The global obesity epidemic has stimulated intense interest in the study of homeostatic mechanisms governing the balance between energy intake and energy expenditure over time, and recent progress in gene targeting and related technologies has pushed mouse models to the forefront of this effort. Although current methods for the measurement of energy expenditure (EE) in mice are sensitive, reproducible, and widely available, their potential to inform the study of obesity remains limited by controversy regarding how best to control for the powerful, independent impact on EE of variation in body size per se (1,2). In this article, we briefly review the recent work that has fueled this controversy and propose an approach to its resolution. We also highlight aspects of EE measurement relevant to obesity pathogenesis that merit additional study. Although we focus on studies in mice, the principles presented can be applied to most other animal models.
Dramatic increases in the prevalence of obesity and its burden of health and economic consequences heighten the need for new insights into mechanisms that govern energy balance. At a superficial level, the problem of obesity resolves to simple math: energy balance, defined as the difference between rates of EE and energy intake (EI), sums over time to determine body energy content stored as fat mass (FM). Hence, obesity can be seen as the consequence of a sustained increase of energy intake relative to energy expenditure. This simplistic thermodynamic explanation, however, belies a far more complex pathophysiology that is crucial for understanding why weight loss is so difficult to achieve and sustain in obese individuals. At the core of this complexity is a biological process termed energy homeostasis, through which energy intake and expenditure are matched over long time intervals to promote the stability of FM. This process is influenced by interactions between genes and environment that affect innumerable physiological and biochemical processes that dictate energy flow and partitioning, and recent progress in understanding these processes is beginning to shed light on the pathogenesis of both common (3,4) and rare forms of obesity (5). Although research has for many years focused on EI as the predominant determinant of obesity risk, emphasis is increasingly being placed on the EE side of the energy balance equation.
To judge the impact of a change of EE on obesity phenotypes, a usual starting point is to compare EE between lean and obese groups. Unlike food intake studies, however, EE measures are typically made over only relatively short time intervals (ranging from a few hours to 2 or 3 days) and hence offer only a snapshot of any differences in EE that might be present. Making matters worse, body size itself is a crucial but complex determinant of EE. Larger animals typically have higher absolute rates of EE owing to an increase in the total amount of metabolically active mass, whereas smaller animals typically have higher per-kg rates of EE (6), in part because their larger body surface area (BSA) relative to volume increases heat loss and thus requires greater heat production to maintain body temperature homeostasis. Consequently, EE data must be adjusted for the influence of body size variation per se to assess whether a change of EE contributed to weight changes in a given experimental model. A traditional (and recurring) remedy for this problem is to divide individual measures of EE by either total body mass (TBM) or lean body mass (LBM) (or fat free mass [FFM]) to yield measures of EE that can be compared between groups. Although these strategies might intuitively be expected to remove the influence of body size disparity on EE, conclusions can differ substantially depending on whether TBM or LBM is the selected denominator (7). Moreover, both choices can produce a confounded variable, undermining confidence in either approach. In this Perspectives in Diabetes article, we 1) review the growing controversy over how best to normalize EE for body size variation in mice, 2) offer a potential resolution to this surprisingly challenging issue, and 3) highlight the implications of this and other aspects of EE biology for basic obesity and diabetes research.
Origins of a controversy.
A recent Perspectives in Diabetes article by Butler and Kozak (1) cogently framed the issue of inappropriate EE normalization in murine models of genetic obesity and illustrated how EE phenotyping can yield flawed conclusions regarding obesity pathogenesis when the confounding influence of body size variation is not effectively controlled. Numerous published examples were cited in which improper normalization of EE for variation in body size undermined the insights gained from genetic or pharmacological interventions that affect EE. The principal concern identified by these authors is that a commonly used method of normalizing EE—division of EE by TBM—can yield seriously confounded results (1), leading them to adopt Himms-Hagen's opinion (8) that since FM is a trivial contributor to whole-body EE, normalization should be performed by dividing EE by LBM. Indeed, FM is comprised primarily of metabolically inert triglyceride, and further, increased weight in many obese animal models primarily reflects an expansion of FM accompanied by a much smaller change of LBM. Using TBM, rather than LBM, as the denominator in ratio-based methods of EE normalization can consequently produce the errant conclusion that obese animals have a lower EE than do lean controls because EE is divided by a denominator that is increased disproportionately relative to their small increase of LBM. It follows that this confounding effect can be averted when EE is divided by either LBM or FFM (LBM contains some essential lipid whereas FFM equals TBM minus all extractable lipid; the two values are nearly identical). Despite this logic, recent work suggests that normalization achieved by dividing EE by FFM (or LBM) is also confounded in mice (2) by factors both physiological and mathematical in nature.
Variation in FM influences EE.
The concept that FM has an inconsequential metabolic energy cost is supported by evidence that adipose tissue has a low mass-specific rate of energy utilization (9). Yet, accumulating data in both mice and humans challenge the conclusion that FM is of little consequence to whole animal EE, and suggest instead that the impact of FM on whole-body EE extends beyond the intrinsic energy costs of adipose tissue (10–14). A recent review (15) concluded that even if the effect of each gram of FM on EE is modest relative to that exerted by each gram of LBM (15–20%), it is nonetheless important to take FM into account in comparisons involving lean and obese animals. These concerns led us (2) to analyze data from a large sample of mice (n = 137) bred onto the C57BL/6J background strain on which both EE (indirect calorimetry) and body composition (high precision quantitative magnetic resonance) were measured at the University of Washington's Mouse Metabolic Phenotyping Center (MMPC). Our analysis revealed an unexpectedly large effect of FM as a determinant of murine EE, with the influence of each gram of FM equaling ∼50% of the per-gram influence of LBM (2). This observation suggests that employing an EE normalization strategy in mice that excludes FM can yield confounded outcomes when comparing lean with obese mice.
An obvious question is: how can FM be a major determinant of EE if adipose tissue itself has a low EE? Although definitive answers to this question are still awaited, we (2) and others (13,15) hypothesize that EE is coupled to FM indirectly via the energy homeostasis system alluded to earlier. According to this concept, negative feedback signaling generated in proportion to FM (via hormones such as leptin and insulin) acts in the central nervous system to induce compensatory adjustments of autonomic and behavioral outputs that affect energy balance so as to promote stability in the amount of body energy stored in the form of fat (16). Thus, if FM is perturbed from its biologically defended level, a corresponding change of negative feedback signaling evokes adaptive changes of both EI and EE that restore FM to its preintervention value. Exceptions to this prediction include situations in which the FM change occurs in response to either a resetting of the defended adiposity level or involves major defects affecting the homeostatic control system as discussed below.
This negative feedback model for the control of FM was first introduced nearly 60 years ago (17) and is valuable for understanding how the control of food intake is linked to changes of body weight and how defects in this control system can cause obesity. Contemporary models recognize that the control system for food intake is complex and that many factors additional to those involved in negative feedback control participate, including diet composition (3,4), environmental influences and learned responses (18), and perhaps even responses triggered by increased body adiposity itself. Based on strong evidence that EE is a modulated effector of the energy homeostasis system (19–23), changes in FM can be expected to trigger compensatory adjustments of EE.
However, uncertainty persists as to how and when EE responds to perturbations of energy storage. Some (19–22,24,25)—but not all (26,27)—studies report that compensatory changes of EE accompany experimental, voluntary, or naturalistic changes of adiposity, and it has been proposed that changes in plasma leptin levels are a key underlying mechanism linking changes in FM to adaptive changes of EE. Consistent with this hypothesis, we found that unlike the situation in normal mice, FM in leptin-deficient ob/ob mice is not a reliable determinant of EE (further work is needed to definitively address this issue), and that during exogenous leptin replacement in ob/ob mice, the plasma leptin level emerges as a key determinant of EE (2). Thus, FM may influence EE at least in part via changes of circulating leptin levels. These observations offer a biological argument in support of the recommendation that FM be included when adjusting for body size variation in comparisons involving lean and obese mice.
Ratio-based EE normalization: mathematical concerns.
The goal of normalizing EE is to eliminate the influence of body size variation per se on EE, such that the normalized EE variable does not systematically vary with body size. Only then can EE be compared across groups to determine the effect of an experimental intervention (e.g., targeted gene knockout or drug treatment). A key to assessing the success of a normalization strategy, therefore, is to formally determine if the normalized EE construct is uncorrelated with variation in body mass and its compartments. This fundamental standard is often not met when EE is divided by TBM or LBM (2,28).
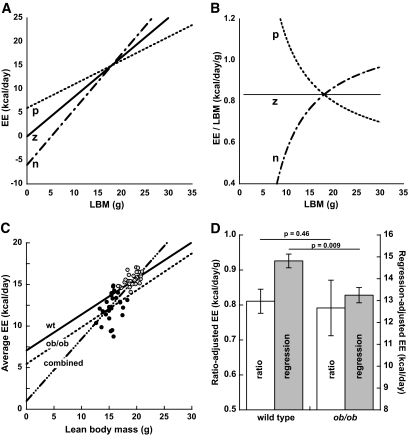
A mathematical digression illustrates why normalization of EE through division by LBM is problematic. For this ratio to be uncorrelated with LBM, EE must scale linearly with LBM such that EE = b LBM where b is a constant; only then does the relationship EE/LBM = b hold across the observed range of LBM. If the linear relationship between EE and LBM involves a nonzero y-intercept term a, such that EE = a + b LBM, then EE/LBM = a/LBM + b, a function that does not have constant value across the observed range of LBM. This concept is illustrated in Fig. 1A and B. Indeed, the relationship between EE and LBM is typically characterized by a positive y-intercept (2), reflecting heterogeneity in the contribution made to EE by individual LBM components (29). Consequently, if normalization involves division of EE by LBM, animals with larger LBM values have a preordained mathematical tendency toward lower normalized EE values (Fig. 1B). This consideration again leads us to caution against dividing EE by LBM as a routine strategy for EE normalization.
FIG. 1.
Illustration of the confounding effect of traditional ratio-based EE normalization contrasted with multiple regression analysis to control for LBM disparity. A and B: Nonzero y-intercept values confound normalization using EE/LBM. Linear regression prediction lines for mean EE values as a function of LBM can potentially have a positive (p), zero (z), or negative (n) y-intercept depending on the data (A). Fits of average daily or resting EE typically entail a positive y-intercept, whereas measures of peak or maximal EE typically entail a negative y-intercept. If the y-intercept is positive, ratios formed by dividing EE by LBM are confounded since they produce normalized EE values that decrease as LBM increases, whereas if the parent relationship has a negative y-intercept, LBM ratio-normalized EE increase as LBM increases (B). Zero-value y-intercepts arguably justify the use of ratio normalization but such relationships are uncommon, and even small departures from zero can significantly confound group comparisons (28). C and D: Comparison of ratio- vs. regression-based analysis of average daily EE controlling for LBM disparity in lean male WT C57BL/6J (circles with dots; aged 8–12 weeks; n = 32) and genetically obese ob/ob mutant mice (filled circles; aged 10–13 weeks; n = 28). EE (indirect calorimetry) was regressed on LBM (quantitative magnetic resonance) using the combined data from both genotypes (C; labeled “combined”). Although the resultant, small positive y-intercept suggests that traditional ratio normalization might be appropriate for analysis of group differences, this analysis indicates no significant EE phenotype difference between groups, whereas analysis by multiple regression discloses a significant reduction of EE in the ob/ob mice after controlling for differences in body composition (D). The offset of the two regression lines (i.e., the difference between their y-intercepts) in C corresponds to the adjusted group difference in EE. For additional analysis details see supplementary materials.
We emphasize that this recommendation is not intended as a blanket repudiation of data generated using LBM ratio–based EE normalization; indeed, regression- and LBM ratio–based approaches can yield qualitatively similar outcomes, especially when body composition is similar between groups and/or the independent variable of interest has a large effect on EE. However, when substantial differences in body composition exist between groups, or when the genetic or other effect on EE is subtle, results obtained using LBM ratio–based EE normalization can be misleading (Fig. 1C and D).
Normalization of EE by allometric scaling.
One approach to EE normalization is to employ allometric scaling in which EE is divided by body mass raised to an exponent (30,31). Specifically, the logarithm (log) of EE is regressed on log(TBM), resulting in an expression of the form: predicted arithmetic mean of log(EE) = log(a) + b log(TBM). Exponentiation yields a power equation of the form: predicted geometric mean of EE = a TBMb where a is the scaling coefficient, and b is the scaling exponent. Normalizing EE by forming the ratio EE to TBMb yields the constant a such that the value of the ratio does not systematically vary with variations in TBM.
This approach originated in the work of Rubner (30) in the late 1800s, yet remains the subject of vigorous contemporary interest (32,33). Rubner argued that resting EE per unit of BSA is essentially constant irrespective of TBM and supported this concept by measuring the BSA of shaved dog carcasses (30). This and the derivation of the BSA-body mass relationship based on Euclidean geometry placed the value of the scaling exponent at 2/3 such that resting EE scaled as TBM2/3. The relationship became known as the surface law, and the concept that metabolism per unit surface area is a constant achieved the status of medical dogma following publication in 1916 of the DuBois BSA formula (34). The surface law implemented in terms of the DuBois BSA profoundly shaped how EE and other aspects of metabolism (e.g., estimates of drug metabolism) were normalized in human studies despite the publication in 1949 of an incisive paper by Tanner (35) (who later introduced the Tanner growth stages paradigm) entitled, “Fallacy of Per-Weight and Per-Surface Area Standards, and Their Relation to Spurious Correlation.” Clearly, few investigators took notice of this aspect of Tanner's work.
Rubner's surface law was also challenged in the 1930s and 1940s by Kleiber (31,36) based on an interspecies regression analysis that encompassed mammals ranging in TBM from 21 g to 600 kg. Kleiber advocated a value for the scaling exponent of 3/4 (31,36) such that resting EE scaled as TBM3/4. Body mass raised to the 3/4 power became known as metabolic body size, and normalizing resting EE to metabolic body size using the fixed 3/4 exponent became a method of choice. Based on a large body of subsequent empirical work, however, it has become clear that there is no universally applicable, within- or between-species value of the scaling exponent for resting EE (e.g., [37] and supplementary materials, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0909/DC1), which both muddies the “meaning” of the allometric construct and has the consequence that if an investigator chooses to employ allometric normalization, the exponent must be identified based on his or her own data. This requirement places limits on the ability to compare EE results obtained across different studies. Taken together, these considerations suggest that allometric scaling is not optimal when used for comparing EE in mice, especially when groups being compared differ substantially in adiposity.
EE normalization using multiple regression to adjust for body size variation.
An alternative strategy for EE normalization that has been widely adopted in human obesity and diabetes research employs multiple regression (11,12,24,26,27,38–42). This approach estimates the unique impact on EE of an independent variable (e.g., ethnicity, sex, age, drug, genetic mutation, change in diet) by statistically controlling for the association between body mass or composition and EE. Although multiple regression has also been employed in some animal studies (10,15,25,43–47), no large-scale comparison of this approach to simpler ratio-based normalization methods had been reported in mice until recently (2).
The severe obesity phenotype of leptin-deficient ob/ob mice has long been attributed to the combined effects of increased EI and decreased EE. Although these mice exhibit robust hyperphagia, the nature of their EE phenotype remains somewhat controversial. On the one hand, leptin clearly stimulates autonomic mechanisms governing EE, and brown adipose tissue (a highly specialized type of fat tissue that dissipates heat through uncoupled mitochondrial oxidation of fatty acids) is atrophic in ob/ob mice, implying an inherent thermogenic defect. Although ob/ob mice exhibit clearly reduced EE compared with wild-type (WT) controls when normalized to total body weight, this analysis is confounded by pronounced increases of body weight and FM in mice lacking leptin. To address this concern, Breslow et al. (7) compared LBM ratio-normalized EE in ob/ob mice with age-matched WT controls and concluded that EE is paradoxically increased in leptin-deficient mice. Butler and Kozak (1) also used LBM ratio normalization to study ob/ob mice and similarly concluded that these animals have higher EE compared with WT controls.
The example of ob/ob mice highlights how different conclusions can be drawn about EE phenotypes when ratio-based normalization is employed, even when LBM is used as the denominator. We submit that an important potential source of confounding using this approach is the aforementioned problem linked to the positive y-intercept (Fig. 1A and B), which is especially concerning when comparing groups of mice that differ markedly in body composition. The use of multiple regression analysis to control for differences in body composition while comparing EE between ob/ob and WT mice effectively addresses this confounding effect. As shown in Fig. 1C and D (and in the supplementary materials), we found in using this approach that ob/ob mice exhibit a significant reduction of EE relative to WT controls, whereas the comparison based on EE/LBM did not. Although additional studies are warranted using larger sample sizes to more fully assess the effects of leptin deficiency on EE, this analysis offers prima facie evidence not only that EE is reduced in ob/ob mice, but supports our previous conclusion (2) that multiple regression analysis is superior to other methods for the analysis of EE data in mice, especially when substantial differences of body composition exist in the groups being compared.
An additional strength of multiple regression is that it does not rely upon a priori assumptions regarding the extent to which LBM or FM (or any other variable included in the model) determines EE in the animals being studied (2), i.e., it “lets the data decide.” Model selection, however, remains a crucial part of the analysis, including decisions as to whether both FM and LBM should universally be included in regression models (supplementary materials). Moreover, given appropriately rigorous measurements of EE and body composition, the sample sizes needed to support regression-based normalization are consistent with those routinely employed in mouse metabolic phenotyping studies.
Remaining questions
Limitations of indirect calorimetry.
As implied by its name, indirect calorimetry (the most common method employed to measure EE in both animal models and humans) does not measure EE directly. Rather, this method mathematically converts the measured rates of oxygen consumption (Vo2) and/or carbon dioxide production (Vco2) into EE data based on equations derived decades ago from studies in which indirect calorimetry was performed concurrently with direct calorimetry, the gold standard method for quantifying EE in animals (48). Direct calorimetry measures heat loss to assess heat production, which will equal EE unless net external work is being performed (heat loss equates with heat production under defined, steady-state conditions [rev. in {48}]).
Although direct calorimetry has been largely supplanted by user-friendly commercial systems that measure EE via indirect calorimetry, it is informative to consider the assumptions that underlie the use of the latter approach to generate EE data. Indirect calorimetry estimates EE based on mathematical relationships that specify the amounts of energy and carbon dioxide that are produced given the amounts of carbohydrate, fat, and protein that are consumed. Indirect calorimetry also provides information on the mix of metabolic fuels being combusted in the form of the respiratory quotient, which is computed from the ratio of Vco2 to Vo2 (49,50).
A limitation to the use of indirect calorimetry is that relationships between measures of gas exchange and both EE and substrate oxidation can vary across different animal models and experimental conditions (51–54). Thus, genetic or pharmacological alterations that markedly alter metabolic processes may in turn alter the stoichiometry that couples respirometric data to EE and consequently violate the assumptions (49,54–56) underlying indirect calorimetry. Others have formally questioned the accuracy of indirect calorimetry in some settings (51–53). Notably, the disparity between directly measured EE and EE predicted from respiratory gas exchange was as high as 38% and averaged 21% in careful studies involving kangaroo rats; similar discrepancies were documented for dove and quail (51). Indirect calorimetry therefore entails greater uncertainty in animals with metabolic phenotypes that differ from more standard laboratory animals.
The equations used routinely (56) for converting respirometric data to EE were derived from studies conducted largely on healthy animals between 1900 and 1940 (51) and assume that net substrate interconversion (e.g., de novo lipogenesis from glucose, ketogenesis from fatty acids, gluconeogenesis from protein) is negligible. Yet this is clearly not the case in mouse models commonly employed today, including genetic models of diabetes (e.g., db/db or NOD mice) and obesity (ob/ob and Ay mice). Since virtually any gene in the mouse genome can now be deleted or overexpressed and the consequences subsequently assessed in vivo, we live in an era of unprecedented opportunity to perturb metabolism in living animals. We suggest that validation of indirect calorimetry against direct calorimetry is once again warranted to instill confidence that experimental interventions producing novel and interesting EE phenotypes do not in and of themselves confound the measurement of EE. The need for validation is underscored by the fact that obesity often develops as a consequence of subtle but sustained states of positive energy balance (48).
Gut flora and obesity pathogenesis.
Of growing interest to the obesity field is the concept that changes of gut flora induced by dietary or other factors can influence energy homeostasis in ways that predispose to or protect against obesity (57,58). Gut bacteria constitute a metabolically significant component of the total living mass contained within human and animal bodies (57–60), exhibit mammal-like mass-specific metabolic rates (61,62), and, unlike mammalian cells, are fueled almost solely by anaerobic metabolism (63). Since the number of gut bacteria is ∼10-fold larger than the number of eukaryotic cells in the body (64), differences in gut bacterial ecology could modify the whole-animal yield of heat production per unit of oxygen consumed, and yet heat produced by gut bacteria is not quantified by indirect calorimetry (which is “blind” to energy-consuming anaerobic processes) (62). So far as we are aware, the impact of gut flora on total body EE has yet to be quantified in any species. If perturbations of gut flora are indeed linked to changes of body fat accumulation, energy balance studies that employ direct, rather than indirect, calorimetry will be important to assess the impact of gut flora on whole-body energy metabolism.
Changes of EE as an adaptive response.
Since EE is typically measured as a snapshot in time, dynamic changes of EE that influence body fat accumulation are often missed. In some experimental models (e.g., in response to a change in diet or drug administration), for example, changes of EE may occur rapidly and hence be missed by measures made only after a new steady-state of body weight has been established. Relevant in this context is the question of whether the experimental intervention evokes a “regulated” change in adiposity or instead imposes a “forced” change that will be resisted by homeostatic responses. Indeed, published evidence (21–23,65) suggests that some interventions that alter body weight do so by changing the defended level of body weight, while many others do not. This is a relevant distinction since the extent to which adaptive changes of EE as well as EI occur in various models depends on whether the new body weight is being actively defended or resisted by the homeostatic control system.
In both human and animal studies, weight loss achieved through caloric restriction (which does not reset the defended level of body weight) is associated with a reduction of EE that exceeds the reduction predicted by loss of body or lean mass alone (19,20,23,25,66), a response that promotes the recovery of lost weight and persists even when weight loss is maintained for long time durations (19,66). Yet a study from the National Weight Control Registry indicates that among subjects who successfully maintain substantial lifestyle-induced weight loss for long time intervals, resting EE was normal after adjustment for LBM, FM, and age (26). This observation raises the interesting and testable hypothesis that successful weight loss maintainers are individuals who undergo little or no regulatory compensation at the level of EE and are thus better able to maintain their body weight at a reduced level. (Although individual differences in EE are documented during overfeeding [67], little is known about individual differences in EE during underfeeding). Alternatively, weight loss maintainers may have previously “forced” their body weight to be above their biologically defended level and thus do not mount an adaptive decrease of EE following the return of weight to its original value.
Of special relevance to obesity pathogenesis is a conundrum surrounding the effects of high-fat feeding on energy balance and body fat stores. Although many factors influence weight gain in this setting, available data suggest that upon switching from standard chow to a high-fat diet, energy intake in healthy mice or rats increases maximally for the first few days and then gradually declines toward baseline values within 1–2 weeks (for a recent example see [4]). Despite this normalization of energy intake during high-fat feeding, body weight often continues to increase (relative to controls fed standard chow) for several weeks thereafter. Accordingly, one infers that EE must decline (relative to chow-fed controls) during this period, yet many studies show that EE increases during high-fat feeding (68), ostensibly as an adaptive response that limits weight gain. Detailed, continuous energy balance studies in animals following the switch to a high-fat diet are therefore warranted to definitively determine both the effect on EE and the extent to which this effect protects against or contributes to weight gain.
Should food intake, like EE, also be normalized?
If EE must be normalized to adjust for variation in body mass, should the same approach pertain to the other side of the energy balance equation? Normalization of food intake data by simple division (by TBM or LBM) is likely to be confounded for the same reason that pertains to EE. Moreover, the fact that intake data can be obtained longitudinally from the beginning of an experiment (when body weights are matched across groups) to its conclusion diminishes the need to normalize such data. If, for example, cumulative intake of one group is greater than another, and if the groups are matched with respect to body weight at study onset, the proper interpretation of this outcome is usually clear without data normalization.
The issue assumes greater complexity when group differences in TBM, body composition, or food intake exist prior to the experimental intervention, especially if they arise from differences of age, sex, or linear growth. In such cases, meaningful comparisons of intake will be confounded unless these variables can be appropriately controlled. Although multiple regression may permit insight into whether an experimental intervention affects intake after adjusting for differences in other variables, this approach has yet to be validated in a large group of mice (or any other species, so far as we are aware). Until such an analysis is undertaken, caution is warranted in efforts to compare intake between groups that differ substantially in age, body composition, or linear growth.
Concluding comments.
Butler and Kozak (1) are to be commended for raising concerns regarding ratio-based methods for normalizing EE to account for body mass variation. Our analysis emphasizes that any ratio-based method for normalizing EE to body mass compartments (whether TBM or LBM is used) can lead to confounded outcomes, leading us to recommend the use of multiple regression to control for variation in body mass and composition in murine studies where EE is an important outcome variable.
Users of indirect calorimetry should also be aware of assumptions that underlie this method and that have yet to be tested in contemporary rodent models of obesity and diabetes and of the limitations inherent in this approach (e.g., failure to detect EE arising from metabolic activity of gut bacteria). Studies that compare direct with indirect calorimetry are warranted to aid in the interpretation of phenotypic data from a growing number of animal models with significant metabolic impairment arising from genetic or other interventions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grants DK068384, DK052989, and DK083042; by the NIH-supported Nutrition Obesity Research Center (DK035816); the Diabetes Endocrine Research Center (DK017047); and the Mouse Metabolic Phenotyping Center (U24 DK076126; U24DK076169) at the University of Washington.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Butler AA, Kozak LP: A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 2010;59:323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaiyala KJ, Morton GJ, Leroux BG, Ogimoto K, Wisse B, Schwartz MW: Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 2010;59:1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Brüning JC: MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 2009;10:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh-I S, Thaler JP, Ogimoto K, Wisse BE, Morton GJ, Schwartz MW: Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am J Physiol Endocrinol Metab 2010;299:E47–E53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqi IS, O'Rahilly S: Monogenic human obesity syndromes. Recent Prog Horm Res 2004;59:409–424 [DOI] [PubMed] [Google Scholar]
- 6.White CR, Seymour RS: Allometric scaling of mammalian metabolism. J Exp Biol 2005;208:1611–1619 [DOI] [PubMed] [Google Scholar]
- 7.Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE: Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol 1999;276:E443–E449 [DOI] [PubMed] [Google Scholar]
- 8.Himms-Hagen J: On raising energy expenditure in ob/ob mice. Science 1997;276:1132–1133 [DOI] [PubMed] [Google Scholar]
- 9.Hallgren P, Sjöström L, Hedlund H, Lundell L, Olbe L: Influence of age, fat cell weight, and obesity on O2 consumption of human adipose tissue. Am J Physiol 1989;256:E467–E474 [DOI] [PubMed] [Google Scholar]
- 10.Selman C, Lumsden S, Bünger L, Hill WG, Speakman JR: Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J Exp Biol 2001;204:777–784 [DOI] [PubMed] [Google Scholar]
- 11.Nelson KM, Weinsier RL, Long CL, Schutz Y: Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr 1992;56:848–856 [DOI] [PubMed] [Google Scholar]
- 12.Bitz C, Toubro S, Larsen TM, Harder H, Rennie KL, Jebb SA, Astrup A: Increased 24-h energy expenditure in type 2 diabetes. Diabetes Care 2004;27:2416–2421 [DOI] [PubMed] [Google Scholar]
- 13.Dulloo AG, Jacquet J: An adipose-specific control of thermogenesis in body weight regulation. Int J Obes Relat Metab Disord 2001;(Suppl 25)5:S22–S29 [DOI] [PubMed] [Google Scholar]
- 14.Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR: Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 2005;82:941–948 [DOI] [PubMed] [Google Scholar]
- 15.Arch JR, Hislop D, Wang SJ, Speakman JR: Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 2006;30:1322–1331 [DOI] [PubMed] [Google Scholar]
- 16.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW: Central nervous system control of food intake and body weight. Nature 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 17.Kennedy GC: The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 1953;140:578–596 [DOI] [PubMed] [Google Scholar]
- 18.Woods SC: The eating paradox: how we tolerate food. Psychol Rev 1991;98:488–505 [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL: Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 2008;88:906–912 [DOI] [PubMed] [Google Scholar]
- 20.Leibel RL, Rosenbaum M, Hirsch J: Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621–628 [DOI] [PubMed] [Google Scholar]
- 21.Keesey RE, Corbett SW: Metabolic defense of the body weight set-point. Res Publ Assoc Res Nerv Ment Dis 1984;62:87–96 [PubMed] [Google Scholar]
- 22.Keesey RE, Corbett SW: Adjustments in daily energy expenditure to caloric restriction and weight loss by adult obese and lean Zucker rats. Int J Obes 1990;14:1079–1084 [PubMed] [Google Scholar]
- 23.Keesey RE, Hirvonen MD: Body weight set-points: determination and adjustment. J Nutr 1997;127:1875S–1883S [DOI] [PubMed] [Google Scholar]
- 24.Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA: Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab 2000;85:1087–1094 [DOI] [PubMed] [Google Scholar]
- 25.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO: Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 2004;287:R1306–R1315 [DOI] [PubMed] [Google Scholar]
- 26.Wyatt HR, Grunwald GK, Seagle HM, Klem ML, McGuire MT, Wing RR, Hill JO: Resting energy expenditure in reduced-obese subjects in the National Weight Control Registry. Am J Clin Nutr 1999;69:1189–1193 [DOI] [PubMed] [Google Scholar]
- 27.Larson DE, Ferraro RT, Robertson DS, Ravussin E: Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr 1995;62:735–739 [DOI] [PubMed] [Google Scholar]
- 28.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB: Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord 1995;19:644–652 [PubMed] [Google Scholar]
- 29.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB: Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol 1998;275:E249–E258 [DOI] [PubMed] [Google Scholar]
- 30.Rubner M: Uber den einfluss der korpergrosse auf stoff- un kraftwechsel. Z Fur Biol 1883;19:535–562[in German] [Google Scholar]
- 31.Kleiber M: Body size and metabolic rate. Physiol Rev 1947;27:511–541 [DOI] [PubMed] [Google Scholar]
- 32.West GB, Brown JH, Enquist BJ: A general model for the origin of allometric scaling laws in biology. Science 1997;276:122–126 [DOI] [PubMed] [Google Scholar]
- 33.Kolokotrones T, Van Savage, Deeds EJ, Fontana W: Curvature in metabolic scaling. Nature 2010;464:753–756 [DOI] [PubMed] [Google Scholar]
- 34.DuBois D, DuBois EF: A formula to estimate the approximate surface area if height and weight be known: 1916. Arch Intern Med 1916;17:863–871 [Google Scholar]
- 35.Tanner JM: Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol 1949;2:1–15 [DOI] [PubMed] [Google Scholar]
- 36.Kleiber M: Body size and metabolism. Hilgardia 1932;6:315–353 [Google Scholar]
- 37.White CR, Cassey P, Blackburn TM: Allometric exponents do not support a universal metabolic allometry. Ecology 2007;88:315–323 [DOI] [PubMed] [Google Scholar]
- 38.Poehlman ET, Toth MJ: Mathematical ratios lead to spurious conclusions regarding age- and sex-related differences in resting metabolic rate. Am J Clin Nutr 1995;61:482–485 [DOI] [PubMed] [Google Scholar]
- 39.Nelson KM, Weinsier RL, James LD, Darnell B, Hunter G, Long CL: Effect of weight reduction on resting energy expenditure, substrate utilization, and the thermic effect of food in moderately obese women. Am J Clin Nutr 1992;55:924–933 [DOI] [PubMed] [Google Scholar]
- 40.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E: Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15:2964–2973 [DOI] [PubMed] [Google Scholar]
- 41.Bandini LG, Must A, Spadano JL, Dietz WH: Relation of body composition, parental overweight, pubertal stage, and race-ethnicity to energy expenditure among premenarcheal girls. Am J Clin Nutr 2002;76:1040–1047 [DOI] [PubMed] [Google Scholar]
- 42.Spadano JL, Bandini LG, Must A, Dallal GE, Dietz WH: Longitudinal changes in energy expenditure in girls from late childhood through midadolescence. Am J Clin Nutr 2005;81:1102–1109 [DOI] [PubMed] [Google Scholar]
- 43.Meyer CW, Klingenspor M, Rozman J, Heldmaier G: Gene or size: metabolic rate and body temperature in obese growth hormone-deficient dwarf mice. Obes Res 2004;12:1509–1518 [DOI] [PubMed] [Google Scholar]
- 44.Meyer CW, Neubronner J, Rozman J, Stumm G, Osanger A, Stoeger C, Augustin M, Grosse J, Klingenspor M, Heldmaier G: Expanding the body mass range: associations between BMR and tissue morphology in wild type and mutant dwarf mice (David mice). J Comp Physiol [B] 2007;177:183–192 [DOI] [PubMed] [Google Scholar]
- 45.Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR: Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev 2005;126:783–793 [DOI] [PubMed] [Google Scholar]
- 46.Pamir N, McMillen TS, Kaiyala KJ, Schwartz MW, LeBoeuf RC: Receptors for tumor necrosis factor-alpha play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology 2009;150:4124–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J: Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab 2003;88:16–23 [DOI] [PubMed] [Google Scholar]
- 48.Kaiyala KJ, Ramsay DS: Direct animal calorimetry, the underused gold standard for quantifying the fire of life. Comp Biochem Physiol A Mol Integr Physiol. 25April2010[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swyer PR: Assumptions used in measurements of energy metabolism. J Nutr 1991;121:1891–1896 [DOI] [PubMed] [Google Scholar]
- 50.Lusk G: The Elements of the Science of Nutrition. 4th ed New York, Johnson Reprint Group; (reprinted 1976), 1928 [Google Scholar]
- 51.Walsberg GE, Hoffman TC: Direct calorimetry reveals large errors in respirometric estimates of energy expenditure. J Exp Biol 2005;208:1035–1043 [DOI] [PubMed] [Google Scholar]
- 52.Webb P: The measurement of energy exchange in man: an analysis. Am J Clin Nutr 1980;33:1299–1310 [DOI] [PubMed] [Google Scholar]
- 53.Webb P: The measurement of energy expenditure. J Nutr 1991;121:1897–1901 [DOI] [PubMed] [Google Scholar]
- 54.Garby L: Enthalpy changes and heat production induced by a meal. Eur J Clin Nutr 1989;43:637–639 [PubMed] [Google Scholar]
- 55.Simonson DC, DeFronzo RA: Indirect calorimetry: methodological and interpretative problems. Am J Physiol 1990;258:E399–E412 [DOI] [PubMed] [Google Scholar]
- 56.Mclean J, Tobin G: Animal and human calorimetry. London, Cambridge University Press, 1987 [Google Scholar]
- 57.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]
- 58.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI: A core gut microbiome in obese and lean twins. Nature 2009;457:480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD: High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009;137:1716–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT: Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science 2010;328:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makarieva AM, Gorshkov VG, Li BL: Energetics of the smallest: do bacteria breathe at the same rate as whales? Proc Biol Sci 2005;272:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gnaiger E: Heat dissipation and energetic efficiency in animal anoxibiosis: economy contra power. J Exp Biol 1983;228:471–490 [Google Scholar]
- 63.Guarner F, Malagelada JR: Gut flora in health and disease. Lancet 2003;361:512–519 [DOI] [PubMed] [Google Scholar]
- 64.Bengmark S: Ecological control of the gastrointestinal tract: the role of probiotic flora. Gut 1998;42:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corbett SW, Stern JS, Keesey RE: Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr 1986;44:173–180 [DOI] [PubMed] [Google Scholar]
- 66.DeLany JP, Hansen BC, Bodkin NL, Hannah J, Bray GA: Long-term calorie restriction reduces energy expenditure in aging monkeys. J Gerontol A Biol Sci Med Sci 1999;54:B5–B11 [DOI] [PubMed] [Google Scholar]
- 67.Levine JA, Eberhardt NL, Jensen MD: Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 1999;283:212–214 [DOI] [PubMed] [Google Scholar]
- 68.Kus V, Prazak T, Brauner P, Hensler M, Kuda O, Flachs P, Janovska P, Medrikova D, Rossmeisl M, Jilkova Z, Stefl B, Pastalkova E, Drahota Z, Houstek J, Kopecky J: Induction of muscle thermogenesis by high-fat diet in mice: association with obesity-resistance. Am J Physiol Endocrinol Metab 2008;295:E356–E367 [DOI] [PubMed] [Google Scholar]