Abstract
OBJECTIVE
A strong association between genetic variants and obesity was found for the fat mass and obesity-associated gene (FTO). However, few details are known concerning the expression and function of FTO in skeletal muscle of patients with metabolic diseases.
RESEARCH DESIGN AND METHODS
We investigated basal FTO expression in skeletal muscle from obese nondiabetic subjects and type 1 and type 2 diabetic patients, compared with age-matched control subjects, and its regulation in vivo by insulin, glucose, or rosiglitazone. The function of FTO was further studied in myotubes by overexpression experiments.
RESULTS
We found a significant increase of FTO mRNA and protein levels in muscle from type 2 diabetic patients, whereas its expression was unchanged in obese or type 1 diabetic patients. Moreover, insulin or glucose infusion during specific clamps did not regulate FTO expression in skeletal muscle from control or type 2 diabetic patients. Interestingly, rosiglitazone treatment improved insulin sensitivity and reduced FTO expression in muscle from type 2 diabetic patients. In myotubes, adenoviral FTO overexpression increased basal protein kinase B phosphorylation, enhanced lipogenesis and oxidative stress, and reduced mitochondrial oxidative function, a cluster of metabolic defects associated with type 2 diabetes.
CONCLUSIONS
This study demonstrates increased FTO expression in skeletal muscle from type 2 diabetic patients, which can be normalized by thiazolidinedione treatment. Furthermore, in vitro data support a potential implication of FTO in oxidative metabolism, lipogenesis and oxidative stress in muscle, suggesting that it could be involved in the muscle defects that characterize type 2 diabetes.
In genomewide association studies, polymorphisms in the fat mass and obesity-associated gene (FTO) were strongly associated with an increased risk of obesity (1–3). The FTO is expressed in a number of tissues relevant to metabolic diseases, including adipose tissue and skeletal muscle (1). Whereas some investigators found that the FTO mRNA level was increased in adipose tissue from obese subjects (4), others reported a negative correlation between FTO expression and BMI (5), and a reduction in FTO gene expression was observed in adipose tissue depots from rodent models of obesity (6). Nevertheless, recent results obtained either in knockout mice (7) or in mice expressing a mutated form of FTO (FTOI367F) (8) demonstrated that a loss of function of FTO is associated with reduced body weight and fat mass, clearly supporting a role of FTO in fat accumulation.
Some FTO polymorphisms have also been associated with type 2 diabetes (9–13), but it could be due to the increased BMI (14). Although several reports suggested that FTO expression could be regulated by environmental factors, such as fasting and feeding (6,15,16), which are known to impact on insulin responsiveness, only a very few studies have specifically investigated FTO expression in relation to insulin sensitivity. A recent study reported that the age-dependant decline of FTO expression is associated with peripheral defects of glucose and lipid metabolism in adipose tissue and skeletal muscle (17). However, to date, little is known regarding the factors and the mechanisms controlling FTO expression, as well as its cellular functions, particularly in human skeletal muscle. Therefore, we investigated FTO gene expression and function in human skeletal muscle and unveiled both in vivo and in vitro a new role of FTO in the pathogenesis of type 2 diabetes.
RESEARCH DESIGN AND METHODS
All participants gave their written consent after being informed of the nature, purpose, and possible risks of the study. The protocol was approved by the ethical committees of the Hospices Civils de Lyon and performed according to French Legislation (Huriet Law). The healthy volunteers were divided into two groups based on their age. None had impaired glucose tolerance or a familial or personal history of diabetes, obesity, dyslipidemia, or hypertension. One group of 10 volunteers (control 50: four women and six men, age: 55.3 ± 3.5 years, BMI: 23.2 ± 0.5 kg/m2, A1C: 5.2 ± 0.2%) was age-matched with 10 nondiabetic obese subjects (three women and seven men, age: 52.0 ± 1.4 years, BMI: 33.3 ± 0.9 kg/m2, A1C: 5.5 ± 0.3%) and 10 obese type 2 diabetic patients (five women and five men, age: 56.2 ± 2.4 years, BMI: 32.1 ± 1.0 kg/m2, A1C: 10.1 ± 0.7%). An unrelated group of healthy lean subjects (control 25: one woman and four men, age: 25.4 ± 1.3 years, BMI: 21.5 ± 0.6 kg/m2, A1C: 5.2 ± 0.3%) served as the control group for type 1 diabetic patients (one woman and four men, age: 28.8 ± 1.5 years, BMI: 24.5 ± 1.3 kg/m2, A1C: 9.1 ± 0.4%). Type 1 diabetic patients had no familial antecedent of type 2 diabetes, and they were all treated with daily injections of insulin (45 ± 5 IU/day) (18). Another group of type 2 diabetic patients (three women and three men, age: 52.6 ± 2.9 years, BMI: 29.5 ± 6.9 kg/m2) were treated for 12 weeks with 8 mg per day of rosiglitazone (Avandia, GlaxoSmithKline, France). Patients had type 2 diabetes for at least one year and a mean duration of diagnosed diabetes of 4 ± 2 years, and all were treated with metformin alone for at least 3 months (between 1.5 and 2 g/day). They have never been treated with thiazolidinediones or insulin. Metformin treatment was not stopped during the study. They did not have complications and their disease state was of mild severity, as assessed by a mean A1C level of 7.0 ± 0.3%.
After an overnight fast, all subjects underwent a 3-h euglycemic hyperinsulinemic clamp, as described previously (18,19). Percutaneous biopsies of the vastus lateralis muscle were performed under local anesthesia, as previously described (18).
Another group of six healthy subjects (six men, age: 21.7 ± 1.5 years, BMI: 22.1 ± 1.4 kg/m2, A1C: 5.1 ± 0.2) were submitted to a 3-h hyperglycemic euinsulinemic clamp with infusion of somatostatin to inhibit endogenous insulin release (20). During this clamp, glycemia roughly doubled (preclamp, 5.1 ± 0.3; postclamp, 9.8 ± 1.2 mmol/l; P < 0.0001) to reach levels classically obtained during postprandial glycemic incursion in diabetic states.
Quantification of mRNAs.
Total RNA samples from muscles and cell cultures were purified as previously described (18,20). mRNA levels were measured by reverse transcription followed by real-time PCR using a Rotor-Gene 6000 (Corbett Research, Mortlake, Australia), as previously described (21,22). Primers were listed in supplementary Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0281/DC1. Values were normalized using hypoxanthine guanine phosphoribosyl transferase (HPRT) or TATA box binding protein mRNA, measured as reference genes, which were similar among subjects or culture conditions.
Western blot.
Muscle biopsies were lysed in PBS containing 1% NP40, 0.5% sodium desoxycholate, 0.1% SDS, an protease inhibitor cocktail, and myotubes in a buffer containing 200 mmol/l NaF, 20 mmol/l NaH2PO4, 150 mmol/l NaCl, 50 mmol/l HEPES, 4 mmol/l NaVO4, 10 mmol/l EDTA, and 2 mmol/l PMSF, with 1% Triton X, 10% glycerol, and protease inhibitor cocktail. Primary antibodies used were FTO (Abcam, ab65366), total Akt (Cell Signaling, no. 9272), phospho-Akt (Cell Signaling, no. 9271), OXPHOS cocktail (Mitosciences, MS604), with tubulin (Santa Cruz, sc-8035) or actin (Sigma A5060) as a loading control.
Protein carbonylation.
Total protein carbonylation was detected, using the Oxidized Protein Detection kit (Chemicon), as previously described (23).
FTO expression vector and recombinant adenovirus.
The cDNA sequence encoding full-length human FTO was PCR-amplified from IMAGE clone 100016381 (Geneservice U.K. Ltd.). The amplified PCR product was cloned into the pGEM-T Easy vector (Promega) and fully sequenced. It was then excised by digestion with EcoRV/NotI and cloned into the pcDNA3 vector (Invitrogen), generating the pcDNA3/FTO plasmid. Recombinant adenoviral genome encoding human FTO was generated by homologous recombination and amplified as described previously (24,25).
Cell culture.
HEK293 cells were grown in Dulbecco modified Eagle's medium (PAA, Germany) supplemented with 10% FBS. Cells were transfected in 6-well plates for 48 h with 3 μg expression plasmid for the FTO gene or with the empty vector as control, using EXGEN 500 transfecting reagent (Euromedex). C2C12 myoblasts were grown in Dulbecco modified Eagle's medium supplemented with 10% FBS. Reducing the serum to 2% induced differentiation. Primary cultures of human myotubes were initiated from satellite cells of muscle (vastus lateralis) obtained from four healthy subjects with normal glucose tolerance and insulin sensitivity (2 men/2 women, age: 47 ± 10 years, BMI: 25 ± 2 kg/m2). These cells were differentiated into myotubes as previously described (22,26). Myotubes were infected for 48 h with recombinant adenovirus expressing green fluorescent protein (GFP) (control) or FTO. Inhibition of FTO expression was performed by RNA interference using small interfering RNA against FTO (Qiagen), using the Hiperfect transfection reagent (Qiagen) for 48 h. A rhodamine-labeled GFP-22 siRNA was used as control.
Lipogenesis.
Postinfected myotubes were cultured for 24 h in a serum-free medium containing 5 mmol/l glucose. The rate of lipogenesis from [2-14C]acetate (0.75 μCi per flask; Perkin Elmer NEC-553) was then measured in the presence of 1 mmol/l acetate during a 24-h period. Labeled fatty acids were extracted according to the Folch Method (27) and then quantified by liquid scintillation counting. Data were normalized by protein concentrations.
Reactive oxygen species production.
Postinfected human myotubes were treated with BSA (0.1%) or palmitate (750 μmol/l) for 16 h, and reactive oxygen species (ROS) production was detected by the nitroblue tetrazolium assay, as previously reported (23).
Microarray analysis.
The procedure used to obtain microarray data has previously been described (7). Briefly, total RNA extracted from GFP and FTO overexpressing myotubes from four different healthy subjects was hybridized on oligonucleotide microarrays produced by the French Genopole network (RNG), consisting of 25,342 oligonucleotides of 50-mers printed on glass slides. The signal intensities of the microarray spots were loaded to R (version 2.9.2), background corrected with a offset of 50 (28), lowess normalized within arrays, quantile normalized between arrays, and log-transformed using the limma package from BioConductor (29). Only spots with a signal-to-noise ratio for Cy3 and Cy5 above 2 and present on the four slides were selected for statistical analysis. The call for differential expression was done by fitting a linear model to each probe (30). The dataset is available from the GEO database (GSE22857).
ATP synthesis.
Mitochondrial ATP synthesis in C2C12 cells was measured according to the protocol of Vives-Bauza et al. (31).
Statistical analysis.
All data are presented as means ± SEM. Normality was tested, and all variables had a Gaussian distribution. Statistical significance was determined using ANOVA when comparing age-matched groups of subjects (lean, obese, and type 2 diabetic subjects). Student paired t test was used when comparing mRNA levels before and after the clamp or rosiglitazone treatment in the same group of subjects. Correlations were analyzed using Spearman rank correlation test. Statistical significance of in vitro results was calculated according to unpaired Student t test. The threshold for significance was set at P < 0.05.
RESULTS
Metabolic characteristics of the subjects.
The metabolic characteristics of the subjects are presented in Table 1. After an overnight fast, insulin, nonesterified fatty acid, and triglyceride plasma concentrations were higher in obese individuals, with or without diabetes, than in lean control subjects. Type 2 diabetic patients also had a higher fasting glycemia. The insulin-stimulated glucose disposal rate was profoundly reduced in both obese and type 2 diabetic patients. The type 1 diabetic patients had fasting hyperglycemia compared with age-matched control subjects and displayed a slight, but nonsignificant, reduction in glucose disposal rate during the clamp, indicating that they were not insulin-resistant in contrast to obese and type 2 diabetic patients.
TABLE 1.
Clinical and metabolic characteristics of the subjects
| Control 50 | Obese | Type 2 diabetes | Control 25 | Type 1 diabetes | |
|---|---|---|---|---|---|
| n | 10 | 10 | 10 | 5 | 5 |
| Men/women | 6/4 | 7/3 | 5/5 | 4/1 | 4/1 |
| Age (years) | 55.3 ± 3.5 | 52.0 ± 1.4 | 56.2 ± 2.4 | 25.4 ± 1.3 | 28.8 ± 1.5 |
| BMI (kg/m2) | 23.2 ± 0.5 | 33.3 ± 0.9‡ | 32.1 ± 1.0‡ | 21.5 ± 0.6 | 24.5 ± 1.3 |
| A1C | 5.2 ± 0.2 | 5.5 ± 0.3 | 10.1 ± 0.7‡* | 5.2 ± 0.3 | 9.1 ± 0.4 |
| Basal | |||||
| Glucose (mmol/l)a | 4.8 ± 0.1 | 5.3 ± 0.1 | 10.5 ± 0.7‡ | 4.6 ± 0.2 | 13.5 ± 0.9† |
| Insulin (pmol/l)a | 34.5 ± 2.9 | 55.9 ± 10.7† | 68.2 ± 6.6‡ | 32.3 ± 1.5 | nd |
| Triglycerides (mmol/l)a | 0.8 ± 0.1 | 1.8 ± 0.2† | 1.9 ± 0.3‡ | 0.4 ± 0.1 | 0.5 ± 0.02 |
| Nonesterified fatty acid (μmol/l)a | 472.1 ± 46.4 | 644.5 ± 41.3† | 619.5 ± 49.1† | 492 ± 48 | 519 ± 97 |
| Glucose disposal rate [mg/(kg·min)] | 2.4 ± 0.1 | 1.8 ± 0.1† | 2.3 ± 0.3 | nd | nd |
| Glucose oxidation rate [mg/(kg·min)] | 1.1 ± 0.1 | 1.0 ± 0.2 | 1.3 ± 0.2 | 1.6 ± 0.3 | 1.5 ± 0.2 |
| Lipid oxidation rate [mg/(kg·min)] | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 | nd | nd |
| Clamp study | |||||
| Glucose (mmol/l) | 5.0 ± 0.2 | 5.2 ± 0.4 | 5.4 ± 0.2 | 4.5 ± 0.1 | 5.6 ± 0.2† |
| Insulin (pmol/l) | 810.0 ± 122.5 | 835.7 ± 150.4 | 1,184.0 ± 67.9‡* | 875 ± 48 | 949 ± 115 |
| Nonesterified fatty acid (μmol/l) | 25.7 ± 3.5 | 77.3 ± 23.8 | 93.7 ± 16.7† | nd | nd |
| Glucose disposal rate [mg/(kg·min)] | 10.6 ± 0.9 | 3.4 ± 0.5‡ | 3.7 ± 0.5‡ | 9 ± 1.4 | 8.2 ± 1.4 |
| Glucose oxidation rate [mg/(kg·min)] | 3.2 ± 0.2 | 2.1 ± 0.1‡ | 2.2 ± 0.1‡ | 3.5 ± 0.2 | 3.4 ± 0.2 |
| Lipid oxidation rate [mg/(kg·min)] | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1† | 6.9 ± 0.7 | 5.3 ± 0.8 |
Data are mean ± SEM. The clamp study refers to an euglycemic hyperinsulinemic clamp as detailed in research design and methods.
aParameters measured after an overnight fast;
†P < 0.05 and
‡P < 0.001 vs. the respective control subjects;
*P < 0.05 when comparing type 2 diabetic with obese subjects; nd, not determined.
Regulation of FTO expression in skeletal muscle.
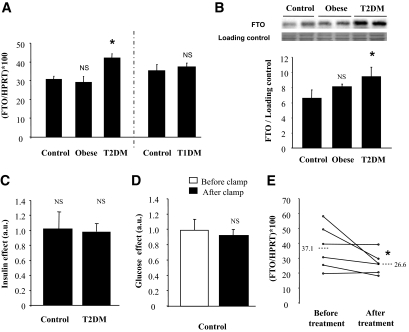
Type 2 diabetic patients had significantly higher skeletal muscle FTO mRNA levels compared either to age-matched lean control subjects or to obese nondiabetic subjects (Fig. 1A). However, there was no difference between lean control subjects and obese nondiabetic subjects (Fig. 1A). It should be noted that the FTO mRNA levels in skeletal muscle were positively correlated with A1C (r = 0.62, P = 0.04) and glycemia (r = 0.5, P = 0.02) and a tendency was observed with the basal rate of lipid oxidation (r = −0.48, P = 0.06). However, we did not observe correlation of FTO mRNA levels with glucose disposal during the clamp (r = 0.22, P = 0.43). In addition, increased FTO expression was also demonstrated at the protein level in muscle from type 2 diabetic patients (Fig. 1B). Interestingly, there was no difference in FTO mRNA levels between type 1 diabetic subjects and age-matched control subjects (Fig. 1A), suggesting that the upregulation of FTO expression observed in type 2 diabetic subjects was not related to chronic hyperglycemia (Fig. 1A).
FIG. 1.
Regulation of FTO expression in human skeletal muscle. A: Basal FTO mRNA levels were measured by real-time RT-PCR in skeletal muscle of age-matched control, obese, type 2 diabetic patients, and type 1 diabetic subjects. Values are means ± SEM (n = 5–10). *P < 0.05 versus age-matched control subjects. The mRNA level of the reference gene HPRT did not differ among groups (3.9 ± 0.5, 2.9 ± 0.1, 3.8 ± 0.5, 3.3 ± 0.3, and 4.2 ± 0.5 amo/μg total RNA, in control 50, obese, type 2 diabetic patients [T2DM], control 25, and type 1 diabetic patients [T1DM], respectively, not significant [NS]). B: Representative Western blot illustrating FTO protein levels in skeletal muscle of age-matched control, obese, and type 2 diabetic patients. Data of the histogram are means ± SEM (n = 3). *P < 0.05 versus age-matched control subjects. C and D: FTO mRNA levels were measured by real-time RT-PCR in skeletal muscle of age-matched control and type 2 diabetic patients, before and after a 3-h euglycemic hyperinsulinemic clamp (C) or a 3-h hyperglycemic euinsulinemic clamp (D). Values are means ± SEM (n = 6). E: FTO mRNA levels were measured by real-time RT-PCR in skeletal muscle of type 2 diabetic patients, before and after a 12-week rosiglitazone treatment. Values are means ± SEM (n = 6). *P < 0.05 versus before treatment. The mRNA level of the reference gene HPRT did not differ before and after rosiglitazone treatment (5 ± 0.6 and 6.6 ± 0.5 amo/μg total RNA, respectively, NS). a.u., arbitrary units.
To get more insight into the regulatory mechanisms of FTO expression, we investigated the effect of 3 h of hyperinsulinemia on FTO mRNA levels in muscle from control and type 2 diabetic patients. FTO expression was not modified during the euglycemic hyperinsulinemic clamp, in both groups of subjects (Fig. 1C). To further test whether FTO expression could be regulated by hyperglycemia, we investigated samples from healthy subjects, submitted to an acute rise of glycemia, independently from insulin (20). Once again, we did not observe significant changes in FTO expression in muscle samples taken before and after a 3-h hyperglycemic euinsulinemic clamp (Fig. 1D).
Because impaired FTO expression was observed only in tissue from type 2 diabetic patients, we investigated the effects of the insulin-sensitizer rosiglitazone on FTO expression in another group of type 2 diabetic subjects. Rosiglitazone treatment for 12 weeks reduced serum insulin levels and significantly improved glucose utilization and oxidation rates (supplementary Table 2, available in an online appendix). The treatment significantly reduced FTO mRNA levels in skeletal muscle (Fig. 1E). There was no correlation between the observed changes in FTO expression and improved insulin sensitivity in this small group of subjects (data not shown).
Effect of FTO overexpression on insulin signaling.
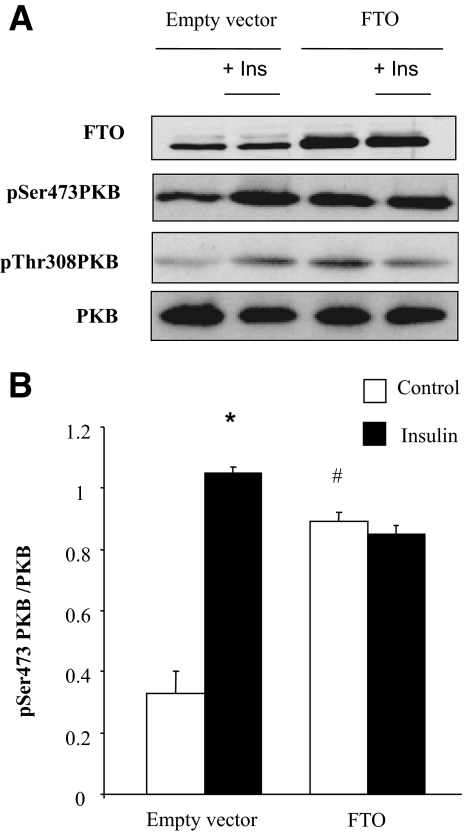
To determine whether the observed upregulation of FTO in muscle from type 2 diabetic patients could contribute to altered insulin action, we transiently overexpressed FTO in HEK293 cells and investigated the consequences on insulin signaling. A twofold increase of FTO protein was associated with a marked increase of basal protein kinase B (PKB) phosphorylation on both Ser473 and Thr308 residues (Fig. 2). Furthermore, the insulin-stimulated phosphorylation of PKB was inhibited in FTO overexpressing cells (Fig. 2).
FIG. 2.
Effect of FTO overexpression on insulin signaling in HEK293 cells. HEK293 were transiently transfected with pcDNA3-FTO or empty pcDNA3 vector (control). Forty-eight h posttransfection, cells were depleted in serum for 3 h and stimulated with insulin (10−7 M, 10 min). A: Representative Western blots of FTO, pSer473PKB, pThr308PKB, and total PKB. B: Histogram illustrates the quantification and normalization of the phosphorylation of PKB in control and FTO-overexpressing cells. Values are means ± SEM (n = 3). *P < 0.001 versus control cells, #P < 0.001 FTO versus GFP. Ins, insulin.
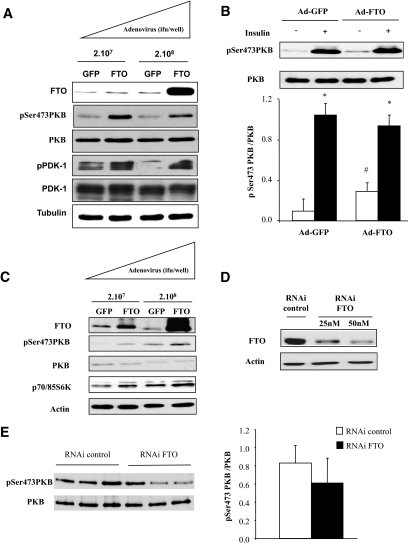
To confirm this effect in muscle cells, we constructed adenoviruses for the overexpression of either GFP (control) or FTO in differentiated myotubes. Overexpression of FTO in C2C12 myotubes (fivefold increase) induced a significant increase of basal PKB phosphorylation (Fig. 3A). In addition, the phosphorylation of PDK1, the kinase mediating the phosphorylation of PKB on Thr308, was also increased after FTO overexpression. Next, we investigated the effect of FTO on insulin-stimulated PKB phosphorylation. At a maximal concentration of insulin (10−7 M), despite a marked reduction in the amplitude of insulin to phosphorylate PKB (3-fold vs. 11-fold increase in GFP-overexpressing cells), insulin was still able to increase PKB phosphorylation in FTO-overexpressing cells (Fig. 3B). In addition, FTO overexpression induced basal phosphorylation of both PKB and p70/85-S6 kinase in human myotubes (Fig. 3C), indicating also an activation of some PKB downstream events. Silencing FTO using a specific RNAi (Fig. 3D) did not significantly modify basal Ser473 PKB phosphorylation in human myotubes (Fig. 3E).
FIG. 3.
Adenoviral overexpression of FTO in differentiated myotubes. Human myotubes or C2C12 cells were infected with recombinant adenovirus encoding human FTO or GFP (control) for 48 h. A: Representative Western blots of FTO, pSer473PKB, PKB, pPDK1, PDK1, and tubulin, in GFP- or FTO-overexpressing C2C12 myotubes. B: Representative Western blots of pSer473PKB and PKB total, in GFP- or FTO-overexpressing C2C12 myotubes (210 [7] ifu/well), under basal conditions or after insulin stimulation. Histogram represents means ± SEM (n = 4). *P < 0.05 versus basal situation, #P < 0.05 FTO versus GFP. C: Representative Western blots of FTO, pSer473PKB, PKB, p70/85S6K, and actin in human myotubes overexpressing either GFP or FTO (210 [7] ifu/well). D and E: Myotubes were transfected with siRNA control or specific for FTO for 48 h. D: Validation of FTO silencing in muscle cells. E: Representative Western blots of pSer473PKB and PKB in myotubes silencing for FTO (50 nmol/l of siRNA). Histogram illustrates the quantification and normalization of the phosphorylation of PKB in myotubes silenced for FTO. Values are means ± SEM (n = 3). ifu, inclusion-forming units.
Effect of FTO overexpression on lipogenesis and oxidative stress.
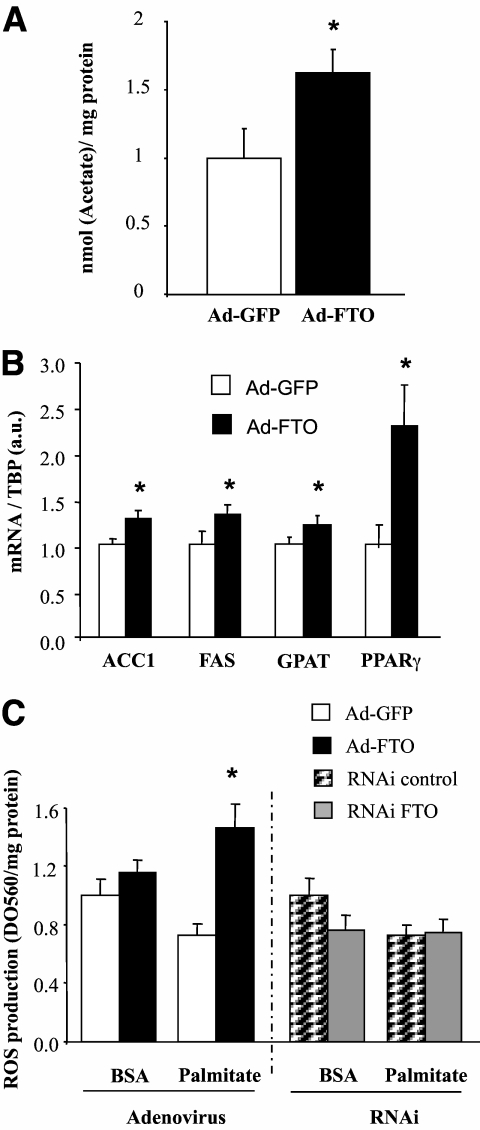
Increased lipids in muscle from type 2 diabetic patients has been associated with insulin resistance (32). In agreement with its implication in obesity, a role for FTO in lipid accumulation has been previously hypothesized (7,8). We therefore investigated the role of FTO on lipid accumulation in human myotubes. FTO overexpression significantly increased the rate of lipogenesis compared with GFP-overexpressing myotubes (Fig. 4A). In addition, the expression of key genes involved in this pathway were induced by FTO overexpression (Fig. 4B), confirming a role for FTO in the control of lipogenesis.
FIG. 4.
Effect of FTO overexpression on de novo lipogenesis. Human myotubes were infected with recombinant adenovirus encoding human FTO genome or GFP (control) for 48 h (210 [7] ifu/well). A: De novo lipogenesis was measured with [2-14C]acetate during 24 h. Values are means ± SEM (n = 3). *P < 0.05. B: mRNA levels of FAS, ACC1, GPAT, and PPARγ were measured by real-time RT-PCR. Data represent means ± SEM (n = 4).*P < 0.05. C: Palmitate-induced ROS production either in human myotubes overexpressing either GFP or FTO, or in myotubes silencing for FTO (siRNA, 50 nmol/l). After 48 h of infection/transfection, myotubes were incubated with BSA or palmitate (750 μmol/l) for 16 h, and ROS production was measured using nitro-blue tetrazolium chloride assay. Values represent means ± SEM (n = 3).*P < 0.05. FAS, fatty acid synthase; ACC1, acetyl-CoA carboxylase 1; GPAT, glycerol-3 phosphate acyltransferase; PPARγ, peroxisome proliferator–activated receptor γ; a.u., arbitrary units; ifu, inclusion-forming units.
Lipids are known to induce the production of ROS in skeletal muscle (23), and ROS have been implicated in insulin resistance and diabetes (33). We therefore investigated whether FTO could modify lipid-induced ROS production in human myotubes. As shown in Fig. 4C, we did not observe a palmitate-induced ROS production in GFP overexpressing myotubes under our experimental conditions. In contrast, a robust increase of palmitate-induced ROS production was observed in cells overexpressing FTO (Fig. 4C). A tendency to increased basal ROS production was also noticeable in response to FTO overexpression (P = 0.07). Silencing FTO did not modify palmitate-induced ROS production, whereas it tended to reduce basal ROS production (Fig. 4C).
Effect of FTO overexpression on mitochondria function.
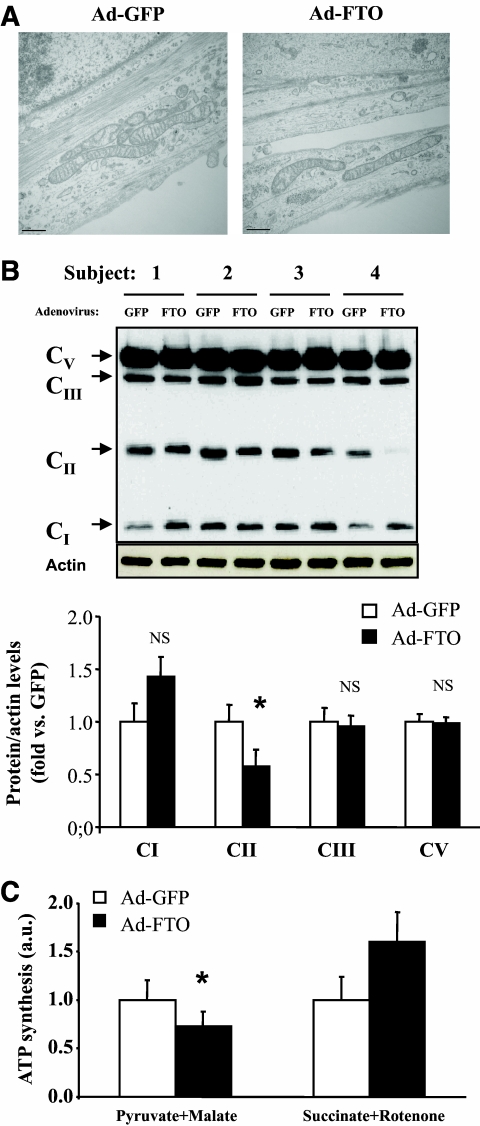
Increases of both lipogenesis and ROS production in myotubes could be related to reduced mitochondria function. This hypothesis is supported by the in vivo negative correlation of FTO expression with lipid oxidation observed in the present study and already reported by others (17). To test this hypothesis, we first performed a transcriptomic analysis to identify genes regulated by FTO overexpression in human myotubes. Microarrays analysis led to the identification of 1,103 regulated spots (for adjusted P value < 0.05 and fold change > 1.2 ) coding for 1,080 unique genes (542 upregulated and 538 downregulated genes). Using David (http://david.abcc.ncifcrf.gov/) to analyze this set of genes, the Gene Ontology class “mitochondrion” showed significant overrepresentation compared with its representation in the human genome (GO:0005739, adjusted P value = 2.13 × 10−3). The corresponding genes (n = 76) are listed in Table 2. Then, we further performed structural and functional analysis of mitochondria. Electronic microscopy analysis of human myotubes overexpressing FTO showed no major structural modification, compared with GFP (Fig. 5A). Analysis of protein levels of individual OXPHOS complexes revealed a significant reduction in complex II 30 kDa subunit, whereas the expression of NDUFB8, CIII-core2, and CV subunit alpha (characteristics of complexes I, III, and V, respectively) was not altered in human myotubes (Fig. 5B). In addition, PPARγ coactivator 1 (PGC1α) mRNA levels were not significantly modified in myotubes overexpressing FTO compared with GFP (PGC1α/TATA box binding protein [fold vs. GFP]: 1.0 ± 0.3 vs. 1.1 ± 0.1, P = 0.64, respectively). Lastly, we investigated the repercussion of FTO overexpression on in vitro substrate-mediated ATP synthesis. As shown in Fig. 5C, FTO overexpression reduced complex I-mediated ATP synthesis in C2C12 cells, whereas ATP synthesis mediated by complex II substrates was not significantly modified.
TABLE 2.
Mitochondria genes regulated by FTO overexpression in human myotubes
| Symbol | Gene name | GeneID | Fold change |
|---|---|---|---|
| ACOT9 | Acyl-CoA thioesterase 9 | 23597 | −1.25 |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 | 2182 | −1.56 |
| AK2 | Adenylate kinase 2 | 204 | −1.47 |
| ALDH7A1 | Aldehyde dehydrogenase 7 family, member A | 501 | 1.2 |
| ATP5B | ATP synthase, H + transporting, mitochondrial F1 complex, beta polypeptide | 506 | −1.47 |
| ATP5I | ATP synthase, H + transporting, mitochondrial F0 complex, subunit E | 521 | 1.35 |
| ATP5J2 | ATP synthase, H + transporting, mitochondrial F0 complex, subunit F2 | 9551 | 1.25 |
| BNIP3 | BCL2/adenovirus E1B 19kDa interacting protein 3 | 664 | 1.36 |
| CABC1 | Chaperone, ABC1 activity of bc1 complex homolog (S. pombe) | 56997 | −1.32 |
| CCDC56 | Coiled-coil domain containing 56 | 28958 | −1.43 |
| CKB | Creatine kinase, brain | 1152 | −1.54 |
| COQ2 | Coenzyme Q2 homolog, prenyltransferase | 27235 | 1.2 |
| COX18 | COX18 cytochrome c oxidase assembly homolog | 285521 | 1.32 |
| COX5B | Cytochrome c oxidase subunit Vb | 1329 | −1.37 |
| CTSB | Cathepsin B | 1508 | −1.35 |
| CYB5B | Cytochrome b5 type B(outer mitochondrial membrane) | 80777 | 1.22 |
| CYBA | Cytochrome b-245, alpha polypeptide | 1535 | −1.27 |
| CYC1 | Cytochrome c-1 | 1537 | −1.27 |
| DGUOK | Deoxyguanosine kinase | 1716 | 1.2 |
| DPYSL2 | Dihydropyrimidinase-like 2 | 1808 | 1.24 |
| FAM82A2 | Family with sequence similarity 82, member A2 | 55177 | 1.21 |
| GATM | Glycine amidinotransferase (L-arginine:glycine amidinotransferase) | 2628 | 1.37 |
| GPX1 | Glutathione peroxidase 1 | 2876 | −1.43 |
| HEBP1 | Heme binding protein 1 | 50865 | 1.27 |
| HIBCH | 3-hydroxyisobutyryl-Coenzyme A hydrolase | 26275 | −1.41 |
| HSD17B10 | Hydroxysteroid (17-beta) dehydrogenase 10 | 3028 | −1.32 |
| IDH3B | Isocitrate dehydrogenase 3 (NAD+) beta | 3420 | 1.3 |
| IDH3G | Isocitrate dehydrogenase 3 (NAD+) gamma | 3421 | 1.2 |
| ISCA1 | Iron-sulfur cluster assembly 1 homolog (S. cerevisiae) | 81689 | −1.27 |
| ISOC2 | Isochorismatase domain containing 2 | 79763 | −7.69 |
| KIF1B | Kinesin family member 1B | 23095 | −1.56 |
| LACTB | Lactamase, beta | 114294 | −1.25 |
| LONP1 | Lon peptidase 1, mitochondrial | 9361 | 1.23 |
| MCAT | Malonyl CoA:ACP acyltransferase | 27349 | 1.28 |
| MLYCD | Malonyl-CoA decarboxylase | 23417 | 1.23 |
| MRPL13 | Mitochondrial ribosomal protein L13 | 28998 | 1.24 |
| MRPL20 | Mitochondrial ribosomal protein L20 | 55052 | −1.32 |
| MRPL27 | Mitochondrial ribosomal protein L27 | 51264 | 1.2 |
| MRPL30 | Mitochondrial ribosomal protein L30 | 51263 | 1.26 |
| MRPL44 | Mitochondrial ribosomal protein L44 | 65080 | 1.25 |
| MRPL47 | Mitochondrial ribosomal protein L47 | 57129 | 1.25 |
| MRPS22 | Mitochondrial ribosomal protein S22 | 56945 | 1.32 |
| MT-COI | Cytochrome c oxidase | 4512 | 1.24 |
| MTIF2 | Mitochondrial translational initiation factor 2 | 4528 | 1.35 |
| NAPG | N-ethylmaleimide-sensitive factor attachment protein, gamma | 8774 | 1.23 |
| NDUFA11 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 11, | 126328 | 1.22 |
| NDUFA12 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 12 | 55967 | 1.2 |
| NDUFA3 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 3, | 4696 | −1.41 |
| NDUFA6 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 6, | 4700 | 1.21 |
| NDUFC1 | NADH dehydrogenase (ubiquinone) 1, subcomplex unknown, 1 | 4717 | −1.25 |
| NDUFS5 | NADH dehydrogenase (ubiquinone) Fe-S protein 5, 15kDa | 4725 | −1.35 |
| PARS2 | Prolyl-tRNA synthetase 2, mitochondrial (putative) | 25973 | −1.43 |
| PHB2 | Prohibitin 2 | 11331 | 1.2 |
| PMPCB | Peptidase (mitochondrial processing) beta | 9512 | 1.22 |
| PPIF | Peptidylprolyl isomerase F | 10105 | 1.38 |
| PRDX4 | Peroxiredoxin 4 | 10549 | −1.33 |
| PTCD3 | Pentatricopeptide repeat domain 3 | 55037 | 1.2 |
| PTRF | Polymerase I and transcript release factor | 284119 | 1.3 |
| RAB32 | RAB32, member RAS oncogene family | 10981 | 1.23 |
| RTN4IP1 | Reticulon 4 interacting protein 1 | 84816 | 1.23 |
| SARS2 | Seryl-tRNA synthetase 2, mitochondrial | 54938 | 1.88 |
| SCP2 | Sterol carrier protein 2 | 6342 | −1.25 |
| SLC27A3 | Solute carrier family 27 (fatty acid transporter), member 3 | 11000 | −1.28 |
| SOD2 | Superoxide dismutase 2, mitochondrial | 6648 | −1.28 |
| SPNS1 | Spinster homolog 1 (Drosophila) | 83985 | 1.22 |
| SUPV3L1 | Suppressor of var1, 3-like 1 (S. cerevisiae) | 6832 | 1.22 |
| TACO1 | Translational activator of mitochondrially encoded cytochrome c oxidase I | 51204 | 1.36 |
| TIMM10B | Racture callus 1 homolog (rat) | 26515 | 1.24 |
| TIMM23 | Translocase of inner mitochondrial membrane 23 homolog (yeast) | 10431 | −1.32 |
| TIMM8A | Translocase of inner mitochondrial membrane 8 homolog A (yeast) | 1678 | 1.28 |
| TIMM8B | Translocase of inner mitochondrial membrane 8 homolog B (yeast) | 26521 | 1.22 |
| TIMM9 | Translocase of inner mitochondrial membrane 9 homolog (yeast) | 26520 | 1.23 |
| TMLHE | Trimethyllysine hydroxylase, epsilon | 55217 | 1.2 |
| TOMM34 | Translocase of outer mitochondrial membrane 34 | 10953 | 1.32 |
| UQCR | Ubiquinol-cytochrome c reductase | 10975 | −1.27 |
| VHL | Von Hippel-Lindau tumor suppressor | 7428 | 1.24 |
FIG. 5.
Effect of FTO overexpression on mitochondria structure and function in human myotubes. Human myotubes were infected with recombinant adenovirus encoding human FTO genome or GFP (control) for 48 h (210 [7] ifu/well). A: Electronic microscopy analysis of human myotubes overexpressing GFP or FTO. B: Analysis of the expression of respiratory chain complexes in human myotubes overexpressing GFP or FTO. It should be noted that complex IV was not detected in our conditions. Histograms represent the means ± SEM (n = 4). *P < 0.05. NS, not significant. C: Analysis of complex I (pyruvate + malate)- and complex II (succinate + rotenone)-mediated ATP synthesis in GFP- and FTO-overexpressing myotubes. Histograms represent the means ± SEM (n = 4). *P = 0.02. a.u., arbitrary units; ifu, inclusion-forming units. (A high-quality color representation of this figure is available in the online issue.)
Decreased expression of FTO-regulated genes in muscle of type 2 diabetic patients.
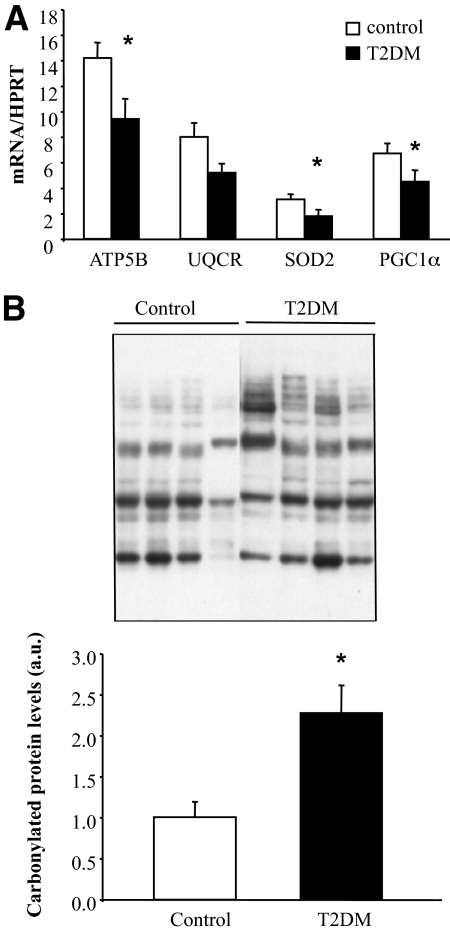
To confirm in vivo the relevance of our in vitro observations, we measured some FTO-regulated mitochondria genes in skeletal muscle of control and type 2 diabetic patients. We measured the expression of three FTO-regulated genes, selected in Table 2 on their implication on oxidative metabolism (ATP5B, UQCR) and oxidative stress (SOD2). As observed in vitro, we found a significant reduction of ATP5B and SOD2 in muscle of type 2 diabetic patients compared with control subjects (Fig. 6A), whereas a tendency to reduction was observed for UQCR (P = 0.06). In addition, PGC1α expression is significantly reduced in muscle of type 2 diabetic patients (Fig. 6A). Lastly, protein carbonylation is induced in muscle of type 2 diabetic patients (Fig. 6B), indicating an increase of oxidative stress.
FIG. 6.
Reduced OXPHOS and antioxidant genes and increased oxidative stress in skeletal muscle of type 2 diabetic patients. A: mRNA levels of ATP5B, UQCR, SOD2, and PGC1α were measured by real-time RT-PCR in skeletal muscle of control and type 2 diabetic patients. Data represent means ± SEM (n = 10). *P < 0.05. B: Immunoblot showing total protein carbonylation in skeletal muscle of control and type 2 diabetic patients. Histogram represents means ± SEM (n = 4).*P < 0.05. ATP5B, ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide; UQCR, ubiquinol-cytochrome c reductase; SOD2, superoxide dismutase 2; a.u., arbitrary units; T2DM, type 2 diabetes.
DISCUSSION
While variations of the FTO gene are associated with obesity and type 2 diabetes, the in vivo regulation of FTO and its function are still largely unknown. Here, we demonstrate that FTO expression is increased in skeletal muscle from type 2 diabetic patients and that rosiglitazone treatment partially reversed this defect. In addition, FTO overexpression in cultured myotubes enhanced basal PKB phosphorylation, increased lipogenesis and lipid-induced ROS production, and reduced mitochondria oxidative function. Because in vitro effects of FTO were partially confirmed in muscle of type 2 diabetic patients, we suggest that increased FTO expression in these patients may contribute to reduced mitochondria oxidative capacities, excessive lipid accumulation, and oxidative stress, a cluster of metabolic defects known to be associated with type 2 diabetes.
We report for the first time a significant increase of both FTO mRNA and protein levels in vastus lateralis muscle from type 2 diabetic patients, compared either to healthy lean control subjects or to BMI-matched obese nondiabetic individuals. However, we found no correlation between FTO expression and insulin sensitivity as estimated by glucose disposal rate during the hyperinsulinemic clamp. This finding may indicate that FTO induction was related to diabetes per se rather than insulin resistance, a conclusion also supported by the fact that FTO mRNA levels were not altered in muscle of insulin-resistant obese subjects. Concerning the regulation of FTO expression, previous data in mice demonstrated that FTO expression could be affected by environmental factors, such as fasting and feeding (6,15,16), suggesting a possible regulation by hormones and/or nutrients. However, we did not observe rapid regulation of FTO mRNA by either insulin or glucose during specific clamp studies, suggesting that FTO expression is not acutely regulated by insulin or glucose in human skeletal muscle, as recently reported (17,34). The unaltered expression of FTO in skeletal muscle from type 1 diabetic patients is in agreement with the absence of a direct regulation of FTO during hyperglycemic clamp. This observation reinforces the concept that increased level of FTO in muscle is a characteristic of type 2 diabetes, independently from obesity and whole-body insulin resistance and also from hyperglycemia. Interestingly, therapy with the antidiabetic agent rosiglitazone produced a significant reduction in the expression of FTO in muscle of type 2 diabetic patients. Unfortunately, up to now, the relationship between insulin sensitivity and loss of FTO function has not been systematically examined in mice or in humans (7,8,35,36), although it is interesting to point out that invalidation of FTO in mice has been associated with a mild improvement of insulin sensitivity (7).
Our in vitro overexpression experiments showed that FTO increased the basal phosphorylation of major actors of the insulin signaling cascade, such as PDK1, PKB, and p70/85S6K. In muscle cells, however, FTO did not appear to inhibit the effect of insulin on PKB activation, suggesting that the upstream steps of the IRS1/PI3-kinase pathway are not acutely affected by FTO in muscle cells. However, FTO inhibited insulin-induced PKB phosphorylation in HEK293 cells, supporting the concept that FTO is able to affect, directly or indirectly, the signaling pathways. While the increased phosphorylation of PKB on Thr308 is probably related to induced PDK1 activity, it is more difficult to explain increased Ser473 PKB phosphorylation because several PKB-Ser473 kinases, including the rictor-mTOR complex, are known. Alternatively, one cannot exclude that PKB-specific phosphatases were inhibited in FTO overexpressing cells.
A new interesting finding is that FTO overexpression induced lipid accumulation, oxidative stress, and mitochondrial dysfunction in myotubes. Alterations of these biological processes have clearly been associated with type 2 diabetes (33,37). Interestingly, expression of multiple components of the mitochondrial respiratory chain were altered by FTO overexpression, including six complex I, two complex III, one complex IV, and three complex V. Whereas no genes of complex II were altered by FTO overexpression in our transcriptomic analysis, we found that FTO induced a significant reduction, at protein level, of the iron sulfur subunit of complex II, indicating that complex II was also controlled by FTO overexpression. We sought to determine whether these changes might be occurring via PGC1α, a transcriptional coactivator that regulates mitochondrial biogenesis and plays a crucial role in the regulation of genes involved in oxidative phosphorylation in muscle (38). Interestingly, FTO encodes a 2-oxoglutarate-dependent nucleic acid demethylase (4), and methylation of the promoter of PGC1α was altered in muscle of type 2 diabetic patients (39). However, we found no modification of PGC1α mRNA levels after FTO overexpression in human myotubes. Nevertheless, PGC1α mRNA levels are barely detectable in myotubes; we cannot exclude that a subtle change of PGC1α expression occurred. Nevertheless, we observed, as previously reported (40–42), a significant reduction of PGC1α expression in muscle of type 2 diabetic patients compared with control subjects, indicating that PGC1α and FTO are regulated in an opposite manner in these patients. In a recent study, a positive correlation between FTO and PGC1α mRNA levels were observed in young and elderly twins (17). This correlation was obtained with nondiabetic subjects and thus cannot be compared with our study. All together, our data suggest that PGC1α is probably not involved in FTO-induced alterations of mitochondria genes under our experimental conditions. Interestingly, the FTO-induced modifications of mitochondria transcriptome is associated with a reduction of oxidative metabolism, as suggested by the decrease of complex I-mediated ATP synthesis. Coupled with the increase of lipid synthesis, these data suggest that FTO induces a shift from oxidation of lipid toward their accumulation in myotubes. These data are in accordance with a recent study showing a negative association between muscle FTO expression and lipid oxidation in a large cohort of young and old subjects (17). It was also recently demonstrated that mice with a dominant mutation in the mouse FTO gene (FTOI367F) showed increased fatty acid gene expression in skeletal muscle (8). Although this data could suggest enhanced lipid synthesis, this latter was not measured and no conclusion could be drawn regarding the relationship between FTO and lipid storage in muscle. In contrast, our data clearly indicated that increased expression of FTO in myotubes is associated with enhanced lipogenesis.
Strong similarities exist between FTO function in myotubes and alterations in skeletal muscle of type 2 diabetes. Diabetic muscle was characterized by decreased expression of oxidative phosphorylation genes (41,42), increased lipid accumulation (37), and increased oxidative stress (33). All these metabolic alterations are reproduced by overexpression of FTO in human myotubes. Interestingly, we found a reduction of OXPHOS and antioxidant genes and an increase of protein carbonylation in muscle of type 2 diabetic patients, confirming partially in vitro data. Taken together, these data suggest that the increased FTO in muscle of type 2 diabetic patients may contribute to altered oxidative metabolism and increased oxidative stress, which characterize the muscle of these patients. Additional studies are clearly needed now to more precisely investigate the in vivo repercussions of skeletal muscle FTO expression.
In conclusion, we demonstrate that type 2 diabetes is associated with increased FTO expression, reduced expression of OXPHOS and antioxidant genes, and increased oxidative stress in human skeletal muscle. Overexpression of FTO in myotubes induces basal PKB phosphorylation, increases lipogenesis and oxidative stress, and reduces mitochondria oxidative function. Therefore, we propose that FTO may contribute to the muscle defects that characterize type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from INSERM and ANR (grant ANR-09-JCJC-0116-01 to J.R.). A.B. is a recipient of a grant from Servier Laboratories and ANRT. GlaxoSmithKline sponsored the rosiglitazone treatment study. No other potential conflicts of interest relevant to this article were reported.
A.B. researched data, contributed to discussion, and reviewed/edited the manuscript. E.L., E.D., J.V., and N.P. contributed to discussion. E.M. and S.P. researched data. R.R.-L., M.L., and H.V. contributed to discussion and reviewed/edited the manuscript. J.R. researched data, contributed to discussion, and wrote the manuscript.
We acknowledge the editorial assistance of Elisabeth Harley, Servier Laboratories. We thank the community imaging center of Laennec and the IFR62 for access to platforms.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI: A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougnères P, Kovacs P, Marre M, Balkau B, Cauchi S, Chèvre JC, Froguel P: Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–726 [DOI] [PubMed] [Google Scholar]
- 3.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orrú M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR: Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 2007;3:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wåhlén K, Sjölin E, Hoffstedt J: The common rs9939609 gene variant of the fat mass- and obesity-associated gene FTO is related to fat cell lipolysis. J Lipid Res 2008;49:607–611 [DOI] [PubMed] [Google Scholar]
- 5.Klöting N, Schleinitz D, Ruschke K, Berndt J, Fasshauer M, Tönjes A, Schön MR, Kovacs P, Stumvoll M, Blüher M: Inverse relationship between obesity and FTO gene expression in visceral adipose tissue in humans. Diabetologia 2008;51:641–647 [DOI] [PubMed] [Google Scholar]
- 6.Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, Zeltser LM, Chung WK, Leibel RL: Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R1185–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U: Inactivation of the Fto gene protects from obesity. Nature 2009;458:894–898 [DOI] [PubMed] [Google Scholar]
- 8.Church C, Lee S, Bagg EA, McTaggart JS, Deacon R, Gerken T, Lee A, Moir L, Mecinović J, Quwailid MM, Schofield CJ, Ashcroft FM, Cox RD: A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet 2009;5:e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herder C, Rathmann W, Strassburger K, Finner H, Grallert H, Huth C, Meisinger C, Gieger C, Martin S, Giani G, Scherbaum WA, Wichmann HE, Illig T: Variants of the PPARG, IGF2BP2, CDKAL1, HHEX, and TCF7L2 genes confer risk of type 2 diabetes independently of BMI in the German KORA studies. Horm Metab Res 2008;40:722–726 [DOI] [PubMed] [Google Scholar]
- 10.Yajnik CS, Janipalli CS, Bhaskar S, Kulkarni SR, Freathy RM, Prakash S, Mani KR, Weedon MN, Kale SD, Deshpande J, Krishnaveni GV, Veena SR, Fall CH, McCarthy MI, Frayling TM, Hattersley AT, Chandak GR: FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia 2009;52:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng MC, Park KS, Oh B, Tam CH, Cho YM, Shin HD, Lam VK, Ma RC, So WY, Cho YS, Kim HL, Lee HK, Chan JC, Cho NH: Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes 2008;57:2226–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legry V, Cottel D, Ferrières J, Arveiler D, Andrieux N, Bingham A, Wagner A, Ruidavets JB, Ducimetière P, Amouyel P, Meirhaeghe A: Effect of an FTO polymorphism on fat mass, obesity, and type 2 diabetes mellitus in the French MONICA Study. Metabolism 2009;58:971–975 [DOI] [PubMed] [Google Scholar]
- 13.Sanghera DK, Ortega L, Han S, Singh J, Ralhan SK, Wander GS, Mehra NK, Mulvihill JJ, Ferrell RE, Nath SK, Kamboh MI: Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet 2008;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freathy RM, Timpson NJ, Lawlor DA, Pouta A, Ben-Shlomo Y, Ruokonen A, Ebrahim S, Shields B, Zeggini E, Weedon MN, Lindgren CM, Lango H, Melzer D, Ferrucci L, Paolisso G, Neville MJ, Karpe F, Palmer CN, Morris AD, Elliott P, Jarvelin MR, Smith GD, McCarthy MI, Hattersley AT, Frayling TM: Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes 2008;57:1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredriksson R, Hägglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, Levine AS, Lindblom J, Schiöth HB: The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 2008;149:2062–2071 [DOI] [PubMed] [Google Scholar]
- 16.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, Galvanovskis J, Rorsman P, Robins P, Prieur X, Coll AP, Ma M, Jovanovic Z, Farooqi IS, Sedgwick B, Barroso I, Lindahl T, Ponting CP, Ashcroft FM, O'Rahilly S, Schofield CJ: The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunnet LG, Nilsson E, Ling C, Hansen T, Pedersen O, Groop L, Vaag A, Poulsen P: Regulation and function of FTO mRNA expression in human skeletal muscle and subcutaneous adipose tissue. Diabetes 2009;58:2402–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducluzeau PH, Perretti N, Laville M, Andreelli F, Vega N, Riou JP, Vidal H: Regulation by insulin of gene expression in human skeletal muscle and adipose tissue. Evidence for specific defects in type 2 diabetes. Diabetes 2001;50:1134–1142 [DOI] [PubMed] [Google Scholar]
- 19.Laville M, Auboeuf D, Khalfallah Y, Vega N, Riou JP, Vidal H: Acute regulation by insulin of phosphatidylinositol-3-kinase, Rad, Glut 4, and lipoprotein lipase mRNA levels in human muscle. J Clin Invest 1996;98:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meugnier E, Rome S, Vidal H: Regulation of gene expression by glucose. Curr Opin Clin Nutr Metab Care 2007;10:518–522 [DOI] [PubMed] [Google Scholar]
- 21.Cozzone D, Fröjdö S, Disse E, Debard C, Laville M, Pirola L, Vidal H: Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia 2008;51:512–521 [DOI] [PubMed] [Google Scholar]
- 22.Cozzone D, Debard C, Dif N, Ricard N, Disse E, Vouillarmet J, Rabasa-Lhoret R, Laville M, Pruneau D, Rieusset J, Lefai E, Vidal H: Activation of liver X receptors promotes lipid accumulation but does not alter insulin action in human skeletal muscle cells. Diabetologia 2006;49:990–999 [DOI] [PubMed] [Google Scholar]
- 23.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J: Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008;118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaussade C, Pirola L, Bonnafous S, Blondeau F, Brenz-Verca S, Tronchère H, Portis F, Rusconi S, Payrastre B, Laporte J, Van Obberghen E: Expression of myotubularin by an adenoviral vector demonstrates its function as a phosphatidylinositol 3-phosphate [PtdIns(3)P] phosphatase in muscle cell lines: involvement of PtdIns(3)P in insulin-stimulated glucose transport. Mol Endocrinol 2003;17:2448–2460 [DOI] [PubMed] [Google Scholar]
- 25.Dif N, Euthine V, Gonnet E, Laville M, Vidal H, Lefai E: Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem J 2006;400:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H: Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 2003;52:1319–1325 [DOI] [PubMed] [Google Scholar]
- 27.Folch J, Lees M, Sloane Stanley GH: A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 28.Silver JD, Ritchie ME, Smyth GK: Microarray background correction: maximum likelihood estimation for the normal-exponential convolution. Biostatistics 2009;10:352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J: Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004;5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth GK: Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004;3:Article3 [DOI] [PubMed] [Google Scholar]
- 31.Vives-Bauza C, Yang L, Manfredi G: Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol 2007;80:155–171 [DOI] [PubMed] [Google Scholar]
- 32.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW: The role of intramuscular lipid in insulin resistance. Acta Physiol Scand 2003;178:373–383 [DOI] [PubMed] [Google Scholar]
- 33.Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L: The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 2001;17:189–212 [DOI] [PubMed] [Google Scholar]
- 34.Grunnet LG, Brøns C, Jacobsen S, Nilsson E, Astrup A, Hansen T, Pedersen O, Poulsen P, Quistorff B, Vaag A: Increased recovery rates of phosphocreatine and inorganic phosphate after isometric contraction in oxidative muscle fibers and elevated hepatic insulin resistance in homozygous carriers of the A-allele of FTO rs9939609. J Clin Endocrinol Metab 2009;94:596–602 [DOI] [PubMed] [Google Scholar]
- 35.Boissel S, Reish O, Proulx K, Kawagoe-Takaki H, Sedgwick B, Yeo GS, Meyre D, Golzio C, Molinari F, Kadhom N, Etchevers HC, Saudek V, Farooqi IS, Froguel P, Lindahl T, O'Rahilly S, Munnich A, Colleaux L: Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet 2009;85:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyre D, Proulx K, Kawagoe-Takaki H, Vatin V, Gutiérrez-Aguilar R, Lyon D, Ma M, Choquet H, Horber F, Van Hul W, Van Gaal L, Balkau B, Visvikis-Siest S, Pattou F, Farooqi IS, Saudek V, O'Rahilly S, Froguel P, Sedgwick B, Yeo GS: Prevalence of loss-of-function FTO mutations in lean and obese individuals. Diabetes 2010;59:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrauwen P, Hesselink MK: Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 2004;53:1412–1417 [DOI] [PubMed] [Google Scholar]
- 38.Puigserver P: Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;1(Suppl.1):S5–S9 [DOI] [PubMed] [Google Scholar]
- 39.Barrès R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K, Krook A, Zierath JR: Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 2009;10:189–198 [DOI] [PubMed] [Google Scholar]
- 40.Debard C, Laville M, Berbe V, Loizon E, Guillet C, Morio-Liondore B, Boirie Y, Vidal H: Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of Type 2 diabetic patients. Diabetologia 2004;47:917–925 [DOI] [PubMed] [Google Scholar]
- 41.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC: PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 [DOI] [PubMed] [Google Scholar]
- 42.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003;100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.