Abstract
OBJECTIVE
Lipotoxicity and ectopic fat deposition reduce insulin signaling. It is not clear whether excess fat deposition in nonadipose tissue arises from excessive fatty acid delivery from adipose tissue or from impaired adipose tissue storage of ingested fat.
RESEARCH DESIGN AND METHODS
To investigate this we used a whole-body integrative physiological approach with multiple and simultaneous stable-isotope fatty acid tracers to assess delivery and transport of endogenous and exogenous fatty acid in adipose tissue over a diurnal cycle in lean (n = 9) and abdominally obese men (n = 10).
RESULTS
Abdominally obese men had substantially (2.5-fold) greater adipose tissue mass than lean control subjects, but the rates of delivery of nonesterified fatty acids (NEFA) were downregulated, resulting in normal systemic NEFA concentrations over a 24-h period. However, adipose tissue fat storage after meals was substantially depressed in the obese men. This was especially so for chylomicron-derived fatty acids, representing the direct storage pathway for dietary fat. Adipose tissue from the obese men showed a transcriptional signature consistent with this impaired fat storage function.
CONCLUSIONS
Enlargement of adipose tissue mass leads to an appropriate downregulation of systemic NEFA delivery with maintained plasma NEFA concentrations. However the implicit reduction in adipose tissue fatty acid uptake goes beyond this and shows a maladaptive response with a severely impaired pathway for direct dietary fat storage. This adipose tissue response to obesity may provide the pathophysiological basis for ectopic fat deposition and lipotoxicity.
The metabolic function of adipose tissue is fat storage. An expansion of adipose tissue mass, in particular abdominal obesity, is associated with an increased risk of type 2 diabetes (1), cardiovascular disease (2), insulin resistance (3), and all-cause mortality (4,5). It is postulated that adipose tissue acts to “buffer” the influx of dietary fat into the circulation (6,7). Thus excess dietary fat should be stored in adipose tissue and not “overflow” to other organs such as the liver and skeletal muscle. Inappropriately stored fat in nonadipose tissue, ectopic fat deposition, has been proposed to underlie obesity-associated insulin resistance (8). Therefore the more efficient the regulation of fat storage in adipose tissue, the less the lipotoxic effects on nonadipose tissues.
The release of nonesterified fatty acids (NEFAs) from upper-body subcutaneous adipose tissue is a major determinant of systemic NEFA plasma concentrations (9), whereas visceral fat may contribute fatty acids specifically to the liver. Increased fasting concentrations of NEFA have been related to adipose tissue mass (10) and the presence of type 2 diabetes (11). However complete absence of such relationships has also been described in nondiabetic people (12–14). After a single meal, the postprandial concentrations of NEFA tend to remain somewhat higher in obese compared with lean people (15,16).
Although adipose tissue masses may vary over a 10-fold range between people, reported fasting NEFA concentrations typically only vary within a 2-fold range. This clearly implies a restriction in the release of NEFA from the adipose tissue of obese individuals. Although the detailed mechanism for this restriction is unknown, it has been observed that adipocytes isolated from overweight subjects respond subnormally to adrenergic lipolytic stimulation (17). However it is less clear if uptake mechanisms of fatty acids by adipose tissue are balanced by similar regulatory mechanisms. In the postprandial period, insulin stimulates fat deposition into adipose tissue. This occurs by hydrolysis of chylomicron triglycerides (TGs) by lipoprotein lipase (LPL) bound to capillary endothelium and subsequent uptake of the released fatty acids by adipose tissue and reesterification (storage). Following ingestion of a single mixed meal, impaired postprandial plasma TG clearance by adipose tissue was reported in obese subjects (18). This is partly explained by a lower functional LPL activity per unit fat mass in combination with the absence of postprandial upregulation of adipose tissue LPL in obesity (19). A pertinent question is therefore if adipose tissue in obese people, despite its enlargement, will induce its fat-storing capacity in the postprandial state as efficiently as in lean people (20).
In line with the known downregulation of lipolysis in obesity (17) and the absence of upregulation of adipose tissue LPL activity (19), we hypothesize that individuals with increased adiposity fail to appropriately upregulate meal-fat storage. Therefore we investigated the impact of obesity on systemic NEFA concentrations and NEFA delivery from adipose tissue, and then did detailed tissue-specific investigation of adipose tissue fatty acid trafficking of endogenous and meal-derived fatty acid over a 24-h period using stable isotope tracer methodology along with transcriptional profiles of the fatty acid trafficking pathways.
RESEARCH DESIGN AND METHODS
Background population.
The Oxford Biobank (21) is a population-based collection of healthy men and women between 30 and 53 years of age (n = 1,900) that was used for the recruitment of participants. We analyzed fasting plasma TG; NEFA; insulin and glucose concentrations in male subjects within two BMI ranges, 19–25 kg/m2 (lean) and 27–32 kg/m2 (abdominally obese); and age range 30–53 years (background cohort for the tissue-specific investigation). We excluded subjects with fasting hyperglycemia (>6.1 mmol/l) and overt hypertriglyceridemia (>3.0 mmol/l). Fat mass was calculated from bioelectric impedance. Subcutaneous adipose tissue biopsies are available from 160 of the participants in the Oxford Biobank, and we selected one lean group (n = 10, BMI 23.4 ± 0.3 kg/m2) and one obese group (n = 10, BMI 33.6 ± 1.7 kg/m2) of men for studies of quantitative mRNA content of genes involved in fatty acid trafficking.
Participants in the tissue-specific investigation.
Nine lean and ten abdominally obese males, comparable for age, were randomly invited from the Oxford Biobank (see above [n = 14]) or recruited locally from Oxford after self-reporting to advertisements (n = 5). Some data from eight of the lean males have been recently reported (20). All volunteers recruited were healthy nonsmokers, not on any medication known to affect lipid metabolism, and fulfilled the above inclusion criteria. We also required the obese participants to have a waist circumference >99 cm. The study was approved by the Oxfordshire Clinical Research Ethics Committee, and all subjects gave written informed consent.
Study protocol.
Subjects arrived to the clinical research unit, having fasted from 10:00 p.m. the night before. They were asked to refrain from strenuous exercise and alcohol for 24 h before the study.
Subcutaneous abdominal adipose tissue metabolism and blood flow was investigated by arterio-venous blood sampling as previously described (20). A constant infusion of [2H2]palmitate (Cambridge Isotopes, CK Gas Products, Cambridge, UK) was given intravenously (0.01 μmol/kg/min) in a total volume of 400 ml human albumin (4.5%) over the 24 h. The infusion was started more than ∼60 min before the first blood sample.
Meals were given at time points breakfast (0), lunch (5 h), and dinner (10 h). The quantity of food was adjusted to individual basic metabolic rate (BMR) estimated by bioimpedance (1.25 × basic metabolic rate) and divided by three to give the number of calories in each meal. Food items were weighed to the nearest gram. Breakfast was tagged with 100 mg [U-13C]linoleic acid, and lunch and dinner were tagged with 100 mg [U-13C]oleic and [U-13C]palmitic acid, respectively. Over the 24-h period, there were 29 blood sampling time points with simultaneous sampling from arterial and adipose venous sites. Further details on the administration, methodology, and calculation using the fatty acid stable isotope tracer as well as the biochemistry methods are essentially identical to a recent report (20) and also provided in the online appendix (available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0867/DC1).
Adipose tissue mRNA quantification.
After local anesthesia with 1% lignocaine, adipose tissue biopsies were taken from subcutaneous abdominal depots using a 12-gauge needle in the fasted state. Tissue samples were immediately extracted using TrizolR reagent (GIBCO-Life Technologies, Grand Island, NY). Between 0.5 and 1 μg of RNA were reverse transcribed to cDNA using random hexamer primers and Invitrogen SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). For the real-time quantitative PCR analyses, 4.5 μl of 1 in 100 or 1 in 20 dilution of cDNA per reaction were used in a final reaction volume of 10 μl. Seventeen genes were analyzed (ANGPTL4, LPL, GPIHBP1, CD36, ACLS1, AGPAT9, GPAM, DGAT1, DGAT2, HSL, PLIN, ATGL, ADBR2, LEP, CD68, CD11b, and CD163) using predesigned TaqMan Assays-on-Demand (Applied Biosystems, Foster City, CA) using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). All the samples were analyzed in quadruplicate and normalized to PPIA and RPLP0.
RESULTS
Relationship between fat mass and NEFA concentration in the population.
We first studied the relationship between fasting plasma NEFA concentrations and fat mass in the background population of 244 lean (BMI 19–25 kg/m2) and 210 abdominally obese (27–32 kg/m2) subjects from the Oxford Biobank (21). Despite an almost twofold difference in total body fat mass between the groups, there was no difference in systemic NEFA concentrations (Table 1). However, compared with the lean group, the abdominally obese men showed the expected higher fasting plasma TG, glucose and insulin concentrations, and lower HDL cholesterol concentration.
TABLE 1.
Age, anthropometric, and biochemical characteristics of participants in the study
| Background population identified from the Oxford Biobank |
Adipose tissue fatty acid trafficking study |
|||
|---|---|---|---|---|
| Lean males | Abdominally obese | Lean males | Abdominally obese | |
| n | 244 | 210 | 9 | 10 |
| Age (years) | 41 ± 6 | 42 ± 5 | 37 ± 11 | 42 ± 7 |
| BMI (kg/m2) | 23.1 ± 1.3 | 29.0 ± 1.4*** | 22.3 ± 1.9 | 30.8 ± 2.9*** |
| Waist circumference (cm) | 84 ± 6 | 100 ± 6*** | 81 ± 4 | 108 ± 7*** |
| Fat mass (kg) | 14.3 ± 2.3 | 25.1 ± 3.6*** | 10.8 ± 3.0 | 25.0 ± 6.4*** |
| Basal metabolic rate (kcal/day) | n/a | n/a | 1,780 ± 200 | 2,140 ± 220** |
| Fasting lipids, insulin, and glucose | ||||
| Total cholesterol (mmol/l) | 5.3 ± 0.9 | 5.7 ± 1.0*** | 4.0 ± 1.2 | 4.7 ± 1.2 |
| HDL cholesterol (mmol/l) | 1.3 ± 0.3 | 1.1 ± 0.2*** | 1.1 ± 0.3 | 1.0 ± 0.1 |
| Triglyceride (mmol/l) | 1.1 ± 0.5 | 1.6 ± 0.6*** | 0.9 ± 0.3 | 1.2 ± 0.3* |
| Nonesterifed fatty acids (μmol/l) | 473 ± 259 | 449 ± 197 | 599 ± 154 | 568 ± 137 |
| Glucose (mmol/l) | 5.2 ± 0.4 | 5.3 ± 0.4** | 5.2 ± 0.4 | 5.2 ± 0.3 |
| Insulin (pmol/l) | 46 ± 20 | 67 ± 29*** | 55 ± 8 | 80 ± 36* |
| Time-averaged postprandial plasma concentrations | ||||
| Triglyceride (mmol/l) | 1.1 ± 0.3 | 1.6 ± 0.3** | ||
| Insulin (pmol/l) | 122 ± 28 | 210 ± 73** | ||
| Glucose (mmol/l) | 5.9 ± 0.6 | 6.1 ± 1.2 | ||
| NEFA (μmol/l) | 283 ± 57 | 309 ± 36 | ||
Values are mean ± SD
*P < 0.05,
**P < 0.01,
***P < 0.001 comparing abdominally obese and lean groups. n/a, not applicable.
Effect of fat mass on expression of genes involved in adipose tissue fatty acid trafficking.
Of 12 selected candidate mRNA for key points in the regulation of fatty acid trafficking genes, 11 were significantly downregulated in the adipose tissue of obese men with the exception of CD36 (Supplementary Table 1). To confirm that the rather uniform downregulation of genes involved in fatty acid trafficking was not part of a universal effect brought about by obesity, we also analyzed leptin (LEP) and CD68 mRNA, CD163, and CD11b as possible positive controls. The expression of the latter three mark macrophage infiltration in adipose tissue and are expected to be increased in obese subjects. All macrophage markers were increased (66–98%) (P < 0.01 to P < 0.001) whereas LEP was unchanged (+4%, not significant). These findings suggest a coordinated downregulation of genes involving fatty acid trafficking in the adipose tissue of abdominally obese men.
Fatty acid trafficking
Subject characteristics.
As in the background population, there was no difference in fasting NEFA concentrations between the lean (n = 9) and the abdominally obese (n = 10) group with expected differences in fasting lipids and lipoproteins for the degree of obesity. Total fat mass was ∼2.5 times higher in the abdominally obese group (Table 1). There were clear signs of insulin resistance in the abdominally obese group; fasting insulin was increased despite near-identical fasting blood glucose between the groups (Table 1). Obese men had slightly higher alanine aminotransferase (P < 0.05), which could be an indicator of increased liver fat. We have recently reported on liver fatty acid partitioning in these groups of people (22).
Diurnal plasma concentrations of TG, NEFA, glucose, and insulin.
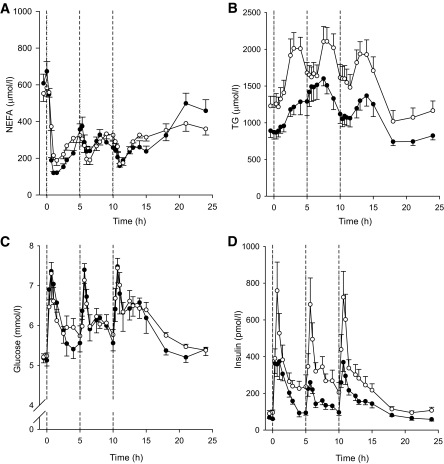
In order to observe diurnal metabolic patterns using a physiologically relevant situation, the participants were given three standardized meals and each was separated by 5 h. The significant differences in fasting TG and insulin between the groups were postprandially augmented (Table 1 and Fig. 1B and D), but the postprandial glucose concentrations were similar between the groups (Fig. 1C). As each meal was labeled with an isotopically labeled fatty acid, the plasma concentration of [13C]-TG reflected the chylomicron/chylomicron remnant TG concentrations; these were marginally higher in the abdominally obese males compared with the lean males (P = 0.08, area under curve [AUC] for the 5-h period after each meal intake, repeated-measures ANOVA).
FIG. 1.
Arterial plasma concentrations of NEFA (A), TG (B), glucose (C), and insulin (D) in lean males (●) and abdominally obese males (○).
There were small differences in the postprandial NEFA concentrations (Fig. 1A) between the groups. Both groups showed distinct suppression of NEFA concentrations immediately after the first meal, but the abdominally obese group remained ∼100 μmol/l higher than the lean group within the first meal period (+40 min to +240 min, P < 0.05 to P = 0.003 for the six consecutive time points measured). However, after this there were no statistically significant differences between the groups over the entire 24-h period. Neither of the groups started to return toward the initial high fasting NEFA concentrations until the early morning.
Rate of appearance of NEFA in the systemic circulation.
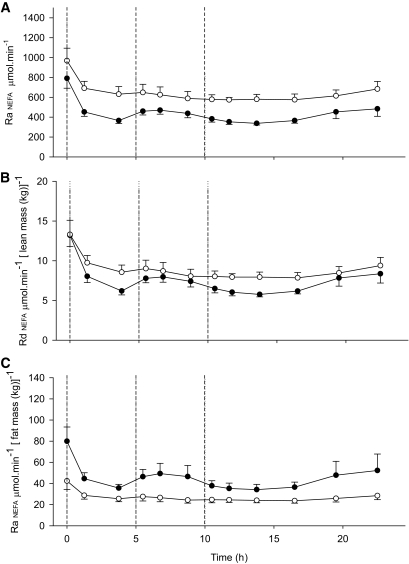
The diurnal NEFA delivery curves were essentially parallel between the groups. Although the fasting rate (790 ± 100 [lean] vs. 970 ± 130 μmol/min [abdominally obese]) was not statistically different between the groups, the time-averaged diurnal effect was very clear (450 ± 35 vs. 650 ± 50 μmol/min, P = 0.009) (Fig. 2A). Both groups showed an immediate reduction in NEFA delivery in response to the breakfast: the NEFA delivery was reduced by 38 ± 6% (P = 0.002) in the lean group with a less distinct response in the abdominally obese group (−19 ± 10%, P = 0.046). Expressed as NEFA disappearance per unit of lean body mass, the difference between the groups disappeared (Fig. 2B). The rationale behind this is based on the assumption that NEFA delivery equals NEFA catabolism and that lean body mass is proportional to the skeletal muscle and the liver. However, normalizing the NEFA delivery to fat mass, the abdominally obese group showed markedly lower fasting as well as time-averaged diurnal NEFA delivery compared with the lean group (Fig. 2C). This highlights the downregulation of NEFA delivery from the expanded adipose tissue in the abdominally obese state.
FIG. 2.
The rate of appearance (Ra) of NEFA in lean and abdominally obese men shown as total body Ra and adjusted for lean and fat mass, respectively. The Ra of NEFA: whole-body (A), expressed per lean mass (B), and per total fat mass (C) in lean (●) and abdominally obese men (○). Three meals were given as indicated by the dotted vertical lines. The Ra of NEFA (μmol/min) was significantly higher in the abdominally obese group compared with the lean group (A) (time × group, P = 0.009). When the data were calculated and expressed as rate of disappearance (Rd) of NEFA, i.e., normalized per lean body mass (μmol · min−1 [lean mass (kg)]−1), this difference disappeared (P = 0.14). The abdominally obese men had significantly lower Ra of NEFA when expressed per total fat mass (μmol · min−1 [fat mass (kg)]−1, P = 0.029).
Adipose tissue blood flow.
The abdominally obese men had a profoundly suppressed baseline adipose tissue blood flow (ATBF) compared with the lean men (2.9 ± 0.5 vs. 6.3 ± 1.5 ml · 100 g−1 · min−1, respectively, P = 0.01) and also displayed an unresponsive postprandial ATBF regulation after meal intake (Fig. 3A).
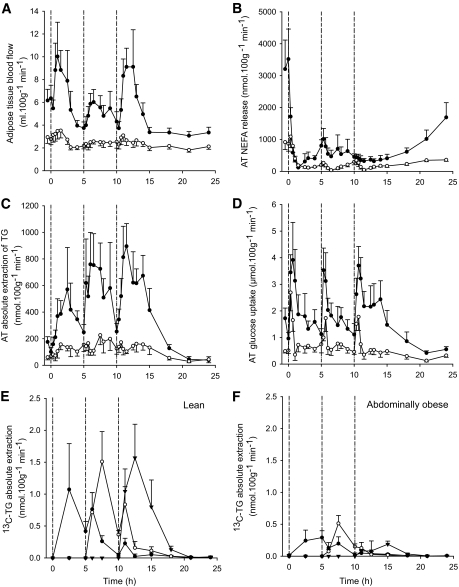
FIG. 3.
Adipose tissue blood flow and abdominal adipose tissue NEFA release, TG extraction and glucose uptake. The abdominally obese men (○) had significantly lower adipose tissue blood flow (time × group, P = 0.001) compared with the lean men (●) (A). The release of NEFA and the extraction of TG from plasma (nmol · 100 g−1 · min−1) was significantly lower in the tissue of the abdominally obese men (○) compared with lean men (●) (B and C) (both time × group, P = 0.001). Glucose uptake by adipose tissue was lower (P < 0.001) in the adipose tissue in the abdominally obese men (○) than in lean men (●) (D). The extraction of 13C-TG from each meal (meal 1 indicated by ●, meal 2 by ○, and meal 3 by ▾) in lean men (E) and in abdominally obese men (F) shows a diminution (P < 0.01) of 13C-TG extraction in the abdominally obese men in line with the net TG extraction (C). AT, adipose tissue.
Adipose tissue fatty acid trafficking.
To more precisely define the fatty acid trafficking in subcutaneous adipose tissue, we established arterio-venous blood sampling together with the ATBF measurements. In line with the NEFA Ra per unit fat mass, the abdominally obese males had significantly lower abdominal subcutaneous adipose tissue NEFA release, expressed per 100 g tissue, in the fasting state (P = 0.008) and across the second and third postprandial periods (P ≤ 0.015) (Fig. 3B), thus confirming the systemic measurements (NEFA Ra normalized for fat mass). The fasting NEFA Ra normalized to fat mass was correlated with net abdominal subcutaneous adipose tissue NEFA release (r = 0.68, P < 0.001, n = 19).
Having established the quiescence in lipolytic function in the abdominally obese adipose tissue, we focused on fatty acid uptake by the tissue with the hypothesis that this function would be equally suppressed. Fasting and postprandial removal of TG from blood across adipose tissue reflects LPL rate of action. A lower fasting absolute TG extraction across adipose tissue was observed in the abdominally obese males compared with the lean males (64 ± 19 vs. 138 ± 34 nmol · 100 g−1 · min−1, respectively, P = 0.07) (Fig. 3C). Postprandially, the abdominally obese males had an accentuated lower TG extraction (P < 0.001 for all meal periods) (Fig. 3C). While postprandial adipose tissue TG extraction increased with each meal in lean males (AUC 360 ± 109, 580 ± 173, 633 ± 114 μmol/100 g, first to third meal, respectively, P ≤ 0.03), it remained unchanged in the abdominally obese males (AUC 122 ± 30, 164 ± 47, 142 ± 31 μmol/100 g, first to third meal, respectively, P = 0.18). The differences between the groups were further augmented when the extraction of TG carried in chylomicrons/chylomicron remnants were studied: the absolute extraction of [13C]-TG across adipose tissue for all the meals was considerably lower per unit tissue in the abdominally obese males compared with the lean males (P < 0.01) (Fig. 3E and F).
As a significant proportion of the plasma TG is carried by VLDL, we explored the effect on VLDL-TG specifically by making use of the [2H2]palmitate accumulation in the whole plasma TG, which represents newly synthesized VLDL. The [2H2]palmitate-TG in plasma rose to a plateau concentration after 150 min with distinctly higher concentrations in the abdominally obese group compared with the lean group (2.1 ± 0.3 vs. 0.9 ± 0.1 μmol/l, P = 0.003) (Supplementary Fig. 1). The fractional extraction of [2H2]palmitate-TG across adipose tissue was similar between the groups (10.0 ± 1.5 vs. 13.3 ± 1.7%, not significant, average of the time period 150–900 min) despite the considerably higher arterial concentration of [2H2]palmitate-TG. These data demonstrate a downregulation of the efficiency of the uptake of fatty acid from all triglyceride-rich lipoproteins in obese adipose tissue. It was therefore also pertinent to note that the glucose uptake by adipose tissue was much lower in the abdominally obese men compared with the lean men as a sign of generally lower metabolic substrate utilization (Fig. 3D).
The arterio-venous model allowed us to study the net movement of fatty acid across the tissue bed, the transcapillary flux of fatty acid (23). In the fasting state, the net movement of fatty acids was directed from adipose tissue to the capillaries and was lower in the abdominally obese males compared with the lean males (−700 ± 210 vs. −2,950 ± 860 nmol · 100 g−1 · min−1, respectively, P = 0.06). In both groups, the direction soon changed after the first meal, and there was net deposition of fatty acids into adipose tissue (Fig. 4A).
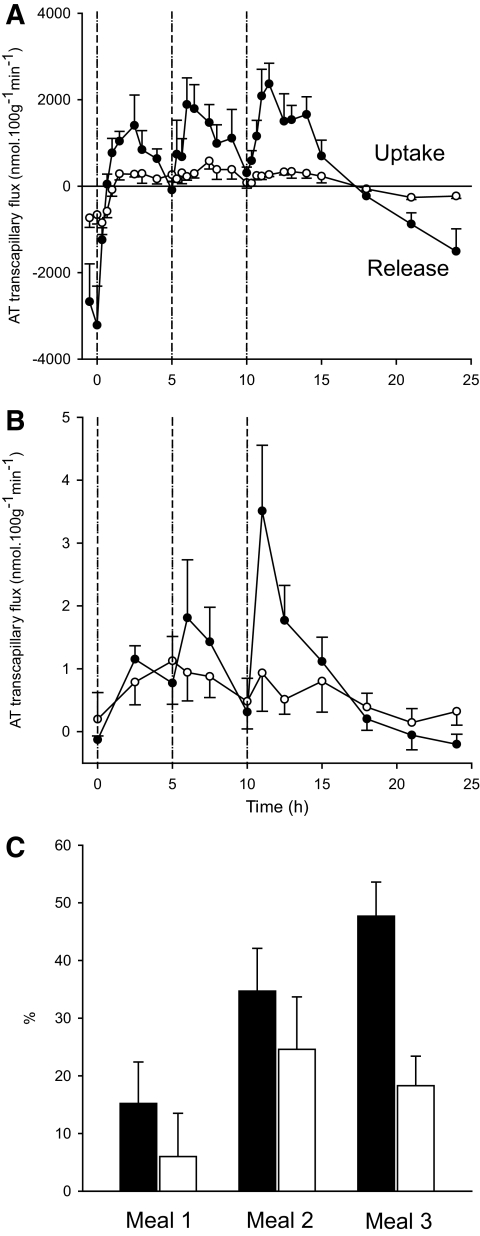
FIG. 4.
Total transcapillary flux of fatty acids across adipose tissue (A) in lean (●) and abdominally obese (○) men following ingestion of three meals (as indicated by the dotted vertical lines). B shows the transcapillary flux of [2H2]-palmitate, which is dominated by the label carried in [2H2]-TG (VLDL). C shows the percentage of meal fat content deposited in adipose tissue after three sequential meals (meal 1, meal 2, meal 3) in lean (■) and abdominally obese (□) males. This was calculated from the transcapillary flux (A) where each 5-h time period (AUC) multiplied for body fat mass to arrive at total body fat being stored. The denominator for each time period is the fat content in the meal. Lean men show a significant meal × fractional uptake effect (P = 0.009), whereas the effect is statistically nonsignificant in the abdominally obese men. AT, adipose tissue.
In the lean men, there was a threefold increase in transcapillary flux of fatty acids in response to sequential meal intake (comparison of first to third meal, Fig. 4A). In the abdominally obese males, the transcapillary flux of fatty acids was overall much lower (AUC for 5-h postprandial periods P = 0.026) and less dynamic with no increase in transcapillary flux with meal sequence (Fig. 4A). These findings were corroborated by studying the transcapillary flux of all [2H2]palmitate-fatty acid (composite measurement of VLDL-TG and direct uptake of NEFA, where the latter is the minor component [24]). This also showed a lower and nondynamic flux of fatty acids in the adipose tissue in the abdominally obese individuals compared with the lean individuals (P = 0.04, RM-ANOVA, Fig. 4B).
A proportion of fatty acid released by LPL escape reesterification, and this pathway has been denoted “spillover” (25), which could make a contribution to the systemic NEFA concentrations (26). We therefore explored the quantitative significance of this pathway. In lean males, the chylomicron-TG–derived ([U-13C]-fatty acid, representing dietary-derived fat) spillover fraction decreased progressively across meal periods as determined at the end of each postprandial period (65, 48, and 37%, first to third meal, P = 0.006) (Supplementary Fig. 2A). However, in the abdominally obese males, it remained unchanged across meal periods and at a lower level than in lean men (35, 24, and 24%, first to third meal, P = 0.13) (Supplementary Fig. 2B). We then calculated the relative proportion of the systemic NEFA concentration that originated from spillover from the chylomicron-carried dietary fat. This shows that the proportion of NEFA derived from adipose tissue spillover is consistently lower (P < 0.01) in the abdominally obese men than their lean counterparts over the meal-feeding period (Supplementary Fig. 2C).
Estimations of whole-body fatty acid deposition into adipose tissue.
It is of importance to consider the expanded adipose tissue mass at the whole-body level in the abdominally obese individuals to understand the adaptation of the system. In the calculation of whole-body effects, we assume that all adipose tissues have similar handling of fatty acid. Thus, accounting for total body fat mass, it was clear that the abdominally obese males had lower total adipose tissue TG extraction compared with the lean men. Over the three consecutive meal periods, they had progressively lower total adipose tissue TG extraction: over the third meal period (meal period AUC data from Fig. 3C multiplied by fat mass), the abdominally obese men's adipose tissue extracted only 53% (P = 0.01) of what was seen in the lean men. The difference between the groups was further amplified when the same calculation was applied to the transcapillary flux of fatty acids (net fat storage) from the abdominal subcutaneous adipose tissue depot. Over the third meal period (meal period AUC data from Fig. 4A multiplied by fat mass), the abdominally obese men stored only 43% of the fat in adipose tissue compared with the lean men (P = 0.03). As previously mentioned, the adipose tissue fatty acid uptake from chylomicrons was particularly low in the abdominally obese men compared with the lean men, whereas the fatty acid uptake from VLDL was less affected (the fractional extraction was similar between the groups, yet the concentration was higher in the abdominally obese men). The total AUC of the transcapillary flux of [2H2]-palmitate (dominated by the [2H2]-palmitate carried by TG in VLDL) factored up for fat mass was twice as high in the abdominally obese men compared with the lean men (178 ± 50 vs. 73 ± 14 μmol/h, P = 0.049) (calculated from Fig. 4B). This implies that a higher proportion of the fatty acid stored in adipose tissue originates from VLDL-TG in abdominally obese men compared with lean men. However for glucose uptake by adipose tissue, the differences between groups disappeared when accounting for total fat mass, and net glucose disposal through adipose tissue is likely to be similar between lean and obese people.
In order to estimate the relative quantity of the meal fat that was stored in adipose tissue, the fat content of each meal was divided by the calculated whole-body net fat storage during the three consecutive 5-h meal periods. In lean males, there was progressive increase in the deposition of meal fat into adipose tissue with each meal (13, 35, and 47%, first to third meal, respectively, P < 0.001) (Fig. 4C). In contrast, the abdominally obese males failed to increase fat storage with sequential feeding (6, 25, and 18%, first to third meal, respectively, P = 0.12) (Fig. 4C). This difference was highly statistically significant for the last meal period (P = 0.001).
DISCUSSION
Upper-body subcutaneous adipose tissue is the main site of storage and release of fatty acid to the systemic circulation (27) and by comparing lean healthy men with moderately abdominally obese men, we describe a remarkable adaptation of the expanded adipose tissue. In terms of delivery of NEFA from the tissue, this adaptation appears to be entirely appropriate, resulting in normal diurnal regulation of NEFA concentrations in abdominally obese men, which is brought about by significantly diminished NEFA release rates per unit of adipose tissue. The storage of fatty acids from plasma TG was correspondingly reduced in subjects with abdominal obesity and in this pathway there were also a clear signs of maladaptation; there was an inability to induce fat storage with sequential meals combined with a failure to effectively store the chylomicron-TG–derived fatty acids originating from the most recent meal. We propose that this is the pathophysiological basis for diversion of fatty acid to be stored in organs not dedicated for fat storage, i.e., ectopic fat deposition.
One such organ is the liver and, in the loops of fatty acid trafficking studied in this article, this became very clear. In lean men, a high proportion of chylomicron-TG was stored immediately in adipose tissue, whereas a lower proportion was seen in abdominally obese men. In contrast, fatty acid originating from VLDL-TG appeared to play a greater proportional role in adipose tissue fat storage in the abdominally obese men. These events are linked, as insufficient extraction of chylomicron-TG produce chylomicron remnants, the TG content of which will be deposited in the liver and thereby provide substrate for VLDL-TG production. The liver in abdominally obese men is therefore burdened by this seemingly “unnecessary” loop of fatty acid trafficking and also showed slightly higher plasma alanine aminotransferase concentrations as an indicator of increased liver fat content.
Obese subjects are often reported to have higher fasting and postprandial NEFA concentrations than lean subjects (10,28), which is thought to be due to the expansion of fat mass (29). However the diurnal systemic NEFA concentrations were essentially not different between lean and abdominally obese males. Although the suppression of systemic NEFA concentrations after the first meal was less marked in the abdominally obese group, which is in line with the observations made by others (16), this was a negligible difference over the 24-h period. Also the fasting concentration of NEFA of much larger numbers of randomly recruited lean and abdominally obese people were not different from each other. As this observation is made in moderate stages of obesity in seemingly healthy individuals, it suggests that NEFA oversupply to the systemic circulation might not be an early or universal feature of the metabolic complications of obesity. Not only does this finding concur with studies of BMI-matched insulin-resistant and insulin-sensitive men (14), but it also concurs with a more extreme example of obesity (13). They are also in line with a recent study by Mittendorfer et al. (12), which describes a similar, but not complete, downregulation of NEFA Ra in a group exhibiting a very wide range of body fat content. It is also noted that very large-scale observations in the Paris Prospective Study (30) showed no association between BMI and fasting NEFA concentrations.
Given the similar systemic NEFA concentrations between the groups in this study, despite a 2- to 2.5-fold difference in the whole-body adipose tissue mass, we interrogated in detail the mechanisms of NEFA delivery from the tissue. In line with earlier observations for LPL (31) and hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) (32), we found lower expression in obese/insulin-resistant men, but our findings on transcript regulation also indicate a profound lowering of all processes involved in fatty acid trafficking in obesity. Most NEFAs delivered from adipose tissue originate from intracellular TG via ATGL/HSL-mediated lipolysis. However, a smaller proportion is originating from spillover of fatty acid derived from the LPL-mediated lipolysis of chylomicron and VLDL-TG and subsequently failing to undergo reesterification. The spillover was generally lower and certainly less dynamic in the abdominally obese group, observed both locally in the adipose tissue after each meal as well as its relative contribution to the systemic NEFA concentration. As the overall quantity of LPL-mediated fatty acid delivery to the tissue was much smaller in the abdominally obese group, we conclude that once fatty acid has been generated through this pathway, they appear to be readily reesterified. Accordingly, the reesterification pathway does not seem to be subject to the same degree of downregulation as the LPL step in obese adipose tissue.
A limitation of this study is the extrapolation of whole-body fat storage from the local measurement in the abdominal subcutaneous tissue knowing that fat depots have different characteristics in this respect. However, from the point of view of NEFA delivery from adipose tissue to the systemic circulation, this extrapolation has validity as the significant majority of NEFAs delivered to the systemic circulation originates from this depot (27). However, with the same argument, we find it difficult to raise support for the argument that excessive NEFA delivery from adipose tissue to the systemic circulation is an early determinant of the insulin resistance in obesity. We have only studied men; extrapolations to women cannot be made.
In summary, adipose tissue shows appropriate adaptation to obesity in terms of systemic NEFA delivery, and this is part of a global response (silencing of metabolic and vascular functions of the tissue), whereas the corresponding downregulation of fat storage exhibit a maladaptive response with insufficient sequestration of dietary fatty acid that may trigger ectopic fat deposition.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by the British Heart Foundation, the Wellcome Trust, the MRC Centre for Obesity and Related Metabolic Disorders, and the National Institute for Health Research, Oxford Biomedical Research Centre. F.K. was a Senior Clinical Fellow of the Wellcome Trust.
No potential conflicts of interest relevant to this article were reported.
S.E.M., K.N.F., and F.K. wrote the manuscript. All the coauthors researched data and reviewed and edited the manuscript.
Parts of this study were presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bjorntorp P: Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabete Metab Rev 1988;4:615–622 [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS: INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–1649 [DOI] [PubMed] [Google Scholar]
- 3.Evans DJ, Murray R, Kissebah AH: Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal, and insulin binding. Effects of obesity and body fat topography. J Clin Invest 1984;74:1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R: Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjønneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quirós JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E: General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–2120 [DOI] [PubMed] [Google Scholar]
- 6.Frayn KN: Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002;45:1201–1210 [DOI] [PubMed] [Google Scholar]
- 7.Lewis GF, Carpentier A, Adeli K, Giacca A: Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002;23:201–229 [DOI] [PubMed] [Google Scholar]
- 8.Unger RH, Orci L: Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord 2000;24(Suppl. 4):S28–S32 [DOI] [PubMed] [Google Scholar]
- 9.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD: Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opie LH, Walfish PG: Plasma free fatty acid concentrations in obesity. N Engl J Med 1963;268:757–760 [DOI] [PubMed] [Google Scholar]
- 11.Fraze E, Donner CC, Swislocki AL, Chiou YA, Chen YD, Reaven GM: Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab 1985;61:807–811 [DOI] [PubMed] [Google Scholar]
- 12.Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S: Relationship between body fat mass and free fatty acid kinetics in men and women. Obesity (Silver Spring) 2009;17:1872–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeds DN, Stuart CA, Perez O, Klein S: Adipose tissue, hepatic, and skeletal muscle insulin sensitivity in extremely obese subjects with acanthosis nigricans. Metabolism 2006;55:1658–1663 [DOI] [PubMed] [Google Scholar]
- 14.Bickerton AS, Roberts R, Fielding BA, Tornqvist H, Blaak EE, Wagenmakers AJ, Gilbert M, Humphreys SM, Karpe F, Frayn KN: Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia 2008;51:1466–1474 [DOI] [PubMed] [Google Scholar]
- 15.Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, Kirk ML, Potts JL, Hockaday TD: Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism 1992;41:264–272 [DOI] [PubMed] [Google Scholar]
- 16.Roust LR, Jensen MD: Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 1993;42:1567–1573 [DOI] [PubMed] [Google Scholar]
- 17.Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P: Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest 1994;93:2590–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potts JL, Coppack SW, Fisher RM, Humphreys SM, Gibbons GF, Frayn KN: Impaired postprandial clearance of triacylglycerol-rich lipoproteins in adipose tissue in obese subjects. Am J Physiol 1995;268:E588–E594 [DOI] [PubMed] [Google Scholar]
- 19.Sadur CN, Yost TJ, Eckel RH: Insulin responsiveness of adipose tissue lipoprotein lipase is delayed but preserved in obesity. J Clin Endocrinol Metab 1984;59:1176–1182 [DOI] [PubMed] [Google Scholar]
- 20.Ruge T, Hodson L, Cheeseman J, Dennis AL, Fielding BA, Humphreys SM, Frayn KN, Karpe F: Fasted to fed trafficking of fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. J Clin Endocrinol Metab 2009;94:1781–1788 [DOI] [PubMed] [Google Scholar]
- 21.Tan GD, Neville MJ, Liverani E, Humphreys SM, Currie JM, Dennis L, Fielding BA, Karpe F: The in vivo effects of the Pro12Ala PPARγ2 polymorphism on adipose tissue NEFA metabolism: the first use of the Oxford Biobank. Diabetologia 2006;49:158–168 [DOI] [PubMed] [Google Scholar]
- 22.Hodson L, McQuaid SE, Humphreys SM, Milne R, Fielding BA, Frayn KN, Karpe F: Greater dietary fat oxidation in obese compared with lean men: an adaptive mechanism to prevent liver fat accumulation? Am J Physiol Endocrinol Metab 2010;299:E584–E592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frayn KN, Shadid S, Hamlani R, Humphreys SM, Clark ML, Fielding BA, Boland O, Coppack SW: Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol 1994;266:E308–E317 [DOI] [PubMed] [Google Scholar]
- 24.Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN: Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 2007;56:168–176 [DOI] [PubMed] [Google Scholar]
- 25.Miles JM, Park YS, Walewicz D, Russell-Lopez C, Windsor S, Isley WL, Coppack SW, Harris WS: Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes 2004;53:521–527 [DOI] [PubMed] [Google Scholar]
- 26.Miles JM, Nelson RH: Contribution of triglyceride-rich lipoproteins to plasma free fatty acids. Horm Metab Res 2007;39:726–729 [DOI] [PubMed] [Google Scholar]
- 27.Koutsari C, Jensen MD: Thematic review series: patient-oriented research: free fatty acid metabolism in human obesity. J Lipid Res 2006;47:1643–1650 [DOI] [PubMed] [Google Scholar]
- 28.Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ, Caprio S: Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab 2001;86:90–96 [DOI] [PubMed] [Google Scholar]
- 29.Flatt JP: Role of the increased adipose tissue mass in the apparent insulin insensitivity of obesity. Am J Clin Nutr 1972;25:1189–1192 [DOI] [PubMed] [Google Scholar]
- 30.Charles MA, Fontbonne A, Thibult N, Claude JR, Warnet JM, Rosselin G, Ducimetière P, Eschwège E: High plasma nonesterified fatty acids are predictive of cancer mortality but not of coronary heart disease mortality: results from the Paris Prospective Study. Am J Epidemiol 2001;153:292–298 [DOI] [PubMed] [Google Scholar]
- 31.Ong JM, Kern PA: Effect of feeding and obesity on lipoprotein lipase activity, immunoreactive protein, and messenger RNA levels in human adipose tissue. J Clin Invest 1989;84:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE: Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab 2007;92:2292–2299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.