Abstract
OBJECTIVE
To assess whether the detection of enterovirus RNA in blood predicts the development of clinical type 1 diabetes in a prospective birth cohort study. Further, to study the role of enteroviruses in both the initiation of the process and the progression to type 1 diabetes.
RESEARCH DESIGN AND METHODS
This was a nested case-control study where all case children (N = 38) have progressed to clinical type 1 diabetes. Nondiabetic control children (N = 140) were pairwise matched for sex, date of birth, hospital district, and HLA-DQ–conferred genetic susceptibility to type 1 diabetes. Serum samples, drawn at 3- to 12-month intervals, were screened for enterovirus RNA using RT-PCR.
RESULTS
Enterovirus RNA–positive samples were more frequent among the case subjects than among the control subjects. A total of 5.1% of the samples (17 of 333) in the case group were enterovirus RNA–positive compared with 1.9% of the samples (19 of 993) in the control group (P < 0.01). The strongest risk for type 1 diabetes was related to enterovirus RNA positivity during the 6-month period preceding the first autoantibody-positive sample (odds ratio 7.7 [95% CI 1.9–31.5]). This risk effect was stronger in boys than in girls.
CONCLUSIONS
The present study supports the hypothesis that enteroviruses play a role in the pathogenesis of type 1 diabetes, especially in the initiation of the β-cell damaging process. The enterovirus-associated risk for type 1 diabetes may be stronger in boys than in girls.
Enterovirus infections are among the major candidates for environmental risk factors for type 1 diabetes. Previous studies have suggested that enterovirus epidemics associate with an increase in the incidence of type 1 diabetes, and an increased frequency of enterovirus antibodies has been reported in patients with type 1 diabetes (1,2). Several studies have detected enterovirus genome in the blood of diabetic patients, but it is unknown whether the finding reflects persistent or acute infection (3). Virus has been detected both in pancreas and in intestinal mucosa and has also shown a tropism for islets (4,5). On some occasions, coxsackievirus B and echoviruses have even been isolated from diabetic children (6). The recent discovery that genetic polymorphism in the IFIH1 gene (innate immune system sensor for enteroviruses) affects diabetes susceptibility has further supported the possible role of enteroviruses (7). Experimental data support these findings because enteroviruses can cause diabetes in mice and damage β-cells in human islet cell cultures in vitro (3).
Type 1 diabetes–associated autoantibodies in peripheral blood reflect initiation of the β-cell–damaging processes. However, the progression toward clinical diabetes is usually slow, and possible triggering infections can occur long before the presentation of clinical type 1 diabetes. Consequently, prospective follow-up series are essential for the identification of such triggers. A few prospective studies have been carried out on the possible role of enterovirus infections, but the results have been conflicting (8–11).
The aim of this study is to test risk effect of enterovirus RNA in blood for the development of type 1 diabetes in a prospective birth cohort study. Blood samples were collected with short intervals, which made it possible to detect enterovirus RNA directly from the serum in different stages of the disease process. We have previously documented the risk effect of enteroviruses in children who developed β-cell autoimmunity. Now, the aim is to confirm these findings in children who have developed type 1 diabetes and to study the role of these viruses in both the initiation of the process and its progression to diabetes.
RESEARCH DESIGN AND METHODS
Study series.
The study series included children who took part in the Finnish type 1 Diabetes Prediction and Prevention (DIPP) study (12). In DIPP, the families of all newborn infants at the University Hospitals of Oulu, Tampere, and Turku are offered a possibility for screening of newborn infants for HLA risk genes for type 1 diabetes. Families with a child who carries increased genetic susceptibility to diabetes are invited to participate in prospective follow-up starting from birth. Blood samples are taken in 3- to 12-month intervals and regularly analyzed for type 1 diabetes–associated autoantibodies. Islet cell antibodies (ICAs) have been used for the primary screening, and all samples of ICA-positive children were tested for autoantibodies against insulin (IAA), glutamate decarboxylase (GADA), and the protein tyrosine phosphatase–related islet antigen 2 (IA-2A). Children who seroconverted to autoantibody positivity were observed subsequently at an interval of 3 months.
The current study is based on a nested case-control design, where the definition of the case status was based on the diagnosis of clinical type 1 diabetes. For every case child, one to six healthy autoantibody-negative control children were matched pairwise for sex, date of birth (±1 month), hospital district, and HLA-DQ–conferred genetic susceptibility to type 1 diabetes. The study population comprised a total of 38 case children (18 boys) and 140 control children (69 boys). Serum samples were collected for virus analyses from November 1994 to April 2003, and the total number of samples analyzed was 1,326. Our earlier study population overlaps with the current study, with 129 samples from 21 case children and 474 samples from 85 control children (11).
The study was approved by the ethics committees of the participating university hospitals. Parents gave written informed consent.
Genetic and autoantibody analyses.
The presence of HLA DR-DQ haplotype associated with type 1 diabetes was determined using typing for DQB1 alleles in the first phase and informative DQA1 and DRB1 alleles in the second phase as previously described (13). ICA, IAA, GADA, and IA-2A were analyzed as previously described (14).
Enterovirus RT-PCR.
RNA was extracted from 140 μl of serum or plasma using a QIAamp viral RNA kit (Qiagen, Hilden, Germany). Screening for enterovirus RNA was done by RT-PCR followed by hybridization of PCR amplicons using an enterovirus-specific probe. The detection limit for the method is <0.015 fg RNA, which is equivalent to fewer than four copies of enteroviral RNA genome (15). All RNA-positive samples were retested twice, and a result of at least two positive tests out of three tests was interpreted as a positive sample.
Statistical analyses.
The significance of difference in the number of enterovirus infections between case and control children was tested using conditional logistic regression analysis (STATA statistical software, College Station, TX). To adjust for more frequent sample collection from autoantibody-positive children than control children, conditional logistic regression analysis was calculated as proportions (%) of positive samples. Effect of HLA type for EV infections was evaluated using Mann Whitney test.
RESULTS
Enterovirus RNA was detected in 2.7% of serum samples (36 of 1,326). In case children, a total of 5.1% of the samples (17 of 333) were enterovirus RNA positive compared with 1.9% (19 of 993) in control children (P < 0.01) (Fig. 1).
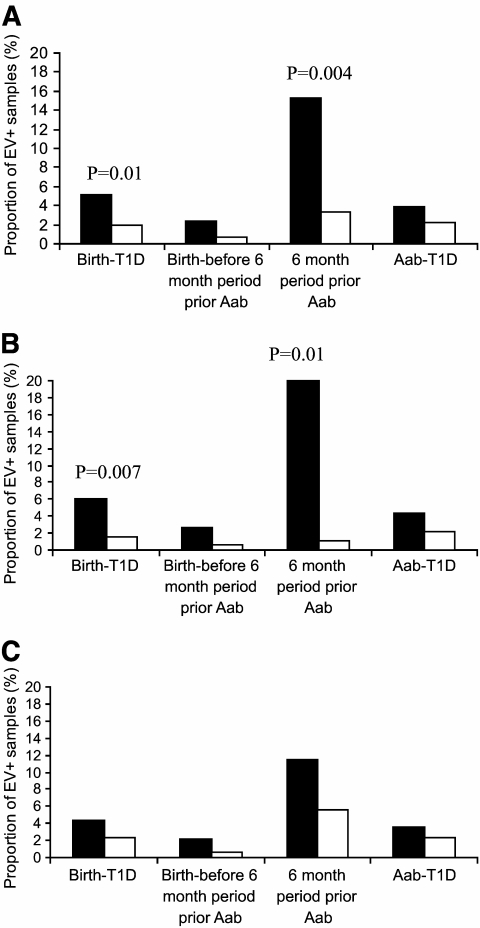
FIG. 1.
Enterovirus (EV) RNA positivity in serum samples during different stages of the diabetic disease process. 6 month period prior AAb, 6-month time window before autoantibody seroconversion; Aab-T1D, period from autoantibody seroconversion to diagnosis of type 1 diabetes; birth-before 6 month period prior Aab, time from birth to 6 months before the seroconversion to positivity for the first autoantibody; birth-T1D, time from birth to diagnosis of type 1 diabetes. A: the whole cohort. B: boys. C: girls. ■, case children; □, control children. The differences between case and control children were tested using conditional logistic regression analysis (P values shown).
The frequency of enterovirus RNA positivity was further analyzed during different stages of the preclinical disease process. The frequency of enterovirus RNA in the case children peaked during the 6-month period before the appearance of the first autoantibody when 15.2% of the samples from the case children and 3.3% of the samples from the control children were positive (odds ratio [OR] 7.7 [95% CI 1.9–31.5], P < 0.004). The lowest frequency of enterovirus RNA was observed during the time period from birth to 6 months before the autoantibody positivity when 2.4% of the samples were positive among the case children and 0.7% in the control group (OR 3.1 [95% CI 0.4–22.4], P < 0.27). After autoantibody seroconversion, 3.9 vs. 2.2% of the samples were enterovirus RNA positive, respectively (Table 1 and Fig. 1).
TABLE 1.
Risk effect of the detection of enterovirus RNA in serum for later development of clinical type 1 diabetes
| Time period | OR | 95% CI | P |
|---|---|---|---|
| Boys and girls | |||
| Birth-before Aab | 6.2 | 1.8–21 | <0.004 |
| Birth-before 6 month period prior Aab | 3.1 | 0.4–22.4 | <0.27 |
| 6 month period prior Aab | 7.7 | 1.9–31.5 | <0.004 |
| Boys | |||
| Birth-before Aab | 18.8 | 2.2–163.7 | <0.008 |
| Birth-before 6 month period prior Aab | 3.9 | 0.2–63.3 | <0.34 |
| 6 month period prior Aab | 18.2 | 2.0–164.5 | <0.01 |
| Girls | |||
| Birth-before Aab | 2.6 | 0.5–12.3 | <0.24 |
| Birth-before 6 month period prior Aab | 2.4 | 0.2–39.7 | <0.53 |
| 6 month period prior Aab | 3.1 | 0.4–21.8 | <0.26 |
6 month period prior AAb, 6-month time window before autoantibody seroconversion; birth-before 6 month period prior Aab, time from birth to 6 months before the seroconversion to positivity for the first autoantibody; birth-before Aab, time from birth to the seroconversion to positivity for the first autoantibody.
The risk effect of enterovirus RNA was stronger among boys than in girls (Table 1). This was similarly seen in infections occurring before and after autoantibody seroconversion. The highest risk was related to infections that occurred in boys during the 6-month period prior to the first autoantibody-positive sample (OR 18.2 [95% CI 2.0–164.5], P < 0.01) (Table 1).
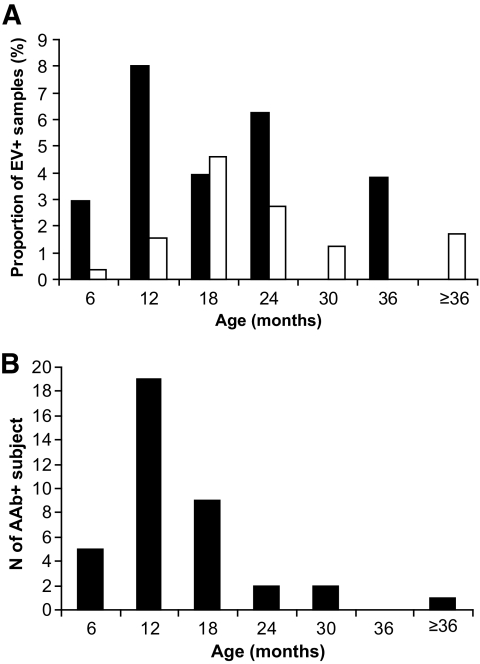
The age of the child had an influence on the frequency of enterovirus RNA in serum. In children who were younger than 6 months of age, only 1.0% of the samples were enterovirus RNA positive compared with 3.5% of samples in 6- to 18-month-old children and 5.0% of the samples in children aged 18–24 months. At the age of 2 years, the frequency of virus-positive samples decreased to 4.3%; the frequency decreased further to 2.0% in children older than 2 years. Case children had the first enterovirus RNA positivity earlier than control children (median 10 vs. 16 months, respectively) (Fig. 2A). The age when autoantibodies were first detected varied from 4 months to 3 years and 5 months (median 12 months) (Fig. 2B.).
FIG. 2.
Age of the first detection of enterovirus (EV) RNA (A) and autoantibodies (B) in serum. ■, case children; □, control children.
In three case children, more than one follow-up sample was enterovirus RNA positive (two positive samples in one child and three positive samples in two children). None of these samples were consecutive, and there were virus-negative samples in between the positive ones. Different virus genotypes were present in repeatedly positive samples in each of these children.
More samples were enterovirus RNA positive in case children with high-risk HLA DR3-DQ2/DR4-DQ8 genotype than in children who carried moderate-risk genotypes with the DR4-DQ8 haplotype (6.8 vs. 2.2%) (P < 0.002).
DISCUSSION
In the present study, enterovirus RNA was detected in serum long before the diagnosis of clinical type 1 diabetes. The frequency of virus peaked during the 6-month window that preceded the first appearance of diabetes-associated autoantibodies. This temporal relationship has also been observed in our previous studies, suggesting that enterovirus infections may play a role in the initiation of the β-cell–damaging process (8,11). Enterovirus RNA was also more common in the case than in the control children after the initial autoantibody seroconversion but was not detected in samples taken close to the presentation of diabetes. The absence of enterovirus RNA at the diagnosis of diabetes is in contrast to the majority of the retrospective case-control studies where altogether an average of 31% of the patients and 6% of the control subjects have been positive for enterovirus RNA (3). The controversy might be due to methodological differences, e.g., the sensitivity of the PCR applied or the type of samples collected (16). On the other hand, it may also reflect a true difference in enterovirus epidemiology. In fact, we have previously observed that enterovirus infections are less frequent in Finland compared with many other countries (17,18). In any case, the present study emphasizes the importance of those infections that occur during the early stages of the diabetogenic pathway, possibly playing a role in the initiation of the β-cell–damaging process rather than the later stages of the process.
As enterovirus viremia usually lasts for a maximum of 2 weeks, the number of enterovirus episodes is largely underestimated if samples are collected at longer intervals. Accordingly, we can estimate the true number of enterovirus RNA–positive episodes (N of positive samples/detection period covered by the collected samples × total follow-up time), which would be 154 episodes in case and 254 in control children (mean 3.7 vs. 1.6 episodes per child). However, it is also possible that the difference between the case and control children reflects prolonged enterovirus episodes in the case group.
Our previous studies among DIPP children suggest that the detection of the first diabetes-associated autoantibody and enterovirus RNA in stools shows similar seasonal variation (19). In the present study, the same seasonal pattern was seen in the detection of enterovirus RNA in serum (frequent in the autumn and winter). In addition, viral RNA was most common in serum at the age when autoantibodies were most frequently induced. Autoantibodies became detectable soon after detection of enterovirus RNA in the serum. In mouse models, this time interval has also been short (20).
It has previously been shown that children are protected against enterovirus infections by maternal antibodies and that this protection is at least partly mediated by antibodies in breast milk. In Finland, children are breastfed for an average of 8 months (range 0.15–23months) (21). This may explain partly the low frequency of viral RNA observed in infants aged <6 months. Viral RNA was most frequent in 12- to 18-month-old children, suggesting that there is a susceptibility period at that age.
Enterovirus persistence has been shown to play a role in chronic cardiomyopathies, and it may also be involved in type 1 diabetes (5,22,23). In the present study, no signs of persistent systemic infection were seen; enterovirus genomes that were detected from repeatedly positive children represented different viral genotypes. However, in persisting infections, the virus replication can occur at a very low level and the virus may not be detectable in peripheral blood even though it may be present in the pancreas or other organs (4,23–25).
The risk effect of enterovirus RNA on autoantibody development was stronger among boys than among girls. Boys are also known to be more susceptible to general complications of enterovirus infections. The higher number of enterovirus RNA–positive samples in children with the DR3-DQ2/DR4-DQ8 genotype merits further investigation to find out whether this genotype is particularly susceptible to diabetogenic effect enteroviruses.
In conclusion, the present study supports the hypothesis that enteroviruses play a role in the pathogenesis of type 1 diabetes. The presence of virus in serum was shown to be a risk factor for the development of β-cell–specific autoimmunity, which progress to clinical diabetes, especially among boys.
ACKNOWLEDGMENTS
This study was supported by the Juvenile Diabetes Research Foundation International, the Academy of Finland, the Medical Research Fund of Tampere University Hospital, and the Tampere Graduate School in Biomedicine and Biotechnology.
H.Hy. and M.K. are minor (<5%) shareholders and members of the board at Vactech Ltd., which develops vaccines against picornaviruses. No other potential conflicts of interest relevant to this article were reported.
S.O. supervised laboratory and data analysis and wrote the manuscript in collaboration with all of the authors. M.M. contributed to the design of the study and writing the manuscript. S.T. contributed to the design of the study and writing the manuscript. H.Hu. was responsible for the statistical analysis of the data. J.I. was responsible for the HLA genotyping and is a member of the Steering Committee of the DIPP study. R.V. is a member of the Steering Committee of the DIPP study. O.S. is a member of the Steering Committee of the DIPP study. M.K. was responsible for the autoantibody analysis and is a member of the Steering Committee of the DIPP study. H.Hy. was responsible for the overall study design and is a member of the Steering Committee of the DIPP study.
We gratefully thank the personnel of the Diabetes Project Laboratory and the DIPP study and highly appreciate the contribution of the families participating in the study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Cabrera-Rode E, Sarmiento L, Tiberti C, Molina G, Barrios J, Hernandez D, Diaz-Horta O, Di Mario U: Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia 2003;46:1348–1353 [DOI] [PubMed] [Google Scholar]
- 2.Wagenknecht LE, Roseman JM, Herman WH: Increased incidence of insulin-dependent diabetes mellitus following an epidemic of Coxsackievirus B5. Am J Epidemiol 1991;133:1024–1031 [DOI] [PubMed] [Google Scholar]
- 3.Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H: Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol [DOI] [PubMed] [Google Scholar]
- 4.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG: The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Oikarinen M, Tauriainen S, Honkanen T, Oikarinen S, Vuori K, Kaukinen K, Rantala I, Maki M, Hyoty H: Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol 2008;151:71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracy S, Drescher KM, Jackson JD, Kim K, Kono K: Enteroviruses, type 1 diabetes and hygiene: a complex relationship. Rev Med Virol 2010;20:106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA: A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet 2006;38:617–619 [DOI] [PubMed] [Google Scholar]
- 8.Lonnrot M, Salminen K, Knip M, Savola K, Kulmala P, Leinikki P, Hyypia T, Akerblom HK, Hyoty H: the Childhood Diabetes in Finland (DiMe) Study Group Enterovirus RNA in serum is a risk factor for beta-cell autoimmunity and clinical type 1 diabetes: a prospective study. J Med Virol 2000;61:214–220 [PubMed] [Google Scholar]
- 9.Fuchtenbusch M, Irnstetter A, Jager G, Ziegler AG: No evidence for an association of Coxsackie virus infections during pregnancy and early childhood with development of islet autoantibodies in offspring of mothers or fathers with type 1 diabetes. J Autoimmun 2001;17:333–340 [DOI] [PubMed] [Google Scholar]
- 10.Graves PM, Rotbart HA, Nix WA, Pallansch MA, Erlich HA, Norris JM, Hoffman M, Eisenbarth GS, Rewers M: Prospective study of enteroviral infections and development of beta-cell autoimmunity: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetes Res Clin Pract 2003;59:51–61 [DOI] [PubMed] [Google Scholar]
- 11.Salminen K, Sadeharju K, Lonnrot M, Vahasalo P, Kupila A, Korhonen S, Ilonen J, Simell O, Knip M, Hyoty H: Enterovirus infections are associated with the induction of beta-cell autoimmunity in a prospective birth cohort study. J Med Virol 2003;69:91–98 [DOI] [PubMed] [Google Scholar]
- 12.Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipila JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyoty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O: Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–1755 [DOI] [PubMed] [Google Scholar]
- 13.Hermann R, Turpeinen H, Laine AP, Veijola R, Knip M, Simell O, Sipila I, Akerblom HK, Ilonen J: HLA DR-DQ-encoded genetic determinants of childhood-onset type 1 diabetes in Finland: an analysis of 622 nuclear families. Tissue Antigens 2003;62:162–169 [DOI] [PubMed] [Google Scholar]
- 14.Kukko M, Kimpimaki T, Korhonen S, Kupila A, Simell S, Veijola R, Simell T, Ilonen J, Simell O, Knip M: Dynamics of diabetes-associated autoantibodies in young children with human leukocyte antigen-conferred risk of type 1 diabetes recruited from the general population. J Clin Endocrinol Metab 2005;90:2712–2717 [DOI] [PubMed] [Google Scholar]
- 15.Lonnrot M, Sjoroos M, Salminen K, Maaronen M, Hyypia T, Hyoty H: Diagnosis of enterovirus and rhinovirus infections by RT-PCR and time-resolved fluorometry with lanthanide chelate labeled probes. J Med Virol 1999;59:378–384 [PubMed] [Google Scholar]
- 16.Schulte BM, Bakkers J, Lanke KH, Melchers WJ, Westerlaken C, Allebes W, Aanstoot HJ, Bruining GJ, Adema GJ, Van Kuppeveld FJ, Galama JM: Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral Immunol 23:99–104 [DOI] [PubMed] [Google Scholar]
- 17.Lonnrot M, Knip M, Marciulionyte D, Rahko J, Urbonaite B, Moore WP, Vilja P, Hyoty H: Enterovirus antibodies in relation to islet cell antibodies in two populations with high and low incidence of type 1 diabetes. Diabetes Care 1999;22:2086–2088 [DOI] [PubMed] [Google Scholar]
- 18.Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Knip M, Hyoty H: Relationship between the incidence of type 1 diabetes and enterovirus infections in different European populations: results from the EPIVIR project. J Med Virol 2004;72:610–617 [DOI] [PubMed] [Google Scholar]
- 19.Salminen KK, Vuorinen T, Oikarinen S, Helminen M, Simell S, Knip M, Ilonen J, Simell O, Hyoty H: Isolation of enterovirus strains from children with preclinical type 1 diabetes. Diabet Med 2004;21:156–164 [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Sheikh S, Martin DL, Chatterjee NK: Coxsackievirus B4 alters pancreatic glutamate decarboxylase expression in mice soon after infection. J Autoimmun 1993;6:529–542 [DOI] [PubMed] [Google Scholar]
- 21.Sadeharju K, Knip M, Virtanen SM, Savilahti E, Tauriainen S, Koskela P, Akerblom HK, Hyoty H: Maternal antibodies in breast milk protect the child from enterovirus infections. Pediatrics 2007;119:941–946 [DOI] [PubMed] [Google Scholar]
- 22.Chapman NM, Kim KS: Persistent coxsackievirus infection: enterovirus persistence in chronic myocarditis and dilated cardiomyopathy. Curr Top Microbiol Immunol 2008;323:275–292 [DOI] [PubMed] [Google Scholar]
- 23.Yin H, Berg AK, Westman J, Hellerstrom C, Frisk G: Complete nucleotide sequence of a Coxsackievirus B-4 strain capable of establishing persistent infection in human pancreatic islet cells: effects on insulin release, proinsulin synthesis, and cell morphology. J Med Virol 2002;68:544–557 [DOI] [PubMed] [Google Scholar]
- 24.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007;104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oikarinen M, Tauriainen S, Honkanen T, Vuori K, Karhunen P, Vasama-Nolvi C, Oikarinen S, Verbeke C, Blair GE, Rantala I, Ilonen J, Simell O, Knip M, Hyoty H: Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia 2008;51:1796–1802 [DOI] [PubMed] [Google Scholar]