Abstract
OBJECTIVE
Increased very-low-density lipoprotein triglycerides (VLDL-TG) concentration is a central feature of diabetic dyslipidemia. The objective was to compare basal and insulin mediated VLDL-TG kinetics, oxidation, and adipose tissue storage in type 2 diabetic and healthy (nondiabetic) men.
RESEARCH DESIGN AND METHODS
Eleven type 2 diabetic and 11 healthy men, matched for BMI and age, were included. Ex vivo-labeled VLDL-TG tracers, blood and breath samples, fat biopsies, indirect calorimetry, and body composition measures were applied to determine VLDL-TG kinetics, VLDL-TG fatty acids (FA) oxidation, and storage in regional adipose tissue before and during a hyperinsulinemic euglycaemic clamp.
RESULTS
VLDL-TG secretion was significantly greater in diabetic compared with healthy men (basal: 86.9 [31.0] vs. 61.9 [30.0] μmol/min, P = 0.03; clamp: 60.0 [26.2] vs. 34.2 [17.9] μmol · min−1, P = 0.01). The insulin mediated suppression of VLDL-TG secretion was significant in both groups. VLDL-TG clearance was lower in diabetic men (basal: 84.6 [32.7] vs. 115.4 [44.3] ml · min−1, P = 0.08; clamp: 76.3 [30.6] vs. 119.0 [50.2] ml · min−1, P = 0.03). During hyperinsulinemia fractional VLDL-TG FA oxidation was comparable, but in percentage of energy expenditure (EE), significantly higher in diabetic men. Basal VLDL-TG storage was similar, but significantly greater in abdominal compared with leg fat.
CONCLUSIONS
Increased VLDL-TG in type 2 diabetic men is caused by greater VLDL-TG secretion and less so by lower VLDL-TG clearance. The ability of hyperinsulinemia to suppress VLDL-TG secretion appears preserved. During hyperinsulinemia VLDL-TG FA oxidation is significantly increased in proportion of EE in type 2 diabetic men. Greater basal abdominal VLDL-TG storage may help explain the accumulation of upper-body fat in insulin-resistant individuals.
Type 2 diabetes is associated with dyslipidemia, which is considered a major risk factor for coronary heart disease (1–3). It is characterized by hypertriglyceridemia, low HDL cholesterol concentrations, small and dense LDL particles, and excessive postprandial lipemia (4–7). Evidence suggests that increased concentration of very-low-density lipoprotein triglycerides (VLDL-TG) is a central pathophysiologic feature of diabetic dyslipidemia (4,5).
Various tracer methods have been applied to study the mechanisms responsible for hypertriglyceridemia. Most techniques are based on in vivo labeling; i.e., infusion of labeled precursors which are eventually incorporated into either apolipoprotein B100 (apoB-100) or the triglyceride content of the very-low-density lipoprotein (VLDL) particles combined with modeling of the subsequent plasma decay data. Because only one apoB-100 molecule exists per VLDL particle, VLDL-apoB-100 reflects particle number, whereas VLDL-TG reflects the major lipid content of the particle. Studies have shown that elevated levels of VLDL-TG in type 2 diabetes is caused by a combination of increased hepatic secretion of VLDL-apoB-100 (8–10) and VLDL-TG (10), specifically in the subfraction of large triglyceride-rich VLDL (VLDL1) (11,12). This has focused attention on the impact of insulin on VLDL kinetics. The acute effect of insulin on VLDL kinetics has been investigated in nondiabetic subjects (13–17). Despite different modeling approaches the results are quite unambiguous, showing that acute hyperinsulinemia decreases hepatic secretion of VLDL-TG (13,14,17) and VLDL-apoB-100 (13–17), primarily VLDL1-apoB-100 (15–17). However, to our knowledge, the effect of insulin on VLDL-TG kinetics has not been studied directly in type 2 diabetic subjects.
These studies were undertaken to compare basal and insulin-mediated VLDL-TG kinetics in type 2 diabetic and healthy men. A secondary objective was to assess peripheral VLDL-TG fatty acids (FA) metabolism, i.e., to what extent VLDL-TG FA are oxidized and stored in regional adipose tissue. This is relevant in the context of VLDL-TG as a potentially important energy source and its contribution to fat accumulation in specific adipose tissue depots.
RESEARCH DESIGN AND METHODS
This study was approved by the local Ethics Committee and informed consent was obtained from all participants.
Eleven type 2 diabetic men and 11 healthy men with no family history of type 2 diabetes, matched for BMI and age, were recruited from the outpatient clinic and through local advertisements. All were nonsmokers, weight stable, and not regularly engaged in vigorous exercise. Current diabetes treatments were lifestyle modifications alone in six patients and either metformin, sulfonylurea, or both in five patients. Oral hypoglycemic agents were discontinued 3 weeks before the study day, and statins and antihypertensive drugs 2 weeks before. All had normal blood count and normal liver and kidney function (Table 1).
TABLE 1.
Subject characteristics
| Healthy men (n = 11) | Type 2 diabetic men (n = 11) | |
|---|---|---|
| Age (years) | 49 ± 10 | 53 ± 12 |
| Weight (kg) | 98.8 ± 9.1 | 93.3 ± 10.7 |
| BMI (kg/m2) | 29.1 (24.4–31.4) | 30.6 (25.8–35.6) |
| FFM (kg) | 68.7 ± 4.9 | 63.2 ± 5.6* |
| Fat mass (kg) | 25.2 ± 6.9 | 26.0 ± 6.2 |
| Visceral fat (kg) | 3.6 ± 1.1 | 4.3 ± 1.1 |
| Abdominal sc fat (kg) | 11.2 ± 3.7 | 12.6 ± 3.4 |
| Leg fat (kg) | 9.3 ± 2.8 | 8.1 ± 2.2 |
| Fat % | 25.7 ± 5.4 | 28.0 ± 4.1 |
| HbA1c (%) | 5.4 ± 0.4 | 6.8 ± 0.8‡ |
| Triglycerides (mmol/l) | 1.10 (0.80–1.88) | 1.68 (1.17–3.77)† |
| Total cholesterol (mmol/l) | 4.6 ± 0.7 | 4.9 ± 0.6 |
| LDL cholesterol (mmol/l) | 3.0 ± 0.6 | 3.0 ± 0.5 |
| HDL cholesterol (mmol/l) | 1.1 (0.8–1.5) | 0.9 (0.7–1.3) |
Data are mean ± SD or median (range). FFM, fat-free mass; sc, subcutaneous.
*P < 0.05,
†P < 0.01,
‡P < 0.001 vs. healthy men.
Protocol.
Screening of potentially eligible subjects was performed after an overnight 12-h fast. A medical history was obtained, and blood samples drawn for lipid profile, A1C, liver and kidney function, and complete blood count.
One week before the study day, subjects meeting the eligibility criteria visited the Clinical Research Center after an overnight fast. Blood was drawn for ex vivo labeling of VLDL-TG as described below. Dual x-ray absorptiometry (DXA) scan and abdominal CT scan at the L2-L3 interspace were performed to obtain anthropometric indexes. Volunteers were interviewed by a dietitian who estimated daily caloric intake, based on which, the participants consumed a weight-maintaining diet (55% carbohydrate, 15% protein, and 30% fat) provided by the hospital kitchen during the 3 days preceding the metabolic study.
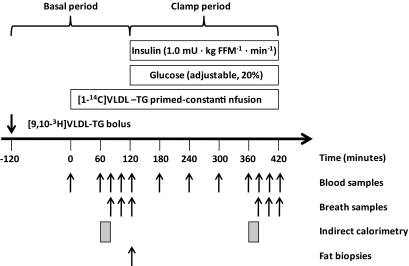
Volunteers were admitted to the Research Center at 22:00 h the evening before the study. From this time and until the end of the study, they fasted and remained in bed. At 05.30 h (t = −120 min) an intravenous catheter was placed in an antecubital vein and a bolus of [9,10-3H]VLDL-TG was infused. Another catheter was placed in a dorsal hand vein for blood sampling. The hand was placed in a heated box to obtain arterialized blood. At 07.30 (t = 0), 20% of the [1-14C]VLDL-TG tracer was infused as a bolus and a constant infusion with the remaining 80% was started. At t = 120 min, a 5-h infusion (1.0 mU · kg FFM−1 · min−1) of human insulin (Actrapid; Novo Nordisk A/S) commenced (FFM represents fat-free mass). Plasma glucose was measured every 10 min and clamped at 5 mmol · l−1 by a variable infusion of 20% glucose. The glucose infusion rate during the last hour of the clamp (M value) was used as an index of insulin sensitivity. Blood samples were drawn to determine VLDL-TG specific activity (SA) at t = 0, 60, 80, 100, and 120 min (basal period) and 180, 240, 300, 360, 380, 400, and 420 min (clamp period). Insulin and metabolite concentrations were determined every 60 min and ApoB-100 concentration at the end of each period. Breath samples to determine 14CO2 SA were obtained at 0, 80, 100, 120, 380, 400, and 420 min. Indirect calorimetry was performed from t = 60 to 80 min and from 360 to 380 min. At t = 120 min, fat biopsies were obtained from the abdominal (periumbilical) and femoral (inner thigh) regions using a liposuction technique. At 420 min, all catheters were removed and the participants discharged. The protocol is illustrated in Fig. 1.
FIG. 1.
Study protocol.
VLDL-TG tracer preparation.
The ex vivo labeling procedure of VLDL-TG with radio labeled triolein has previously been described in detail (18,19). Briefly, each half of the plasma obtained from a 120-ml blood sample was mixed with either [1-14C]triolein or [9,10-3H]triolein and sonicated in a cell incubator at 37°C for 6 h. The plasma was transferred to sterile Optiseal centrifuge tubes (Beckman Instruments), covered with a saline solution (d = 1.006 g · ml−1), and centrifuged (Ti 50.3 rotor; Beckman Instruments) for 18 h at 40,000 rpm and 10°C. The supernatant containing the labeled VLDL particles was removed using a sterile Pasteur pipette, filtered, and stored at 5°C. Samples were tested for bacterial growth to ensure sterility.
Body composition.
Total body fat, leg fat, fat percentage, and fat-free mass (FFM) were examined by dual X-ray absorptiometry (DXA) scanning (QDR-2000; Hologic). Upper-body fat and visceral fat mass were assessed using the CT measures of intra-abdominal and subcutaneous adipose tissue combined with abdominal fat mass measured by DXA as previously described (20). Abdominal subcutaneous fat was taken as upper body fat minus visceral fat. Leg fat was measured using the region of interest program with the DXA instrument.
Indirect calorimetry.
Energy expenditure (EE) and substrate oxidation rates were measured by indirect calorimetry (Deltatrac monitor; Datex Instruments) and net lipid and glucose oxidation rates were calculated by using the nonprotein respiratory quotient from the above measurements (21).
Laboratory procedures.
Plasma glucose was analyzed using a YSI 2,300 STAT Plus glucose analyzer (YSI). Blood samples were placed on ice and separated as quickly as possible by centrifugation (3,600 rpm at 4°C for 10 min). Aliquots of plasma (3 ml) were stored at 4°C for isolation of VLDL immediately after completion of the examination as described below. Remaining samples were stored at −20°C for later analysis. Triglyceride concentrations were analyzed using a Cobas Fara II (F. Hoffmann-La Roche). Serum insulin concentrations were measured with an immunoassay (DAKO Denmark A/S). Serum free fatty acid (FFA) concentrations were determined by a colorimetric method using a commercial kit (Wako Pure Chemical Industries). ApoB-100 concentration in the supernatant following ultracentrifugation (see below) was determined using an ELISA kit (Mabtech). Duplicate samples were diluted to ensure reading on the linear part of the standard curve.
Adipose tissue 3H-TG SA.
Adipose tissue lipid SA (dpm · g−1) was measured after lipid extraction from adipose tissue biopsies as previously described (22). In brief, extracted lipid was weighed, scintillation cocktail (Optiphase HiSafe 2; PerkinElmer) was added, mixed thoroughly, and 3H activity was counted.
Breath 14CO2 SA.
Breath samples were obtained using breath bags (IRIS breath bags, Wagner Analysen Technik). Expired air was passed through a solution containing benzethonium hydroxide (Sigma-Aldrich) with thymolphthalein (Sigma-Aldrich) in a scintillation vial. A color change occurred when exactly 0.25 mmol CO2 was trapped in the solution. Scintillation fluid was added and 14C activity was counted. Total CO2 production rate was obtained from the indirect calorimetry measurements.
Plasma VLDL-TG concentration and SA.
VLDL was isolated from ∼3 ml of each plasma sample by ultracentrifugation as described above. The supernatant containing the VLDL fraction was obtained by tubeslicing (Beckman Instruments) and transferred to a scintillation vial. A small sample was analyzed for triglyceride concentration, and the plasma concentration of VLDL-TG was calculated. Scintillation fluid was added, and 14C activity was counted.
Calculations
VLDL-TG secretion rate.
VLDL-TG SA steady state was effectively reached during the last hour of each of the basal and the clamp period. VLDL-TG secretion rate (μmol · min−1) was calculated by dividing the infusion rate by the plateau SA in each period:
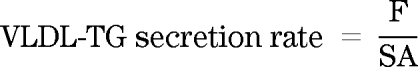 |
VLDL-TG clearance.
VLDL-TG clearance rate (ml · min−1) was calculated by dividing the secretion rate by the average VLDL-TG concentration in each period:
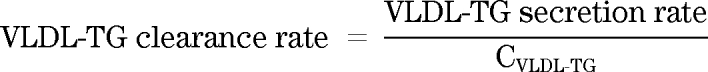 |
VLDL-TG FA oxidation.
Fractional oxidation (percentage) of the infused [1-14C]VLDL-TG was calculated as follows:
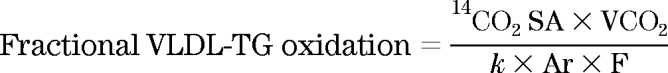 |
Here, k is the volume of CO2 at 20°C and 1 atm pressure (22.4 l · mol−1) and Ar is the fractional acetate carbon recovery factor in breath CO2, and F is the tracer infusion rate. Sidossis et al. (23) calculated Ar to be 0.56 for resting conditions and 0.5 for hyperinsulinemia, respectively. The total VLDL-TG FA oxidation rate (μmol · min−1) was calculated as follows and multiplied by three to allow for three FAs in each triglyceride:
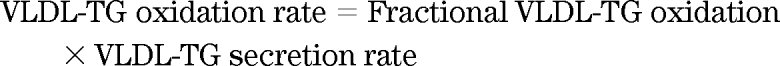 |
To calculate energy production (kcal · day−1) from VLDL-TG FA oxidation, the oxidation rate was converted to its weight equivalent using the molecular weight of oleic acid (282 g · mol−1) and multiplied by the caloric density of 9.1 kcal · g−1 and 1,440 min · day−1.
Adipose tissue VLDL-TG FA storage.
Fractional adipose tissue VLDL-TG FA storage (percentage) in abdominal and leg fat was calculated using the regional (abdomen or thigh) adipose lipid SA multiplied by the total amount of lipid in the region divided by the injected dose. The VLDL-TG storage rates (μmol · min−1) in specific regions were calculated as the fractional storage multiplied by VLDL-TG secretion rate.
Statistics.
Data are mean (SD) or median (range). Between groups, comparisons were performed using the Student t test or Mann-Whitney test. Within groups, comparisons were performed using Student t test for paired comparisons or Wilcoxon test. Adipose tissue storage values were log transformed to obtain normal distribution before statistical processing. Correlations were tested using the Pearson r or Spearman ρ. P < 0.05 was considered significant.
RESULTS
Subject characteristics.
Subject characteristics are summarized in Table 1. The groups were well matched for age and BMI, and there was no significant difference in body composition indexes except FFM. As expected, greater A1C (6.8 [0.8] vs. 5.4 [0.4]% P < 0.001) and triglycerides (1.68 [1.17–3.77 ] vs. 1.10 [0.80–1.88 ] mmol · l−1, P < 0.01) was noted in type 2 diabetic men.
Circulating metabolites and insulin.
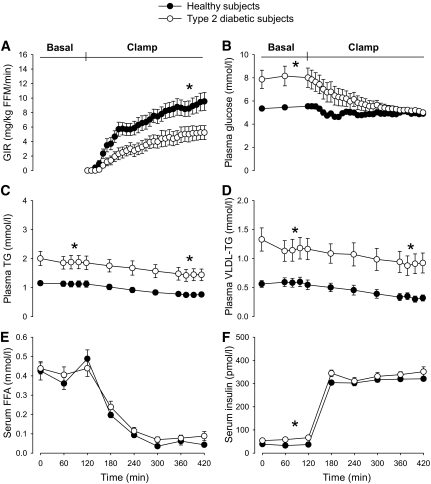
Concentrations of glucose, triglycerides, VLDL-TG, FFA, and insulin are shown in Fig. 2. In the basal period, glucose, VLDL-TG, and insulin were significantly greater in diabetic men compared with healthy men, whereas there was no significant difference in FFA concentrations. During the clamp, plasma glucose gradually decreased in diabetic men to the target of ∼5 mmol · l−1. Although hyperinsulinemia resulted in near complete suppression of FFA (P < 0.001, both groups), the decrease in plasma VLDL-TG was more modest, but still highly significant (P = 0.001 in both groups). In absolute numbers the decrease in VLDL-TG was comparable (−0.24 [0.15] vs. −0.25 [0.11] mmol · l−1, ns); however, the relative decrease was significantly greater in healthy subjects (−23.9 [18.2] vs. −44.1 [15.7]% P = 0.01). The resulting VLDL-TG concentration was significantly greater in diabetic men compared with healthy men, whereas there was no significant difference in glucose, insulin, or FFA concentrations between the groups during the clamp.
FIG. 2.
Glucose infusion rate during the hyperinsulinemic clamp (A), concentrations of glucose (B), triglycerides (TG) (C), VLDL-TG (D), FFA (E), and insulin (F) in the basal state and during the hyperinsulinemic clamp. *P < 0.05 between groups (60–120 min and 360–420 min). Black circles, healthy subjects; open (white) circles, type 2 diabetic subjects. Data are presented as mean ± SEM.
Metabolic parameters.
Metabolic parameters are summarized in Table 2. In the basal state, glucose oxidation rates were similar, but lipid oxidation rates were significantly lower in diabetic men compared with healthy men (0.57 [0.19] vs. 0.74 [0.14] mg · kg−1 · min−1, P = 0.02). Conversely, there were no significant differences in glucose and lipid oxidation rates or respiratory quotient (RQ) during hyperinsulinemia. As expected, insulin-mediated glucose disposal rate was significantly reduced in diabetic men (5.1 [3.3] vs. 9.0 [3.7] mg · kg−1 · min−1, P = 0.02).
TABLE 2.
Circulating metabolites, insulin, and metabolic parameters in the basal state and during hyperinsulinemia
| Healthy men (n = 11) |
Type 2 diabetic men (n = 11) |
|||
|---|---|---|---|---|
| Basal | Clamp | Basal | Clamp | |
| Glucose (mmol/l) | 5.5 (4.5–6.7) | 4.9 (4.6–5.3) | 6.8 (5.8–14.7)‡ | 5.0 (4.9–6.7)‖ |
| FFA (mmol/l) | 0.43 ± 0.12 | 0.03 (0.02–0.16) | 0.42 ± 0.13 | 0.05 (0.01–0.23) |
| VLDL-TG (mmol/l) | 0.54 (0.31–1.10) | 0.28 (0.13–0.63) | 0.92 (0.64–2.70)† | 0.70 (0.25–2.15)† |
| Insulin (pmol/l) | 27 (18–66) | 320 ± 31 | 65 (22–118)† | 345 ± 46 |
| EE (kcal/day) | 1,956 ± 125 | 2,021 ± 131 | 1,876 ± 197 | 1,819 ± 190†§ |
| RQ | 0.81 ± 0.04 | 0.89 ± 0.05 | 0.85 ± 0.04* | 0.88 ± 0.05 |
| Glucose oxidation (mg/kg/min) | 1.02 ± 0.52 | 2.09 ± 0.72 | 1.42 ± 0.55 | 1.80 ± 0.68§ |
| Lipid oxidation (mg/kg/min) | 0.74 ± 0.14 | 0.37 ± 0.23 | 0.57 ± 0.19* | 0.38 ± 0.23 |
| GIR (mg/kg/min) | 9.0 ± 3.7 | 5.1 ± 3.3* | ||
Data are mean ± SD or median (range). EE, energy expenditure; FFA, free fatty acids; GIR, glucose infusion rate; RQ, respiratory quotient; VLDL-TG, very low density lipoprotein triglyceride.
*P < 0.05;
†P < 0.01;
‡P < 0.001 vs. healthy men.
§P < 0.05;
‖P < 0.01 difference (basal-clamp) vs. difference (basal-clamp) in healthy men.
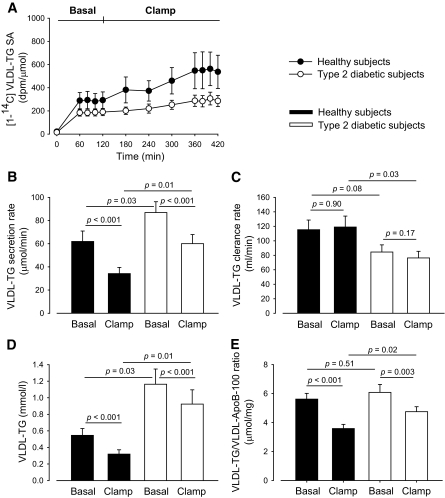
VLDL-TG secretion and clearance.
VLDL-TG SA steady state was effectively reached in the last hour of both the basal and the clamp period (Fig. 3A). VLDL-TG secretion (Fig. 3B) was significantly greater in diabetic men compared with healthy men in both the basal state (86.9 [31.0] vs. 61.9 [30.0] μmol · min−1, P = 0.03) and during hyperinsulinemia (60.0 [26.2] vs. 34.2 [17.9] μmol · min−1, P = 0.01). The suppression of VLDL-TG secretion was highly significant in both groups (P < 0.001, both groups). Moreover, the suppression was comparable between groups both in absolute numbers (−26.9 [17.2] vs. −27.7 [16.9] μmol · min−1, ns) and in percentage (−30.6 [20.7] vs. −43.6 [15.4]%, ns). VLDL-TG clearance (Fig. 3C) was lower in diabetic men compared with healthy men in both periods, although significant only during the clamp (84.6 [32.7] vs. 115.4 [44.3] ml · min−1, P = 0.08) and (76.3 [30.6] vs. 119.0 [50.2] ml · min−1, P = 0.03), respectively. There was no significant change in VLDL-TG clearance in either group or in the change between the groups.
FIG. 3.
VLDL-TG–specific activity (A), VLDL-TG secretion rate (B), VLDL-TG clearance rate (C), VLDL-TG concentration (D), and VLDL-TG/VLDL-ApoB-100 ratio (E) in the VLDL-TG SA steady state periods. Black circles and bars, healthy subjects; open (white) circles and bars, type 2 diabetic subjects. Data are mean ± SEM.
VLDL-apoB-100 concentration and VLDL-TG/VLDL-apoB-100 ratio.
Plasma VLDL-apoB-100 concentration was significantly greater in diabetic men compared with healthy men in both the basal state (16.3 [10.1–42.2 ] vs. 12.2 [4.6–17.1 ] mg · dl−1, P = 0.02) and during hyperinsulinemia (12.8 [9.7–40.0 ] vs. 7.7 [3.5–17.6 ] mg · dl−1, P = 0.01). A slight, nonsignificant decrease was noted in both groups during hyperinsulinemia. The decrease was not significantly different between the groups. The VLDL-TG/VLDL-apoB-100 ratio was comparable in the two groups in the basal state (6.1 [2.0] vs. 5.6 [1.3] × 10−3 mmol · mg−1, ns) but significantly higher in diabetic men during hyperinsulinemia (4.8 [1.1] vs. 3.6 [0.9] × 10−3 mmol · mg−1, P = 0.02) (Fig. 3E). The decrease in the ratio during the clamp was significant in both groups but less so in diabetic men, although only the relative decrease was significantly different between the groups (−19.4 [16.9] vs. −35.3 [14.6]%, P = 0.03).
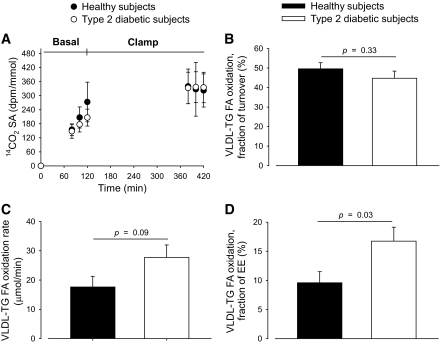
VLDL-TG FA oxidation.
Breath 14CO2 SA steady state was reached in the clamp period, but not in the basal period, allowing calculation of VLDL-TG FA oxidation only during the clamp. VLDL-TG FA oxidation during the clamp is depicted in Fig. 4B–D. The fraction of VLDL-TG secretion that was oxidized was comparable in diabetic and healthy men (44.8 [12.1] vs. 49.6 [10.4]%, ns). However, total VLDL-TG FA oxidation tended to be greater in diabetic compared with healthy men (27.7 [14.2] vs. 17.6 [12.2] μmol · min−1, P = 0.09). Expressed as a fraction of EE, the contribution from VLDL-TG FA to total substrate oxidation was significantly greater in diabetic men (16.7 [7.9] vs. 9.6 [6.3]%, P = 0.03).
FIG. 4.
Breath 14CO2 SA steady state was reached in the clamp period, but not in the basal period (A). Therefore, only VLDL-TG FA oxidation data from the clamp period are illustrated and analyzed statistically. VLDL-TG FA oxidation expressed as fraction of secretion (B) and as oxidation rate (fractional oxidation × VLDL-TG secretion rate) (C). VLDL-TG FA oxidation as fraction of EE (D). Black circles and bars, healthy subjects; open (white) circles and bars, type 2 diabetic subjects. Data are mean ± SEM.
VLDL-TG FA adipose tissue storage.
At 120 min (4 h after bolus infusion of [9,10-3H]VLDL-TG), the remaining 3H plasma activity was (6.1 [2.2–20.1]% vs. 5.8 [2.3–8.0], ns, diabetic vs. healthy men, respectively). This was calculated by dividing plasma 3H activity (dpm · ml−1) at 120 min by the initial activity estimated by dividing infused activity (dpm) by plasma volume (55 ml · kg FFM−1) (24). Fractional VLDL-TG FA storage was comparable in the groups both in abdominal (4.2 [2.9] vs. 4.2 [2.3]%, ns) and leg fat (1.0 [0.3–4.8 ] vs. 0.8 [0.2–5.5]%, ns) but significantly greater in abdominal compared with leg fat (Fig. 5). Since practically all 3H activity had disappeared from plasma at 120 min, it is possible to estimate the rate of VLDL-TG FA storage in the tissues by multiplying fractional storage by turnover rate. Despite the difference in VLDL-TG secretion rate, there was no significant difference between diabetic and healthy men in VLDL-TG FA storage rate in abdominal (2.7 [1.3–7.0 ] vs. 2.0 [0.5–5.6 ] μmol · min−1, ns) or in leg fat (1.0 [0.2–3.4 ] vs. 0.4 [0.1–4.7 ] μmol · min−1, ns). However, storage rate was significantly greater in abdominal fat compared with leg fat in both groups (Fig. 5).
FIG. 5.
Storage of VLDL-TG FA in abdominal (A and B) and leg (C and D) subcutaneous adipose tissue expressed as fraction of VLDL-TG secretion (A and C) and as storage rate (fraction storage × VLDL secretion rate) (B and D). Black bars, healthy subjects; white bars, type 2 diabetic subjects. Data are presented as mean ± SEM.
Correlations.
No significant within-group correlations were found between VLDL-TG secretion rates and M-value or concentrations of glucose, FFA, or insulin in the basal period or between the relative decrease in VLDL-TG secretion and clearance rates and M-value in the clamp period.
DISCUSSION
These studies were undertaken to perform comprehensive comparisons of VLDL-TG kinetics and metabolism in type 2 diabetic men and healthy, age- and BMI-matched men. We report several novel findings. First, VLDL-TG secretion is significantly increased in type 2 diabetic men, both postabsorptively and during hyperinsulinemia, and in addition, the ability of hyperinsulinemia to suppress VLDL-TG secretion in type 2 diabetic men appears preserved. Second, fractional VLDL-TG FA oxidation is similar and quantitatively important during hyperinsulinemia in both type 2 diabetic and healthy men. Third, storage pattern of VLDL-TG FA is similar in both groups, with significantly greater storage in abdominal subcutaneous fat compared with leg fat.
Our findings support and extend results from previous reports showing that increased postabsorptive VLDL-TG concentrations in type 2 diabetes results from hepatic hypersecretion (8–12) and adds new information regarding the effect of insulin on VLDL kinetics (13–17). In addition to increased VLDL-TG secretion, we found that VLDL-TG clearance is lower in diabetic men compared with healthy men, although the difference was significant only during hyperinsulinemia. Therefore, we conclude that the increased plasma VLDL-TG concentrations observed in type 2 diabetic men is primarily explained by increased VLDL-TG secretion and only to a lesser degree by decreased VLDL-TG clearance.
To our knowledge, the effect of experimental hyperinsulinemia on VLDL-TG kinetics in type 2 diabetic patients and healthy subjects has not previously been compared directly. In one study, the effect of insulin on VLDL kinetics was compared in subjects with high liver fat (80% with type 2 diabetes) and low liver fat (all nondiabetic) (17). Hyperinsulinemia resulted in a rapid decline in VLDL1-TG and -apoB-100 secretion in the group with low liver fat, whereas there was no significant change in VLDL1 secretion in the group with high liver fat. However, the study was designed to study the effect of liver fat content rather than type 2 diabetes on VLDL kinetics. The effect of hyperinsulinemia on VLDL-apoB-100 kinetics in type 2 diabetes has been studied in a few studies, but results are conflicting (25,26). However, lack of a healthy control group in the first study may explain the difference. In the present study VLDL-apoB-100 concentration was significantly greater in type 2 diabetic men compared with healthy men, and there was no change in concentrations during hyperinsulinemia. In addition, the VLDL-TG/VLDL-apoB-100 ratio, a measure of VLDL particle size, was comparable in the basal state, but decreased significantly in both groups during hyperinsulinemia. This indicates that postabsorptively, type 2 diabetic men secrete more—not larger—VLDL particles than healthy men and that hyperinsulinemia decreases the average particle triglyceride content without changing the particle number. This effect was, however, attenuated in type 2 diabetic men since the relative decrease in VLDL-TG/VLDL-apoB-100 ratio was significantly greater in healthy men.
As a novel observation we found that VLDL-TG FA oxidation during hyperinsulinemia accounted for 50% of VLDL-TG turnover, equivalent to 16.7% and 9.6% of total EE, which was significantly greater in type 2 diabetic men compared with healthy men. In the basal state, the potential VLDL-TG energy yield, that is, if all circulating VLDL-TG is oxidized, was ∼40% of EE. Since we did not achieve steady state in breath 14CO2 in the basal period, we cannot estimate basal VLDL-TG FA oxidation. However, we recently reported basal VLDL-TG FA oxidation rates of ∼20% of EE in obese, insulin-resistant, and lean women (27). In the present study, the fractional oxidation of VLDL-TG FA during hyperinsulinemia was comparable in the two groups. However, because of the greater VLDL-TG secretion rate, VLDL-TG oxidation rates tended to be greater in type 2 diabetic men. The greater VLDL-TG oxidation in relation to EE in type 2 diabetic men compared with healthy men suggests a decreased capacity of insulin to suppress VLDL-TG oxidation in type 2 diabetic men. This is interesting in the context of “metabolic inflexibility” in type 2 diabetes, i.e., reduced capacity to shift appropriately between lipid and carbohydrate fuels for oxidation (28). Thus, we propose to extend the concept of metabolic inflexibility in type 2 diabetes to include the failure to suppress VLDL-TG FA oxidation during hyperinsulinemia.
Finally, we report that the fractions of circulating VLDL-TG FA that are stored in abdominal and leg fat are similar in type 2 diabetic and healthy men who have a similar amount of fat tissue. However, the VLDL-TG FA storage rate is somewhat greater, although not significantly, in type 2 diabetic men compared with healthy men in both abdominal and leg fat (Fig. 5). Both the fractional storage and the storage rate were significantly greater in abdominal fat compared with leg fat in both groups. This difference cannot be ascribed to a greater amount of abdominal fat compared with leg fat. Thus, abdominal subcutaneous fat mass was only ∼25% greater than leg fat mass, whereas storage was ∼3 times greater in abdominal fat. Therefore, storage per kg fat was significantly greater in abdominal fat compared with leg fat in both groups. In a recent study of obese and lean women, we reported similar VLDL-TG factional storage and storage rates in abdominal and leg fat in upper-body obese women. Both fractional storage and storage rates in abdominal fat were similar to the values found in men in the present study. However, leg fat storage was much less in men in the present study compared with the obese women of the previous study (27). We believe that the present study is the first to report postabsorptive VLDL-TG storage rates in regional fat in type 2 diabetic men in comparison with matched healthy men, and that the results offer new information to explain the preferential fat accumulation in abdominal fat in upper-body obese subjects.
We acknowledge the limitations of our study. The inability to detect a significant difference between groups in suppression of VLDL-TG secretion may represent a type 2 error. At least our data indicate that insulin affects VLDL-TG kinetics differently in the two groups. Basal VLDL-TG secretion rates are significantly greater in type 2 diabetic men despite significantly higher insulin concentrations. Moreover, the relative decrease in VLDL-TG concentration and VLDL-TG/VLDL-apoB-100 ratio was significantly lower in type 2 diabetic men. In addition, the capacity of insulin to reduce VLDL-TG secretion could be proportionally less pronounced at lower (physiologic) insulin levels in type 2 diabetic than in healthy men. In comparison, suppression of glucose production is impaired in type 2 diabetic subjects compared with weight-matched healthy subjects at physiologic, but not supraphysiologic, insulin concentrations (29). VLDL-TG oxidation rates were calculated by multiplying fractional oxidation by VLDL-TG turnover. Obviously, if either parameter is overestimated, the oxidation rates are overestimated. The optimal approach would be measurement of acetate recovery in each experiment, since the recovery dependents on, e.g., EE and tracer infusion duration. The VLDL-TG secretion rates reported in our study are higher than values reported in most studies based on precursor labeling and compartmental modeling (30,31). However, traditional tracer dilution technique and noncompartmental modeling is a conceptually more simple approach that allows calculation of kinetic parameters of interest without the assumptions inherent to methods based on compartmental modeling. Importantly, the VLDL-TG secretion rates reported in the present study are in reasonable agreement with values from studies using other model-independent methods, e.g., splanchnic differences and studies using primed-constant reinfusion of endogenously labeled VLDL-TG tracer as discussed in detail previously (19). We also acknowledge the use of a VLDL-TG tracer prepared from VLDL-TG particles during fasting, which may not be representative to the VLDL-TG particle composition during hyperinsulinemia. Ideally, VLDL-TG sampling for tracer preparation should be performed during hyperinsulinemia. Using this approach, Lewis et al.(13) reported unaltered VLDL-ApoB-100 kinetics. Moreover, we found no published information regarding the impact of altered plasma particle composition on the peripheral fate of VLDL-TG FA. Finally, we cannot extend our findings to type 2 diabetic women.
In conclusion, this study demonstrates that the increased VLDL-TG concentration in type 2 diabetic men results from increased hepatic VLDL-TG secretion, and that the ability of hyperinsulinemia to suppress VLDL-TG secretion is preserved in type 2 diabetic men at this relatively high insulin dose. Moreover, VLDL-TG FA oxidation accounts for 50% of VLDL-TG turnover, and constitutes a significantly greater proportion of EE in type 2 diabetic men (∼17%) compared with age- and weight-matched men (10%). Finally, both type 2 diabetic and healthy men store more VLDL-TG in abdominal fat than in leg fat, which may provide a mechanism whereby some individuals develop a preferential upper-body fat distribution.
ACKNOWLEDGMENTS
This work was supported by grants (to S.N.) from the Danish Medical Research Council, the Novo Nordic Foundation, and the Danish Diabetes Foundation.
No potential conflicts of interest relevant to this article were reported.
L.P.S. and S.N. researched data, contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript. I.R.A. researched data, and reviewed/edited the manuscript. E.S. researched data, contributed to discussion, and reviewed/edited the manuscript. L.G. and J.S.C. contributed to discussion and reviewed/edited the manuscript. O.S. contributed to discussion.
The authors acknowledge the excellent technical assistance of Lone Kvist and Susanne Sørensen, Department of Endocrinology and Internal Medicine, Aarhus University Hospital.
Footnotes
Clinical trial reg. no. NCT01037647, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM: Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988;260:1917–1921 [PubMed] [Google Scholar]
- 2.Fontbonne A, Eschwege E, Cambien F, Richard JL, Ducimetiere P, Thibult N, Warnet JM, Claude JR, Rosselin GE: Hypertriglyceridaemia as a risk factor of coronary heart disease mortality in subjects with impaired glucose tolerance or diabetes. Results from the 11-year follow-up of the Paris Prospective Study. Diabetologia 1989;32:300–304 [DOI] [PubMed] [Google Scholar]
- 3.Manninen V, Tenkanen L, Koskinen P, Huttunen JK, Manttari M, Heinonen OP, Frick MH: Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation 1992;85:37–45 [DOI] [PubMed] [Google Scholar]
- 4.Taskinen MR: Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia 2003;46:733–749 [DOI] [PubMed] [Google Scholar]
- 5.Adiels M, Olofsson SO, Taskinen MR, Boren J: Diabetic dyslipidaemia. Curr Opin Lipidol 2006;17:238–246 [DOI] [PubMed] [Google Scholar]
- 6.Verges B: New insight into the pathophysiology of lipid abnormalities in type 2 diabetes. Diabete Metab 2005;31:429–439 [DOI] [PubMed] [Google Scholar]
- 7.Packard CJ: Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans 2003;31:1066–1069 [DOI] [PubMed] [Google Scholar]
- 8.Ouguerram K, Magot T, Zair Y, Marchini JS, Charbonnel B, Laouenan H, Krempf M: Effect of atorvastatin on apolipoprotein B100 containing lipoprotein metabolism in type-2 diabetes. J Pharmacol Exp Ther 2003;306:332–337 [DOI] [PubMed] [Google Scholar]
- 9.Cummings MH, Watts GF, Umpleby AM, Hennessy TR, Naoumova R, Slavin BM, Thompson GR, Sonksen PH: Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetologia 1995;38:959–967 [DOI] [PubMed] [Google Scholar]
- 10.Kissebah AH, Alfarsi S, Evans DJ, Adams PW: Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in non-insulin-dependent diabetes mellitus. Diabetes 1982;31:217–225 [DOI] [PubMed] [Google Scholar]
- 11.Adiels M, Boren J, Caslake MJ, Stewart P, Soro A, Westerbacka J, Wennberg B, Olofsson SO, Packard C, Taskinen MR: Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 2005;25:1697–1703 [DOI] [PubMed] [Google Scholar]
- 12.Taskinen MR, Packard CJ, Shepherd J: Effect of insulin therapy on metabolic fate of apolipoprotein B-containing lipoproteins in NIDDM. Diabetes 1990;39:1017–1027 [DOI] [PubMed] [Google Scholar]
- 13.Lewis GF, Uffelman KD, Szeto LW, Steiner G: Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes 1993;42:833–842 [DOI] [PubMed] [Google Scholar]
- 14.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G: Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 1995;95:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malmström R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Järvinen H, Shepherd J, Taskinen MR: Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes 1998;47:779–787 [DOI] [PubMed] [Google Scholar]
- 16.Malmström R, Packard CJ, Watson TD, Rannikko S, Caslake M, Bedford D, Stewart P, Yki-Järvinen H, Shepherd J, Taskinen MR: Metabolic basis of hypotriglyceridemic effects of insulin in normal men. Arterioscler Thromb Vasc Biol 1997;17:1454–1464 [DOI] [PubMed] [Google Scholar]
- 17.Adiels M, Westerbacka J, Soro-Paavonen A, Hakkinen AM, Vehkavaara S, Caslake MJ, Packard C, Olofsson SO, Yki-Järvinen H, Taskinen MR, Boren J: Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia 2007;50:2356–2365 [DOI] [PubMed] [Google Scholar]
- 18.Gormsen LC, Jensen MD, Nielsen S: Measuring VLDL-triglyceride turnover in humans using ex vivo-prepared VLDL tracer. J Lipid Res 2006;47:99–106 [DOI] [PubMed] [Google Scholar]
- 19.Sørensen L, Gormsen L, Nielsen S: VLDL-TG kinetics: a dual isotope study for quantifying VLDL-TG pool size, production rates and fractional oxidation in humans. Am J Physiol Endocrinol Metab 2009 [DOI] [PubMed] [Google Scholar]
- 20.Jensen MD, Kanaley JA, Reed JE, Sheedy PF: Measurement of abdominal and visceral fat with computed tomography and dual-energy X-ray absorptiometry. Am J Clin Nutr 1995;61:274–278 [DOI] [PubMed] [Google Scholar]
- 21.Frayn KN: Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 22.Marin P, Rebuffe-Scrive M, Björntorp P: Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 1990;20:158–165 [DOI] [PubMed] [Google Scholar]
- 23.Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR: A new correction factor for use in tracer estimations of plasma fatty acid oxidation. Am J Physiol Endocrinol Metab 1995;269:E649–E656 [DOI] [PubMed] [Google Scholar]
- 24.Boer P: Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol Endocrinol Metab 1984;247:F632–F636 [DOI] [PubMed] [Google Scholar]
- 25.Cummings MH, Watts GF, Umpleby AM, Hennessy TR, Kelly JM, Jackson NC, Sonksen PH: Acute hyperinsulinemia decreases the hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetes 1995;44:1059–1065 [DOI] [PubMed] [Google Scholar]
- 26.Malmström R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Järvinen H, Shepherd J, Taskinen MR: Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia 1997;40:454–462 [DOI] [PubMed] [Google Scholar]
- 27.Gormsen LC, Nellemann B, Sørensen LP, Jensen MD, Christiansen JS, Nielsen S: Impact of body composition on very-low-density lipoprotein-triglycerides kinetics. Am J Physiol Endocrinol Metab 2009;296:E165–E173 [DOI] [PubMed] [Google Scholar]
- 28.Kelley DE, Mandarino LJ: Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000;49:677–683 [DOI] [PubMed] [Google Scholar]
- 29.Firth R, Bell P, Rizza R: Insulin action in non-insulin-dependent diabetes mellitus: the relationship between hepatic and extrahepatic insulin resistance and obesity. Metabolism 1987;36:1091–1095 [DOI] [PubMed] [Google Scholar]
- 30.Magkos F, Sidossis LS: Measuring very low density lipoprotein-triglyceride kinetics in man in vivo: how different the various methods really are. Curr Opin Clin Nutr Metab Care 2004;7:547–555 [DOI] [PubMed] [Google Scholar]
- 31.Horowitz JF, Klein S: Lipid metabolism during endurance exercise. Am J Clin Nutr 2000;72:558S–563S [DOI] [PubMed] [Google Scholar]