Abstract
OBJECTIVE
We have previously shown that overnight fasted women have higher insulin-stimulated whole body and leg glucose uptake despite a higher intramyocellular triacylglycerol concentration than men. Women also express higher muscle mRNA levels of proteins related to lipid metabolism than men. We therefore hypothesized that women would be less prone to lipid-induced insulin resistance.
RESEARCH DESIGN AND METHODS
Insulin sensitivity of whole-body and leg glucose disposal was studied in 16 young well-matched healthy men and women infused with intralipid or saline for 7 h. Muscle biopsies were obtained before and during a euglycemic-hyperinsulinemic clamp (1.42 mU · kg−1 · min−1).
RESULTS
Intralipid infusion reduced whole-body glucose infusion rate by 26% in women and 38% in men (P < 0.05), and insulin-stimulated leg glucose uptake was reduced significantly less in women (45%) than men (60%) after intralipid infusion. Hepatic glucose production was decreased during the clamp similarly in women and men irrespective of intralipid infusion. Intralipid did not impair insulin or AMPK signaling in muscle and subcutaneous fat, did not cause accumulation of muscle lipid intermediates, and did not impair insulin-stimulated glycogen synthase activity in muscle or increase plasma concentrations of inflammatory cytokines. In vitro glucose transport in giant sarcolemmal vesicles was not decreased by acute exposure to fatty acids. Leg lactate release was increased and respiratory exchange ratio was decreased by intralipid.
CONCLUSIONS
Intralipid infusion causes less insulin resistance of muscle glucose uptake in women than in men. This insulin resistance is not due to decreased canonical insulin signaling, accumulation of lipid intermediates, inflammation, or direct inhibition of GLUT activity. Rather, a higher leg lactate release and lower glucose oxidation with intralipid infusion may suggest a metabolic feedback regulation of glucose metabolism.
Whole-body insulin resistance plays a major role in the pathogenesis of type 2 diabetes and has generally been related to high plasma concentrations of lipids. Intralipid infusion increases plasma lipid concentrations and has been used as a model to investigate lipid-induced insulin resistance in rodents (1,2) and humans (3–5). The studies uniformly demonstrate that intralipid infusion reduces whole-body insulin sensitivity markedly within 3–4 h. Various mechanisms have been suggested to explain this phenomenon, including lipid-induced interaction with proximal insulin-signaling capacity, accumulation of lipid intermediates, and inflammation (5). The classic lipid-induced inhibition of the insulin-signaling pathway has, however, been challenged in recent studies where insulin receptor substrate (IRS)-1 tyrosine phosphorylation, IRS-1–associated phosphatidylinositol (PI) 3-kinase activity, Akt, and AS160 phosphorylation were unaltered after 2- to 6-h intralipid infusion in rats (1) and in lean (6) and obese (7) men.
Only one of the human studies included women and, in that study, a matching of sexes with respect to important matching criteria (i.e., aerobic fitness levels) was not performed (8). In a rodent study, 2 h of intralipid infusion reduced insulin-stimulated whole-body glucose uptake in male rats but not in females rats (9) and, in accordance, phosphorylation of IRS-1 and PI 3-kinase activity was reduced only in male rats (9). We have previously reported that women have greater insulin-stimulated whole-body and leg glucose uptake than matched men despite higher intramyocellular triacylglycerol (IMTG) concentration (10). Also, women have higher muscle mRNA levels of several proteins involved in muscle lipid metabolism including fatty acid translocase/CD36 (FAT/CD36), membrane-bound fatty acid–binding protein (FABPpm), cytosolic fatty acid–binding protein (FABPc), lipoprotein lipase (11), and a higher percentage of myosin heavy chain type 1 muscle fibers (10,12,13). Therefore, the aims of this study were 1) to test the hypothesis that women are less prone to intralipid-induced insulin resistance on a whole-body level and in skeletal muscle than men and 2) to investigate the molecular mechanisms responsible for the intralipid-induced decrease in insulin sensitivity.
RESEARCH DESIGN AND METHODS
Women (n = 8) and men (n = 8) were recruited for the study after gaining written informed consent of the study protocol and possible risks. The study was approved by the Copenhagen Ethics Committee (number KF 01 261127) and performed in accordance with the Declaration of Helsinki II.
All subjects were moderately fit, and women and men were matched with respect to maximal oxygen uptake (Vo2peak) expressed relative to lean body mass (LBM), habitual physical activity level, and exercise training history (Table 1). LBM and lean leg mass (LLM) were calculated from their body composition determined by dual-energy X-ray absorptiometry (DPX-IQ Lunar; Lunar Corporation, Madison, WI) and by hydrostatic weighing (14). Women were eumenorrheic, and none were taking oral contraceptives. All experiments in women were performed in the midfollicular phase of their menstrual cycle (days 7–11).
TABLE 1.
Subject characteristics
| Women | Men | |
|---|---|---|
| n | 8 | 8 |
| Age (years) | 25 ± 1 | 25 ± 1 |
| Height (m) | 1.74 ± 0.03 | 1.81 ± 0.03 |
| Body mass (kg) | 65.3 ± 3.0* | 77.9 ± 2.8 |
| BMI (kg/m2) | 21.5 ± 0.5 | 23.5 ± 0.5 |
| Body fat (%) | 23.4 ± 0.9* | 17.4 ± 1.4 |
| LBM (kg) | 50.0 ± 2.5† | 64.3 ± 2.6 |
| LLM (kg) | 8.6 ± 0.4† | 11.8 ± 0.5 |
| Vo2peak | ||
| l/min | 3.1 ± 0.1† | 4.1 ± 0.1 |
| ml · kg−1 body mass · min−1 | 47.3 ± 1.0† | 52.3 ± 0.6 |
| ml · kg−1 LBM · min−1 | 61.9 ± 1.5 | 63.4 ± 1.3 |
| Training history | ||
| Frequency (workouts/week) | 2.9 ± 0.4 | 2.7 ± 0.3 |
| Duration (h/week) | 2.8 ± 0.2 | 3.9 ± 0.6 |
Data are means ± SE.
*P < 0.05;
†P < 0.001 vs. women.
Experimental protocol.
All subjects underwent two experimental trials including infusion of either intralipid (20%, containing 200 g soy oil and 12 g egg-lecithin per liter; Fresenius-Kabi, Copenhagen, Denmark) plus heparin or saline (control) for 7 h in randomized order. After 8 days on a controlled diet (60 energy percent [E%] carbohydrate, 15 E% protein, and 25 E% fat), the subjects arrived at the laboratory at 7:00 a.m. after a breakfast (20% of the daily energy intake) given 3 h (5:00 a.m.) before the experiment started. Subjects had abstained from exercise training 48 h before the experimental day. After 45 min of rest in the supine position, expired air was collected in Douglas bags for determination of resting metabolic rate. A venous catheter was inserted into an antecubital arm vein, and blood was drawn for determination of sex hormones. Then infusion of saline (1.15 ml · kg−1 · h−1) or intralipid (1.15 ml · kg−1 · h−1) plus heparin (0.2 units · kg−1 · min−1) was initiated. A catheter was inserted into an antecubital vein of the contralateral arm for infusion of stable isotopes. Teflon catheters were inserted into the femoral artery and vein and a thermistor (EDSLAB T.D. Model 94-030-2.F; Baxter Healthcare CA) was inserted through the femoral vein catheter for blood flow determination. After 3 h of infusion of intralipid plus heparin or saline, a bolus injection of [6,6-2H]glucose was given (3.203 mg/kg body mass) within 1 min followed by a constant infusion (0.055 ml · kg−1 · min−1) for the remaining experimental period (4 h). After 5 h of saline or intralipid plus heparin infusion, subjects underwent a 120-min hyperinsulinemic-euglycemic clamp (1.42 mU · kg body mass−1 · min−1) initiated with a bolus injection of insulin (9.0 mU/kg) (Actrapid, Novo Nordisk, Bagsvaerd, Denmark). Blood was sampled simultaneously from the femoral artery and vein, femoral venous blood flow was determined (15), and expired air was sampled in Douglas bags frequently during the experiment.
Biopsies were obtained from the vastus lateralis muscle and from the subcutaneous adipose tissue near the umbilicus before the clamp (after 5 h of intralipid or saline infusion), 30 min after initiation of the clamp, and at the end of the clamp. One part of the muscle biopsy and the adipose tissue biopsies were immediately frozen in liquid nitrogen and stored at −80°C. Another part of the muscle biopsy was mounted in embedding medium, frozen in precooled isopentane, and stored at −80°C. Before biochemical analysis, muscle samples were freeze-dried and dissected free of all connective tissue and blood under a microscope. Analysis and calculations are described in the supplementary data, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0698/DC1.
Glucose transport in giant sarcolemmal vesicles in rats.
To study the hypothesis that the effect of intralipid infusion is a direct effect of fatty acids on sarcolemmal glucose transport, giant sarcolemmal vesicles were prepared from rat gastrocnemius muscle (16) and incubated in vitro with linoleic acid because intralipid contains 52% linoleic acid. 2-Deoxy glucose transport into giant vesicles was measured as previously described (16). More details are given in the online appendix.
Statistical analysis.
All data are expressed as means ± SE. Data were evaluated using two-way ANOVA with repeated measures for both time and sex. For variables independent of time, a two-way ANOVA was used to determine influences of sex and trials. For variables independent of time and trial (delta values), an unpaired t test was performed to test for differences between men and women. A Tukey test was used as a post hoc test. Correlation was investigated using the Pearson product moment correlation. A significance of P < 0.05 was chosen.
RESULTS
Characteristics of the subjects are given in Table 1.
Resting metabolic rate and respiratory exchange ratio.
Basal oxygen uptake per kilogram LBM was 4.9 ± 0.3 and 4.74 ± 0.2 ml · min−1 · kg LBM−1 in women and men, respectively, in the control trial and remained unchanged preclamp (after 5 h infusion) and during the clamp in both sexes. In the intralipid trial, oxygen uptake was similar compared with control at basal and preclamp and remained unchanged during the clamp in women, whereas it was increased to 5.2 ± 0.1 ml · min−1 · kg LBM−1 (P < 0.05) in men and was higher (P < 0.05) compared with the control trial.
Respiratory exchange ratio (RER) was ∼0.78 in women and men in both trials preclamp. In the intralipid trial, RER remained at this level during the clamp in both sexes, whereas RER increased (P < 0.05) in the control trial in both women and men and was higher (P < 0.05) than in the intralipid trial (Table 2).
TABLE 2.
Femoral venous blood flow, arterial blood concentration, plasma substrate concentration, hormone concentration, and serum cytokine concentration in the control and intralipid trial in women and men
| Control |
Intralipid |
|||
|---|---|---|---|---|
| Preclamp (300 min) | End of clamp (420 min) | Preclamp (300 min) | End of clamp (420 min) | |
| RER | ||||
| Women | 0.80 ± 0.02 | 0.89 ± 0.01‖ | 0.77 ± 0.01 | 0.79 ± 0.01† |
| Men | 0.78 ± 0.02 | 0.87 ± 0.01‖ | 0.76 ± 0.01 | 0.78 ± 0.01* |
| Blood flow (ml/min) | ||||
| Women | 523 ± 44 | 607 ± 55 | 626 ± 91 | 564 ± 93 |
| Men | 520 ± 90 | 670 ± 58 | 472 ± 83 | 491 ± 58 |
| Arterial blood glucose (mmol/l) | ||||
| Women | 5.4 ± 0.1 | 5.4 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 |
| Men | 5.2 ± 0.1 | 5.1 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 |
| Plasma LCFA (μmol/l) | ||||
| Women | 541 ± 50§ | 9 ± 4‖ | 1,706 ± 197† | 1,415 ± 236† |
| Men | 519 ± 92‖ | 15 ± 6‖ | 2,020 ± 216† | 1,572 ± 111† |
| Plasma triacylglycerol (μmol/l) | ||||
| Women | 484 ± 24 | 409 ± 26 | 1,706 ± 209* | 2,038 ± 351† |
| Men | 540 ± 45 | 471 ± 47 | 2,478 ± 180†‡ | 2,765 ± 253† |
| Plasma insulin (μU/ml) | ||||
| Women | 8.0 ± 0.8 | 87.7 ± 5.7‖ | 9.4 ± 0.7 | 88.8 ± 2.9‖ |
| Men | 7.8 ± 0.5 | 94.3 ± 9.5‖ | 10.1 ± 1.0 | 86.9 ± 7.6‖ |
| Plasma epinephrine (nmol/l) | ||||
| Women | 0.36 ± 0.06 | 0.40 ± 0.09 | 0.21 ± 0.04 | 0.47 ± 0.11 |
| Men | 0.34 ± 0.07 | 0.45 ± 0.09 | 0.26 ± 0.05 | 0.38 ± 0.09 |
| Plasma norepinephrine (nmol/l) | ||||
| Women | 1.2 ± 0.23 | 1.33 ± 0.28 | 0.98 ± 0.11 | 1.34 ± 0.17 |
| Men | 1.46 ± 0.36 | 1.48 ± 0.35 | 1.14 ± 0.18 | 1.38 ± 0.21 |
| Serum TNF-α (pg/ml) | ||||
| Women | 2.21 ± 0.66 | 2.08 ± 0.66 | 2.27 ± 0.85 | 2.28 ± 0.82 |
| Men | 1.50 ± 0.32 | 1.44 ± 0.45 | 1.42 ± 0.36 | 1.49 ± 0.45 |
| Serum adiponectin (pg/ml) | ||||
| Women | 26.5 ± 3.1 | 25.0 ± 2.9 | 28.5 ± 2.2 | 27.1 ± 2.6 |
| Men | 10.5 ± 1.3‡ | 9.9 ± 1.2‡ | 11.1 ± 1.3‡ | 10.1 ± 1.2‡ |
| Lactate release (μmol/min) | ||||
| Women | 5 ± 2 | 74 ± 6‖ | 11 ± 2* | 111 ± 11*‖ |
| Men | 6 ± 2 | 63 ± 9‖ | 11 ± 1* | 95 ± 16*‖ |
Data are means ± SE of eight determinations in both women and men.
*P < 0.05,
†P < 0.001 vs. control trial;
‡P < 0.05 vs. women,
§P < 0.05,
‖P < 0.001 vs. previous time point.
Insulin sensitivity.
In response to insulin infusion, the arterial insulin concentration reached ∼90 μU/ml in both sexes in both trials (Table 2).
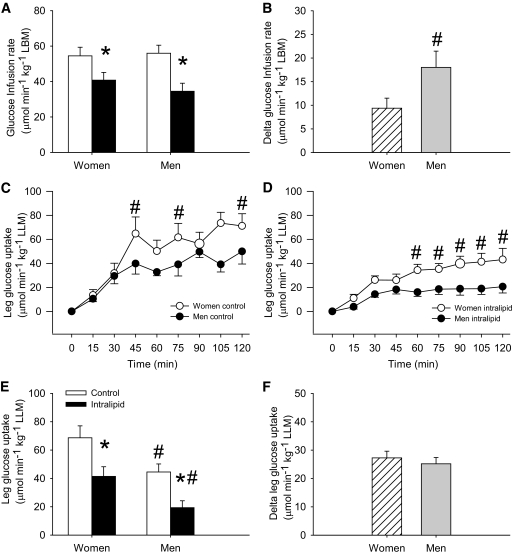
In the control trial, insulin-stimulated whole-body glucose infusion rate to maintain euglycemia was 54 ± 5 and 56 ± 5 μmol · min−1 · kg−1 LBM in women and men, respectively (Fig. 1A). Glucose infusion rate was decreased (P < 0.05) by intralipid infusion and significantly more in men (−38%) than in women (−24%) also when expressed in absolute numbers (Fig. 1B).
FIG. 1.
Glucose infusion rate and insulin-stimulated leg glucose uptake in women and men in the control (□) and intralipid (■) trial. A: Glucose infusion rate the last 30 min of the clamp. B: Delta glucose infusion rate the last 120 min of the clamp, expressed as the difference between the control and the intralipid trial. C: Insulin-stimulated leg glucose uptake during the 120-min clamp in the control trial. D: Insulin-stimulated leg glucose uptake during the 120-min clamp in the intralipid trial. E: Insulin-stimulated leg glucose uptake the last 30 min of the clamp. F: Delta insulin-stimulated leg glucose uptake the last 30 min of the clamp expressed as the difference between the control and the intralipid trial. *P < 0.05 vs. control trial, #P < 0.05 vs. women. Data are means ± SE; n = 16.
Glucose infusion rate expressed per kilogram body mass was 42 ± 4 and 47 ± 4 μmol · min−1 · kg−1 body mass in women and men, respectively, in the control trial and was reduced (P < 0.05) to 31 ± 3 and 29 ± 4 μmol · min−1 · kg−1 body mass, respectively, in the intralipid trial.
Insulin-stimulated leg glucose uptake increased continuously from basal to 120 min of the clamp and was higher (P < 0.05) in women than in men in both trials (Fig. 1C and D). In the control trial, insulin-stimulated leg glucose uptake expressed per kilogram LLM was higher (P < 0.05) in women (69 ± 8 μmol · min−1 · kg−1 LLM) than in men (45 ± 6 μmol · min−1 · kg−1 LLM) and reduced (P < 0.05) by 43% in women and 60% in men (P < 0.05) in the intralipid trial (Fig. 1E). However, in absolute numbers, the intralipid-induced suppression of insulin-stimulated leg glucose uptake (delta glucose uptake) was not different between women and men (Fig. 1F).
Hepatic glucose production.
Hepatic glucose production preclamp was 10.0 ± 0.4 and 11.4 ± 0.6 μmol · min−1 · kg−1 in women and men, respectively, in the control trial and 10.6 ± 0.6 and 10.5 ± 0.5 μmol · min−1 · kg−1 in the intralipid trial, respectively. Hepatic glucose production decreased similarly to slightly negative values in both sexes during the clamp in both trials (data not shown).
Blood flow and blood parameters.
Venous blood flow, epinephrine, and norepinephrine remained unchanged during the clamp in both trials and was not changed with intralipid infusion in either sex (Table 2).
In response to insulin, the concentration of long-chain fatty acids (LCFAs) was completely suppressed (P < 0.05) in the control trial in both sexes, whereas triacylglycerol concentrations remained unchanged. Intralipid infusion markedly elevated (P < 0.05) arterial plasma LCFAs (1,706 ± 197 and 2,020 ± 216 μmol/l in women and men, respectively) and triacylglycerol concentrations (1,706 ± 209 and 2,478 ± 180 μmol/l in women and men, respectively, P < 0.05), compared with the control trial and remained unchanged during the clamp in both sexes (Table 2).
Leg lactate release was higher preclamp (P < 0.05) in the intralipid trial than in the control trial in both sexes and increased during the clamp to a larger extent in the intralipid trial than in the control trial (Table 2).
Basal serum estradiol concentration was 0.28 ± 0.05 and 0.11 ± 0.01 nmol/l (P < 0.05) in women and men, respectively, in the control trial and 0.21 ± 0.03 and 0.11 ± 0.01 nmol/l (P < 0.05) in the intralipid trial. Basal serum progesterone concentration was 1.92 ± 0.15 and 2.08 ± 0.15 nmol/l in women and men, respectively, in the control trial and 2.17 ± 0.25 and 2.07 ± 0.10 nmol/l in the intralipid trial.
Muscle substrates.
Preclamp muscle glycogen concentration was 374 ± 23 and 433 ± 33 mmol/kg dry weight (d.w.) in women and men, respectively, in the control trial and 455 ± 39 and 444 ± 37 mmol/kg d.w. in the intralipid trial and was not changed during the clamp in either trial.
Proximal insulin signaling in skeletal muscle.
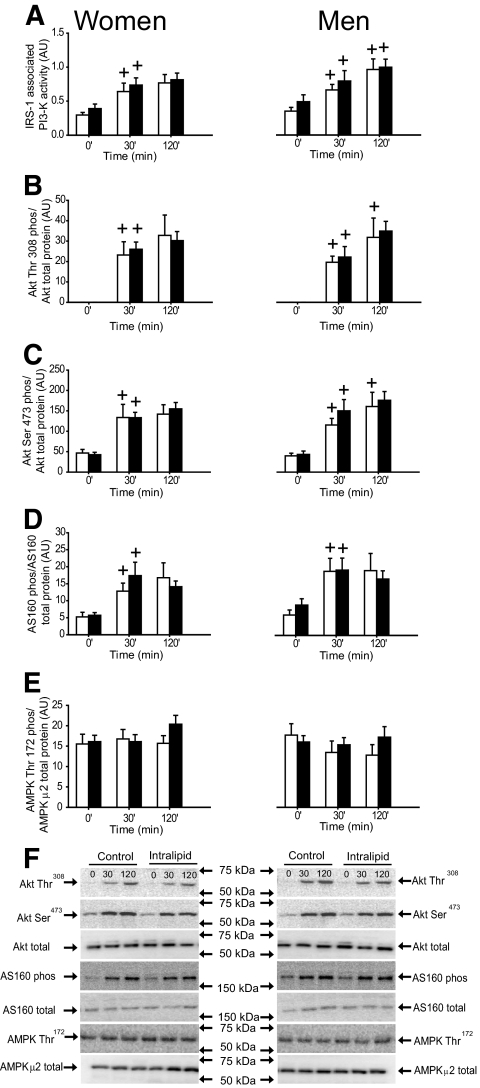
IRS-1–associated PI 3-kinase activity, Akt Thr308, Ser473, and AS160 phosphorylation were similar in both trials and sexes preclamp and increased (P < 0.05) similarly during the clamp in both trials and sexes (Fig. 2). AMPK Thr172 phosphorylation was not different between trials and sexes preclamp and remained unchanged during the clamp (Fig. 2E).
FIG. 2.
IRS-1–associated PI 3-kinase activity, Akt Thr308 and Ser473 phosphorylation/Akt total protein expression, AS160 phosphorylation/total AS160 protein expression, and AMPK Thr172 phosphorylation/AMPK α2 total protein expression in the vastus lateralis muscle in women and men in the control (□) and intralipid (■) trial. A: IRS-1–associated PI 3-kinase activity. B: Akt Thr308 phosphorylation/Akt total protein expression. C: Akt Ser473 phosphorylation/Akt total protein expression. D: AS160 phosphorylation/total AS160 protein expression. E: AMPK Thr172 phosphorylation/AMPK α2 total protein expression. F: Representative immunoblots. +P < 0.05 vs. previous time point. Values are means ± SE; n = 16. AU, arbitrary units.
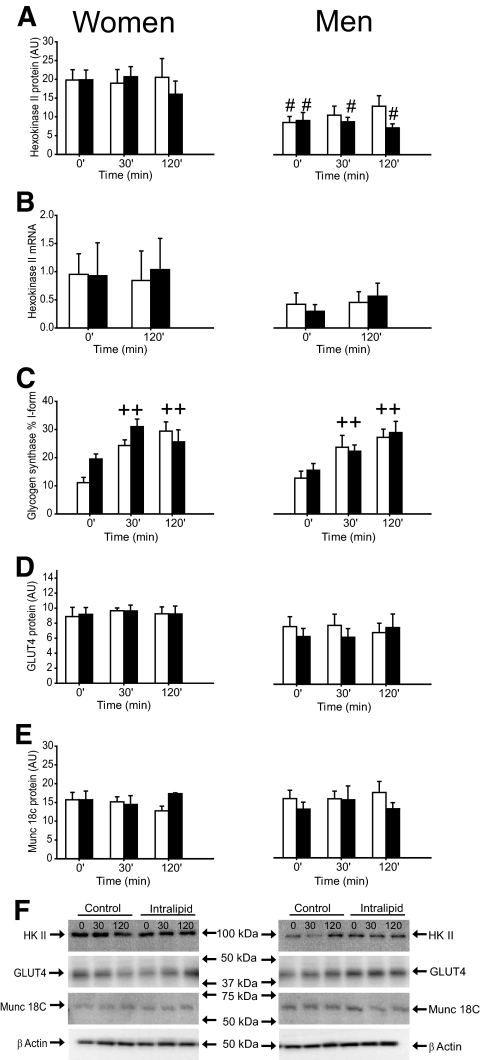
Glycogen synthase (GS) activity expressed as the I-form (Fig. 3C) and fractional velocity (%FV) (data not shown) was similar in both trials and sexes preclamp and increased during the clamp in both sexes in both trials.
FIG. 3.
HKII protein expression, HKII mRNA, glycogen synthase activity, GLUT4, and Munc 18c protein expression in the vastus lateralis muscle in women and men in the control (□) and intralipid (■) trial. A: HKII protein expression. B: HKII mRNA. C: Glycogen synthase activity. D: GLUT4 protein expression. E: Munc 18c protein expression. F: Representative immunoblots. ++P < 0.001 vs. previous time point. #P < 0.05 vs. women. Values are means ± SE; n = 16. AU, arbitrary units.
Protein content of HKII, GLUT4, and Munc 18C.
Preclamp hexokinase II (HK II) protein expression was higher (P < 0.05) and HKII mRNA expression tended to be higher (P = 0.1) in women than in men and remained unchanged during the clamp in both trials and sexes (Fig. 3A and B). Insulin-stimulated leg glucose uptake correlated with HKII protein expression (r = 0.58, P = 0.02, n = 16) when combining values from women and men in the saline trial. This was due to a significant correlation in the women alone (r = 0.74, P = 0.035, n = 8) but not in the men alone (supplementary Fig. 1). GLUT4 and Munc 18C protein expression were similar in both trials and sexes before and during the clamp (Fig. 3D and E).
IMTG and lipid intermediates.
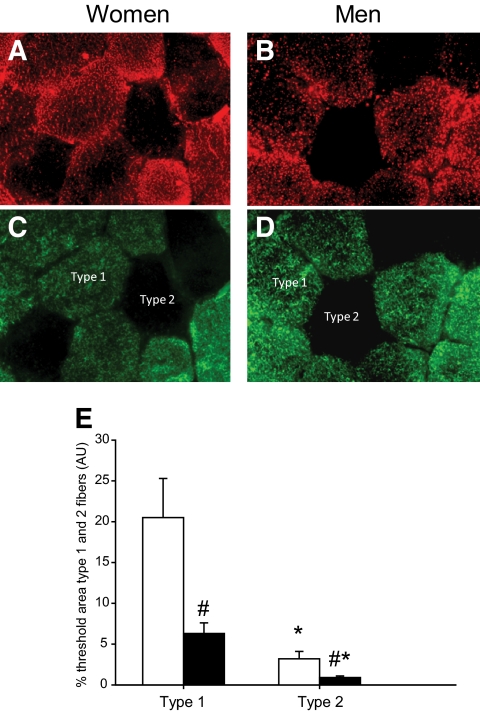
In the basal state, the content of IMTG in vastus lateralis muscle was greater (P < 0.05) in women than in men in both type 1 and type 2 fibers (Fig. 4). Preclamp mean IMTG concentration was ∼85 mmol/kg d.w. in women in both trials, which was 42% higher than in men (∼50 mmol/kg d.w. in both trials). IMTG content remained unchanged during the clamp in both trials and sexes.
FIG. 4.
IMTG measured with Oil Red O (ORO) in type 1 and type 2 fibers in women (□) and men (■). A: ORO signal in women. B. ORO signal in men. C. Myosin heavy chain (MHC) staining in women. Type 1 fibers are colored green. D: MHC staining in men. E: IMTG content (measured by ORO) in type 1 and type 2 fibers of the vastus lateralis muscle in women and men. Type 1 fibers are colored green. *P < 0.05 vs. type 1 fibers, #P < 0.05 vs. women. Values are means ± SE; n = 16. AU, arbitrary units. (A high-quality digital representation of this figure is available in the online issue.)
In the control trial, the preclamp content of LCFA-CoA was 51 ± 8 and 52 ± 6 nmol/g d.w., DAG was 140 ± 12 and 167 ± 36 nmol/mg d.w., and ceramide was 26 ± 10 and 28 ± 9 nmol/mg d.w. in women and men, respectively, and was not changed in the intralipid trial (LCFA-CoA was 47 ± 6 and 42 ± 4 nmol/g d.w., DAG was 185 ± 29 and 167 ± 25 nmol/mg d.w., and ceramide was 27 ± 8 and 32 ± 9 nmol/mg d.w. in women and men, respectively). LCFA-CoA, DAG, and ceramide content remained unchanged during the clamp in both trials.
Cytokines.
Serum tumor necrosis factor (TNF)-α concentration was similar in both trials and sexes before and during the clamp (Table 2). Serum adiponectin concentration was higher (P < 0.05) in women than in men before and during the clamp in both trials (Table 2). Preclamp plasma resistin concentration was 10.9 ± 0.77 and 10.6 ± 1.11 ng/ml in the control trial in women and men, respectively, and was not different from the intralipid trial. Plasma resistin concentration remained unchanged during the clamp in both trials (data not shown).
Fiber type composition, fiber area, and capillary density can be found in supplementary Table 1 (online appendix).
Adipose tissue.
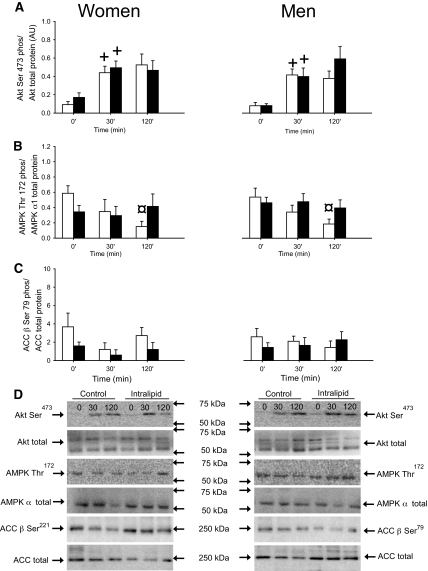
Akt Ser473 phosphorylation preclamp was similar in women and men in both trials and increased significantly during the clamp in both sexes and trials (Fig. 5A).
FIG. 5.
Akt Ser473 phosphorylation/Akt total protein expression, AMPKthr172 phosphorylation/AMPK α1 total protein expression, and ACC β Ser79/ACC β total protein expression in subcutaneous adipose tissue in women and men in the control (□) and intralipid (■) trial. A: Akt Ser473 phosphorylation/Akt total protein expression. B: AMPKthr172 phosphorylation/AMPK α1 total protein expression. C: ACC β Ser79/ACC β total protein expression. D: Representative immunoblots. +P < 0.05 vs. previous time point, ¤P < 0.05 vs. time point 0. Values are means ± SE; n = 16. AU, arbitrary units.
Phosphorylation of AMPK decreased during the control clamp in both women and men, and this was prevented by intralipid infusion. Phosphorylation of acetyl-CoA-carboxylase (ACC) was similar in both sexes and trials before and during the clamp (Fig. 5B and C).
Glucose transport in giant sarcolemmal vesicles in rats.
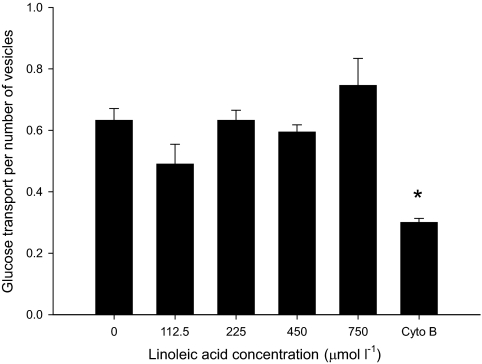
Glucose transport was similar when giant sarcolemmal vesicles were incubated with linoleic acid in concentrations from 0 to 700 μmol/l and 190 μmol/l BSA and decreased with incubation of cytochalasin B (Fig. 6). Interestingly, we also observed that unbound fatty acid concentration did not interfere with glucose uptake into giant sarcolemmal vesicles until it reached 400 μmol/l using both linoleic and palmitic acid (data not shown). This unbound fatty acid concentration was >200-fold higher than the estimated unbound interstitial fatty acid concentration in the subjects that were infused with intralipid.
FIG. 6.
Glucose transport in giant sarcolemmal vesicles prepared from rat muscle after incubation of different concentrations of linoleic acid related to the number of vesicles present after the incubation and the glucose transport after inhibition with cytochalasin B (Cyto B). *P < 0.05 vs. glucose uptake when incubated with 0, 112.5, 225, 450, and 750 μmol/l linoleic acid. Values are means ± SE; n = 4.
DISCUSSION
In agreement with our hypothesis, women were less prone to acute lipid-induced insulin resistance than men on the whole-body level and measured as percentage reduction of leg glucose uptake. In contrast to the current dogma linking the effect of acute lipid-induced insulin resistance to decreased tyrosine phosphorylation of IRS-1 (17), insulin-stimulated IRS-1–associated PI 3-kinase activity (17–19), Akt Ser473 phosphorylation (17), and nPKC activity (5), intralipid-induced insulin resistance of skeletal muscle glucose uptake was not accompanied by changes in markers of the insulin-signaling cascade (Fig. 3) or AMPK. Akt phosphorylation in subcutaneous adipose tissue was also unimpaired (Fig. 5). Furthermore, intralipid infusion did not increase muscle content of LCFA-CoA, DAG, ceramide, and IMTG in women or men. In contrast to the effect of intralipid on glucose uptake, but supporting the lack of effect on the insulin-signaling cascade, intralipid infusion did not decrease activation of GS in muscle. Infusion of intralipid, furthermore, did not change plasma concentrations of inflammatory cytokines and did not change the intrinsic activity of glucose transporters, as judged by the lack of effect of fatty acids on glucose transport in giant sarcolemmal vesicles. On the other hand, there was indirect evidence for a decrease in pyruvate dehydrogenase (PDH) activity with intralipid such as increased leg lactate release and decreased RER, which, as proposed by Randle et al. (20), might decrease glucose uptake. Consistent with our observations, a few other studies have also failed to observe intralipid-induced interactions with parts of the insulin-signaling cascade. These include studies where insulin-mediated whole-body glucose disposal was reduced after 2–6 h of intralipid infusion in lean (6) and obese (7) subjects without any changes in IRS-1 tyrosine phosphorylation (7), insulin-stimulated IRS-1–associated PI 3-kinase activity (7), or Akt Ser473 phosphorylation (6,7). In addition, Hoy et al. (1) recently reported no changes in IRS-1, Akt, or AS160 phosphorylation after 3 and 5 h intralipid infusion in rats. Clearly changes in insulin sensitivity of glucose uptake can be induced independent of changes in insulin signaling. For instance, glucose infusion (21) and short-term high-fat feeding (22) caused whole-body insulin resistance without changes in Akt and AS160 phosphorylation in rats (21,22). In aerobically fit humans, increased insulin sensitivity of glucose uptake after exercise is not accompanied by increased activation of the proximal insulin-signaling cascade (23,24). We therefore contend that intralipid-induced insulin resistance of glucose uptake in human skeletal muscle is unrelated to impairment of the canonical insulin-signaling pathway, a contention that is supported by the finding that insulin-induced activation of GS was also unimpaired. This latter observation indicates that the effect of intralipid is specific to glucose transport and is not a general impairment of insulin effects. Supporting our interpretation, it has been shown that 2 weeks of high-fat feeding in C57BL6J mice impaired glucose uptake in cardiomyocytes without changes in Akt phosphorylation, AS160, and PDH activity (22). In that study, it was found that total GLUT4 content and translocation measured by subcellular fractionation and immunofluorescence were significantly decreased after high-fat feeding (22). Although GLUT4 translocation could not be assessed in the present human study, it is possible that intralipid did interfere with GLUT4 translocation, docking, or fusion and that this is unrelated to defects in the canonical insulin-signaling cascade.
An accumulation of lipid intermediates such as LCFA-CoA, DAG, and ceramide as well as high IMTG levels has been reported to be associated with intralipid-induced suppression of insulin signaling. For instance, in healthy men, 5- to 6-h intralipid infusion resulted in accumulation of ceramide (25), LCFA-CoA (6), and a threefold increased DAG content accompanied by decreased protein kinase C activity (5). However, in the latter study, the intralipid infusion was carried out on different subjects than the controls, which may explain the large difference in DAG content between trials. Furthermore, the subjects were 30–44 years old, physical activity level was not mentioned, and BMI ranged from 22 to 27 kg/m2. Absolute fitness level and BMI could probably play a role in whole-body substrate handling during intralipid infusion and may contribute to explain the discrepant findings in that study compared with our study. With regard to ceramide accumulation, palmitate serves as a precursor for ceramide synthesis (26) and because intralipid consists primarily of soybean oil, which contains unsaturated fatty acids, one would not expect ceramide accumulation during intralipid infusion. In the present study, no difference was observed in LCFA-CoA, DAG, or ceramide content in the control trial compared with the intralipid trial in women or men. Our findings are supported by studies where young healthy subjects infused with intralipid decreased whole-body insulin sensitivity without any changes in ceramide content (5,26).
In the present study, no significant change in IMTG was found with intralipid infusion. Others have reported remarkably large increases in IMTG content during 4- to 6-h intralipid infusion, e.g., 20 and 60% in the soleus and tibialis anterior muscles, respectively (27), and 56% in the vastus lateral muscle (4). However, to increase IMTG concentrations by 50% in a total muscle mass of 45 kg would require 280 mmol triacylglycerol, provided that the uptake in all muscles would be equal, which is 2.2 times more than what reportedly was infused in the quoted studies (4,27).
In an attempt to elucidate the mechanisms impairing insulin action in the present study, we also evaluated inflammatory cytokines that have been suggested to inhibit insulin signaling (28). Inflammatory cytokines such as TNF-α and resistin levels were not different in the intralipid trial compared with the control trial in women or men. Furthermore, because the insulin-signaling pathway was not inhibited by intralipid infusion, it is unlikely that insulin resistance in the present study was caused by inflammation.
AMPK is an important energy sensor in skeletal muscle and a decrease in its activity and/or protein expression in muscle has been found in several rodent models of insulin resistance (29) as well as in some (30) but not all (31) studies of type 2 diabetic patients. Furthermore, infusion of glucose to rats leads to insulin resistance and decreased AMPK activity (32). Finally, activation of AMPK in rat muscle increased insulin sensitivity (33). Together, these observations led us to examine whether intralipid infusion, which is also an acute energy overload, decreased AMPK activity. This was not the case in either muscle or subcutaneous adipose tissue, but interestingly the clamp procedure resulted in a slight decrease in AMPK phosphorylation in adipose tissue, which, however, was prevented by intralipid infusion (Figs. 3E and 5B).
In the present study, we measured lactate release and found that intralipid increased lactate release from the leg in both sexes. This finding might suggest that PDH activity was inhibited in the intralipid trial in accordance with the Randle cycle (20). It was previously found that intralipid inhibited the insulin-mediated decrease in PDK4 mRNA in humans (6) and in rats (1,34), which would be expected to impair the insulin-mediated increase in PDH activity. However, in a study by Pilegaard et al. (35), 4 h of intralipid infusion increased PDK4 mRNA and PDH-E1α phosphorylation without corresponding changes in PDHa activity measured in vitro. This finding might indicate that PDHa activity measured in vitro may not accurately reflect activity in vivo. Alternatively, changes in PDHa activity might be too small to be detectable in an in vitro assay. At any rate, the lower RER values recorded during intralipid infusion during the clamp in the present study indicate a lower carbohydrate oxidation rate consistent with lower conversion of pyruvate to acetyl-CoA. Whether the Randle cycle could also contribute to decreased glucose uptake is more doubtful, since no accumulation of glucose-6-phophate or free glucose (data not shown) was observed in accord with earlier findings using nuclear magnetic resonance spectroscopy (18).
The present finding that intralipid infusion decreased whole-body insulin sensitivity 38% in men and only 26% in women compared with the control situation was observed despite similar insulin-stimulated reduction in hepatic glucose production and similar impairment in insulin-stimulated leg glucose uptake in both sexes when expressed in absolute numbers (Fig. 1F). This allowed for the speculation that impaired whole-body insulin sensitivity also relates to defects in adipose tissue. However, similar to the findings in skeletal muscle, Akt Ser473 phosphorylation in adipose tissue was not different between women and men and was not affected by intralipid (Fig. 5A). Previously, sex differences in intralipid-induced insulin resistance were investigated showing that 5 h of intralipid infusion reduced insulin-stimulated whole-body glucose uptake in men but not in women (8). Findings reported in that study were, however, conducted in women and men matched only with respect to BMI with no considerations of aerobic physical activity level, which is important when comparing sexes (10,36). The study included both pre- and postmenopausal women, and the premenopausal women were studied twice in the follicular phase of their menstrual cycle with 5 to 10 days between the trials (8). Therefore, serum estradiol concentrations likely differed between the control and the intralipid trial, which may confound the results. In this study, insulin-stimulated whole-body insulin sensitivity was not different between women and men in contrast to our previous findings showing that women were more insulin sensitive than well-matched men (10). In that study, subjects fasted overnight before the experiment, whereas in the present study, a breakfast was given, which perhaps can influence the results. Still, women were more insulin sensitive in leg muscle glucose uptake than men in agreement with previous findings (10). An explanation for the higher insulin-stimulated leg glucose uptake in women than in men could be linked to 28% higher type 1 fibers and 28% higher capillary density as discussed previously (10). Furthermore, in the present study, a 56% higher HKII protein expression was found in women than in men, and HKII protein expression correlated (r = 0.74, P < 0.035) with insulin-stimulated leg glucose uptake in women (supplementary Fig. 1), suggesting that increased HKII may play a role in increased muscle insulin sensitivity in women. This has also been suggested by experiments in mouse muscle where increased HKII expression increased insulin-stimulated glucose uptake at high uptake rates (37).
In conclusion, while women are less prone to acute lipid-induced insulin resistance than men, insulin resistance was not accompanied by accumulation of lipid intermediates, signs of inflammation, or decreased signaling in the canonical insulin-signaling pathway in muscle or subcutaneous adipose tissue or decreased AMPK phosphorylation. The lack of inhibition of signaling is supported by the finding that insulin-induced activation of GS was not affected by intralipid. Moreover, glucose transport measured across sarcolemmal vesicles in vitro was not reduced when vesicles were incubated with different fatty acid levels, indicating that glucose transport or transporter activity is not directly impaired by the presence of lipids. Intralipid increased leg lactate release during insulin infusion and others have found that a fat-rich diet in mice decreases GLUT4 translocation in cardiomyocytes in the absence of changes in insulin signaling. Thus, taken together with our findings, the currently accepted mechanism for acute intralipid-induced insulin resistance of glucose uptake is challenged. A higher leg lactate release and lower glucose oxidation with intralipid infusion may rather suggest a metabolic feedback regulation of glucose metabolism.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Danish Ministry of Food, Agriculture and Fisheries, the Danish Medical Research Council, The Lundbeck Research Foundation, The Novo Nordisk Research Foundation, and an integrated project funded by the European Union (LSHM-CT-2004-005272). This work was carried out as part of the research program of the UNIK: Food, Fitness and Pharma for Health and Disease (see www.foodfitnesspharma.ku.dk). The UNIK project is supported by the Danish Ministry of Science, Technology and Innovation.
No other potential conflicts of interest relevant to this article were reported.
L.D.H., K.A.S., J.F.P.W., E.A.R., and B.K. designed the study, carried out the experiments, and wrote the manuscript. L.D.H., K.A.S., J.J., T.E.J., C.F., J.B.B., B.B., N.H., and H.P. performed assays. All authors contributed to the final version of the manuscript.
We acknowledge the skilled technical assistance of Irene Bech Nielsen and Betina Bolmgren (Molecular Physics Group, Denmark) and Duncan Talbot (Unilever Discover, Colworth).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Hoy AJ, Brandon AE, Turner N, Watt MJ, Bruce CR, Cooney GJ, Kraegen EW: Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am J Physiol Endocrinol Metab 2009;297:E67–E75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI: Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002;277:50230–50236 [DOI] [PubMed] [Google Scholar]
- 3.Boden G, Chen X, Ruiz J, White JV, Rossetti L: Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 1994;93:2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeks J, Hesselink MK, Russell AP, Mensink M, Saris WH, Mensink RP, Schrauwen P: Peroxisome proliferator-activated receptor-gamma coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia 2006;49:2419–2426 [DOI] [PubMed] [Google Scholar]
- 5.Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2005;51:2005–2011 [DOI] [PubMed] [Google Scholar]
- 6.Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D: Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme A. J Clin Endocrinol Metab 2007;92:3967–3972 [DOI] [PubMed] [Google Scholar]
- 7.Storgaard H, Jensen CB, Bjornholm M, Song XM, Madsbad S, Zierath JR, Vaag AA: Dissociation between fat-induced in vivo insulin resistance and proximal insulin signaling in skeletal muscle in men at risk for type 2 diabetes. J Clin Endocrinol Metab 2004;89:1301–1311 [DOI] [PubMed] [Google Scholar]
- 8.Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT: Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes 2001;50:1344–1350 [DOI] [PubMed] [Google Scholar]
- 9.Hevener A, Reichart D, Janez A, Olefsky J: Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes 2002;51:1907–1912 [DOI] [PubMed] [Google Scholar]
- 10.Hoeg L, Roepstorff C, Thiele M, Richter EA, Wojtaszewski JF, Kiens B: Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J Appl Physiol 2009;107:824–831 [DOI] [PubMed] [Google Scholar]
- 11.Kiens B, Roepstorff C, Glatz JF, Bonen A, Schjerling P, Knudsen J, Nielsen JN: Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol 2004;97:1209–1218 [DOI] [PubMed] [Google Scholar]
- 12.Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B: Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol 2006;574:125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steffensen CH, Roepstorff C, Madsen M, Kiens B: Myocellular triacylglycerol breakdown in females but not in males during exercise. Am J Physiol Endocrinol Metab 2002;282:E634–E642 [DOI] [PubMed] [Google Scholar]
- 14.Siri WE: The gross composition of the body. Adv Biol Med Phys 1956;4:239–280 [DOI] [PubMed] [Google Scholar]
- 15.Andersen P, Saltin B: Maximal perfusion of skeletal muscle in man. J Physiol 1985;366:233–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ploug T, Wojtaszewski J, Kristiansen S, Hespel P, Galbo H, Richter EA: Glucose transport and transporters in muscle giant vesicles: differential effects of insulin and contractions. Am J Physiol 1993;264:E270–E278 [DOI] [PubMed] [Google Scholar]
- 17.Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, DeFronzo RA, Cusi K: Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 2005;54:1640–1648 [DOI] [PubMed] [Google Scholar]
- 18.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI: Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999;103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM: Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J Clin Endocrinol Metab 2002;87:226–234 [DOI] [PubMed] [Google Scholar]
- 20.Randle PJ, Garland PB, Hales CN, Newsholme EA: The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 21.Hoy AJ, Bruce CR, Cederberg A, Turner N, James DE, Cooney GJ, Kraegen EW: Glucose infusion causes insulin resistance in skeletal muscle of rats without changes in Akt and AS160 phosphorylation. Am J Physiol Endocrinol Metab 2007;293:E1358–E1364 [DOI] [PubMed] [Google Scholar]
- 22.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED: Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 2009;82:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojtaszewski JF, Hansen BF, Gade J, Kiens B, Markuns JF, Goodyear LJ, Richter EA: Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 2000;49:325–331 [DOI] [PubMed] [Google Scholar]
- 24.Hoehn KL, Hohnen-Behrens C, Cederberg A, Wu LE, Turner N, Yuasa T, Ebina Y, James DE: IRS1-independent defects define major nodes of insulin resistance. Cell Metab 2008;7:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J: Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 2004;53:1215–1221 [DOI] [PubMed] [Google Scholar]
- 26.Serlie MJ, Meijer AJ, Groener JE, Duran M, Endert E, Fliers E, Aerts JM, Sauerwein HP: Short-term manipulation of plasma free fatty acids does not change skeletal muscle concentrations of ceramide and glucosylceramide in lean and overweight subjects. J Clin Endocrinol Metab 2007;92:1524–1529 [DOI] [PubMed] [Google Scholar]
- 27.Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen CD, Schick F, Haring HU, Jacob S: Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 2001;50:2579–2584 [DOI] [PubMed] [Google Scholar]
- 28.Barnes KM, Miner JL: Role of resistin in insulin sensitivity in rodents and humans. Curr Protein Pept Sci 2009;10:96–107 [DOI] [PubMed] [Google Scholar]
- 29.Richter EA, Ruderman NB: AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J 2009;418:261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM: Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis: thiazolidinedione treatment reverses these defects. Diabetes 2006;55:2277–2285 [DOI] [PubMed] [Google Scholar]
- 31.Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF: AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am J Physiol Endocrinol Metab 2004;286:E239–E244 [DOI] [PubMed] [Google Scholar]
- 32.Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB: Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab 2006;290:E471–E479 [DOI] [PubMed] [Google Scholar]
- 33.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA: Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 2002;282:E18–E23 [DOI] [PubMed] [Google Scholar]
- 34.Kim YI, Lee FN, Choi WS, Lee S, Youn JH: Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes 2006;55:2311–2317 [DOI] [PubMed] [Google Scholar]
- 35.Pilegaard H, Birk JB, Sacchetti M, Mourtzakis M, Hardie DG, Stewart G, Neufer PD, Saltin B, van Hall G, Wojtaszewski JF: PDH-E1alpha dephosphorylation and activation in human skeletal muscle during exercise: effect of intralipid infusion. Diabetes 2006;55:3020–3027 [DOI] [PubMed] [Google Scholar]
- 36.Blaak E: Sex differences in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care 2008;11:500–504 [DOI] [PubMed] [Google Scholar]
- 37.Fueger PT, Lee-Young RS, Shearer J, Bracy DP, Heikkinen S, Laakso M, Rottman JN, Wasserman DH: Phosphorylation barriers to skeletal and cardiac muscle glucose uptakes in high-fat fed mice: studies in mice with a 50% reduction of hexokinase II. Diabetes 2007;56:2476–2484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.