Abstract
OBJECTIVE
The “portal hypothesis” proposes that the liver is directly exposed to free fatty acids and cytokines increasingly released from visceral fat tissue into the portal vein of obese subjects, thus rendering visceral fat accumulation particularly hazardous for the development of hepatic insulin resistance and type 2 diabetes. In the present study, we used a fat transplantation paradigm to (artificially) increase intra-abdominal fat mass to test the hypothesis that venous drainage of fat tissue determines its impact on glucose homeostasis.
RESEARCH DESIGN AND METHODS
Epididymal fat pads of C57Bl6/J donor mice were transplanted into littermates, either to the parietal peritoneum (caval/systemic venous drainage) or, by using a novel approach, to the mesenterium, which confers portal venous drainage.
RESULTS
Only mice receiving the portal drained fat transplant developed impaired glucose tolerance and hepatic insulin resistance. mRNA expression of proinflammatory cytokines was increased in both portally and systemically transplanted fat pads. However, portal vein (but not systemic) plasma levels of interleukin (IL)-6 were elevated only in mice receiving a portal fat transplant. Intriguingly, mice receiving portal drained transplants from IL-6 knockout mice showed normal glucose tolerance.
CONCLUSIONS
These results demonstrate that the metabolic fate of intra-abdominal fat tissue transplantation is determined by the delivery of inflammatory cytokines to the liver specifically via the portal system, providing direct evidence in support of the portal hypothesis.
Several hypotheses have been formulated and pursued to explain the deteriorating impact of intra-abdominal (visceral) adipose tissue accumulation on glucose metabolism: intra-abdominal fat tissue may be inherently different from subcutaneous fat, e.g., in cellular composition, tissue dynamics (tendency to respond to excess calories with a hypertrophic rather than a hyperplastic response), adipokine release, and hormonal responses. Alternatively (or complementarily), the venous drainage of subcutaneous fat differs from that of visceral fat: secreted products such as adipocytokines and free fatty acids (FFAs) from subcutaneous fat are drained systemically into the vena cava, whereas secreted products from mesenteric or visceral fat are drained into the portal system. Exaggerated hepatic delivery of FFAs and proinflammatory cytokines originating from adipose tissue (and/or the gastrointestinal tract) result in hepatic insulin resistance and metabolic deterioration constituting “the portal theory” (1–4).
We previously found that increasing fat mass by intra-abdominal transplantation of fat pads isolated from healthy non–insulin-resistant littermates could not recapitulate the effects of fat accumulation in obesity in healthy recipient mice. Instead, increased insulin sensitivity and improved glucose tolerance were observed (5). Yet, blood from these fat pads is drained into the vena cava inferior and thus reaches the systemic circulation while circumventing the liver. A prominent hypothesis linking visceral/intra-abdominal adiposity to cardio-metabolic diseases involves the tight association with fatty liver diseases, which is explained by the portal theory. Thus, it is plausible that the metabolic effect of intra-abdominal adipose tissue transplantation depends on the venous drainage of the transplant. Indeed, in a recent study, no difference was reported in glucose tolerance between mice receiving an intra-abdominal fat transplant and sham-operated mice (6), potentially because transplants were drained to both the caval as well as the portal circulation.
The aim of the present study was to test the hypothesis that venous drainage of fat tissue determines its impact on glucose homeostasis. To this end, we developed a novel approach to mimic visceral fat accumulation by transplanting fat pads from a donor mouse to the mesenterium of a recipient littermate. These pads are drained solely to the portal vein. In contrast to mice receiving caval drained fat pad transplantation, mice receiving a portal drained fat pad developed (hepatic) insulin resistance and were glucose intolerant. Furthermore, we present evidence that interleukin (IL)-6 is at least partly responsible for impaired glucose metabolism in these mice. Thus, our results hint toward an important influence of venous drainage of fat pads on glucose homeostasis, supporting the portal hypothesis.
RESEARCH DESIGN AND METHODS
Animals.
Male C57BL6JOlaHsd mice were purchased from Harlan Netherlands (Harlan, Horst, the Netherlands), and male IL-6 KO mice and respective wild-type mice were purchased from Charles River Laboratories (Wilmington, MA). All mice were housed in a pathogen-free environment on a 12-h light-dark cycle, with free access to standard rodent diet (Provimi Kliba, Kaiseraugst, Switzerland). All protocols conformed to the Swiss animal protection laws and were approved by the Cantonal Veterinary Office in Zurich, Switzerland.
Surgical procedure.
Transplantation procedure was performed at 6 weeks of age. Mice were anesthetized with isoflurane (Abbott, Baar, Switzerland). Both epididymal fat pads (average size per pad 75 mg [range 65–90 mg]) were removed from the donor mouse, rinsed with 0.9% saline, and thereafter stitched to the visceral side of the peritoneum (caval transplantation) or to the mesenterium of the recipient mouse (portal transplantation) using Monocryl 6.0 (Johnson-Johnson, Spreitenbach, Switzerland). Sham-operated control mice underwent the same surgical procedure without receiving a fat transplant. Subcutaneous injection of buprenorphine every 12 h for 2 days was used for analgesia. To prevent transplant rejection, donor and recipient mice were littermates.
Glucose tolerance tests.
For the intraperitoneal glucose tolerance test, mice were fasted overnight. Glucose (2 g/kg body wt) was injected intraperitoneally and blood was collected from the tail vein at different time points, as indicated (5). Plasma glucose was measured using a glucose meter (Ascensia Contour; Bayer, Zurich, Switzerland).
Histology.
Fat tissues were fixed in 4% buffered formalin and embedded in paraffin. Sections were obtained at the time of death and stained with hematoxylin and eosin. For each fat pad of a mouse, at least 100 adipocytes were analyzed. Images were analyzed using ImageJ software for quantification (National Institutes of Health, Bethesda, MD). Macrophages were immunostained with mac2 antibody. Mac2-positive cells were counted per nuclei of seven randomly selected fields per section containing at least 90 cells.
Hyperinsulinemic-euglycemic clamps.
Clamps were performed in freely moving mice 5 weeks after transplantation as described previously (7). Glucose infusion rate was calculated once glucose infusion reached a more or less constant rate with blood glucose levels at 5 mmol/l. This was reached in most mice 80–90 min after the start of insulin infusion. Thereafter, blood glucose was kept constant at 5 mmol/l for 20 min (with no or minimal adjustment of the glucose infusion rate), and glucose infusion rate was calculated. The glucose disposal rate was calculated by dividing the rate of [3-3H]glucose infusion by the plasma [3-3H]glucose specific activity (8,9). Endogenous glucose production during the clamp was calculated by subtracting the glucose infusion rate from the glucose disposal rate (8,9). Insulin-stimulated glucose disposal rate was calculated by subtracting basal endogenous glucose production (equal to basal glucose disposal rate) from glucose disposal rate during the clamp (10).
Determination of plasma adiponectin, leptin, IL-6, insulin, and FFA.
Plasma adiponectin, leptin, and IL-6 levels were determined with mouse LINCOplex kits from Linco Research (Labodia, Yens, Switzerland); plasma insulin and FFA levels were measured as previously described (5).
Total liver lipid extraction.
Liver tissue (30 mg) was homogenized in PBS, and lipids were extracted in a chloroform-methanol (2:1) mixture. Total liver lipids were determined by a sulfophosphovanillin reaction as previously described (7).
RNA extraction and quantitative RT-PCR.
Total RNA from fat pads was extracted with the RNeasy lipid tissue mini-kit (Qiagen). A total of 0.75 μg RNA was reverse-transcribed with Superscript III Reverse Transcriptase (Invitrogen) using random hexamer primer (Invitrogen). Taqman system (Applied Biosystems) was used for real-time PCR amplification. Relative gene expression was obtained after normalization to 18sRNA (Applied Biosystems), using the formula 2−ΔΔcp (11). The following primers were used: TNF-α Mm004483258_m1, IL-6 Mm00446190_m1, KC Mm00433859_m1, IL-1β Mm0043422/_m1, MCP-1 Mm00441242_m1, Fas Mm00433237_m1, FasL Mm00438864_m1, cd11c Mm00498698_m1, Hif-1α Mm00468869_m1, SOCS.3 Mm 00545913_s1, CD68 Mm03047343_m1, F4/80 Mm00802529_m1, and cd11b Mm00434455_m1 (Applied Biosystems).
Western blot.
Western blotting was performed as previously described (7). The following antibodies were used: anti–phospho-Akt (S473) and anti-Akt were purchased from Cell Signaling; secondary horseradish peroxidase–conjugated antibody were purchased from either Santa Cruz Biotechnology or Alexis Biochemicals.
Data analysis.
Data are presented as means ± SEM and were analyzed by Student t test or ANOVA with a Turkey correction for multiple group comparisons.
RESULTS
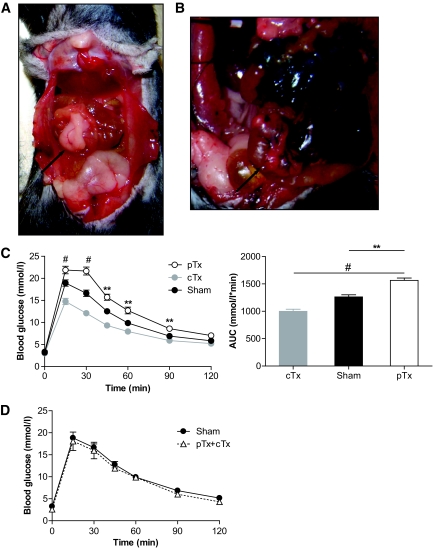
To estimate potential metabolic effects of an artificial increase in fat tissue mass drained uniquely into the portal system, epididymal fat pads of a regular diet–fed donor mouse were transplanted to the mesenterium of a regular diet–fed littermate mouse using a novel surgical approach. At the time of death (5 weeks after transplantation), transplanted fat pads were adherent to the mesenterium and well vascularized (Fig. 1A). Sham operation was performed in control littermates. Mice tolerated the surgical procedure and the fat transplantation well; no adverse events were noted. To confirm venous drainage of transplanted fat pads to the portal vein, Evans Blue was injected retrogradely into the portal vein. As depicted in Fig. 1B, transplanted fat pads were stained with Evans Blue, as was endogenous mesenteric fat tissue, but not the caval-drained epididymal fat tissue, confirming the portal drainage of the mesenteric-transplanted fat tissue.
FIG. 1.
Transplantation was performed at 6 weeks of age. Both epididymal fat pads were removed from a donor mouse and stitched to the mesenterium (pTx) of a littermate recipient. A: Six weeks after transplantation, the abdominal cavity was opened and the transplanted fat pad was visualized. B: To confirm portal drainage of transplanted fat pads, Evans blue dye (5 mg/ml) was injected into the portal vein. Arrow: portal transplanted fat pad. C: Intraperitoneal glucose tolerance test (2 g glucose/kg body weight) was performed 4 weeks after surgical procedure in mice receiving portal drained transplants (pTx; ○), in mice receiving caval drained transplants (cTx;  ), or in sham-operated mice (●) after an overnight fast. Results are expressed as mean blood glucose concentration (left panel) or area under curve (AUC) (right panel) ± SEM of 5 (pTx), 16 (cTx), or 23 (sham) animals. **P < 0.01, #P < 0.001 (left panel: Student t test [pTx vs. sham]; right panel: ANOVA). D: Both epididymal fat pads were removed from a donor mouse. One was stitched to the peritoneum (caval drained; cTx) and the other to the mesenterium (portal drained; pTx) of a littermate. Intraperitoneal glucose tolerance test (2 g glucose/kg body wt) was performed 4 weeks after surgical procedure in mice receiving a double transplant (cTx + pTx; ▵) or in sham-operated mice (●) after an overnight fast. Results are expressed as mean blood glucose concentration ± SEM of three to eight animals. (A high-quality digital representation of this figure is available in the online issue.)
), or in sham-operated mice (●) after an overnight fast. Results are expressed as mean blood glucose concentration (left panel) or area under curve (AUC) (right panel) ± SEM of 5 (pTx), 16 (cTx), or 23 (sham) animals. **P < 0.01, #P < 0.001 (left panel: Student t test [pTx vs. sham]; right panel: ANOVA). D: Both epididymal fat pads were removed from a donor mouse. One was stitched to the peritoneum (caval drained; cTx) and the other to the mesenterium (portal drained; pTx) of a littermate. Intraperitoneal glucose tolerance test (2 g glucose/kg body wt) was performed 4 weeks after surgical procedure in mice receiving a double transplant (cTx + pTx; ▵) or in sham-operated mice (●) after an overnight fast. Results are expressed as mean blood glucose concentration ± SEM of three to eight animals. (A high-quality digital representation of this figure is available in the online issue.)
We have previously shown that mice receiving fat pads drained to the inferior vena cava (caval-drained fat transplantation [cTx]) had improved glucose tolerance (5). Four weeks after transplantation, body weight gain was not different between sham-operated mice and mice receiving a portal-drained fat transplant (3.0 ± 0.4 vs. 2.9 ± 0.5 g; P = 0.85). However, as depicted in Fig. 1C, increase in blood glucose concentrations after an intraperitoneal glucose load was significantly higher in mice receiving a fat transplant drained to the portal vein (portal-drained fat pad transplantation [pTx]) compared with mice receiving cTx, as well as to sham-operated mice, indicating impaired glucose tolerance in mice receiving a pTx.
To determine whether the effect on glucose homeostasis of one transplantation site predominates over the other, a double transplantation approach was performed, i.e., one epididymal fat pad of the donor mouse was stitched to the peritoneum (and thus drained to the inferior caval vein), and the other pad was stitched to the mesenterium (and thus drained to the portal vein). Transplanted fat pads were similar in size and originated from the same donor mouse, which was a littermate of the recipient mouse. As depicted in Fig. 1D, glucose tolerance in such transplanted mice was not different from sham-operated mice 4 weeks after surgery.
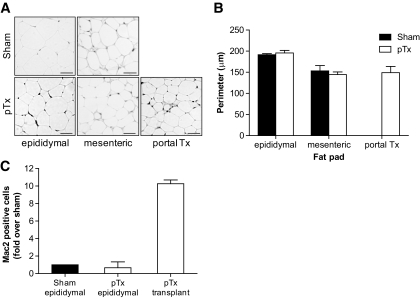
We next assessed adipose tissue morphology of endogenous and transplanted fat pads in sham-operated and pTx mice (Fig. 2A). As depicted in Fig. 2B, perimeters of endogenous epididymal and mesenteric adipocytes were similar in both groups. However, epididymal adipocytes were larger than mesenteric adipocytes. Moreover, adipocytes in portal-transplanted fat pads were similar in size as endogenous mesenteric adipocytes but smaller than endogenous epididymal adipocytes. Furthermore, macrophage infiltration rate was much higher in portal-transplanted fat pads than endogenous epididymal fat pads of transplanted and sham-operated mice (Fig. 2C).
FIG. 2.
A: Representative hematoxylin- and eosin-stained histological sections of endogenous epididymal, endogenous mesenteric, or portal-transplanted fat pads from sham-operated and pTx mice are shown 5 weeks after surgery. Adipocyte cell perimeter was measured using ImageJ. B: Up to 100 cells for each fat pad were analyzed, and the results represent the mean ± SEM of three or four different mice. Black bar: sham-operated mice; white bar: mice receiving a portal fat transplant. C: Histological sections of endogenous epididymal and portal-transplanted tissue were immunostained with mac2 antibody. Mac2-positive cells were counted in seven randomly selected fields per section (about 90 cells) and expressed relative to total cell nucleus content. Results represent the mean ± SEM of three to five different mice. All values are expressed relative to Mac2-positive cells of epididymal fat pad of sham-operated mice.
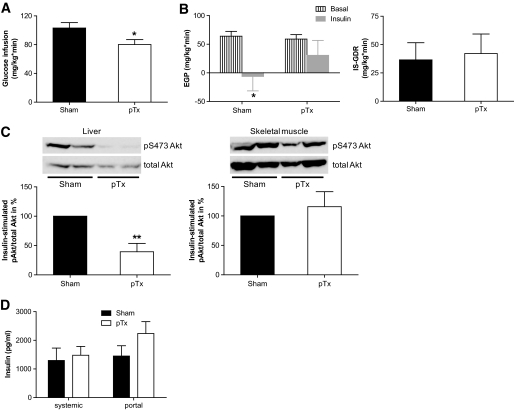
Consistent with the portal theory, we hypothesized that portal drainage of fat transplant would cause hepatic insulin resistance, and to assess this possibility, a hyperinsulinemic-euglycemic clamp was performed 5 weeks after surgery. Glucose infusion rate was significantly lower in portal-transplanted mice than in sham-operated mice (Fig. 3A and supplementary Figure S1 [available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0697/DC1). Moreover, during a hyperinsulinemic-euglycemic clamp, insulin-induced suppression of endogenous glucose production was blunted in mice receiving a fat pad transplant drained to the portal vein, but was clearly evident in sham-operated mice (Fig. 3B, left panel). Therefore, mice receiving portal drained fat pad transplants developed hepatic insulin resistance. Furthermore, insulin-induced phosphorylation of Akt was markedly reduced in livers of portal-transplanted mice compared with that of sham-operated mice (Fig. 3C, left panel). This eminent reduction in hepatic insulin signaling confirmed the development of hepatic insulin resistance in portal-transplanted mice. In contrast, glucose disposal rate (Fig. 3B, right panel) as well as insulin signaling in skeletal muscle were not affected by the portal-drained transplant (Fig. 3C, right panel), suggesting maintained insulin sensitivity of skeletal muscle. Intriguingly, insulin levels (3 h after food withdrawal) were only slightly increased in systemic samples but clearly elevated in portal blood samples of mice receiving a portal fat transplantation compared with sham-operated mice (Fig. 3D), matching the clamp results presented above. Further metabolic characteristics of sham-operated and portal-transplanted mice are presented in Table 1.
FIG. 3.
A and B: Hyperinsulinemic-euglycemic clamps were performed 5 weeks after transplantation. A: Mean glucose infusion rate (see research design and methods). B: Endogenous glucose production (EGP; left panel) as well as insulin-stimulated glucose disposal rate (IS-GDR; right panel) were calculated in the basal period and in response to insulin infusion (18 mU/kg*min) during the clamp study. Results are the mean ± SEM of five to six animals. *P < 0.05 (Student t test). C: Insulin (1 U/kg) was injected intraperitoneally in overnight fasted mice 5 weeks after transplantation. Liver and skeletal muscle samples were harvested 15 min later, and phosphorylation of Akt at S473 was determined by Western blotting. A representative blot is shown in the upper panel. Results are the mean ± SEM of three to four animals. **P < 0.01 (Student t test). D: Blood samples were obtained as indicated, and insulin was determined as described in research design and methods. Results are expressed as mean plasma insulin concentration ± SEM of five to six animals.
TABLE 1.
Metabolic characteristics of sham-operated and portal-transplanted mice
| Sham | pTx | |
|---|---|---|
| Before transplantation | ||
| Body weight (g) | 22.3 ± 0.7 | 23.0 ± 0.4 |
| Fat pad weight (mg) | ||
| Transplant | 150 ± 7 | |
| At sacrifice | ||
| Body weight (g) | 25.2 ± 0.4 | 26.0 ± 0.5 |
| Fat pad weight (mg) | ||
| Transplant | 117 ± 12 | |
| Epididymal | 215 ± 22 | 222 ± 29 |
| Mesenteric | 135 ± 12 | 138 ± 11 |
| Retroperitoneal | 68 ± 11 | 59 ± 10 |
| Inguinal | 193 ± 21 | 188 ± 16 |
| Glucose (mmol/l) | 5.6 ± 0.7 | 5.7 ± 0.2 |
| Insulin (pg/ml) | 1,295 ± 486 | 1,479 ± 338 |
| Adiponectin (μg/ml) | 27.5 ± 3.7 | 33.0 ± 4.5 |
| Leptin (pg/ml) | 66.9 ± 14.6 | 81.6 ± 23.7 |
| IL-6 (pg/ml) | 6.5 ± 0.9 | 6.8 ± 1.6 |
| FFA (mmol/l) | 0.31 ± 0.04 | 0.39 ± 0.08 |
Data are means ± SE of 5–10 animals. Blood samples were taken 3 h after fasting.
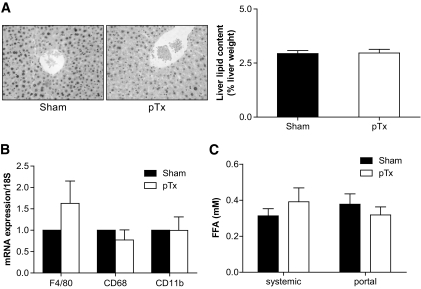
The development of hepatic insulin resistance is often associated with hepatic steatosis and Kupffer cell activation (12). Thus, hepatic lipid contents as well as expression levels of tissue macrophage markers were assessed. Histological examinations and biochemical determination of total hepatic lipid content was similar in sham-operated and peritoneal-transplanted mice (Fig. 4A). Moreover, no increased infiltration/activation of hepatic Kupffer cells was detected (Fig. 4B). Consistent with the absence of hepatic steatosis in portal-transplanted mice, no differences in systemic and portal plasma FFA levels were found in both groups of mice (Fig. 4C). Similarly, no difference in circulating adiponectin and leptin levels occurred (Table 1.). Thus, transplantation of fat pads to sites drained by the portal vein induces hepatic insulin resistance independently of hepatic steatosis and Kupffer cell infiltration/activation.
FIG. 4.
A: Left panel: Hematoxylin- and eosin-stained liver sections from sham-operated and pTx mice. Right panel: Total liver lipids were determined and expressed relative to liver weight. Results represent the mean ± SEM of four to six mice of each group. B: mRNA expression of indicated markers in liver tissue of sham-operated and pTx mice was analyzed. Results are the mean ± SEM of five mice per group, expressed relative to sham-operated mice and normalized to expression of 18S. C: FFA levels were determined in systemic and portal plasma samples of mice receiving a portal drained fat transplant (pTx) and in sham-operated mice after 3 h of fasting. Results are the mean ± SEM of five to six animals.
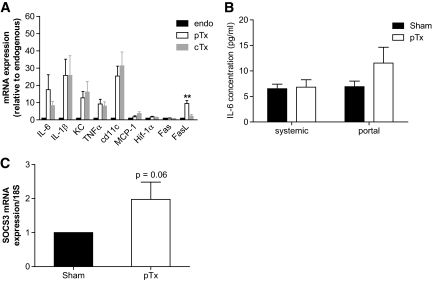
We next sought to investigate potential mediators of hepatic insulin resistance observed in mice receiving a pTx. Transplanted fat tissue may not acquire the exact biological function as the endogenous fat tissue of the same depot, and its function may be affected by a combination of host and donor factors. Thus, to determine potential differences between caval- and portal-transplanted fat pads, we used endogenous and transplanted fat depots from the double-transplanted mice. Such an approach allowed us to exclude different host factors, as well as secondary metabolic effects induced by cTx versus pTx, as shown in Fig. 1. As depicted in Fig. 5A, transplanted fat tissue of either localization exhibited a much more inflammatory gene expression profile than endogenous epididymal fat pads, as assessed by mRNA expression of proinflammatory cytokines and cd11c. Yet intriguingly, when comparing portal-transplanted to caval-transplanted fat pads, the former exhibited higher expression levels of IL-6 and Fas ligand (FasL).
FIG. 5.
A: Five weeks after transplantation, endogenous epididymal fat pads (endo), the portal-drained transplant (pTx), and the caval-drained transplant (cTx) were collected and real-time PCR was performed. Results are the mean ± SEM of two to seven animals. **P < 0.01 pTx vs. cTx (ANOVA). B: IL-6 was measured in systemic and portal plasma samples of mice receiving a portal-drained fat transplant (pTx) and in sham-operated mice. Results are the mean ± SEM of 8–10 animals. C: Five weeks after transplantation, liver tissue was collected and rtPCR was performed. Results are the mean ± SEM of four to five animals.
Because IL-6 was previously associated with hepatic insulin resistance (7,13), we postulated that increased IL-6 expression and secretion from portal-transplanted fat pads might be responsible for hepatic insulin resistance. Thus, IL-6 was determined in systemic and portal vein plasma samples of sham-operated and portal-transplanted mice. Systemic and portal IL-6 plasma levels were similar in sham-operated mice, whereas IL-6 concentration was >70% higher in portal than systemic blood samples of mice receiving a pTx (Fig. 5B). Even though this difference was not significant, these data suggest increased delivery of IL-6 to the liver of mice receiving a portal transplant, potentially originating from the transplanted fat pad. Consistent with an increased delivery of IL-6 to the liver, hepatic SOCS3 mRNA expression, which is induced by IL-6 (14,15), was enhanced in mice receiving a portal-transplanted fat pad (Fig. 5C).
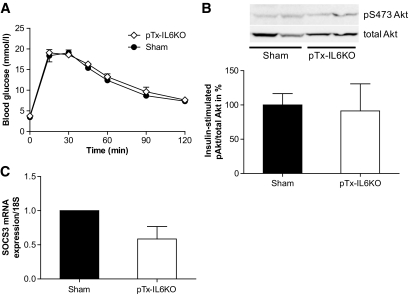
To substantiate the potential contribution of IL-6 to the induction of insulin resistance in portal-transplanted mice, epididymal fat pads of an IL-6 knockout donor were transplanted into a wild-type recipient (pTx-IL6KO). Characteristics of these mice are given in supplementary Table S1. As shown in Fig. 6A, glucose tolerance of such transplanted mice was not impaired compared with sham-operated wild-type mice. Moreover, the insulin-induced increase in Akt phosphorylation in liver samples was completely maintained in these mice (Fig. 6B) in contrast to mice receiving a pTx from wild-type mice (Fig. 3C). In addition, hepatic SOCS-3 mRNA expression was not increased, likely because of IL-6 deficiency (Fig. 6C). These results point toward a causative role for IL-6 in the development of insulin resistance in portal-transplanted mice.
FIG. 6.
Both epididymal fat pads were removed from an IL-6 knockout mouse (donor) and stitched to the mesenterium of a wild-type mouse (recipient; pTx-IL6KO). A: Intraperitoneal glucose tolerance test (2 g glucose/kg body wt) was performed 4 weeks after surgical procedure in mice receiving portal transplants (pTx-IL6KO; ♢) or in sham-operated mice (●). B: Insulin (1 U/kg) was injected intraperitoneally in overnight fasted mice 5 weeks after transplantation. Liver samples were harvested 15 min later, and phosphorylation of Akt at S473 was determined by Western blotting. A representative blot is shown in the upper panel. Results are the mean ± SEM of three animals. C: Five weeks after transplantation, liver tissue was collected and real-time PCR was performed. Results are the mean ± SEM of two to three animals.
DISCUSSION
The “portal hypothesis” proposes that the liver is directly exposed to FFAs and cytokines increasingly released from visceral fat tissue into the portal vein of obese subjects, thus rendering visceral fat accumulation particularly hazardous for the development of hepatic insulin resistance and type 2 diabetes (2,3). In the present study, we used a fat transplantation paradigm to (artificially) increase fat mass depot specifically. In accordance with the portal hypothesis, we demonstrate that fat transplant localization through its venous drainage determines its effect on glucose homeostasis.
When using a fat pad transplantation paradigm, several factors need to be taken into account. First, the source of the transplanted tissue, i.e., which fat depot is used, may impact the metabolic outcome. Second, the functional outcome of the transplant may depend on a myriad of host factors, which include (but are not limited to) the location (and thus venous drainage) of the transplant. Previous studies by different groups including our own have examined the effect of adipose tissue transplantation into the abdominal cavity (5,6,16). Yet, whereas our group only used epididymal fat pads for transplantation, the other groups implemented both subcutaneous and epididymal fat pads (6,16). In contrast to the study presented here, Tran et al. (6) observed no changes in glucose tolerance when epididymal fat pads were transplanted into the abdominal cavity. However, as discussed by the authors, fat pads were drained both to the caval as well as to the portal vein. Similarly, we show here that such dual drainage of transplanted fat tissue may in fact result in no net alteration in glucose tolerance (Fig. 1D). One potential explanation might be the fact that the beneficial metabolic effect of the systemically drained fat transplant (5) was balanced by the detrimental effect of the portal-drained transplant. On the other hand, the lack of deterioration in glucose homeostasis of double-transplanted mice or in the study by Tran et al. might be due to other reasons.
We further used the pTx paradigm to determine potential mediators of hepatic insulin resistance in conditions with increased visceral adiposity. Adipose tissue inflammation was previously correlated with hepatic insulin resistance, and IL-6 was suggested as a potential candidate (7,13,17). Indeed, IL-6 treatment of hepatocytes induced insulin resistance in vitro (18), and it seems to be generally accepted that IL-6 causes hepatic insulin resistance (19). Particularly, IL-6 was shown to activate the negative insulin-signaling regulator SOCS3 in C57BL/6J mice (14,15), resulting in reduced activation of phosphatidylinositol 3-kinase and Akt (18). Furthermore, IL-6 plasma concentration was 50% greater in the portal vein than in systemic circulation in obese subjects, and portal vein IL-6 concentration correlated directly with systemic C-reactive protein concentrations (20). Accordingly, IL-6 expression in portal-transplanted adipose tissue and IL-6 levels in the portal vein of such transplanted mice were increased (Fig. 5A and B), whereas portal IL-6 concentration was not increased in caval-transplanted mice (supplementary Figure S2). Moreover, hepatic SOCS3 expression was induced (Fig. 5C) and, consistently, insulin-stimulated Akt-phosphorylation was reduced in liver samples of mice receiving portal transplants (Fig. 3C). Both changes could be prevented in mice receiving transplants from IL-6 knockout mice (Fig. 6B and C). Interestingly, IL-6 mRNA expression was also significantly increased in caval-transplanted fat pads compared with endogenous (epididymal) fat pads (Fig. 5A), consistent with the transplanted fat depot being the source of the elevated portal IL-6 levels.
Remarkably, fat pads transplanted to both sites were characterized by increased expression of proinflammatory cytokines (Fig. 5A). Potential explanations might be a transplant rejection reaction (even though recipient and donor mice were littermates of inbred colonies) or an inflammatory reaction due to transient transplant hypoxia. Intriguingly, increased production of proinflammatory cytokines by the transplanted fat pads only deteriorated glucose metabolism when they were drained to the portal vein. Moreover, mesenteric transplantation of peritoneal tissue that did not contain adipose tissue had no effect on glucose tolerance (supplementary Figure S3). These data suggest that the transplantation procedure on its own does not deteriorate glucose homeostasis, for example, by altering the delivery of inflammatory mediators from the gastrointestinal tract. Such a finding emphasizes the importance of fat tissue localization and thus vascular drainage and suggests that the increased production of proinflammatory cytokines as it is found in obesity is particularly detrimental when released into the portal circulation supporting the portal hypothesis (3). However, it needs to be emphasized that we examined an artificial system herein, and, even though increased expression of proinflammatory cytokines in adipose tissue of obese subjects was previously demonstrated (21), the findings presented here may not be extrapolated to the situation of mesenteric fat accumulation. Therefore, we presently cannot exclude that the impaired glucose tolerance observed in the portally transplanted mice may not be due to the additional adipose tissue per se but rather due to the increased inflammatory products related to the transplantation procedure.
Moreover, besides IL-6, other factors may have contributed to the deterioration of glucose tolerance and hepatic insulin resistance. Interestingly, lack of IL-6 also reduced expression of other proinflammatory factors such as IL-1β in adipose tissue (supplementary Figure S4). In addition, reduced cd11c expression levels point toward reduced macrophage infiltration. Rotter et al. (22) suggested that IL-6 induce insulin resistance in concert with other cytokines that are also upregulated in adipocytes. Especially IL-6 and IL-1β were shown to mediate the change of hepatic glucose metabolism, influencing the glucoregulatory action of insulin through their signal pathways involving protein phosphorylation (21,23). Interestingly, we found increased levels of FasL in portal-transplanted fat pads. We previously reported that Fas activation by FasL treatment induced secretion of IL-6 in 3T3-L1 adipocytes (7). Conversely, adipocyte-specific Fas-knockout mice showed decreased IL-6 expression in adipose tissue and reduced circulating IL-6 levels (7). Thus, FasL-induced Fas activation might be responsible for increased IL-6 secretion of portal-transplanted fat pads, ultimately inducing hepatic insulin resistance. In support of the latter, FasL expression was not reduced in portal-transplanted IL-6–deficient fat pads, suggesting that Fas activation may be indeed upstream of IL-6 release (supplementary Figure S4).
Strikingly, hepatic insulin resistance in our model was not associated with hepatic steatosis (Fig. 4A) or increased hepatic Kupffer cell activation (Fig. 4B), since it is often observed in high-fat diet–induced hepatic insulin resistance (12). These changes might be due to a direct effect of high-fat diet on liver metabolism or due to an increased release of FFAs from (mesenteric) adipose tissue. Consistent with the latter notion, FFA levels were found to be increased in high fat–fed mice, which was not the case in our study.
In conclusion, our results demonstrate that the metabolic fate of intra-abdominal fat tissue transplantation is determined by the delivery of inflammatory cytokines to the liver specifically via the portal system providing clear direct support for the portal hypothesis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Swiss National Science Foundation (310000-112275), the Zurich Center for Integrative Physiology, University of Zurich (Cooperative Project Grant), the Novartis Foundation for Biomedical Research, and the European Foundation for the Study of Diabetes (EFSD-Lilly) (all to D.K.).
No other potential conflicts of interest relevant to this article were reported.
J.M.R. researched data and wrote the manuscript. S.W. researched data, contributed to discussion, and reviewed/edited the manuscript. E.J.S. contributed to discussion and reviewed/edited the manuscript. D.K. researched data and wrote the manuscript.
We acknowledge Matthias Blueher, University of Leipzig, Germany, for helping us establish hyperinsulinemic-euglycemic clamping in mice; Maggy Arras, University Hospital Zurich, Switzerland, for expert veterinary advice; and Assaf Rudich, Ben-Gurion University of the Negev, Israel, for helpful and highly appreciated discussion.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Catalano KJ, Stefanovski D, Bergman RN: Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 2010;59:1416–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MD: Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93:S57–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN: Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab 2005;288:E454–E461 [DOI] [PubMed] [Google Scholar]
- 4.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R: Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 2007;30:1647–1652 [DOI] [PubMed] [Google Scholar]
- 5.Konrad D, Rudich A, Schoenle EJ: Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia 2007;50:833–839 [DOI] [PubMed] [Google Scholar]
- 6.Tran TT, Yamamoto Y, Gesta S, Kahn CR: Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 2008;7:410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wueest S, Rapold RA, Schumann DM, Rytka JM, Schildknecht A, Nov O, Chervonsky AV, Rudich A, Schoenle EJ, Donath MY, Konrad D: Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest 2010;120:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher SJ, Kahn CR: Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. J Clin Invest 2003;111:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI: Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest 2000;105:1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saberi M, Bjelica D, Schenk S, Imamura T, Bandyopadhyay G, Li P, Vargeese C, Wang W, Bowman K, Zhang Y, Polisky B, Olefsky JM: Novel liver-specific TORC2 siRNA corrects hyperglycemia in rodent models of type 2 diabetes. Am J Physiol Endocrinol Metab 2009August25[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acid Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM: Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010;59:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ: A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008;322:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA: Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 2003;278:13740–13746 [DOI] [PubMed] [Google Scholar]
- 15.Weigert C, Hennige AM, Lehmann R, Brodbeck K, Baumgartner F, Schauble M, Haring HU, Schleicher ED: Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem 2006;281:7060–7067 [DOI] [PubMed] [Google Scholar]
- 16.Hocking SL, Chisholm DJ, James DE: Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdominal compartment. Diabetologia 2008;51:900–902 [DOI] [PubMed] [Google Scholar]
- 17.Cancello R, Clement K: Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 2006;113:1141–1147 [DOI] [PubMed] [Google Scholar]
- 18.Senn JJ, Klover PJ, Nowak IA, Mooney RA: Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002;51:3391–3399 [DOI] [PubMed] [Google Scholar]
- 19.Carey AL, Febbraio MA: Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia 2004;47:1135–1142 [DOI] [PubMed] [Google Scholar]
- 20.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S: Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007;56:1010–1013 [DOI] [PubMed] [Google Scholar]
- 21.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotter V, Nagaev I, Smith U: Interleukin-6 (IL-6) induces insulin resistance in 3T3–L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 2003;278:45777–45784 [DOI] [PubMed] [Google Scholar]
- 23.Kanemaki T, Kitade H, Kaibori M, Sakitani K, Hiramatsu Y, Kamiyama Y, Ito S, Okumura T: Interleukin 1beta and interleukin 6, but not tumor necrosis factor alpha, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology 1998;27:1296–1303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.