Abstract
OBJECTIVE
Previous studies have observed disruptions in brain white and gray matter structure in individuals with type 1 diabetes, and these structural differences have been associated with neurocognitive testing deficiencies. This study investigated the relationship between cerebral cortical thickness reductions and white matter microstructural integrity loss in a group of patients with type 1 diabetes and in healthy control subjects using diffusion tensor imaging (DTI).
RESEARCH DESIGN AND METHODS
Twenty-five subjects with type 1 diabetes for at least 15 years and 25 age- and sex-matched control subjects underwent structural T1 and proton-density and DTI on a 3.0 Tesla scanner. Fractional anisotropy measurements were made on major cerebral white matter tracts, and DTI tractography was performed to identify cortical regions with high connectivity to these tracts.
RESULTS
Posterior white matter tracts with reduced fractional anisotropy (optic radiations, posterior corona radiata, and the splenium region of the corpus callosum) were found to have high connectivity with a number of posterior cortical regions, including the cuneus, precuneus, fusiform, and posterior parietal cortical regions. A significant reduction in cortical thickness in the diabetic group was observed in the regions with high connectivity to the optic radiations and posterior corona radiata tracts (P < 0.05).
CONCLUSIONS
The direct relationship between white and gray matter structural pathology has not been previously demonstrated in subjects with long-standing type 1 diabetes. The relationship between posterior white matter microstructural integrity disruption and lower cortical thickness demonstrated using a novel DTI connectivity technique suggests a common or interrelated pathophysiological mechanism in type 1 diabetes.
The effects of type 1 diabetes on the central nervous system have only recently begun to be identified. A wide range of cognitive deficits have been reported, including reduced performance on tests that measure working memory, learning, attention, information processing speed, and visual-spatial memory (1). Neurofunctional changes such as deficits in electroencephalography (2) and visually evoked potentials (3) have also been found. In addition, patients with type 1 diabetes have been found to have structural differences in both white (4–6) and gray matter (7,8). Mild ventricular atrophy has also been observed (9,10). Ferguson et al. (11), however, observed no relationship between gray matter structure and disease severity. More recent studies using automated voxel-based morphometric analyses have been interesting but inconsistent. Although one report identified reduced density of bilateral frontal, left cerebellum, and right occipital gray matter (8), another report identified reduced gray matter density in only the left parietal, temporal, and frontal and right parietal lobes (7). A recent study noted significant increases in hippocampal volumes in youth with type 1 diabetes experiencing recurrent episodes of hypoglycemia compared with sibling control subjects (12). We are unaware of any studies linking abnormalities in gray and white matter structure in subjects with diabetes.
In this study, we used a novel technique to overcome the inherent alignment issues that occur with voxel-based morphometric analyses (13) to better define the changes in gray matter density seen in patients with type 1 diabetes. Our study aim was to determine the connectivity between the abnormalities in white matter microstructure we previously reported in a population of subjects with long-standing type 1 diabetes and any identified reductions in gray matter density. We hypothesized that gray matter structural deficits would be associated with white matter structural deficits previously reported for these subjects (4).
RESEARCH DESIGN AND METHODS
Twenty-five adults with type 1 diabetes for >15 years were recruited from the University of Minnesota Diabetes Clinic and were matched by sex and age with 25 healthy volunteers. The exclusion criteria included history of or current substance abuse disorder other than tobacco and/or caffeine dependence, severe psychiatric disorder, history of seizure disorder (not related to hypoglycemia), transient ischemic attack/stroke, head injury, or other disease of the central nervous system, or standard contraindication to magnetic resonance imaging (MRI). All procedures were approved by the University of Minnesota's Institutional Review Board.
A previous publication reported measurements of fractional anisotropy and cognitive function in this population (4). As noted in that publication, three subjects with diabetes reported a history of retinopathy, two reported a history of gastroparesis, and three reported histories of both retinopathy and neuropathy. No subjects with diabetes reported a history of nephropathy. Fifteen of the 25 subjects with diabetes reported a history of severe hypoglycemia, defined as having seizures, loss of consciousness, or needing another person's help to treat the symptoms of low blood glucose. There were no differences between the subjects with diabetes and the control subjects on the performance on the Wechsler Abbreviated Scale of Intelligence (WASI). Ten of the 25 subjects with diabetes and 3 of the 25 control subjects were taking antihypertensive medications at the time of study. Twelve of the 25 subjects with diabetes and 2 of the control subjects were taking drugs to lower cholesterol.
Prior to undergoing the imaging protocol, blood glucose values were measured in subjects with type 1 diabetes using a reflectance meter to ensure values were between 100 and 250 mg/dl. If the blood glucose level was outside this range, it was corrected or the study was scheduled for another day. Table 1 details clinical information about these subjects.
TABLE 1.
Demographic information of subjects
| Type 1 diabetes | Control | |
|---|---|---|
| n | 25 | 25 |
| Age (years) | 45.1 ± 10.5 | 45.6 ± 10.8 |
| Sex (M/F) | 17/8 | 17/8 |
| Education (years) | 16.7 ± 1.9 | 16.1 ± 2.3 |
| Duration of diabetes (years) | 30.3 ± 10.8 | na |
| A1C (%) | 7.4 ± 1.0 | na |
| BMI (kg/m2) | 26.6 ± 3.5 | 26.7 ± 5.2 |
| Blood glucose before MRI (mg/dl) | 168 ± 64 | na |
Data are means ± SD unless otherwise indicated. na, not available.
MRI data acquisition.
Imaging data were acquired on a Siemens Trio 3 Tesla scanner (Erlangen, Germany) at the University of Minnesota's Center for Magnetic Resonance Research. Axial diffusion tensor imaging (DTI) was performed using the following parameters: The field of view was positioned to cover the entire cerebrum. Whole brain DTI was performed using a dual spin echo, single shot, echo planar, diffusion-weighted sequence with these parameters: repetition time (TR) = 8,000 ms echo time (TE) = 83 ms, 128 × 128 matrix, 32 cm field of view, 2-mm thick slices skip 0, 64 slices, and b value = 1,000. Diffusion was measured along 12 noncollinear directions. A 3D T1 magnetization-prepared rapid acquisition of gradient echo (MPRAGE) sequence was acquired with the following parameters: TR = 2530ms, TE = 3.63 ms, imaging time (TI) = 1,100 ms, 1 mm isotropic, field of view = 256 mm, and 256 × 256 matrix. No grossly abnormal findings that would have required exclusion were observed in structural MRI scans.
DTI data processing.
In our previous report on this subject group (4), individual white matter tracts were reconstructed using diffusion-weighted images. This procedure is discussed in depth in the Supplementary Data section in the online appendix available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0598/DC1. Briefly, for each subject, bilateral forceps minor, cingulum bundle, medial corona radiata, superior longitudinal fasciculus, and optic radiation regions of interest were generated in addition to six corpus callosum regions of interest: genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium using Tract-Based Spatial Statistics (14) and custom software. Figure 1 demonstrates these segmented white matter tracts on a composite T1 structural brain image. Groupwise differences in fractional anisotropy, a commonly used metric of white matter microstructural integrity, were calculated. Those tracts that were found to have significantly different fractional anisotropy between the diabetic and control subject groups were then used for starting points for the fiber tract projection analysis described below.
FIG. 1.
Automated white matter image analysis technique resulting in tract-specific regions of interest: corona radiata (red), superior longitudinal fasiciculus (yellow), cungulum bundle (green) (left), six corpus callosum subdivisions (middle), and the optic radiations (right). (A high-quality digital representation of this figure is available in the online issue.)
Fiber tract projection analysis.
The connectivity of these tracts, which included the optic radiations, posterior corona radiata, and splenium tracts, was determined using Probtrack and Bedpost software (15). The Tract-Based Spatial Statistics–generated skeletonized tracts described above were used as region-of-interest starting masks, and a connectivity distribution for each tract was calculated using the FSL-FDT software package (16). This distribution represents the degree of connectivity from each starting voxel in the region of interest to every other voxel on the basis of the diffusion data and relies on a probabilistic white matter tractography approach. Voxels with high measured connectivity to the starting voxel tend to be connected by white matter tracts with consistent diffusion directions.
This technique relied on a probabilistic Markov Chain Monte Carlo sampling approach to determine which voxels throughout the brain are most closely linked to these starting points on the basis of the diffusion-weighted imaging data. This connectivity distribution was thresholded in the same way for each starting mask using a sum of the distribution's mean and SD thus removing all insignificant connectivity results from the distribution. The average cortical thickness of the gray matter regions that intersected with each of these connectivity distributions was then calculated as is discussed below.
Cortical thickness MRI analyses.
Individual T1 MRI images were processed using the FreeSurfer software package (13). T1 images from each subject were registered to a common template based on the interface between gray and white matter using a standard Talairach atlas. Cortical thickness (the distance between the pial surface and white matter) was calculated for each point throughout the cortex as the distance between the pial and white matter boundaries. The gyral surface for each subject was labeled using a probabilistic algorithm, and the cortical thickness was calculated for these named gyri and sulci (17). The cortical segmentation and labeling method generates results that have similar reliability to manual methods (18).
RESULTS
Connectivity analysis.
Using this dataset, we have previously reported that the subjects with type 1 diabetes had significantly lower measures of fractional anisotropy than the control subjects in the optic radiations, posterior corona radiata, and splenium. In the current analysis, connectivity seeds were generated for these three tracts for each diabetic and control subject. Figure 2A shows this process of connectivity mapping for a representative subject. The white matter tract regions of interest (in red) are used as starting seeds for connectivity mapping, and voxels found to have high connectivity on the basis of probabilistic tractography with these starting seeds are marked in yellow. Subsequent analysis determined which gray matter regions had high connectivity with these white matter seeds. Figure 2B demonstrates in a representative subject this method of using the overlap of the connectivity map with cortical boundaries to determine the connectivity of the original white matter region of interest to specific cortical regions. The upper image shows the lateral occipital gyral cortical boundary in blue, and the overlap with the optic radiations connectivity map in yellow is shown in the lower image. In this case, there are a number of voxels that overlap, and connectivity is established between the optic radiations region of interest and the lateral occipital cortical region for further statistical analysis of the thickness in this cortical region. The overlap analysis was performed in each subject's native imaging space instead of warping to a common template in order to increase connectivity mapping accuracy, and the images presented in Fig. 2 demonstrate results in native imaging space for this representative subject.
FIG. 2.
A: Connectivity maps taken from a representative subject. The white matter tract regions of interest in red are used as starting seeds for connectivity mapping, and diffusion MRI data are used to generate connectivity maps. Yellow voxels in these images indicate high connectivity to the original region of interest. The connectivity maps and starting seeds are shown for the optic radiations white matter tract (top left), posterior corona radiata (top right), and splenium of corpus callosum (bottom). A representative axial (on the left) and coronal view (on the right) is shown for each case. B: The overlap between the connectivity map and cortical regions is used to determine which cortical regions have high connectivity with the original white matter regions of interest. In this representative subject, the lateral occipital gyral cortical boundary is shown in blue in the upper image, and overlap with the optic radiations connectivity map is shown in yellow in the lower image. (A high-quality digital representation of this figure is available in the online issue.)
The connectivity analysis was undertaken for each white matter region of interest and all cortical regions for each control and type 1 diabetic subject. Table 2 lists the cortical regions that had a cortical-connectivity map overlap of at least one voxel on average across all subjects for each of the three white matter seeds.
TABLE 2.
White matter connectivity with gyral interface masks
| Optic radiations | Posterior corona radiata | Splenium |
|---|---|---|
| Cuneus | Inferior parietal | Precuneus |
| Fusiform | Paracentral | |
| Lateral occipital | Pericalcarine | |
| Lingual | Postcentral | |
| Pericalcerine | Precentral | |
| Superior parietal | Precuneus |
Relationship between gray matter cortical thickness and water matter regions with reduced fractional anisotropy.
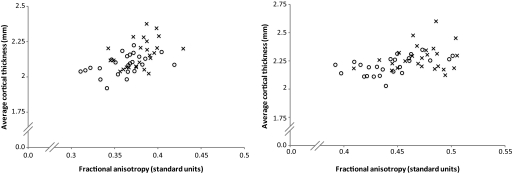
Significantly lower average cortical thickness was found in the subjects with type 1 diabetes for cortical regions with high connectivity to the optic radiations (P = 0.012, d = −0.86) and posterior corona radiata tracts (P = 0.031, d = −0.74). Figure 3 shows the relationship between average fractional anisotropy in the white matter seed regions of interest and the average cortical thickness in cortical regions found to have high connectivity with these seeds. These plots show that subjects with type 1 diabetes cluster with lower fractional anisotropy and lower cortical thickness. A Pearson correlation analysis performed across all subjects between fractional anisotropy for the posterior corona radiata and connected cortex resulted in r = 0.427 (P = 0.002), and for fractional anisotropy in the optic radiations and connected cortex data r = 0.503 (P < 0.001).
FIG. 3.
The average fractional anisotropy for the posterior corona radiata white matter region of interest (left) and optic radiations (right) is plotted against average cortical thickness for cortical regions found to have high connectivity to each of these seed regions. Diabetic subjects are denoted by ○; control subjects are denoted by ×.
Average cortical thickness in regions without high connectivity (the remainder) was not significantly lower than in control subjects (P > 0.45). Overall brain size was not significantly different between groups. Of note, in the subjects with diabetes a trend toward reduced cortical thickness was observed in the cortical regions found to have high connectivity to the splenium, but this did not reach statistical significance (P = 0.11).
Relationships between gray matter cortical thickness and subject characteristics.
Subject age and thickness in the cortex with high connectivity to the posterior corona radiata were found to be significantly related (Spearman correlation = −0.597, P = 0.002 after Bonferroni correction). Diabetes duration, A1C at the time of the testing, and BMI were not significantly related to gray matter cortical thickness. Performance on the Grooved Peg Board with the dominant hand was the only neuropsychometric measure that correlated with gray matter thickness in regions with high connectivity to the posterior corona radiata (Spearman correlation = −0.51, P = <0.0001 after Bonferroni correction) or the optic radiations (Spearman correlation = −0.41, P = <0.0001 after Bonferroni correction).
DISCUSSION
In this study, we determined that there was linkage between the white matter tracts we had previously found to have reduced fractional anisotropy (4) and regions with reduced cortical thickness. Together these findings suggest that long-standing type 1 diabetes may cause widespread microstructural alterations in the posterior cerebrum.
Although a number of neurological and neuropsychiatric diseases have been shown to alter gray matter morphometric measurements based on structural MR, diabetes has not been consistently associated with gray matter pathology. Musen et al. (7), using a voxelwise analysis, found that relative to control subjects, subjects with type 1 diabetes had asymmetric reductions in gray matter density in the frontal and parietal lobes. On the other hand, Wessels et al. (8) identified an asymmetric reduction in gray matter density in the occipital and frontal lobes in subjects with diabetic retinopathy. The voxelwise comparisons used in these investigations warped images onto each other and relied on blurring to overcome the problems with anatomic variation that occur when gyri are aligned among subjects. As a result, voxel-based morphological techniques are exquisitely sensitive to alignment errors, and discrepant conclusions between studies are the frequent result (19). In our study, we overcame the limitation of voxel-based analyses by using techniques that measured the thickness of the cortex at the individual sulcus and gyrus level based on T1 MRI images (13).
It is interesting that we found a predominantly posterior pattern of microstructual alterations in patients with type 1 diabetes because other diseases such as adrenoleukodystrophy, posterior reversible encephalopathy syndrome (PRES), and posterior cortical atrophy are known to display a similar pattern of change. The progressive dysmyelination present in adrenoleukodystropy, which results from accumulation of very-long-chain fatty acids, classically initiates in posterior white matter (20) before gray matter is affected. The reason for this predilection is not known and may be the result of local immunological or biochemical sensitivities. The posterior PRES presents with posterior white matter edema and often occurs transiently in the face of hypertension, immunosuppressive therapy, or renal disease. The etiology of PRES is unknown, although it is speculated that endothelial dysfunction (21) or cerebral autoregulation (22) may be involved. Whether common mechanisms are responsible for the posterior changes seen in these diseases is unknown but should be the topic of subsequent research.
We observed there to be connectivity between the gray matter regions with a reduction in cortical thickness and the white matter regions with a reduced fractional anisotropy in the subjects with type 1 diabetes. Although it is intuitive to expect that cortical thickness and the neuronal process that caused this thinning would result in pathology in the axons that lead to and from these areas of cortex, there have been few human studies that have addressed this issue. In patients with posterior cortical atrophy, reduced fractional anisotropy in the splenium of the corpus callosum was found to be correlated with volume loss in the occipital cortex in patients (23). Similarly, white matter hyperintensities located in frontal or parieto-occipital white matter were found to correlate with reduced hippocampal volume in patients with Alzheimer's disease (24). The mechanism for the concurrent white and gray matter structural findings in our study is not known, but it could involve a causal event starting in either tissue type resulting in pathology in the other or else pathology occurring in both white and gray matter simultaneously. It has been proposed that white matter may be more susceptible to microvascular disease than cortical gray matter (25), and it is plausible that diabetic microvascular disease may be a causative factor leading to first to white and then to gray matter structural deficits.
In our previous study, we found a significant correlation between reduced fractional anisotropy and reduced performance on the copy portion of the Rey-Osterreith Complex Figure Drawing Test and the Grooved Peg Board Test (4). Both tests are believed to assess white matter function, but our finding of a significant relationship between cortical thickness in posterior gray matter regions and performance on the Grooved Peg Board Tests suggests this region may also participate in performing the tasks of executive function and psychomotor speed. Age was the only subject characteristic that related significantly to cortical thickness in areas with high connectivity to the posterior corona radiata and the optic radiations. A prospective evaluation of a larger sample size will be necessary to determine if diabetes-related characteristics such as glycemic control have an impact on gray matter cortical thickness.
One limitation of our study is that we cannot control for the effect of diabetic retinopathy and other related ophthalmological complications on the posterior regions in which we identified microstructural changes. However, as reported previously (4), only two members of this group had received photocoagulation therapy for diabetic retinopathy in the years prior to the imaging study. None of our subjects reported vision loss, but it is not known if subtle retinopathy results in general in structural brain changes.
In conclusion, we found that patients with long-standing type 1 diabetes have concurrent structural deficits in both the gray and the white matter located in the posterior region of the brain. Future investigation will be necessary to identify both the cause and the long-term effects of these findings.
Supplementary Material
ACKNOWLEDGMENTS
This work was undertaken with the support from General Clinical Research Center (GCRC) Grant MO1 RR00400, National Center for Research Resources (NCRR) Biotechnology Research Resource Grant P41-RR-08079, and University of Minnesota Neuroscience Core Center Grant P30 NS057091.
No potential conflicts of interest relevant to this article were reported.
D.T.F. collected and analyzed data and wrote the manuscript. C.T.K., B.A.M., R.L.M., K.O.L., and E.R.S. collected and analyzed data and reviewed and edited the manuscript.
The authors are grateful for the participation of their research volunteers.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kodl CT, Seaquist ER: Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008;29:494–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howorka K, Pumprla J, Saletu B, Anderer P, Krieger M, Schabmann A: Decrease of vigilance assessed by EEG-mapping in type I diabetic patients with history of recurrent severe hypoglycaemia. Psychoneuroendocrinology 2000;25:85–105 [DOI] [PubMed] [Google Scholar]
- 3.Pozzessere G, Rizzo PA, Valle E, Mollica MA, Meccia A, Morano S, Di Mario U, Andreani D, Morocutti C: Early detection of neurological involvement in IDDM and NIDDM: multimodal evoked potentials versus metabolic control. Diabetes Care 1988;11:473–480 [DOI] [PubMed] [Google Scholar]
- 4.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER: Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes 2008;57:3083–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, Werther GA, Cameron FJ: Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care 2009;32:445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinger K, Jacobson AM, Musen G, Lyoo IK, Ryan CM, Jimerson DC, Renshaw PF: The effects of type 1 diabetes on cerebral white matter. Diabetologia 2008;51:417–425 [DOI] [PubMed] [Google Scholar]
- 7.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM: Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 2006;55:326–333 [DOI] [PubMed] [Google Scholar]
- 8.Wessels AM, Simsek S, Remijnse PL, Veltman DJ, Biessels GJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ, Rombouts SA: Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia 2006;49:2474–2480 [DOI] [PubMed] [Google Scholar]
- 9.Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, Deary IJ: Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 2005;28:1431–1437 [DOI] [PubMed] [Google Scholar]
- 10.Lunetta M, Damanti AR, Fabbri G, Lombardo M, Di Mauro M, Mughini L: Evidence by magnetic resonance imaging of cerebral alterations of atrophy type in young insulin-dependent diabetic patients. J Endocrinol Invest 1994;17:241–245 [DOI] [PubMed] [Google Scholar]
- 11.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM: Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156 [DOI] [PubMed] [Google Scholar]
- 12.Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH: Hippocampal volumes in youth with type 1 diabetes. Diabetes 2010;59:236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischl B, Dale AM: Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 2000;97:11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.