Abstract
OBJECTIVE
Reactive oxygen species (ROS) is one of most important factors in impaired metabolism secretion coupling in pancreatic β-cells. We recently reported that elevated ROS production and impaired ATP production at high glucose in diabetic Goto-Kakizaki (GK) rat islets are effectively ameliorated by Src inhibition, suggesting that Src activity is upregulated. In the present study, we investigated whether the glucagon-like peptide-1 signal regulates Src activity and ameliorates endogenous ROS production and ATP production in GK islets using exendin-4.
RESEARCH DESIGN AND METHODS
Isolated islets from GK and control Wistar rats were used for immunoblotting analyses and measurements of ROS production and ATP content. Src activity was examined by immunoprecipitation of islet lysates followed by immunoblotting. ROS production was measured with a fluorescent probe using dispersed islet cells.
RESULTS
Exendin-4 significantly decreased phosphorylation of Src Tyr416, which indicates Src activation, in GK islets under 16.7 mmol/l glucose exposure. Glucose-induced ROS production (16.7 mmol/l) in GK islet cells was significantly decreased by coexposure of exendin-4 as well as PP2, a Src inhibitor. The Src kinase–negative mutant expression in GK islets significantly decreased ROS production induced by high glucose. Exendin-4, as well as PP2, significantly increased impaired ATP elevation by high glucose in GK islets. The decrease in ROS production by exendin-4 was not affected by H-89, a PKA inhibitor, and an Epac-specific cAMP analog (8CPT-2Me-cAMP) significantly decreased Src Tyr416 phosphorylation and ROS production.
CONCLUSIONS
Exendin-4 decreases endogenous ROS production and increases ATP production in diabetic GK rat islets through suppression of Src activation, dependently on Epac.
In pancreatic β-cells, glucose metabolism regulates exocytosis of insulin granules through metabolism secretion coupling, in which glucose-induced ATP production in mitochondria plays an essential role (1). Impairment of mitochondrial ATP production causes reduced glucose-induced insulin secretion.
Reactive oxygen species (ROS) is one of the most important factors that impair metabolism secretion coupling in β-cells. Exposure to exogenous hydrogen peroxide (H2O2), the most abundant ROS, reduces glucose-induced insulin secretion by impairing mitochondrial metabolism in β-cells (2,3). However, little is known of the role of endogenous ROS in impaired glucose-induced insulin secretion from β-cells. Some studies (4,5) have shown that endogenous ROS is produced in mitochondria by exposure to high glucose. In Zucker diabetic fatty rats, the superoxide content of islets at basal glucose levels is higher than that in Zucker lean control rats (4). Furthermore, we recently reported that high glucose–induced ROS production in islet cells is elevated in diabetic Goto-Kakizaki (GK) rats compared with control Wistar rats (6). Thus, endogenous ROS production is elevated in β-cells under diabetic pathophysiological conditions.
Although the mechanism of endogenous ROS production in β-cells in the diabetic state remains largely unknown, we have reported that Src (c-Src) plays an important role in the signal transduction that produces ROS (6). Src is a nonreceptor tyrosine kinase that is associated with the cell membrane and plays important roles in various signal transductions, and its activity is regulated by intramolecular interactions that depend on tyrosine phosphorylation (7,8). Phosphorylation of Tyr527 (Tyr529 in humans), which is located near the C terminus of Src, is brought about by COOH terminal Src kinase (Csk), a negative regulator of Src (9), and holds the kinase in the inactive form. Dephosphorylation of Tyr527 followed by disruption of the intramolecular interaction allows phosphorylation of Tyr416 (Tyr418 in humans) at the kinase domain, resulting in Src activation. In our previous report (6), PP2, a selective Src inhibitor, decreased high-glucose–induced ROS production in GK islet cells, in contrast to the lack of any effect of the agent in Wistar islet cells, suggesting that Src may be activated in the diabetic condition and cause elevation of ROS production in the presence of high glucose.
Glucagon-like peptide (GLP)-1 is one of the incretin peptides released from the intestine in response to nutrient ingestion that augments glucose-induced insulin secretion from β-cells (10,11). GLP-1 binding to the GLP-1 receptor, a member of the G protein–coupled receptor (GPCR) superfamily, induces activation of adenylyl cyclase and elevation of intracellular cAMP levels, which elicits protein kinase A (PKA)-dependent signal transduction. Recently, Epac (also known as cAMP-GEF [guanine nucleotide exchange factor]) has been shown to be a novel cAMP sensor in the PKA-independent pathway (12,13). In β-cells, one member of the Epac family, Epac2, has an important role in insulin secretion, especially in regulation of exocytosis of insulin granules (14,15). Previous studies have shown that GLP-1 also has beneficial long-term effects on diabetic β-cells, including induction of β-cell proliferation (16,17), enhanced resistance to apoptosis (17,18), and amelioration of endoplasmic reticulum stress (19). Furthermore, increased ROS in diabetic db/db mouse islets is decreased by treatment with an inhibitor of dipeptidyl peptidase IV that delays the degradation of GLP-1 (20).
In the present study, we investigated whether the GLP-1 signal directly ameliorates endogenous ROS production in diabetic GK islets using exendin-4, a GLP-1 receptor agonist. In particular, we focused on clarifying regulation of Src activity by GLP-1 signaling. We describe here both a novel effect and a mechanism of GLP-1 signaling that acutely decreases ROS production by high glucose through suppression of Src activation PKA independently and Epac dependently.
RESEARCH DESIGN AND METHODS
Male Wistar and GK rats were obtained from Shimizu (Kyoto, Japan). All experiments were carried out with rats that were aged ∼7–8 weeks. Nonfasting blood glucose levels were ∼160–240 mg/dl in the GK rats and ∼70–120 mg/dl in the Wistar rats used in the experiments. The animals were maintained and used in accordance with the guidelines of the animal care committee of Kyoto University.
Islet preparation.
Pancreatic islets were isolated from Wistar and GK rats by the collagenase digestion technique (6). Isolated islets were washed with Krebs Ringer bicarbonate buffer (KRBB) (in mmol/l: 129.4 NaCl, 5.2 KCl, 2.7 CaCl2, 1.3 KH2PO4, 1.3 MgSO4, and 24.8 NaHCO3 [equilibrated with 5% CO2/95% O2, pH 7.4]) containing 2.8 mmol/l glucose and cultured for ∼20 h in RPMI-1640 medium containing 5.5 mmol/l glucose and 10% FCS. Cultured islets were preincubated for 30 min at 37°C in KRBB supplemented with 0.2% BSA and 10 mmol/l HEPES (KRBB medium) containing 2.8 mmol/l glucose and incubated for the indicated times at 37°C in KRBB medium containing 16.7 mmol/l glucose with or without test materials.
Retroviral-mediated gene transfer.
Production of retroviral vectors with pCX4 was performed as previously described (21). Src kinase–negative mutant (K295M) was subcloned into pCX4pur (22). Gene transfer experiments of islets were carried out by an in vivo gene transduction method (23). Briefly, after rats were anesthetized and subjected to laparotomy, the hepatic artery with the portal vein and the splenic artery were ligated. The upper side of the celiac artery that branches from the abdominal aorta was clamped, and 100 μl of retroviral vector suspension was injected into the lower side of the clamped point of the artery. The pancreatic islets were then isolated and cultured for 48 h before the experiment. Gene expression using green fluorescent protein–expressing vector was effective in the inside of the islets, as previously reported (23).
Immunoprecipitation and immunoblotting.
Fresh or incubated islets were lysed in ice-cold lysis buffer (10 mmol/l Tris [pH 7.2], 100 mmol/l NaCl, 1 mmol/l EDTA, 1% Nonidet P-40, and 0.5% sodium deoxycholate) containing protease inhibitor cocktail (Complete; Roche, Mannheim, Germany), phosphatase inhibitor cocktail (Calbiochem, Darmstadt, Germany), and 5 mmol/l sodium pyrophosphate. For determination of Src activation, lysates were centrifuged at 560,000g for 10 min at 4°C, and the supernatant (∼2 mg of protein content/2,500 islets) was mixed with 4 μg mouse monoclonal anti-Src antibody (clone GD11; Upstate, Billerica, MA) and 30 μl washed protein G Sepharose (GE Healthcare, Uppsala, Sweden) followed by gentle rotation for 4 h at 4°C. Immunoprecipitates or islet lysates (50 μg) were subjected to immunoblotting as previously described (23). Primary antibodies used were rabbit anti–phospho-Src (Tyr416) and anti–phospho-Src (Tyr527) from Biosource (Camarillo, CA); rabbit anti-Src, anti-Csk, anti-Epac2, extracellular signal–regulated kinase (ERK) 1/2, and mouse anti–phospho-ERK1/2 (Thr202/Tyr204) from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit anti-Rap1 from Upstate; rabbit anti–phospho-Akt (Ser473) and anti-Akt from Cell Signaling (Danvers, MA); and mouse anti–β-actin from Sigma (St. Louis, MO). Secondary antibodies used were horseradish peroxidase–conjugated anti-rabbit and mouse antibody (GE Healthcare). Band intensities were quantified with Multi Gauge software (Fujifilm, Tokyo, Japan).
Measurement of ROS production.
ROS production in islet cells was measured by 2′,7′-dichlorofluorescein fluorescence (6). Briefly, cultured islets were dispersed using 0.05% trypsin/0.53 mmol/l EDTA (Invitrogen, Carlsbad, CA) and PBS. Dispersed islet cells were preincubated in KRBB medium containing 2.8 mmol/l glucose and 10 μmol/l 5-(and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Invitrogen) for 20 min at 37°C. After a 60-min incubation in 400 μl KRBB medium containing 16.7 mmol/l glucose with or without test materials, fluorescence was measured using a spectrofluorophotometer (RF-5300PC; Shimadzu, Kyoto, Japan), with excitation wavelength at 505 nm and emission wavelength at 540 nm. Fluorescence was corrected by subtracting parallel blanks and represented by fold increases of the value at time zero.
Measurement of ATP content.
ATP content in islets was determined by luminometry as previously described (6). Briefly, after preincubation, groups of 10 islets were batch incubated for 30 min in KRBB medium containing 2.8 or 16.7 mmol/l glucose with or without test materials. Incubation was stopped immediately by addition of HClO4 and sonication in ice-cold water for 10 min. They were then centrifuged, and a fraction of the supernatant was mixed with HEPES and Na2CO3. The ATP content in the supernatant of islet lysates was measured using ENLITEN luciferase/luciferin reagent (Promega, Madison, WI) with a luminometer (GloMax 20/20n; Promega).
Materials.
Exendin-4 and forskolin were purchased from Sigma. PP2 was purchased from Tocris (Ellisville, MO). PP3, H-89, myristoylated PKA inhibitor amide14–22 (PKI), LY294002, wortmannin, PD98059, and AG1478 were purchased from Calbiochem. Dibutyryl cAMP was purchased from Daiichisankyo (Tokyo, Japan). 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8CPT-2Me-cAMP) was purchased from Biolog Life Science (Bremen, Germany).
Statistical analysis.
Data are expressed as means ± SE. Statistical significance of difference was evaluated by the unpaired Student t test. P < 0.05 was considered significant.
RESULTS
Comparison of expression of Src between Wistar and GK islets.
To examine whether the expression levels of Src in GK islets differ from those in Wistar islets, immunoblotting using fresh islets was performed. As shown in Fig. 1A, the level of Src pY416, which indicates activation of Src, in GK islets was significantly higher than that in Wistar islets. The levels of Src pY527, total Src, and Csk in GK islets were significantly lower than those in Wistar islets. The levels of other Src family kinases (SFKs) were similar in Wistar and GK islets, whereas the expression of Fgr was very low and that of Fyn was undetectable (supplementary Fig. 1 in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0021/DC1). Results of immunoblotting using islets cultured for 20 h in the presence of 5.5 mmol/l glucose (supplementary Fig. 2) were similar to those shown in Fig. 1A.
FIG. 1.
Comparison of expression of Src between fresh Wistar and GK islets. Fresh islets were lysated and subjected to immunoblot analyses. Blots (50 μg of protein) were probed with anti–phospho-Src (Tyr416), anti–phospho-Src (Tyr527), anti-Src, or anti-Csk. The same blots were stripped and reprobed with anti–β-actin, respectively. Intensities of the bands were quantified with densitometric imager. The bar graphs are expressed relative to Wistar islet value corrected by β-actin level (means ± SE). *P < 0.05; †P < 0.01; ‡P < 0.001. Representative blot panels of three to five independent experiments are shown.
Exendin-4 suppresses Src activity in GK islets.
To investigate whether exendin-4 regulates Src activity, phosphorylation of Src was examined by immunoprecipitation and immunoblotting. As shown in Fig. 2A, Src pY416 was significantly decreased by 100 nmol/l exendin-4 in the presence of 16.7 mmol/l glucose in GK islets. Exendin-4 also significantly increased Src pY527 in GK islets in the same condition. On the other hand, exendin-4 did not affect Src pY416 or pY527 at high glucose in Wistar islets (Fig. 2B). Both Src pY416 and pY527 were not altered by change in glucose concentration in GK or Wistar islets (supplementary Fig. 3).
FIG. 2.
Exendin-4 suppresses Src activity at high glucose in GK islets. Effects of exendin-4 on Src activity at high glucose in GK (A) and Wistar (B) islets. After preincubation in the presence of 2.8 mmol/l glucose for 30 min, islets were incubated in the presence of 16.7 mmol/l glucose with or without 100 nmol/l exendin-4 for 10 min. Islet lysates (∼2 mg of protein) were immunoprecipitated with anti-Src antibody and subjected to immunoblot analyses. Blots were probed with anti–phospho-Src (Tyr416), anti–phospho-Src (Tyr527), or anti-Src by stripping and reprobing of the same blots. Intensities of the bands were quantified with densitometric imager. The bar graphs are expressed relative to control value corrected by Src level (means ± SE). *P < 0.05; †P < 0.01. Representative blot panels of four (A) or three (B) independent experiments are shown.
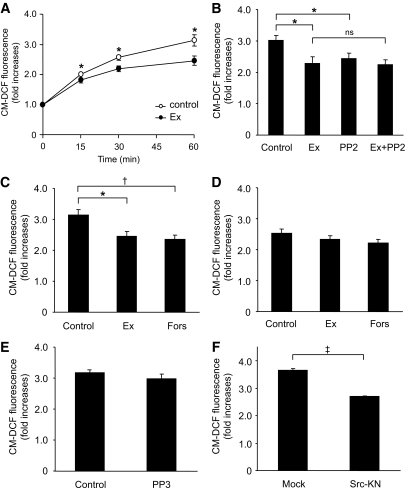
Exendin-4 decreases ROS production in GK islet cells.
We then investigated whether exendin-4 ameliorates endogenous ROS production at high glucose in GK islet cells. A total of 16.7 mmol/l glucose exposure induced ROS production in GK islet cells (Fig. 3A). Coexposure of exendin-4 significantly decreased ROS production in the presence of 16.7 mmol/l glucose at 15, 30, and 60 min. A total of 10 μmol/l PP2, a Src inhibitor, significantly decreased high-glucose–induced ROS production (Fig. 3B), but PP3, the inactive PP2 analog, did not affect it (Fig. 3E). Exendin-4 did not further decrease ROS production in the presence of PP2 (Fig. 3B), suggesting that the effect of exendin-4 is via the Src signal. The decrease in high-glucose–induced ROS production also was observed in the presence of 10 μmol/l forskolin, an adenylyl cyclase activator (Fig. 3C). High-glucose–induced ROS production in Wistar islet cells was lower than that in GK islet cells and was not changed by addition of exendin-4 or forskolin (Fig. 3D). To confirm that Src is actually involved in ROS production, we measured ROS production in GK islets expressing a kinase-negative form of Src (Src-KN) by retroviral vector. ROS production in Src-KN–expressing islets was significantly lower than that in control (Fig. 3F), demonstrating that Src regulates ROS production in GK islets.
FIG. 3.
Exendin-4 decreases ROS production at high glucose in GK islet cells. A: Time course of high-glucose–induced ROS production with or without 100 nmol/l exendin-4 in GK islet cells. After preincubation in the presence of 2.8 mmol/l glucose and 10 μmol/l CM-H2DCFDA for 20 min, dispersed islet cells were incubated in the presence of 16.7 mmol/l glucose with (●) or without (○) 100 nmol/l exedin-4 for 60 min. Fluorescence is represented as fold increases against the value at time zero. Data are expressed as means ± SE (n = 5–7). *P < 0.05 vs. control. B: Effects of exendin-4 and PP2 on high-glucose–induced ROS production at 60 min in GK islet cells. Data are expressed as means ± SE (n = 4–6). *P < 0.05. C: Effects of exendin-4 and forskolin on high-glucose–induced ROS production at 60 min in GK islet cells. Data are expressed as means ± SE (n = 5–6). *P < 0.05; †P < 0.01. D: Effects of exendin-4 and forskolin on high-glucose–induced ROS production at 60 min in Wistar islet cells. Data are expressed as means ± SE (n = 3–4). E: Effects of PP3 on high-glucose–induced ROS production at 60 min in GK islet cells. Data are expressed as means ± SE (n = 3). F: Effect of Src-KN on high-glucose–induced ROS production at 60 min in GK islet cells. Retroviral (empty vector and Src-KN vector)-mediated gene transfer to islets was carried out by in vivo gene transduction method, as described in research design and methods. Data are expressed as means ± SE (n = 3). ‡P < 0.001.
Exendin-4 increases ATP content in GK islets.
In Wistar islets, 16.7 mmol/l glucose-exposure significantly increased ATP content compared with that in the presence of 2.8 mmol/l glucose, as shown in Fig. 4B. Exendin-4, PP2, or exendin-4 plus PP2 did not affect the ATP content in the presence of 16.7 mmol/l glucose in Wistar islets. The ATP content in GK islets exposed to 16.7 mmol/l glucose was not increased compared with that in the presence of 2.8 mmol/l glucose (Fig. 4A). Exendin-4 as well as PP2 significantly increased the ATP content in the presence of 16.7 mmol/l glucose. Further increase of ATP content by combined exendin-4 and PP2 was not observed.
FIG. 4.
Exendin-4 increases ATP content at high glucose in GK islets. Effects of exendin-4 and PP2 on ATP content in the presence of high glucose for 30 min in GK (A) and Wistar (B) islets. After preincubation in the presence of 2.8 mmol/l glucose for 30 min, islets were incubated in the presence of 2.8 or 16.7 mmol/l glucose with or without 100 nmol/l exendin-4, 10 μmol/l PP2, or both for 30 min. Data are expressed as means ± SE (n = 7–8). *P < 0.05; †P < 0.01.
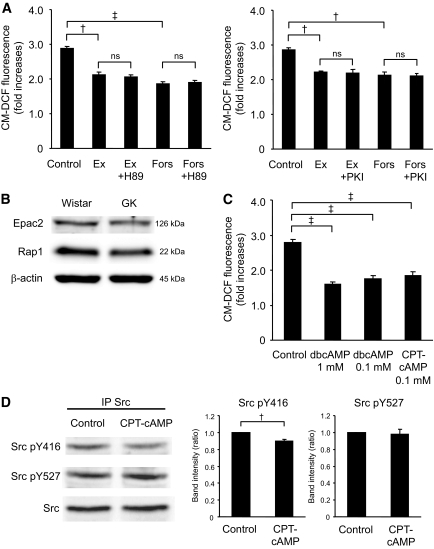
The effects of exendin-4 are dependent on Epac.
We then investigated whether the decrease in ROS production by exendin-4 is dependent on PKA. As shown in Fig. 5A, decreased ROS production by exendin-4 or forskolin was not affected by 10 μmol/l H-89 or PKI, a PKA inhibitor, indicating that the effect is PKA independent. Not only dibutyryl cAMP, a general cAMP analog, but also 8CPT-2Me-cAMP, an Epac-specific cAMP analog, decreased ROS production (Fig. 5C). Epac possesses guanine nucleotide exchange factor activity toward Rap1, a member of the Ras superfamily of small GTPases. Epac2 and Rap1 proteins were expressed similarly in both Wistar and GK islets (Fig. 5B). To determine involvement of Epac in Src activation, Src phosphorylation was examined. Src pY416 was significantly decreased by 8CPT-2Me-cAMP (Fig. 5D).
FIG. 5.
The effects of exendin-4 are dependent not on PKA but on Epac. A: Effects of H-89 or PKI on the decrease in high-glucose–induced ROS production by exendin-4 or forskolin at 60 min in GK islet cells. After preincubation in the presence of 2.8 mmol/l glucose and 10 μmol/l CM-H2DCFDA for 20 min, dispersed islet cells were incubated in the presence of 16.7 mmol/l glucose with or without 100 nmol/l exendin-4 or 10 μmol/l forskolin with or without 10 μmol/l H-89 or 10 μmol/l PKI for 60 min. Fluorescence is represented as fold increases against the value at time zero. Data are expressed as means ± SE (n = 3). †P < 0.01; ‡P < 0.001. B: Expression of Epac2 and Rap1 in Wistar and GK islets. Fresh islets were lysated and subjected to immunoblot analyses. Blots (50 μg of protein) were probed with anti-Epac2 or anti-Rap1. The same blots were stripped and reprobed with anti–β-actin, respectively. Representative blot panels of three independent experiments are shown. C: Effects of cAMP analogs on high-glucose–induced ROS production at 60 min in GK islet cells. Data are expressed as means ± SE (n = 3–4). ‡P < 0.001. D: Epac-specific cAMP analog suppresses Src activity at high glucose in GK islets. After preincubation in the presence of 2.8 mmol/l glucose for 30 min, islets were incubated in the presence of 16.7 mmol/l glucose with or without 0.1 mmol/l 8CPT-2Me-cAMP for 8 min. Islet lysates (∼2 mg of protein) were immunoprecipitated with anti-Src antibody and subjected to immunoblot analyses. Blots were probed with anti–phospho-Src (Tyr416), anti–phospho-Src (Tyr527), or anti-Src by stripping and reprobing of the same blots. Intensities of the bands were quantified with densitometric imager. The bar graphs are expressed relative to control value corrected by Src level (means ± SE). †P < 0.01. Representative blot panels of four independent experiments are shown.
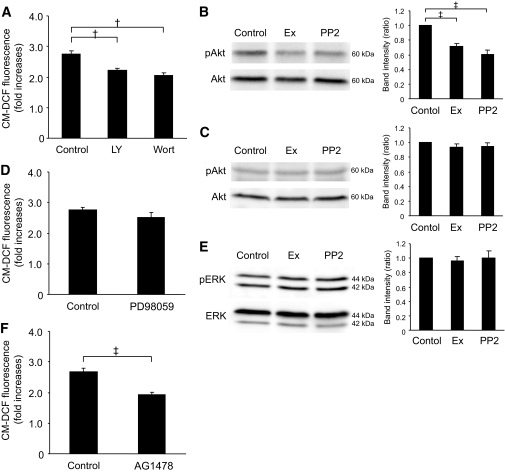
A downstream pathway of Src is PI3K/Akt signaling.
Src signalings toward downstream proteins are complex, but one of the typical pathways is phosphatidylinositol 3 kinase (PI3K)/Akt signaling (8). We therefore examined the involvement of PI3K/Akt signaling on ROS production. A total of 50 μmol/l LY294002 and 0.5 μmol/l wortmannin, both of which are PI3K inhibitors, significantly decreased ROS production in GK islets (Fig. 6A). Exendin-4 and PP2 both significantly decreased phosphorylation of Akt in GK islets (Fig. 6B) but not in Wistar islets (Fig. 6C). Considering these findings together, PI3K/Akt signaling that produces ROS is located downstream of Src activation. We also examined the involvement of mitogen-activated protein kinase signaling, another downstream pathway of Src. A total of 50 μmol/l PD98059, a MAPK-ERK kinase inhibitor, did not affect ROS production in GK islets (Fig. 6D), and neither exendin-4 nor PP2 affected phosphorylation of ERK (Fig. 6E). Several GPCR agonists have been shown to induce transactivation of epidermal growth factor receptor (EGFR) (24,25) by a mechanism involving Src (25–27) and frequently subsequent PI3K/Akt signaling (25,28). Therefore, involvement of EGFR transactivation on regulation of ROS production was examined. A total of 0.5 μmol/l AG1478, an EGFR kinase inhibitor, significantly decreased ROS production (Fig. 6F).
FIG. 6.
The downstream pathway of Src is involved in PI3K/Akt signaling. A: Effects of LY294002 or wortmannin on high-glucose–induced ROS production at 60 min in GK islet cells. After preincubation in the presence of 2.8 mmol/l glucose and 10 μmol/l CM-H2DCFDA for 20 min, dispersed islet cells were incubated in the presence of 16.7 mmol/l glucose with or without 50 μmol/l LY294002 or 0.5 μmol/l wortmannin for 60 min. Fluorescence was represented as fold increases against the value at time zero. Data are expressed as means ± SE (n = 3–5). †P < 0.01. B: Akt phosphorylation in the presence of high glucose with or without exendin-4 or PP2 in GK islets. After preincubation in the presence of 2.8 mmol/l glucose for 30 min, islets were incubated in the presence of 16.7 mmol/l glucose with or without 10 μmol/l forskolin or 10 μmol/l PP2 for 10 min. Islets were lysated and subjected to immunoblot analyses. Blots (50 μg of protein) were probed with anti–phospho-Akt or anti-Akt by stripping and reprobing of the same blots. Intensities of the bands were quantified with densitometric imager. The bar graphs are expressed relative to control value corrected by Akt level (means ± SE). ‡P < 0.001. Representative blot panels of five independent experiments are shown. C: Akt phosphorylation in the presence of high glucose with or without exendin-4 or PP2 in Wistar islets. Representative blot panels of three independent experiments are shown. D: Effects of PD98059 on high-glucose–induced ROS production at 60 min in GK islet cells. Data are expressed as means ± SE (n = 4). E: ERK phosphorylation in the presence of high glucose with or without exendin-4 or PP2 in GK islets. Blots (50 μg of protein) were probed with anti–phospho-ERK or anti-ERK by stripping and reprobing of the same blots. The bar graphs are expressed relative to control value corrected by ERK level (means ± SE). Representative blot panels of three independent experiments are shown. F: Effects of AG1478 on high-glucose–induced ROS production at 60 min in GK islet cells. Data are expressed as means ± SE (n = 5). ‡P < 0.001.
DISCUSSION
We previously reported that endogenous ROS production by high glucose in diabetic GK islets is elevated compared with that in control Wistar islets and is effectively ameliorated by Src inhibition, suggesting that Src may be activated in GK islets (6). In the present study, we first investigated whether Src activity is altered in GK islets. Immunoblotting analysis revealed that the level of Src pY416, which indicates the level of Src activation, is higher in GK islets than that in Wistar islets, despite lower levels of total Src, Src pY527, and Csk. The lower level of total Src seems to be a consequence of Src activation. Targeted degradation of active forms of Src is brought about by ubiquitination (29). The protooncogene c-Cbl, recently found to be an E3 ubiquitin ligase, mediates ubiquitination of activated Src (30). These reports suggest that increased degradation of activated Src may result in a lower level of total Src in GK islets. In addition, a lower level of Csk might cause a lower activity of the kinase in GK islets. However, Src activity is not directly regulated through phosphorylation of Tyr527 by Csk (8), and a subtle decrease in Csk activity is not believed to contribute to regulation of Src activity because of the excess amount of expression of Csk. This is supported by the findings that heterozygous disruption of ubiquitously expressed Csk does not affect the phenotype in mice, contrary to neural tube defects and embryonic lethality in homozygous deficient mice (31). Moreover, the localization of Csk in the cytosol before recruitment to the membrane for Src regulation dose not differ in Wistar and GK islets (supplementary Fig. 4). Thus, the lower expression level of Csk found in our results is not likely to play a role in the Src activation in GK islets. Activation of Src as well as elevated endogenous ROS production at high glucose in GK islets was clearly suppressed by exendin-4, which did not affect Src phosphorylation or ROS production in Wistar islets. Thus, the GLP-1 signal might well suppress activation of Src and excessive ROS production under diabetic conditions in addition to other beneficial long-term effects on β-cells.
GLP-1 induces elevation of intracellular cAMP levels and subsequent activation of PKA after binding to the GLP-1 receptor. In the present study, the effect of GLP-1 signaling, which suppresses Src activation and ROS production, was found to be independent of PKA. Epac is a PKA-independent cAMP sensor; Epac2 is expressed mainly in neuroendocrine cells including pancreatic β-cells. Epac2 regulates exocytosis of insulin granules in β-cells by mobilizing intracellular Ca2+ and interacting granule-associated proteins (14,15). Although the relationship between Epac and Src is not well known, a recent report (32) has shown that cAMP protects against hepatocyte apoptosis Epac dependently through Src and PI3K/Akt activation. Further evaluation of the role of cAMP in regulation of Src and PI3K/Akt signaling is required.
In the present study, we have shown that one of these Src signals, the PI3K/Akt signal, regulates ROS production. Furthermore, GLP-1 induces β-cell proliferation through PI3K signaling via Src and EGFR transactivation (33). Our finding that the EGFR kinase inhibitor decreases ROS production suggests that EGFR transactivation may be involved in the ROS-reducing effect of exendin-4 via Src. Under normal conditions, GPCR stimulation generally activates Src toward EGFR transactivation, frequently followed by PI3K activation (25). The present study reveals that Src and PI3K activities are upregulated in islets under diabetic conditions, which are suppressed by the GLP-1 signal. Many studies in oncology have shown that several growth factors including EGF and platelet-derived growth factor induce ROS through PI3K activation (34–36). Thus, EGFR transactivation/PI3K signaling should be activated under pathophysiologically disordered conditions. In the various states between normal and diabetic conditions, the ameliorative effects of the GLP-1 signal may differ (37). Further elucidation of these signals in the pathophysiology of diabetes should be helpful in future development of therapeutic strategies.
Previous studies have shown that the antioxidant capacity in β-cells is very low because of weak expression of antioxidant enzymes in pancreatic islets compared with that in various other tissues (38). The superoxide anion is converted by superoxide dismutase (SOD) into hydrogen peroxide that is eventually removed by glutathione peroxidase (Gpx). The expression level of MnSOD, which is localized in mitochondria, was significantly lower in GK islets than in Wistar islets, and that of of Gpx was similar in Wistar and GK islets (supplementaty Fig. 5A). However, an enzymatic assay revealed that MnSOD activity in GK islets was similar to that in Wistar islets and that it was not affected by exendin-4 or PP2 (supplementary Fig. 5B and C). These results indicate that regulation of MnSOD activity does not play a role in the suppressive effects of ROS production by exendin-4.
One of the important sites of ROS generation in β-cells is the mitochondrial electron transport chain, in which ROS generation increases according to the hyperpolarization of mitochondrial inner membrane derived from accelerated glucose metabolism (39). However, in pathophysiological conditions, NADPH oxidase may play an important role in ROS generation in β-cells. Chronic exposure to proinflammatory cytokines and abundant nutrients including glucose and palmitate augments the expression of a phagocyte-like NADPH oxidase in β-cells (40). Moreover, the expression of NADPH oxidase is increased in islets of diabetic Otsuka Long Evans Tokushima Fatty rats (41). Because Src is involved in regulation of NADPH oxidase activity (42), further examination to elucidate the site of ROS generation related to Src activation in β-cells is needed. On the other hand, previous reports have shown that ROS itself regulates Src activity (43,44) in addition to Src activity regulation of ROS production (45). To clarify this mutual causal relationship between Src and ROS, we examined ROS production in GK islets expressing Src-KN, which was found to cause a distinct decrease in high-glucose–induced ROS production. This finding demonstrates that Src activity regulates ROS production and does not contradict the possibility of a feedback regulation mechanism of ROS on Src activity (45).
The high-glucose–induced increase in ATP production is impaired in GK rats (6,46) as well as in patients with type 2 diabetes (47). In addition, islets in GK rats and human type 2 diabetes are oxidatively stressed (48–50). In the present study, exendin-4 was able to recover this impaired increase in ATP production by high glucose in GK islets as well as to decrease excessive ROS production. Thus, GLP-1 signaling may improve β-cell function in the diabetic state not only because it enhances Ca2+ efficacy of the exocytotic system of insulin granules but also because it improves impaired metabolism-secretion coupling. GLP-1 receptor agonists are widely used in treatment of type 2 diabetes for their ability to improve glucose intolerance. Their clinical beneficial effect seems to be provided not only by their insulinotropic action but also by their reduction of β-cell apoptosis and induction of β-cell proliferation (16–18). Further elucidation of endogenous ROS regulation by GLP-1 may help to clarify the mechanism of the various beneficial effects of these agents.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a research grant on Nanotechnical Medicine from the Ministry of Health, Labor, and Welfare of Japan; by scientific research grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and also by the Kyoto University Global Center of Excellence Program Center for Frontier Medicine.
No potential conflicts of interest relevant to this article were reported.
E.M. researched data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. S.F. contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. H.S., C.O., R.K., Y.S., M.S., and Y.N. researched data. M.O. contributed to the discussion and reviewed/edited the manuscript. N.I. contributed to the discussion and reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
We acknowledge the editorial assistance of Dalmen Mayer. We thank C. Kotake for excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Maechler P, Wollheim CB: Mitochondrial function in normal and diabetic beta-cells. Nature 2001;414:807–812 [DOI] [PubMed] [Google Scholar]
- 2.Krippeit-Drews P, Kramer C, Welker S, Lang F, Ammon HP, Drews G: Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol 1999;514(Pt 2):471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maechler P, Jornot L, Wollheim CB: Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem 1999;274:27905–27913 [DOI] [PubMed] [Google Scholar]
- 4.Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH: Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem 2003;278:9796–9801 [DOI] [PubMed] [Google Scholar]
- 5.Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, Ichinose K, Brownlee M, Araki E: Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun 2003;300:216–222 [DOI] [PubMed] [Google Scholar]
- 6.Kominato R, Fujimoto S, Mukai E, Nakamura Y, Nabe K, Shimodahira M, Nishi Y, Funakoshi S, Seino Y, Inagaki N: Src activation generates reactive oxygen species and impairs metabolism-secretion coupling in diabetic Goto-Kakizaki and ouabain-treated rat pancreatic islets. Diabetologia 2008;51:1226–1235 [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Harrison SC, Eck MJ: Three-dimensional structure of the tyrosine kinase c-Src. Nature 1997;385:595–602 [DOI] [PubMed] [Google Scholar]
- 8.Martin GS: The hunting of the Src. Nat Rev Mol Cell Biol 2001;2:467–475 [DOI] [PubMed] [Google Scholar]
- 9.Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H: Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 1991;351:69–72 [DOI] [PubMed] [Google Scholar]
- 10.Baggio LL, Drucker DJ: Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ: The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 12.Seino S, Shibasaki T: PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 2005;85:1303–1342 [DOI] [PubMed] [Google Scholar]
- 13.Roscioni SS, Elzinga CR, Schmidt M: Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol 2008;377:345–357 [DOI] [PubMed] [Google Scholar]
- 14.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S: cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2000;2:805–811 [DOI] [PubMed] [Google Scholar]
- 15.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG: Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 2003;278:8279–8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu G, Stoffers DA, Habener JF, Bonner-Weir S: Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–2276 [DOI] [PubMed] [Google Scholar]
- 17.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R: Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 2002;143:4397–4408 [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ: Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 2003;278:471–478 [DOI] [PubMed] [Google Scholar]
- 19.Tsunekawa S, Yamamoto N, Tsukamoto K, Itoh Y, Kaneko Y, Kimura T, Ariyoshi Y, Miura Y, Oiso Y, Niki I: Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J Endocrinol 2007;193:65–74 [DOI] [PubMed] [Google Scholar]
- 20.Cheng Q, Law PK, de Gasparo M, Leung PS: Combination of the dipeptidyl peptidase IV inhibitor LAF237 [(S)-1-[(3-hydroxy-1-adamantyl)ammo]acetyl-2-cyanopyrrolidine] with the angiotensin II type 1 receptor antagonist valsartan [N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-4-yl]methyl]-L-valine] enhances pancreatic islet morphology and function in a mouse model of type 2 diabetes. J Pharmacol Exp Ther 2008;327:683–691 [DOI] [PubMed] [Google Scholar]
- 21.Akagi T, Sasai K, Hanafusa H: Refractory nature of normal human diploid fibroblasts with respect to oncogene-mediated transformation. Proc Natl Acad Sci U S A 2003;100:13567–13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florio M, Wilson LK, Trager JB, Thorner J, Martin GS: Aberrant protein phosphorylation at tyrosine is responsible for the growth-inhibitory action of pp60v-src expressed in the yeast Saccharomyces cerevisiae. Mol Biol Cell 1994;5:283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukai E, Fujimoto S, Sakurai F, Kawabata K, Yamashita M, Inagaki N, Mizuguchi H: Efficient gene transfer into murine pancreatic islets using adenovirus vectors. J Control Release 2007;119:136–141 [DOI] [PubMed] [Google Scholar]
- 24.Daub H, Weiss FU, Wallasch C, Ullrich A: Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996;379:557–560 [DOI] [PubMed] [Google Scholar]
- 25.Rozengurt E: Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 2007;213:589–602 [DOI] [PubMed] [Google Scholar]
- 26.Eguchi S, Iwasaki H, Inagami T, Numaguchi K, Yamakawa T, Motley ED, Owada KM, Marumo F, Hirata Y: Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension 1999;33:201–206 [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Tang S, Zhou S, Ware JA: The thromboxane A2 receptor activates mitogen-activated protein kinase via protein kinase C-dependent Gi coupling and Src-dependent phosphorylation of the epidermal growth factor receptor. J Pharmacol Exp Ther 2001;296:426–433 [PubMed] [Google Scholar]
- 28.Chiu T, Santiskulvong C, Rozengurt E: EGF receptor transactivation mediates ANG II-stimulated mitogenesis in intestinal epithelial cells through the PI3-kinase/Akt/mTOR/p70S6K1 signaling pathway. Am J Physiol Gastrointest Liver Physiol 2005;288:G182–G194 [DOI] [PubMed] [Google Scholar]
- 29.Harris KF, Shoji I, Cooper EM, Kumar S, Oda H, Howley PM: Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc Natl Acad Sci U S A 1999;96:13738–13743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokouchi M, Kondo T, Sanjay A, Houghton A, Yoshimura A, Komiya S, Zhang H, Baron R: Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J Biol Chem 2001;276:35185–35193 [DOI] [PubMed] [Google Scholar]
- 31.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S: Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell 1993;73:1125–1135 [DOI] [PubMed] [Google Scholar]
- 32.Gates A, Hohenester S, Anwer MS, Webster CR: cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide-3-kinase p110 beta/alpha in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 2009;296:G764–G774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buteau J, Foisy S, Joly E, Prentki M: Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes 2003;52:124–132 [DOI] [PubMed] [Google Scholar]
- 34.Zhu QS, Xia L, Mills GB, Lowell CA, Touw IP, Corey SJ: G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood 2006;107:1847–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P, Konkol C, Hescheler J, Vantler M, Rosenkranz S: Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem 2008;283:7864–7876 [DOI] [PubMed] [Google Scholar]
- 36.Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI: EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun 2009;379:445–450 [DOI] [PubMed] [Google Scholar]
- 37.Peyot ML, Gray JP, Lamontagne J, Smith PJ, Holz GG, Madiraju SR, Prentki M, Heart E: Glucagon-like peptide-1 induced signaling and insulin secretion do not drive fuel and energy metabolism in primary rodent pancreatic beta-cells. PLoS One 2009;4:e6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiedge M, Lortz S, Drinkgern J, Lenzen S: Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997;46:1733–1742 [DOI] [PubMed] [Google Scholar]
- 39.Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R: Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol 2007;583:9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, Curi R, Newsholme P, Carpinelli AR: Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia 2007;50:359–369 [DOI] [PubMed] [Google Scholar]
- 41.Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, Tsubouchi H, Sonoda N, Kobayashi K, Sumimoto H, Nawata H: Increased expression of NAD(P)H oxidase in islets of animal models of Type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun 2005;332:927–933 [DOI] [PubMed] [Google Scholar]
- 42.Chowdhury AK, Watkins T, Parinandi NL, Saatian B, Kleinberg ME, Usatyuk PV, Natarajan V: Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J Biol Chem 2005;280:20700–20711 [DOI] [PubMed] [Google Scholar]
- 43.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P: Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol 2005;25:6391–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Xing D, Gao X: Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J Cell Physiol 2008;217:518–528 [DOI] [PubMed] [Google Scholar]
- 45.Xie Z, Cai T: Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv 2003;3:157–168 [DOI] [PubMed] [Google Scholar]
- 46.Hughes SJ, Faehling M, Thorneley CW, Proks P, Ashcroft FM, Smith PA: Electrophysiological and metabolic characterization of single β-cells and islets from diabetic GK rats. Diabetes 1998;47:73–81 [DOI] [PubMed] [Google Scholar]
- 47.Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Rabuazzo AM, Purrello F, Marchetti P: Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 2005;48:282–289 [DOI] [PubMed] [Google Scholar]
- 48.Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y: Hyperglycemia causes oxidative stress in pancreatic β-cells of GK rats, a model of type 2 diabetes. Diabetes 1999;48:927–932 [DOI] [PubMed] [Google Scholar]
- 49.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S: Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002;45:85–96 [DOI] [PubMed] [Google Scholar]
- 50.Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P: Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 2005;54:727–735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.