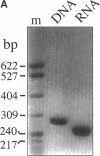
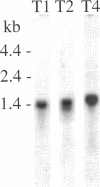
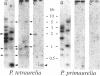
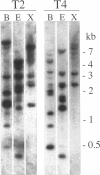
Abstract
The secretory granules (trichocysts) of Paramecium are characterized by a highly constrained shape that reflects the crystalline organization of their protein contents. Yet the crystalline trichocyst content is composed not of a single protein but of a family of related polypeptides that derive from a family of precursors by protein processing. In this paper we show that a multigene family, of unusually large size for a unicellular organism, codes for these proteins. The family is organized in subfamilies; each subfamily codes for proteins with different primary structures, but within the subfamilies several genes code for nearly identical proteins. For one subfamily, we have obtained direct evidence that the different members are coexpressed. The three subfamilies we have characterized are located on different macronuclear chromosomes. Typical 23-29 nucleotide Paramecium introns are found in one of the regions studied and the intron sequences are more variable than the surrounding coding sequences, providing gene-specific markers. We suggest that this multigene family may have evolved to assure a microheterogeneity of structural proteins necessary for morphogenesis of a complex secretory granule core with a constrained shape and dynamic properties: genetic analysis has shown that correct assembly of the crystalline core is necessary for trichocyst function.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., de Loubresse N. G., Beisson J. Proteolytic cleavage and maturation of the crystalline secretion products of Paramecium. J Mol Biol. 1984 Dec 25;180(4):1065–1081. doi: 10.1016/0022-2836(84)90271-7. [DOI] [PubMed] [Google Scholar]
- Amar L. Chromosome end formation and internal sequence elimination as alternative genomic rearrangements in the ciliate Paramecium. J Mol Biol. 1994 Feb 18;236(2):421–426. doi: 10.1006/jmbi.1994.1154. [DOI] [PubMed] [Google Scholar]
- Black S. D., Coon M. J. P-450 cytochromes: structure and function. Adv Enzymol Relat Areas Mol Biol. 1987;60:35–87. doi: 10.1002/9780470123065.ch2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Karrer K. M. Genomic reorganization in ciliated protozoans. Annu Rev Genet. 1986;20:501–521. doi: 10.1146/annurev.ge.20.120186.002441. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Caron F. A high degree of macronuclear chromosome polymorphism is generated by variable DNA rearrangements in Paramecium primaurelia during macronuclear differentiation. J Mol Biol. 1992 Jun 5;225(3):661–678. doi: 10.1016/0022-2836(92)90393-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. G. Invasion and maintenance of a gene duplication. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2950–2954. doi: 10.1073/pnas.91.8.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis P. The beta-tubulin genes of Paramecium are interrupted by two 27 bp introns. EMBO J. 1992 Oct;11(10):3713–3719. doi: 10.1002/j.1460-2075.1992.tb05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Desbruyères E., Gros F., Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990 Jan 5;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Eperon S., Viguès B., Peck R. K. Immunological characterization of trichocyst proteins in the ciliate Pseudomicrothorax dubius. J Eukaryot Microbiol. 1993 Jan-Feb;40(1):81–91. doi: 10.1111/j.1550-7408.1993.tb04886.x. [DOI] [PubMed] [Google Scholar]
- Forney J. D., Blackburn E. H. Developmentally controlled telomere addition in wild-type and mutant paramecia. Mol Cell Biol. 1988 Jan;8(1):251–258. doi: 10.1128/mcb.8.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gautier M. C., Garreau de Loubresse N., Madeddu L., Sperling L. Evidence for defects in membrane traffic in Paramecium secretory mutants unable to produce functional storage granules. J Cell Biol. 1994 Mar;124(6):893–902. doi: 10.1083/jcb.124.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. R., Kafatos F. C. Developmentally regulated genes in silkmoths. Annu Rev Genet. 1984;18:443–487. doi: 10.1146/annurev.ge.18.120184.002303. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Maley M. E., Ling K. Y., Kanabrocki J. A., Kung C. Efficient expression of the Paramecium calmodulin gene in Escherichia coli after four TAA-to-CAA changes through a series of polymerase chain reactions. J Protozool. 1991 Sep-Oct;38(5):441–447. doi: 10.1111/j.1550-7408.1991.tb04814.x. [DOI] [PubMed] [Google Scholar]
- Madeddu L., Gautier M. C., Le Caer J. P., Garreau de Loubresse N., Sperling L. Protein processing and morphogenesis of secretory granules in Paramecium. Biochimie. 1994;76(3-4):329–335. doi: 10.1016/0300-9084(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Ohta T. Multigene families and the evolution of complexity. J Mol Evol. 1991 Jul;33(1):34–41. doi: 10.1007/BF02100193. [DOI] [PubMed] [Google Scholar]
- Redeker V., Levilliers N., Schmitter J. M., Le Caer J. P., Rossier J., Adoutte A., Bré M. H. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science. 1994 Dec 9;266(5191):1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C. B., Fraga D., Hinrichsen R. D. Extremely short 20-33 nucleotide introns are the standard length in Paramecium tetraurelia. Nucleic Acids Res. 1994 Apr 11;22(7):1221–1225. doi: 10.1093/nar/22.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn T. M. The Determination of Hereditary Antigenic Differences in Genically Identical Paramecium Cells. Proc Natl Acad Sci U S A. 1948 Aug;34(8):413–418. doi: 10.1073/pnas.34.8.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling L., Keryer G., Ruiz F., Beisson J. Cortical morphogenesis in Paramecium: a transcellular wave of protein phosphorylation involved in ciliary rootlet disassembly. Dev Biol. 1991 Nov;148(1):205–218. doi: 10.1016/0012-1606(91)90330-6. [DOI] [PubMed] [Google Scholar]
- Sperling L., Tardieu A., Gulik-Krzywicki T. The crystal lattice of Paramecium trichocysts before and after exocytosis by X-ray diffraction and freeze-fracture electron microscopy. J Cell Biol. 1987 Oct;105(4):1649–1662. doi: 10.1083/jcb.105.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall S. H., DeVito L. D., Nelson D. L. Biochemical characterization of the proteins of Paramecium secretory granules. J Cell Sci. 1989 Mar;92(Pt 3):441–447. doi: 10.1242/jcs.92.3.441. [DOI] [PubMed] [Google Scholar]
- Tindall S. H. Selection of chemical spacers to improve isoelectric focusing resolving power: implications for use in two-dimensional electrophoresis. Anal Biochem. 1986 Dec;159(2):287–294. doi: 10.1016/0003-2697(86)90345-3. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Larkins B. A. Zein gene organization in maize and related grasses. J Mol Evol. 1984;20(3-4):330–340. doi: 10.1007/BF02104739. [DOI] [PubMed] [Google Scholar]