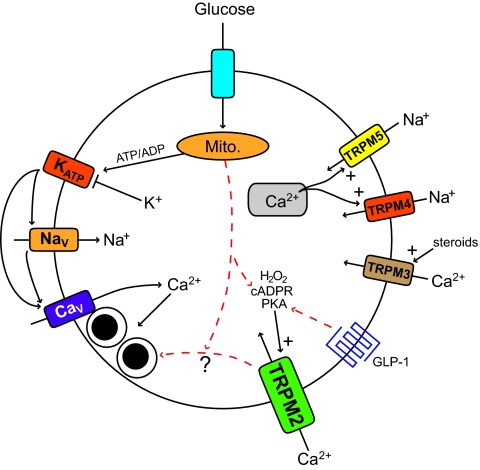
The means by which glucose stimulates insulin secretion from pancreatic β-cells has been studied for decades. Yet we still do not fully understand the cellular machinery underlying this process, the complexity of which continues to surprise. The triggering pathway for glucose-induced insulin secretion is generally well described, and the current model, shown on the left-side of Fig. 1, has been accepted for more than 20 years (1,2). However, it is abundantly clear that the ion channels currently included in this consensus model are insufficient to describe the complicated electrophysiological and intracellular Ca2+ responses of the β-cell to glucose and other secretagogues. Furthermore, the contribution of an ion channel-based component to the well-known “amplifying” effects of glucose (3) remains unclear. Recent studies, including that of Uchida et al. (4) in the present issue, are beginning to elucidate roles for multiple additional ion channels in the β-cell electrical and intracellular Ca2+ responses, particularly the contribution of the transient receptor potential (TRP) channels. Moreover, there are hints that these channels may play a more complex role in β-cells than we suspect.
FIG. 1.
TRPM channels in insulin secretion. On the left is the consensus triggering pathway for insulin secretion in which a glucose-stimulated rise in the ATP/ADP ratio closes ATP-sensitive K+ (KATP) channels, depolarizing the β-cell and activating voltage-gated Na+ (NaV) and Ca2+ (CaV) channels. The latter mediates Ca2+ influx that triggers insulin granule exocytosis. On the right are TRPM channels shown to contribute to β-cell Ca2+ and insulin responses. TRPM4 and (perhaps to a greater degree) TRPM5 mediate an inward Na+ current in response to increases in intracellular Ca2+, perhaps through depletion of Ca2+ stores, contributing to membrane depolarization and control of Ca2+ oscillations. TRPM3 activation by external steroidal signals allows influx of Ca2+. TRPM2 is activated by internal signals that include PKA phosphorylation, cADPR, and hydrogen peroxide (H2O2). Uchida et al. demonstrate a role for TRPM2 in glucose and GLP-1 stimulated Ca2+ responses and insulin secretion. Also intimated in their data is a role for TRPM2 as a regulator of the “amplifying” effects of glucose independent from the channel's role in mediating Ca2+ influx. Putative interactions are shown as red dashed arrows. Mito., mitochondria.
In mammals there are 28 members of the TRP channel family, and these generally show a selective permeability to cations such as Na+ and Ca2+ (5). Thus, activation of these channels could contribute to β-cell depolarization and intracellular Ca2+ responses. TRP channels play exceptionally diverse roles in many different tissues, acting as sensors of signals that include temperature, mechanical stress, pheremones, Ca2+, and intracellular messengers. Several studies have now suggested that numerous TRP channels are expressed in β-cells (6–12). Therefore, TRP channels in the β-cell may integrate a variety of stimuli to modulate glucose-stimulated electrical and Ca2+ responsiveness. Recent work has focused on the melastatin-related family of channels (TRPM) and suggested roles for these in the control of islet Ca2+ oscillations (12) and responses to steroid hormones (9), intracellular protein kinase A (PKA) and cyclic ADP–ribose (cADPR) (8), and hydrogen peroxide (13) (Fig. 1). Uchida et al. have now examined mice lacking TRPM2, demonstrating impaired glucose homeostasis and reduced islet intracellular calcium concentration and secretory responses to glucose and glucagon-like peptide 1 (GLP-1).
TRPM2 contributes to the intracellular Ca2+ response of islets by mediating Ca2+ influx, and it is interesting that several potential mechanisms, including metabolic and hormonal signals, may regulate this. The authors' previous work demonstrating that β-cell TRPM2 channels are activated by cADPR and PKA (8) may explain why loss of this channel impairs glucose and GLP-1 stimulated insulin secretion, respectively. Consistent with a metabolic requirement for the involvement of TRPM2, insulin secretion stimulated by tolbutamide was preserved in TRPM2−/− islets. However, the role for TRPM2 may be more complex than first thought, because it could be argued that the reduction in glucose-stimulated insulin secretion is greater than can be accounted for by reduced intracellular Ca2+ alone. Furthermore, under conditions designed to “clamp” intracellular calcium concentration with KCl and diazoxide, glucose triggers insulin secretion through activation of an “amplifying” pathway (3,14). This well-known effect is completely lost in the TRPM2−/− islets even though no difference was observed in intracellular Ca2+ under these conditions (supplementary Fig. 4 in Uchida et al.). One could possibly invoke a role for TRPM2 modulating Ca2+ just under the plasma membrane, which would be undetectable by cytosolic Ca2+ measurement. However, a recent article by Gilon and colleagues (15) demonstrates that glucose has no effect on submembrane Ca2+ under identical conditions. Thus, the present data are suggestive of a role for TRPM2 that is independent of its ability to mediate membrane depolarization or Ca2+ entry!
Such a role for ion channels in hormone secretion, separate from their ability to conduct ions, is not without precedent. It has been known for some time that interaction of Ca2+ channels with exocytotic soluble N-ethylmaleimide attachment protein receptor (SNARE) proteins acts to localize insulin granules close to sites of Ca2+ entry, and that disruption of this interaction impairs insulin exocytosis without affecting Ca2+ influx (16). The localization of ATP-sensitive K+ channels to secretory granules suggests a role in insulin secretion, independent of their plasma membrane K+ conductance (17). More recently, the voltage-dependent K+ channel Kv2.1, thought to play an important role in β-cell action potential repolarization (18), is proposed to play a direct role in exocytosis in PC12 and chromaffin cells independent of its K+ conductance through its interaction with the SNARE proteins (19,20). A role for this in insulin secretion, however, has yet to be shown. Indeed, the related TRPM5 channel has been implicated in the direct control of insulin secretion, distinct from the channel's Na+ conductance, because arginine-stimulated insulin secretion from islets lacking TRPM5 is reported to be impaired (11). Thus, a novel and perhaps direct function for TRPM2 in glucose-stimulated insulin secretion is indicated by the fact that it appears to play a role under conditions in which it is not affecting either membrane depolarization or Ca2+ to any detectable degree.
A hallmark of provocative and interesting papers is that they often raise many questions. The work of Uchida et al. succeeds in this respect because it raises several issues with respect to the role of TRPM2 in insulin secretion. Is this channel activated by GLP-1? Is it regulated by β-cell glucose metabolism, and does cADPR act as a signal for this? Does TRPM2 play a role in the amplifying effects of glucose? If so, is this through some as yet undetected effect on Ca2+, or is it through some unsuspected role independent from its ability to conduct Ca2+ ions? These questions can only be answered by a more detailed investigation of the interaction of TRPM2 with the insulin secretory machinery and the regulation of this interaction by intracellular cues. Nonetheless, it is clear that a number of TRP channels are important contributors to pancreatic islet function, and we will continue our “TRP” down the path toward a fuller understanding of the complexity underlying the physiological mechanism of insulin secretion.
ACKNOWLEDGMENTS
Ion channel research in P.E.M.'s laboratory is supported by a Discovery Grant from the National Science and Engineering Research Council (NSERC) of Canada. P.E.M. is Canada Research Chair in Islet Biology and holds scholarships from Alberta Innovates - Health Solutions and the Canadian Diabetes Association (CDA).
No potential conflicts of interest relevant to this article were reported.
The author thanks Dr. Jocelyn Manning Fox (University of Alberta) for critical reading of the manuscript.
Footnotes
See accompanying original article, p. 119.
REFERENCES
- 1.Ashcroft FM, Rorsman P: Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 1989;54:87–143 [DOI] [PubMed] [Google Scholar]
- 2.MacDonald PE, Joseph JW, Rorsman P: Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 2005;360:2211–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henquin JC: Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000;49:1751–1760 [DOI] [PubMed] [Google Scholar]
- 4.Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, Yada T, Minokoshi Y, Tominaga M: Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes 2011;60:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gees M, Colsoul B, Nilius B: The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2010;2:a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakura H, Ashcroft FM: Identification of four trp1 gene variants murine pancreatic beta-cells. Diabetologia 1997;40:528–532 [DOI] [PubMed] [Google Scholar]
- 7.Qian F, Huang P, Ma L, Kuznetsov A, Tamarina N, Philipson LH: TRP genes: candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes 2002;51(Suppl. 1):S183–S189 [DOI] [PubMed] [Google Scholar]
- 8.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M: TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 2006;25:1804–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Düfer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J: Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol 2008;10:1421–1430 [DOI] [PubMed] [Google Scholar]
- 10.Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T: Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun 2004;321:219–225 [DOI] [PubMed] [Google Scholar]
- 11.Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, Zabel BU, Prawitt D: TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch 2010;460:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R: Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5-/- mice. Proc Natl Acad Sci U S A 2010;107:5208–5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bari MR, Akbar S, Eweida M, Kühn FJ, Gustafsson AJ, Lückhoff A, Islam MS: H2O2-induced Ca2+ influx and its inhibition by N-(p-amylcinnamoyl) anthranilic acid in the beta-cells: involvement of TRPM2 channels. J Cell Mol Med 2009;13:3260–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gembal M, Gilon P, Henquin JC: Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest 1992;89:1288–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravier MA, Cheng-Xue R, Palmer AE, Henquin JC, Gilon P: Subplasmalemmal Ca2+ measurements in mouse pancreatic beta cells support the existence of an amplifying effect of glucose on insulin secretion. Diabetologia 2010;53:1947–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiser O, Trus M, Hernández A, Renström E, Barg S, Rorsman P, Atlas D: The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci U S A 1999;96:248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng X, Li L, Watkins S, Robbins PD, Drain P: The insulin secretory granule is the major site of KATP channels of the endocrine pancreas. Diabetes 2003;52:767–776 [DOI] [PubMed] [Google Scholar]
- 18.MacDonald PE, Wheeler MB: Voltage-dependent K+ channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia 2003;46:1046–1062 [DOI] [PubMed] [Google Scholar]
- 19.Singer-Lahat D, Sheinin A, Chikvashvili D, Tsuk S, Greitzer D, Friedrich R, Feinshreiber L, Ashery U, Benveniste M, Levitan ES, Lotan I: K+ channel facilitation of exocytosis by dynamic interaction with syntaxin. J Neurosci 2007;27:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinshreiber L, Singer-Lahat D, Ashery U, Lotan I: Voltage-gated potassium channel as a facilitator of exocytosis. Ann N Y Acad Sci 2009;1152:87–92 [DOI] [PubMed] [Google Scholar]