectively; Invitrogen, Barcelona, Spain). pSer79ACC1 is inversely associated with ACC activity. The analytical sensitivity of these assays are <0.5 units/ml of human ACC1 [pS79] and human ACC1, respectively. These were determined by adding two SDs to the mean O.D. obtained when the zero standards was assayed 30 times. The percentage recovery was calculated as an average of 93 and 97%, respectively. The specificity of this assay for phosphorylated ACC1 [pS79] was confirmed by peptide competition. Intra- and interassay coefficients of variation for all these determinations were between 5 and 10%.
Oil Red staining and analysis.
Differentiation was monitored by morphologic assessment and Oil red O staining. For Oil Red staining, cells were washed twice with PBS, fixed in 4% formaldehyde for 1 h, and stained for 30 min with 0.2% Oil Red O solution in 60% isopropanol. Cells were then washed several times with water, and excess water was evaporated by placing the stained cultures at ∼32°C. To determine the extent of adipose conversion, 0.2 ml of isopropanol was added to the stained culture dish. The extracted dye was immediately removed by gentle pipeting and its optical density was monitored spectrophotometrically at 500 nm using a multiwell plate reader (Model Anthos Labtec 2010 1.7 reader).
Cell counting and LDH activity assay.
Cell counting was assessed by trypan blue dye exclusion using a Neubauer hemacytometer, after 14 days differentiation of human subcutaneous preadipocytes, in triplicate.
To evaluate cell integrity, LDH activity released from damaged cells was analyzed by Cytotoxicity Detection Kit (LDH; Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions.
Statistical analyses.
Statistical analyses were performed using SPSS 12.0 software. Unless otherwise stated, descriptive results of continuous variables are expressed as mean and SD for Gaussian variables, or median and interquartile range. Parameters that did not fulfill normal distribution were mathematically transformed to improve symmetry for subsequent analyses. The relation between variables was analyzed by simple correlation (Spearman test). Unpaired and paired t tests were used to compare clinical variables and OCT-1 and −2 gene expressions according to obesity status. Nonparametric tests, Mann Whitney U and Wilcoxon's tests, were used to evaluate the effects of metformin and cimetidine treatments in vitro and ex vivo. The experiments were performed in triplicate.
RESULTS
OCT-1 and OCT-2 expression during differentiation of human preadipocytes.
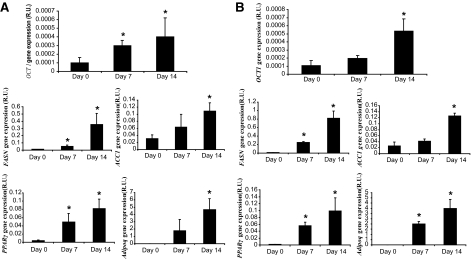
The differentiation process was monitored through FASN, ACC1, PPARγ, and Adipoq gene expression (Fig. 1), with accumulation of lipid droplets in the cytoplasm.
FIG. 1.
OCT1, FASN, ACC1, PPARγ and adiponectin gene expression during differentiation of human preadipocytes from obese (A) and lean subjects (B). *P < 0.05 vs. day 0.
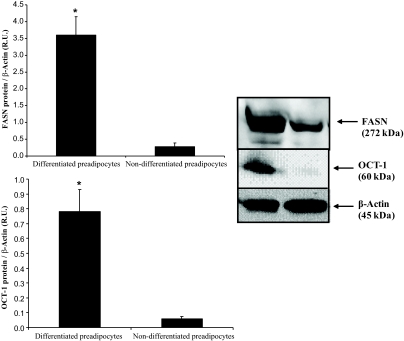
In isolated preadipocytes from lean and obese subjects, OCT1 gene expression was significantly increased during the differentiation process in parallel to adipogenic genes (Fig. 1). In differentiated preadipocytes OCT1 protein levels were significantly higher than in nondifferentiated preadipocytes (Fig. 2). OCT2 gene expression was very small and disappeared during adipogenesis.
FIG. 2.
OCT1 and FASN protein levels in differentiated and nondifferentiated preadipocytes at day 14. *P < 0.05 vs. nondifferentiated preadipocytes.
OCT1 gene expression was positively associated with FASN (r = 0.8, P < 0.001), ACC1 (r = 0.66, P = 0.003), PPARγ (r = 0.65, P = 0.004) and Adipoq (r = 0.73, P = 0.001) gene expressions and negatively with IL-6 gene expression (r = −0.7, P = 0.002).
Metformin effects on adipogenesis and AMPK activity.
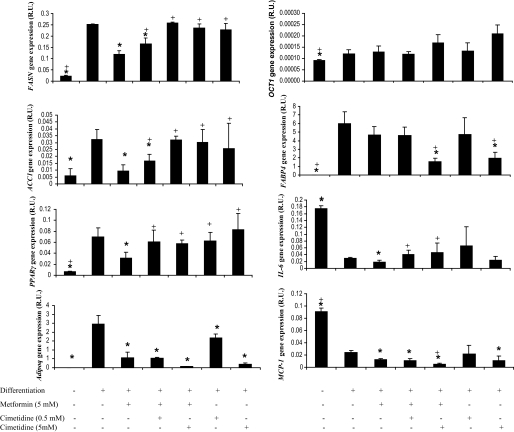
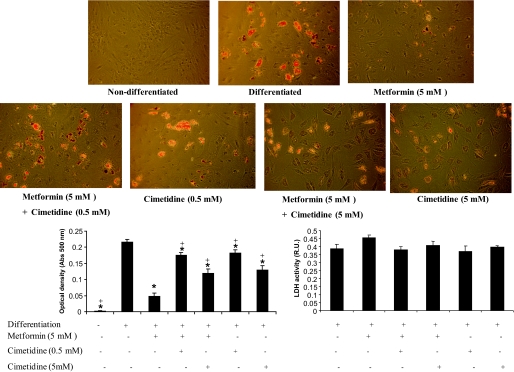
Metformin (5 mmol/l) decreased the expression of lipogenic genes (Fig. 3) and lipid droplets accumulation (Fig. 4), leading to impaired differentiation of human preadipocytes. Cotreatment with cimetidine restored adipogenesis (Fig. 3).
FIG. 3.
FASN, ACC1, PPARγ, adiponectin, OCT1, FABP4, IL-6, MCP-1 gene expressions after metformin (5 mmol/l) and cimetidine (0.5 and 5 mmol/l) cotreatments in differentiated human adipocytes. *P < 0.05 vs. differentiated adipocytes; +P < 0.05 vs. metformin (5 mmol/l) treatment.
FIG. 4.
Oil red O staining and LDH activity in differentiated human adipocytes, and after metformin (5 mmol/l) and cimetidine (0.5 and 5 mmol/l) cotreatment. *P < 0.05 vs. differentiated adipocytes; +P < 0.05 vs. metformin (5 mmol/l) treatment. (A high-quality color representation of this figure is available in the online issue.)
Proinflammatory molecules, such as IL-6 and MCP-1, significantly decreased during adipocyte differentiation. However, metformin administration did not increase IL-6 and MCP-1 gene expression in comparison with differentiated adipocytes. The decreased levels of expression of proinflammatory molecules were maintained with cimetidine cotreatment. High doses of cimetidine (5 mmol/l) significantly decreased adiponectin gene expression (Fig. 3).
OCT1 gene expression did not change significantly after metformin or cimetidine treatments (Fig. 3).
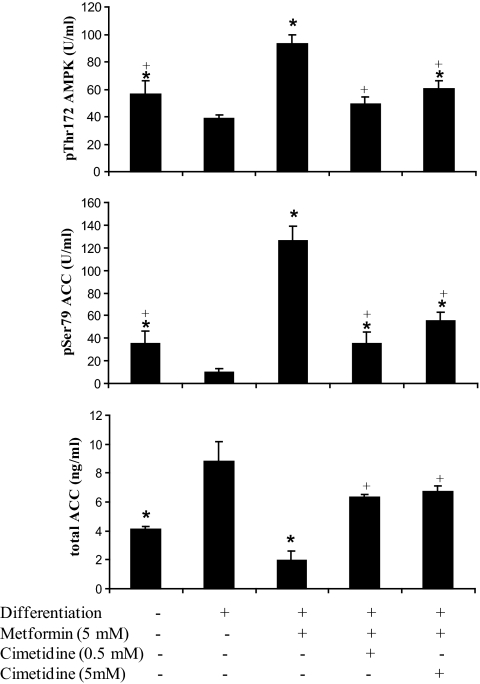
Metformin treatment increased significantly AMPK activity, increasing p172ThrAMPK and consequently p79SerACC1. In cimetidine coincubation, the increase of AMPK activity was blunted (Fig. 5). The total ACC1 quantification showed a similar expression pattern to ACC1 gene expression.
FIG. 5.
Effects of metformin (5 mmol/l) and cimetidine (0.5 and 5 mmol/l) cotreatment on p172ThrAMPK, p79SerACC1, and ACC1 concentrations. *P < 0.05 vs. differentiated adipocytes; +P < 0.05 vs. metformin (5 mmol/l) treatment.
LDH activity was measured to evaluate the cytotoxicity in each of the treatments. No significant difference was found comparing undifferenciated and differenciated adipocytes, whether treated or untreated with cimetidine (0.5 mmol/l and 5 mmol/l). After metformin (5 mmol/l) treatment, LDH activity tended to be higher compared with differentiated adipocytes (Fig. 4).
Metformin effects in subcutaneous adipose tissue explants.
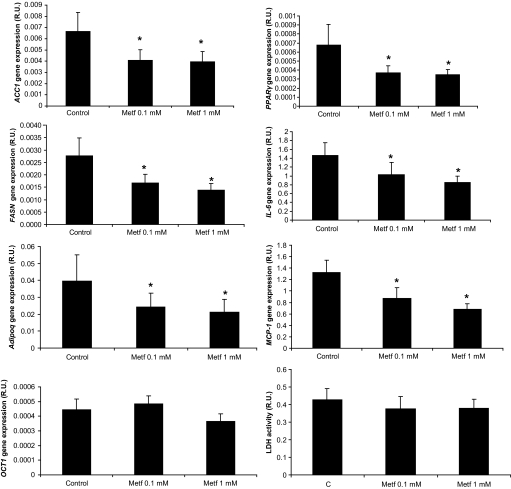
Metformin (0.1 and 1 mmol/l) led to decreased adipogenic and inflammatory gene expression (Fig. 6). OCT1 gene expression was not affected by metformin administration (Fig. 6).
FIG. 6.
FASN, ACC1, PPARγ, adiponectin, OCT1, IL-6, MCP-1 gene expressions after metformin (0.1 and 1 mmol/l) in subcutaneous adipose tissue explants. *P < 0.05 vs. control (vehicle) treatment.
In addition, baseline OCT1 gene expression was associated with the metformin-induced adipogenic gene expression reduction (ACC1, Adipoq, FASN, and PPARγ), suggesting that the higher the OCT-1 gene expression, the higher the effects of metformin (for 0.1 mmol/l, r = −0.81, P = 0.04, r = −0.94, P = 0.004, r = −0.96, P = 0.002 and r = −0.68, P = 0.1, respectively; and for 1 mmol/l, r = −0.76, P = 0.07, r = −0.94, P = 0.004, r = −0.88, P = 0.02, and r = −0.64, P = 0.17, respectively).
Treatment of adipose tissue with metformin (0.1 and 1 mmol/l) did not change lactate dehydrogenase activity (cell integrity) in comparison with control treatment (Fig. 6).
OCT1 and OCT2 expression in human adipose tissue.
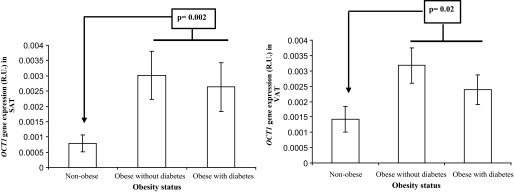
Anthropometric and clinical characteristics of all participants are shown in Table 1. The OCT1 gene was similarly expressed in subcutaneous and visceral adipose tissue [0.002 (0.0005–0.0034) versus 0.0015 (0.0004–0.003) R.U., P = 0.4, n = 31]. In fact, the expression of OCT1 in both fat depots correlated significantly (r = 0.54, P < 0.001). The relative OCT1 gene expression was lower compared with lipogenic genes (100- and 10-fold decrease in comparison with FASN and ACC1 gene expression). In both subcutaneous and visceral fat depots, OCT1 gene expression correlated significantly with BMI (r = 0.46, P < 0.001 and r = 0.47, P < 0.001, respectively) and percent fat mass (r = 0.36, P = 0.005, and r = 0.49, P < 0.001, respectively) (Fig. 7). In addition, OCT1 gene expression correlated significantly with diastolic blood pressure (r = 0.35, P = 0.04) in visceral adipose tissue. No associations were detected with other metabolic parameters (age, systolic blood pressure, fasting glucose, fasting triglycerides, HDL cholesterol, and LDL cholesterol).
TABLE 1.
Anthropometrical and biochemical variables of study participants
| Not obese | Obese | Obese with type 2 diabetes | P | |
|---|---|---|---|---|
| N | 15 | 32 | 14 | |
| Sex (men/women) | 5/10 | 9/23 | 2/12 | |
| Age (years) | 47.4 ± 10.4 | 44.1 ± 9.7 | 46.6 ± 12.4 | 0.4 |
| BMI (Kg/m2) | 26.57 ± 2.34 | 41.7 ± 7.6 | 41.4 ± 4.1 | <0.001 |
| Fat mass (%) | 33.5 ± 4.98 | 51.3 ± 10.6 | 53.5 ± 4.9 | <0.001 |
| SBP (mmHg) | 125 ± 15.5 | 133.4 ± 20.4 | 141.4 ± 26.8 | 0.05 |
| DBP (mmHg) | 78.5 ± 9.8 | 77.1 ± 10.55 | 77.47 ± 9.98 | 0.4 |
| Fasting glucose (mg/dl) | 87.2 ± 11.5 | 93.29 ± 11.3 | 132.57 ± 46.2 | 0.001 |
| Fasting triglycerides (mg/dl) | 112.3 ± 54.04 | 112.6 ± 66.9 | 123.85 ± 39.1 | 0.7 |
| HDL cholesterol | 69.07 ± 29.3 | 52.9 ± 18.14 | 54.9 ± 13.5 | 0.01 |
| LDL cholesterol | 98.4 ± 37.1 | 110.02 ± 30.9 | 121.5 ± 25.1 | 0.1 |
| OCT1 gene expression in SAT (relative units) | 0.0005 (0.00008–0.005) | 0.0015 (0.00001–0.004) | 0.002 (0.0003–0.012) | 0.002* |
| OCT1 gene expression in VAT (relative units) | 0.0006 (0.0002–0.006) | 0.0026 (0.0001–0.015) | 0.0027 (0.0002–0.005) | 0.02* |
Values are mean ± SD.
*Comparing obese subjects vs. nonobese. Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
FIG. 7.
OCT1 relative gene expression in both visceral and subcutaneous adipose tissue according to obesity status.
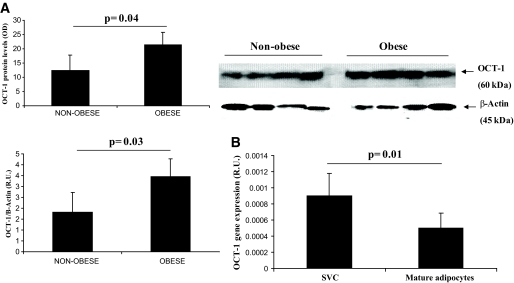
There is evidence indicating that mRNA levels may not necessarily predict the translated protein levels. In this regard, we measured OCT1 protein in adipose tissue by Western blot. OCT1 protein was significantly increased in obese subjects (Fig. 8A).
FIG. 8.
A: OCT1 protein levels in subcutaneous adipose tissue according to obesity status. Non-normalized OCT1 protein and normalized for β-Actin values (relative levels) are shown. B: OCT1 gene expression in stromovascular cells and mature adipocytes from subcutaneous adipose tissue.
To gain insight about the type of cells from adipose tissue that expressed OCT1, we analyzed OCT1 gene expression in stromo-vascular cells and mature adipocytes from subcutaneous adipose tissue. In stromo-vascular cells OCT1 gene expression was significantly higher than in mature adipocytes (1.8-fold increase, P = 0.01; Fig. 8B).
The OCT2 gene was not significantly expressed in human adipose tissue.
DISCUSSION
Metformin has been described to be more effective in obese subjects, and the degree of obesity has been found to constitute a strong predictor of metformin effects (3,4). The main findings of this study are: 1) OCT1 gene expression was detectable in whole adipose tissue (similarly in the subcutaneous and visceral fat depots) and in isolated adipocytes; 2) this expression increased significantly with adipocyte differentiation in association with lipogenic (FASN, ACC, PPARg) and adipogenic (Adipoq) genes; 3) metformin (5 mmol/l) blunted the adipocyte differentiation of human preadipocytes in parallel to decreasing significantly the expression of proinflammatory mediators; 4) blocking metformin action using cimetidine reversed these effects; and 5) In ex-vivo experiments, metformin led to decreased adipogenic and proinflammatory gene expression. To the best of our knowledge, this is the first study evaluating metformin effects and OCT1 gene expression in human adipocytes. The increased OCT1 gene expression in stromo-vascular cells compared with adipocytes suggests that metformin action on stromo-vascular cells might contribute to systemic effects. Considering the importance of OCT1 in the metformin response (9), we suggest that the higher OCT1 gene expression in obese subjects is behind the increased metformin effects in these subjects. Interestingly we found that baseline OCT1 gene expression was associated with the metformin-induced adipogenic gene expression reduction (ACC1, Adipoq, FASN and PPARγ), suggesting that the higher the OCT-1 gene expression, the higher the effects of metformin.
The inhibitory effects of metformin on adipogenesis have been previously shown in the 3T3-L1 cell line (11–13) via AMPK activation. However, to our knowledge, these actions have not been explored in human preadipocytes. In the current study, metformin (5 mmol/l) significantly decreased the expression of adipogenic (FASN, ACC, PPARg, adipoq) genes and the formation of lipid droplets, increasing AMPK activity (p172ThrAMPK and consequently p79SerACC1). The adipocyte differentiation (increasing lipogenic gene expression and decreasing AMPK activity) was restored dose-dependently when the OCT1 blocker agent cimetidine was used as a cotreatment.
In agreement with our data, the response to metformin was inhibited in mice models in which the OCT1 gene was deleted (OCT1−/−). Subjects carrying a single nucleotid polymorphism associated with a decrease in OCT1 gene expression also showed decreased metformin effects (4,10,16).
Importantly, metformin administration significantly decreased IL-6 and MCP-1 gene expression in adipocytes and in adipose tissue explants. Recently, metformin has been shown to display anti-inflammatory effects in endothelial cells by inhibiting TNF-α–induced IKKα/β phosphorylation, IkappaB-α degradation, and IL-6 production (17,18).
The mode of action of metformin has yet to be fully established. In muscle, liver, and endothelial cells, the metabolic changes induced by metformin appear to be mediated by the AMP-activated protein kinase (AMPK). AMPK acts as a sensor of the cellular energy status, being switched on by an increased ATP demand or by processes that interfere with ATP production such as ischemia. The activated form of AMPK switches on catabolic pathways while switching off ATP-consuming processes (19). It has been reported that metformin binds to complex I of the mitochondrial respiratory chain, and this could in part explain how this drug acts (20). The inhibition of complex I would cause a decrease in energy supply that would in turn lead to a higher AMP/ATP ratio, and the concomitant activation of AMPK. It seems counterintuitive that metformin is providing a beneficial effect by attenuating adipocyte differentiation. However, although metformin has been associated with weight loss, glitazones lead to increased adipocyte differentiation and weight gain. Metformin stimulates catabolic pathways in white adipose tissue through the activation of AMPK, reducing the triglyceride stores as reflected by the smaller size of the adipocytes (13,21). These effects are achieved through an increase in lipolysis and β-oxidation, which would imply that there is no release of fatty acids and that they are oxidized within the adipocyte. Other authors have described that metformin inhibited adipocyte differentiation (using rat mesenchymal stem cells) (22). We propose here that OCT1 density in adipose tissue is a factor that can significantly influence all these effects of metformin. Furthermore, Fisher et al. (14) have shown that metformin induces glucose uptake independent of insulin in subcutaneous and visceral human adipocytes.
In conclusion, OCT1 gene expression and protein levels are detectable in adipose tissue. The increased OCT1 gene expression in adipose tissue of obese subjects might contribute to increased metformin action in these subjects.
ACKNOWLEDGMENTS
This work was partially supported by research grants from the Ministerio de Educación y Ciencia (SAF2008-0273).
No potential conflicts of interest relevant to this article were reported.
J.M.F.-R. and J.M.M.-N. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
J.M.M.-N. researched data and wrote the manuscript. F.J.O., J.-I.R.-H., M.S., and G.P. researched data. W.R. contributed to discussion. J.M.F.-R. designed the study, contributed to discussion, and wrote the manuscript.
The authors acknowledge the clinical help of Oscar Rovira.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bailey CJ, Turner RC: Metformin. N Engl J Med 1996;334:574–579 [DOI] [PubMed] [Google Scholar]
- 2.Kirpichnikov D, McFarlane SI, Sowers JR: Metformin: an update. Ann Intern Med 2002;137:25–33 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shikata E, Yamamoto R, Takane H, Shigemasa C, Ikeda T, Otsubo K, Ieiri I: Human organic cation transporter (OCT1 and OCT2) gene polymorphisms and therapeutic effects of metformin. J Hum Genet 2007;52:117–122 [DOI] [PubMed] [Google Scholar]
- 5.Koepsell H, Lips K, Volk C: Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 2007;24:1227–1251 [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM: Cloning and functional expression of a human liver organic cation transporter. Mol Pharmacol 1997;51:913–921 [DOI] [PubMed] [Google Scholar]
- 7.Dresser MJ, Leabman MK, Giacomini KM: Transporters involved in the elimination of drugs in the kidney: organic anion transporters and organic cation transporters. J Pharm Sci 2001;90:397–421 [DOI] [PubMed] [Google Scholar]
- 8.Dresser MJ, Xiao G, Leabman MK, Gray AT, Giacomini KM: Interactions of n-tetraalkylammonium compounds and biguanides with a human renal organic cation transporter (hOCT2). Pharm Res 2002;19:1244–1247 [DOI] [PubMed] [Google Scholar]
- 9.Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH, Sugiyama Y: Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther 2002;302:510–515 [DOI] [PubMed] [Google Scholar]
- 10.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM: Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 2007;117:1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenhard JM, Kliewer SA, Paulik MA, Plunket KD, Lehmann JM, Weiel JE: Effects of troglitazone and metformin on glucose and lipid metabolism: alterations of two distinct molecular pathways. Biochem Pharmacol 1997;54:801–808 [DOI] [PubMed] [Google Scholar]
- 12.Huypens P, Quartier E, Pipeleers D, Van de Casteele M: Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Eur J Pharmacol 2005;518:90–95 [DOI] [PubMed] [Google Scholar]
- 13.Alexandre KB, Smit AM, Gray IP, Crowther NJ: Metformin inhibits intracellular lipid accumulation in the murine pre-adipocyte cell line, 3T3–L1. Diabetes Obes Metab 2008;10:688–690 [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Timper K, Radimerski T, Dembinski K, Frey DM, Zulewski H, Keller U, Müller B, Christ.Crain M, Grisouard J: Metformin induces glucose uptake in human preadipocyte-derived adipocytes from various fat depots. Diabetes Obes Metab 2010;12:356–359 [DOI] [PubMed] [Google Scholar]
- 15.Fernández.Real JM, Moreno JM, Ricart W: Circulating retinol-binding protein-4 concentration might reflect insulin resistance.associated iron overload. Diabetes 2008;57:1918–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM, Giacomini KM: Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 2008;83:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang NL, Chiang SH, Hsueh CH, Liang YJ, Chen YJ, Lai LP: Metformin inhibits TNF-alpha-induced IkappaB kinase phosphorylation, IκB-α degradation and IL. 6 production in endothelial cells through PI3K-dependent AMPK phosphorylation. Int J Cardiol 2009;134:169–175 [DOI] [PubMed] [Google Scholar]
- 18.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schönbeck U, Libby P: Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells. Arterioscler Thromb Vasc Biol 2006;26:611–617 [DOI] [PubMed] [Google Scholar]
- 19.Hardie DG, Hawley SA, Scott JW: AMP-activated protein kinase.development of the energy sensor concept. J Physiol 2006;574:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen MR, Doran E, Halestrap AP: Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348:607–614 [PMC free article] [PubMed] [Google Scholar]
- 21.An Z, Wang H, Song P, Zhang M, Geng X, Zou MH: Nicotine.induced activation of AMP.activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role for oxidant stress. J Biol Chem 2007;282:26793–26801 [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Xue J, Li X, Jia Y, Hu J: Metformin regulates osteoblast and adipocyte differentiation of rat mesenchymal stem cells. J Pharm Pharmacol 2008;60:1695–1700 [DOI] [PubMed] [Google Scholar]