Abstract
BTBR mice are potentially useful tools for autism research because their behavior parallels core social interaction impairments and restricted-repetitive behaviors. Altered regulation of central serotonin (5-HT) neurotransmission may underlie such behavioral deficits. To test this, we compared 5-HT transporter (SERT), 5-HT1A and 5-HT2A receptor densities among BTBR and C57 strains. Autoradiographic [3H] cyanoimipramine (1nM) binding to SERT was 20–30% lower throughout the adult BTBR brain as compared to C57BL/10J mice. In hippocampal membrane homogenates [3H] citalopram maximal binding (Bmax) to SERT was 95 ± 13 fmol/mg protein in BTBR and 171 ± 20 fmol/mg protein in C57BL/6J mice, and the BTBR dissociation constant (KD) was 2 ± 0.3 nM vs. 1.1 ± 0.2 in C57BL/6J mice. Hippocampal 5-HT1A and 5-HT2A receptor binding was similar among strains. However, 8-OH-DPAT-stimulated [35S] GTPγS binding in the BTBR hippocampal CA1 region was 28% higher, indicating elevated 5-HT1A capacity to activate G-proteins. In BTBR mice, the SERT blocker, fluoxetine (10 mg/kg) and the 5-HT1A receptor partial-agonist, buspirone (2 mg/kg) enhanced social interactions. The D2/5-HT2 receptor antagonist, risperidone (0.1 mg/kg) reduced marble burying but failed to improve sociability. Overall, altered SERT and/or 5-HT1A functionality in hippocampus could contribute to the relatively low sociability of BTBR mice.
Keywords: Sociability, buspirone, 5-HT1A receptor, fluoxetine, SERT, CA1 of hippocampus
Introduction
Autism spectrum disorders (ASDs) are complex developmental psychiatric conditions in which multiple genetic and environmental risk factors, and their interactions, appear to be involved. Dysfunction of several neurotransmitter systems have been implicated in ASD etiology (Bartlett et al. 2005; Pardo and Eberhart 2007). The serotonin (5-HT) system is chief among them and may underlie characteristic ASD social interaction impairments (Santangelo and Tsatsanis 2005; Lam et al. 2006; Moy et al. 2006; Brune et al. 2006). The 5-HT system plays many critical roles in brain development, and its perturbation in rodents can result in behavioral phenotypes reminiscent of autism (Whitaker-Azmitia 2005; Murrin et al. 2007; Borue et al. 2007; Boylan et al. 2007). About 20–45% of ASD patients have elevated platelet 5-HT levels, which might suppress 5-HT terminal formation if it also occurs early in brain development (Anderson et al. 1990; Whitaker-Azmitia 2005). At critical postnatal stages, capacity for brain 5-HT synthesis could be reduced or peak 5-HT levels may be mistimed (Chandana et al. 2005). Such processes may impair 5-HT system function and impede therapeutic intervention persistently.
In some studies, ASD susceptibility appears to correlate with function-impairing polymorphisms of 5-HT transporter (SERT), such as the short allele form of the 5-HTTLPR, and 5-HT receptor genes (Chugani 2002; Sutcliffe et al. 2005; Cho et al. 2007; Orabona et al. 2008; Richardson-Jones et al. 2010). Consistent with this scenario, reduced cortical SERT density, as measured by tomography, has been reported in autistic children and adults (Makkonen et al. 2008; Nakamura et al. 2010). In other reports, the long allele form of the 5-HTTLPR appears to be overtransmitted in autistic patients and/or is associated with increased aggression and repetitive behaviors in the disorder (Devlin et al. 2005; Brune et al. 2006). Thus, evidence from clinical studies suggests altered expression and/or function of the SERT may contribute to behavioral deficits in subpopulations of autistic patients.
From early juvenile stages (PD 21) through adulthood, the inbred BTBR T+tf/J (BTBR) mouse exhibits deficits in play and social approach and engages in excessive self-grooming and other repetitive behaviors relative to other strains, such as C57 or FVB lines, that can be analogized to core symptoms of ASD (Moy et al. 2007; Bolivar et al. 2007; Crawley 2007; Yang et al., 2007a; McFarlane et al. 2008). Aberrant social behaviors in BTBR mice must have some genetic basis, since they remain after cross-fostering with C57 mothers (Yang et al. 2007a; Benno et al. 2008). Further, the behavioral response of BTBR mice to the SERT blocker citalopram is more pronounced than that of C57BL/6 mice (Crowley et al. 2006). Based on these findings, we hypothesized that altered SERT or 5-HT receptor function could contribute to the aberrant social behavior of BTBR mice, and compared ligand binding properties of its SERT, 5-HT1A and 5-HT2A receptors to more “sociable” C57 strains.
Few effective therapeutic interventions are available for ASD, and hardly any of them improve social behavior. Selective 5-HT reuptake inhibitors (SSRIs) such as fluoxetine (Prozac) improve symptoms for some autistic patients, but they have limited effectiveness as a comprehensive ASD therapeutic, and their use in juveniles is controversial (West et al. 2009; Kirsch et al. 2008; Henry et al. 2009; Daws 2009; Richardson-Jones et al. 2010). Risperidone, a D2/5-HT2 antagonist, is often used to control aggression and self-injury in ASD, but it is less efficacious in some patient groups and does not enhance sociability (West et al. 2009; Marek et al., 2003; Dolzan et al. 2008). Buspirone, an anxiolytic 5-HT1A partial agonist is reported to enhance rodent social interaction at low doses (File and Seth, 2003). Since the relatively low sociability of BTBR mice bears some face validity to autism, and its utility for pharmacological testing has been suggested (Moy et al. 2006), we examined the effects of acute fluoxetine, risperidone and buspirone administration on their social and repetitive behaviors. The combined neurochemical and behavioral approach of this study was designed to reveal how key regulators of 5-HT transmission in emotional centers of the brain could be involved in mammalian social interaction and repetitive behaviors.
Materials and Methods
Animals
All animal procedures were performed in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committees of the University of Texas Health Science Center at San Antonio, TX (UTHSCSA), and William Paterson University, Wayne, NJ (WPU). Mice at both facilities were housed and bred under standard conditions: 12h light/dark cycle, 20–22°C, ad libitum access to food (Teklad rodent diet, Harlan, Indianapolis, IN) and water in ventilated racks with plastic housing cages lined with chipped or shaved wood bedding. Water was changed every 2 days and cages refreshed every 7–10 days. Adult (4-month old) male BTBR and C57BL/10J mice used in autoradiography were second generation offspring from colony founders obtained from the Jackson Laboratory (Bar Harbor, ME) and bred at WPU. Mice were sacrificed by cervical dislocation and decapitation. Whole brains were removed, rinsed in saline and fresh-frozen in powdered dry ice and express shipped the same day from WPU to UTHSCSA for the quantitative autoradiography experiments. There were a total of 8 BTBR and 8 C57BL/10J mouse brains used in autoradiography.
C57BL/6J mice are often used as a control for BTBR mice in social interaction tests (e.g. McFarlane et al. 2008; Silverman et al. 2010), and their binding properties at brain 5-HT sites have been well-described because they are a common background strain for the SERT knock-out mouse (e.g. Li et al. 2000; 2003; Montañez et al. 2003). However, we compared SERT and 5-HT1A and 5-HT2A receptor binding site densities of BTBR mice to C57BL/10J mice instead, because the literature indicated that 10J mice are better matched for brain size during juvenile development, and like BTBR mice they are also prone to hippocampal anatomy deficits (Wahlsten et al. 2003; Kusek et al. 2007; Deacon et al. 2007). Thus C57BL/10J mice may more appropriate behavioral controls than C57BL/6J for BTBR mice for brain development studies.
Male BTBR mice used in behavior (3–4 month old) and saturation binding (4 month old) at UTHSCSA were second generation offspring from colony founders obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in the UTHSCSA facility. Adult (4-month old) C57BL/6J mice used in saturation binding and 6–8 week old C57BL/10J and C57BL/6J used in social interaction testing were purchased directly from the Jackson Laboratory (Bar Harbor, ME), and were housed in UTHSCSA facilities for at least one week prior to use in experiments. Mice were sacrificed by cervical dislocation and decapitation, brains for saturation binding were rinsed in ice cold saline, hippocampi were dissected out and used immediately in experiments.
Quantitative Autoradiography
Tissue Preparation
Brains from BTBR and C57BL/10J mice were coronally sectioned at a thickness of 20 μm in a cryostat (Leica, Bannockburn, IL), at −20°C at the levels of prefrontal cortex, hippocampus, and dorsal raphe. Sections were thaw-mounted onto gelatin-coated microscope slides, desiccated and stored at −80°C until their use in binding assays. Sections were collected from 8 animals per strain for all autoradiography studies.
Serotonin Transporter (SERT)
For SERT binding, the method of Kovachich et al. (1988) was used. Sections on slides were incubated for 18 h in 4°C 50 mM Tris, 120 mM NaCl, 5 mM KCl buffer containing 1 nM [3H] cyanoimipramine (American Radiolabeled Chemicals, St. Louis, MO). Non-specific binding was defined by incubating adjacent sections in assay buffer containing 10 μM sertraline (Pfizer, Groton, CT). A post-incubation wash was carried out in at 4°C Tris-NaCl-KCl buffer for 1h. [3H] cyanoimipramine has high affinity and specificity for the SERT, and due to its slow dissociation under these conditions, it is a useful radioligand for high volume quantitative autoradiography experiments (Kovachich et al. 1988).
5-HT1A receptor
5-HT1A receptor binding was performed at room temperature in Tris-HCl buffer for 1 hour using 2 nM [3H] 8-OH-DPAT (GE Healthcare, Piscataway, NJ) as we have done previously (Rossi et al., 2008). 1 μM WAY100,635 (Tocris, Ellisville, MO) was added to the incubation solution to determine non-specific binding in adjacent sections on slides.
5-HT1A receptor agonist-stimulated GTPγS binding
For 5-HT1A receptor-stimulated [35S] GTPγS binding, the methods of Rossi et al. (2008) were used. Brain sections on slides were equilibrated in buffer containing dithiothreitol (2 mM), then pre-incubated in buffer containing 2 mM GDP, and finally incubated in buffer containing 40 pM [35S] GTPγS (Perkin-Elmer NEN, Boston, MA) in the absence (basal) or presence (agonist-stimulated) of a maximal (Emax) concentration of 8-OH-DPAT (1 μM). Nonspecific binding was defined under basal conditions with 10 μM GTPγS. All reagents were from Sigma, St. Louis, MO.
5-HT2A receptor
5-HT2A binding involved incubating sections for 30 min in Tris-HCl buffer containing 1 nM [3H] ketanserin (Perkin-Elmer NEN), with 100 nM prazosin, 100 nM pyrilamine, and 1 μM tetrabenazine to block non 5-HT2A binding as previously described (Valdez et al. 2002). 10 μM methysergide was used to determine non-specific binding. All reagents were from Sigma.
Quantitative Image Analysis
From SERT, 5-HT1A and 5-HT2A assays, [3H] labeled sections were opposed to Kodak Biomax MR film along with [3H] calibration standards (American Radiolabeled Chemicals), calibrated to brain mash, for 6 weeks. For 8-OH-DPAT-stimulated GTPγS binding, [35S] GTPγS labeled sections were opposed to Kodak Biomax MR film along with [14C] calibration standards (American Radiolabeled Chemicals) for 48 hours.
Autoradiograms were captured with a digital imaging system: Nikon lens, Kaiser copy stand, “Northern Lights” precision illuminator (all from InterFocus Imaging Ltd., Linton, England), camera and frame grabber card (Scion Corporation, Frederick, MD). Digital brain images were calibrated to units of fmol/mg protein for [3H] ligands (per Geary et al. 1985) or nCi/mg for [35S] ligands using [14C] standards, and measured using NIH Image, version 1.47 (NIH, Bethesda, MD) on a Macintosh with OS 9. Specific agonist-stimulated 5-HT1A [3H]GTPγS binding was expressed as percent above basal binding.
Saturation Radioligand Binding to Serotonin Transporters in Hippocampal Homogenates
Saturation binding of [3H] citalopram in membrane homogenate preparations from mouse hippocampi was performed following the methods of D’Amato et al. (1987), with minor modifications. Five independent, replicate experiments were performed to compare SERT saturation curves for each mouse strain. Fresh hippocampi were pooled from either two BTBR or two C57BL/6J mice (4 month old males) for each preparation. Hippocampal membrane homogenates were incubated at 26°C for 1 hour in buffer containing 0.1 – 12 nM of [3H] citalopram (PerkinElmer, Boston, MA). Non-specific binding was defined by 50 μM sertraline (Pfizer, Groton, CT). Incubation was terminated by addition of 4 ml of buffer, pH 7.4 at 4°C, and rapid filtration under vacuum onto Whatman GF/B filter paper strips (Brandel, Gaithersburg, MD) pre-soaked in 5% polyethyleneimine (Sigma). Filters were washed twice and radioactivity trapped on the filters was measured by liquid scintillation counting. Binding data were analyzed by non-linear regression using DeltaGraph software (Red Rock Software, Salt Lake City, UT). Unlike [3H] cyanoimipramine binding (Kovachich et al. 1988), [3H] citalopram binding requires neither an extended incubation at 4°C nor a 1 hour post incubation wash, and is therefore better suited for homogenate binding experiments (D’Amato et al. 1987).
Behavioral Tests Following Acute Treatment with Drugs Affecting the Serotonergic System
Social Interaction
Male BTBR mice, bred in the UTHSCSA-LAR facility, and C57BL/6J and C57BL/10J mice purchased from Jackson laboratory and housed for one week in the UTHSCSA-LAR facility were utilized at 3 months of age as subjects in social interaction, social sniff and social novelty behavior tests. BTBR mice do not exhibit any preference for a stranger mouse over a novel object in these sociability tests, which have been extensively validated in previous studies (Nadler et al. 2004; Ryan et al. 2010; Mc Farlane et al. 2008; Moy et al. 2004; 2007; 2009; Yang et al. 2007a,b; Silverman et al. 2010). “Stranger” mice, male mice of the same strain and age as the subjects but with different parents and no prior contact with the subject mice, were habituated to wire cup cages in arenas during three 30 min exposures conducted over two days prior to testing. Risperidone (0.1 mg/kg), buspirone (2 mg/kg) and fluoxetine (10 mg/kg), from Sigma Chemical Co. (St. Louis MO), were dissolved in saline with the aid of heat and/or sonication as necessary, and administered at room temperature to BTBR mice by intraperitoneal (i.p.) injection 30 min prior to commencing the test protocols. Drug doses were selected based on prior studies in mice to produce behavioral effects without sedation (Holmes et al. 2002; File and Seth 2003; Dulawa et al. 2004; Wang et al. 2007; Silverman et al. 2010). C57BL/6J and C57BL/10J mice were not injected prior to testing, but the testing procedure was otherwise the same.
Social interaction and social sniff tests were conducted between 0900 and 1600 h under dim red light (16 lux), since similar baseline results were obtained for BTBR mice irrespective of whether conducted in light vs. dark phase of housing light cycles (Yang et al. 2007b). We utilized 4 custom-made three-chambered rectangular plastic testing arenas, with dimensions and properties similar to arenas described in Moy et al. (2007), including slide-in doors and transparent interior walls. Before testing, subject mice were introduced into the central chamber of the empty arena first with doors to side compartments closed for 10 min, then with the doors opened so the subjects could explore the entire arena for another 10 min. Subjects were then confined in the central chamber, while either an empty wire cup-cage or cup cage containing a novel stranger mouse (stranger 1), which the subject had no prior contact with, were introduced into opposite ends of the arena. The doors were re-opened for the subject to explore the testing arena, novel cage and stranger for 10 min of testing.
Following the social interaction test, subjects were again confined to the central chamber, while a new stranger mouse (stranger 2) was placed under the empty cup cage for the social novelty test. The original stranger (stranger 1) remained under the same cup cage in the same end of the arena. The doors were opened for another 10 min testing session. Behavior in the testing arenas was filmed from above with digital camera (R742 Photosmart, Hewlett Packard, Palo Alto, CA) mounted on a tripod (Targus, Anaheim, CA). Chamber entries and social sniff time (sniffing of a stranger by the subject mouse) was monitored by observers unaware of treatments.
Marble burying
Marble burying behavior was assessed after social novelty, utilizing previously described general procedures for this test in mice (Matsushita et al. 2005; Briuns-Slot et al. 2008). At 70 min post-injection, subjects were introduced into a clean, sterilized large plastic rat housing cage filled with bedding to a depth of 5 cm and topped with 16 blue marbles evenly spaced apart in three rows of 5–6 marbles and topped with a filter lid for 30 min. The number of marbles buried by each mouse was tallied at the conclusion of the test. Buried is defined by having over two thirds of the total top surface of the marble covered by bedding.
Statistical analyses
For autoradiography, one-way MANOVA (when multiple brain regions were measured) or ANOVA (for single brain regions) statistical comparisons were performed. Wilks λ or F values reaching significance (p <0.05) were evaluated post hoc by Newman Keul’s test. For satuaration analysis, maximal binding (Bmax) and dissociation constant (KD) values were compared by one-way ANOVA, and Tukey’s HSD post hoc test. Comparison of the social interaction and social novelty behavior among drug treatment groups was performed using repeated measures ANOVA, with one way ANOVA and Fishers LSD post hoc analyses where significant main effects or interactions were observed. Social sniff time and marble burying were also compared among groups by ANOVA and Fisher’s LSD post hoc. Statistical analyses were performed using Statistica software (StatSoft, Tulsa, OK).
Results
Serotonin transporter binding comparison in BTBR and C57 mouse brains
Serotonin transporter (SERT) density was significantly reduced by 20–30% in BTBR mice relative to C57BL/10J mice in most brain regions measured (Wilks’ λ(10,5)=0.08, p=0.03, F(1,14) ≥8, Newman-Keul’s post hoc p<0.05, N=8). Representative autoradiograms illustrating [3H] cyanoimipramine binding in brain terminal fields from 4-month old male BTBR and C57BL/10J mice are shown in Figure 1. Specific SERT binding densities in several brain regions for BTBR and C57BL/10J mice are shown in Table 1.
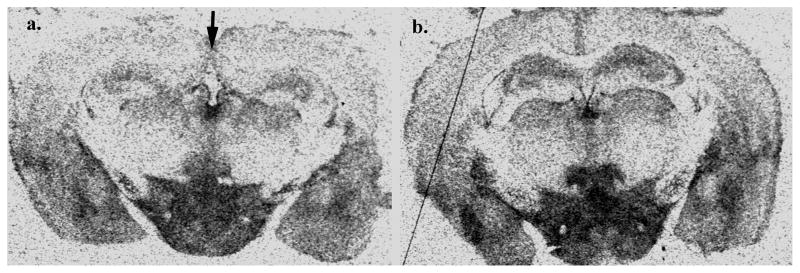
Figure 1. Representative autoradiograms of [3H] cyanoimipramine (1 nM) binding to serotonin transporters in (a.) BTBR and (b.) C57BL/10J adult male mouse brains.
Shown are autoradiograms of total binding. Note the missing corpus callosum and hippocampal commissure in BTBR brain (arrow), as described by Wahlsten et al. (2003).
Table 1. Binding of [3H] cyanoimipramine (1 nM) to serotonin transporters in brain regions of adult male BTBR and C57BL/10J mice.
Non-specific binding was defined in the presence of 10 μM sertraline, and was < 10% of total binding.
| Brain Region | BTBR | C57BL/10J |
|---|---|---|
| Specific Binding | fmol/mg pr. | fmol/mg pr. |
| Medial prefrontal cortex | 435 ± 22a | 515 ± 32 |
| Parietal cortex | 323 ± 11* | 402 ± 25 |
| Caudate putamen | 367 ± 23 | 402 ± 38 |
| Hippocampus: | ||
| CA1 | 359 ± 25* | 540 ± 28 |
| CA2 | 400 ± 29* | 528 ± 34 |
| CA3 | 429 ± 19* | 608 ± 36 |
| Dentate Gyrus | 472 ± 35* | 630 ± 36 |
| Basolateral nu. amygdala | 865 ± 16* | 1073 ± 35 |
| Ventromed. hypothal. nu. | 968 ± 39* | 1182 ± 68 |
| Dorsal raphe nu. | 1342 ± 47* | 1675 ± 60 |
Mean ± standard error, N=8.
Indicates significantly less than C57BL/10J (p<0.05).
SERT saturation binding to [3H] citalopram in BTBR hippocampal homogenates revealed maximal binding (Bmax) of 95.4 ± 13.2 fmol/mg protein and a dissociation constant (KD) of 2.0 ± 0.3 nM that differed significantly from the Bmax and KD of C57BL/6J hippocampi (171.2 ± 20 fmol/mg protein and 1.1 ± 0.2 nM) (F(1,8) ≥ 8.4, p< 0.025 for Bmax and KD), as figure 2 shows.
Figure 2. Specific binding of [3H] citalopram to SERT in hippocampal membrane homogenates from C57BL/6J and BTBR mice.
Membrane preparations were incubated with increasing concentrations of [3H] citalopram. Non-specific binding was defined in the presence of 50 μM sertraline. Specific binding was obtained by subtracting non-specific binding from total binding at each ligand concentration. Experiments were performed in triplicate, with hippocampal homogenates pooled from two mice per replicate, N=5 replicates.
Serotonin 5-HT1A receptor binding and agonist-stimulated G-protein coupling
The density of 5-HT1A receptors in brain, as measured by the binding of [3H] 8-OH-DPAT, did not differ significantly between BTBR and C57BL/10J strains in any of the ten regions wherein we examined SERT binding (λ(10,5)=0.2, p=0.24), and no trends were observed. For example, BTBR vs. C57BL/10 5-HT1A binding density in the dentate gyrus of hippocampus was 348±22 vs. 370±20 fmol/mg protein, in the ventromedial hypothalamus it was 123±9 vs. 117±6 fmol/mg protein, in the medial prefrontal cortex it was 149±9 vs. 160±12 fmol/mg protein, and in the dorsal raphe nucleus it was 392 ± 20 vs. 376 ± 30 fmol/mg protein.
However, 8-OH-DPAT-stimulated [35S] GTPγS binding in the CA1 region of hippocampus was significantly higher in BTBR than C57BL/10J mice (F(1,14)=5.8, Newman-Keul’s post hoc p<0.05, N=8). CA1 basal binding for BTBR was 149 ± 9 vs. 151 ± 10 tissue equivalent values (nCi/g) in C57BL/10J mice, and 8-OH-DPAT-stimulated [35S] GTPγS binding in this region was 28% higher in BTBR mice than in C57BL/10J mice. No other significant differences were observed in 8-OH-DPAT-stimulated [35S] GTPγS binding in any of the ten other brain regions measured (λ(11,4)=0.2, p=0.45), but there was a non-significant trend for 8-OH-DPAT-stimulated binding to be higher in other regions of the dorsal hippocampus. In the dorsal raphe nucleus, there was no difference in basal binding (394 ± 29 vs. 348 ± 31 nCi/g) or 8-OH-DPAT-stimulated [35S] GTPγS binding (31 ± 7 % vs. 28 ± 6 % above basal) among BTBR or C57BL/10J mice, respectively. Figure 3 illustrates the relationship between 5-HT1A receptor density and 8-OH-DPAT stimulated G-protein coupling in the CA1 region of the hippocampus.
Figure 3. 5-HT1A receptor binding and function in CA1 region of hippocampus of adult BTBR and C57BL/10J mice.
a. The specific binding of [3H] 8-OH-DPAT (2 nM) to 5-HT1A receptors. Nonspecific binding was defined in the presence of 1 μM WAY100635. b. [35S]GTPγS binding stimulated by the agonist 8-OH-DPAT (1 μM). Nonspecific binding was defined in the presence of 10 μM GTPγS. Specific binding is expressed as % above basal. Bars represent mean and lines standard error of the mean, N=8. *Indicates significantly higher 8-OH-DPAT-induced stimulation than in C57BL/10J.
Serotonin 5-HT2A receptor binding
There was no significant difference observed in [3H] ketanserin binding to 5-HT2A sites between BTBR and C57BL/10J adult mice in any region measured (F(1,14)<0.8, p>0.39, N=8). Representative 5-HT2A receptor densities for BTBR and C57BL/10J mice were 112±24 vs. 96±15 fmol/mg protein in the CA1 region of the hippocampus, 74±17 vs. 92±10 fmol/mg protein in the ventromedial hypothalamus, and 345±32 vs. 372±38 fmol/mg protein in layer IV of the frontal-parietal cortex.
Behavioral test outcomes following acute drug administration to BTBR mice
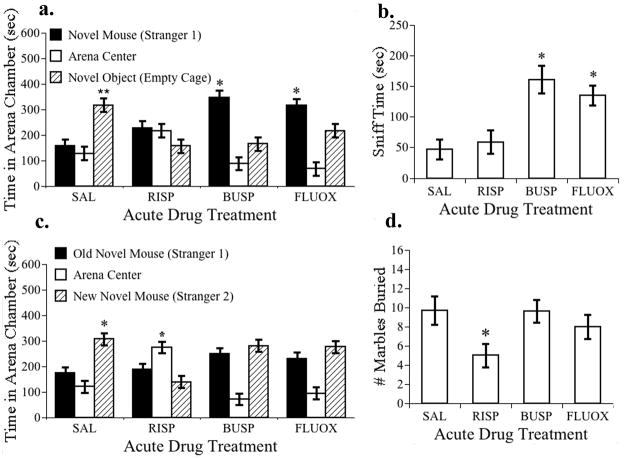
In the social interaction test, there was a significant drug treatment × social preference interaction effect in the repeated measures ANOVA (F(3,28)=3.5, p=0.03). Saline-treated BTBR controls exhibited no preference for social interaction; they spent significantly less time in the box with the stranger mouse and more time in the arena box containing the empty cage than either risperidone or buspirone-treated mice (F(3,28)=3.13, p<0.05, indicated by ** in Fig 4 panel a). While risperidone treatment reduced dwelling in the novel cage box as compared to saline treated controls, it failed to improve the lack of preference for sociability in BTBR mice since they instead spent more time in the center box. Buspirone and fluoxetine treatments significantly increased preference for sociability, defined as spending proportionally more time in the arena box containing the novel stranger (F(3,28)=3.24, p<0.05 signified by * in Fig. 4 panel a). Both buspirone and fluoxetine treatments significantly increased the amount of time spent sniffing the stranger mouse over saline treated control values (F(3,28)=9, p<0.01, Fig. 4 panel b), while risperidone treatment failed to do so. The number of box entries did not differ for any drug treatment group (F(3,28)=0.9, p=0.42), the mean was 37 ± 4 entries for all groups.
Figure 4. Effect of acute drug treatments on the behavior of BTBR mice in tasks relevant to core behavioral symptoms of ASD.
The effects of acute saline (SAL), risperidone (RISP, 0.1 mg/kg), buspirone (BUSP, 2 mg/kg) or fluoxetine (FLUOX, 10 mg/kg) administration on BTBR mouse behavior. N=8 per treatment. (a) Buspirone and fluoxetine significantly increased sociability in BTBR mice. Time spent by subjects in the arena box containing the stranger mouse was significantly greater than the time spent in the box containing a novel empty cage (*, p < 0.05). Saline-treated BTBR mice exhibited no preference for sociability and spent significantly more time in the box with the novel object and less time in the box with the stranger than either buspirone or fluoxetine treated mice (**, p< 0.05). (b) Buspirone and fluoxetine treatments increased the time spent by BTBR mice sniffing the stranger mouse in the social interaction test relative to saline-treated controls (*, p<0.05). (c) In the test for social novelty, only the saline-treated mice spent proportionally more time in the box containing the new mouse (stranger 2) relative to the box containing the old novel mouse (stranger 1) (*, p<0.05). (d) Marble burying by BTBR mice was reduced by risperidone treatment (*, p<0.05).
In the social novelty test, there was a significant drug treatment effect in the repeated measures ANOVA (F(3,28)=3.2, p=0.04). BTBR mice in all drug treatment groups exhibited a trend toward reduced preference for social novelty as compared to the saline control group (F(3,28)=2.4, p=0.08). This is because they spent proportionately less time in the box containing the new mouse (stranger 2) relative to the time they spent in the box containing the original stranger mouse (stranger 1) (p<0.05, * in Fig. 4 panel c). While time spent in the arena center is not typically included in sociability data analysis (Moy et al., 2007), we observed that risperidone treated mice spent significantly more time in the arena center than the other treatment groups (F3,28)= 3.18, p=0.039, Fisher’s LSD p<0.05). These mice were not sedated by risperidone (0.1 mg/kg) treatment, all of them were active and explored the central arena, engaged in self-grooming, sniffing, head movements, and entered the two side chambers. They just spent more of the test time occupying the central arena.
In the marble burying test there was no significant difference in number of marbles buried among BTBR mice in all drug treatment groups (F(3,28)=1.8, p=0.17), when all mice were included. However, one of the eight saline-treated mice was an outlier that did not bury any marbles. When this control was dropped, the risperidone-treated mice buried significantly fewer marbles than the saline-treated controls (F(3,27)=2.9, p<0.05, Fig. 4 panel d).
Outcomes of behavioral tests comparing C57BL/6J and C57BL/10J mice
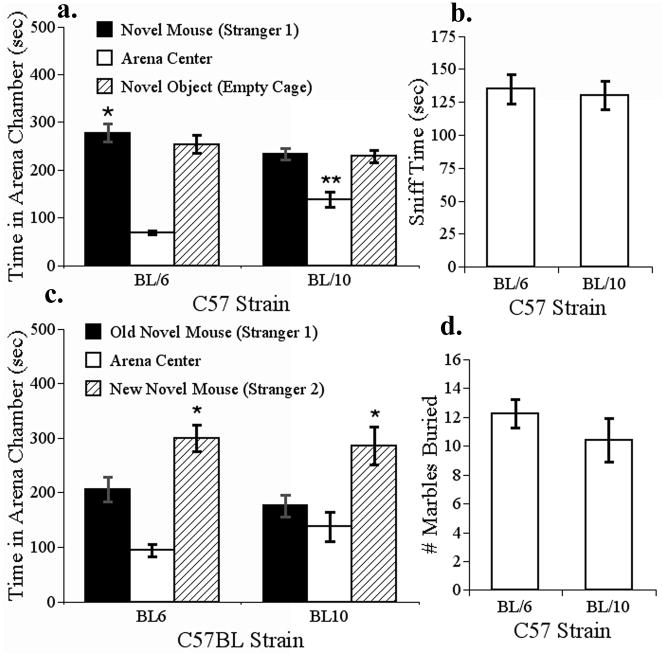
In the social interaction test, there was a significant main effect of strain (F(1,14)=16.7, p=0.001), but not of the repeated factor (no proportional preference for social novelty for either strain) or the interaction in the repeated-measures ANOVA (F(1,14) ≤0.43, p>0.5). C57BL/10J mice spent significantly less time than C57BL/6J mice in the box containing the stranger mouse (F(1,14)=4.3, p=0.05). Interestingly, the C57BL/10J mice also spent significantly more time in the middle of the testing arena than the C57BL/6J mice (F(1,14)=16.7, p=0.001), and made fewer box entries (47 ± 4 vs. 68 ± 4 entries, F(1,14)=16, p=0.001) than C57BL/6J mice (Fig. 5 panel a). There was no difference among strains in the amount of time spent sniffing the stranger C57BL/6J mice in the social interaction test (F(1,14)=0.097, p=0.75, Fig. 5 panel b), but as compared to saline treated BTBR mice (figure 4 panel b) both C57 strains spent far more time sniffing the stranger C57BL/6 mice in this test. In the preference for social novelty test, there was no effect of strain or interaction (F(1,14)≤ 2.3, p> 0.15), but there was a significant repeated measures factor, which indicates that both mice spent more time in the box containing the new stranger (stranger 2) vs. the old stranger that remained in the arena from the social interaction test (stranger 1) (F(1,14)=9.1, p=0.01). There was no significant difference between the C57 strains in the amount of time spent in each of the three boxes of the testing arena in the social novelty test (Fig. 5 panel c). There was no difference among the C57 strains in the number of marbles buried (F(1,14)=0.9, p>0.3), as shown in Fig. 5 panel d.
Figure 5. C57 strain comparison for behavior in tasks relevant to core ASD symptoms.
The social interaction, social novelty and marble burying behavior of 8 week old C57BL/10J and C57BL/6J mice were compared, N=8 per strain. (a) C57BL/6J mice exhibited a significant preference for social novelty that was not shared by C57BL/10J mice (*, p< 0.001). This difference was largely due to an increase in time spent in the middle chamber of the testing arena, which was significantly greater in C57BL/10J mice (**, p< 0.05). (b) Time spent sniffing a novel stranger mouse (C57BL/6J) did not differ among C57 subject strains. (c) In the test for social novelty, both C57 strains exhibited a significant preference for social novelty, since subject mice mice spent proportionally more time in the box containing the new mouse (stranger 2) relative to the box containing the old novel mouse (stranger 1) (*, p<0.01). (d) C57BL/6J and C57BL/10J mice buried a similar number of blue marbles with wood-chip bedding over 30 min in this test of compulsive behavior.
Discussion
BTBR mice exhibit sociability deficits and engage in repetitive behaviors that are analogous to core behavioral symptoms of autism (Bolivar et al. 2007; McFarlane et al. 2008), and have been used in other studies aiming to characterize the effects of potential or extant therapeutic interventions on social behavior and other relevant behaviors (Silverman et al. 2010; Chadman 2010). The present study compared serotonin transporter (SERT) and 5-HT2A and 5-HT1A receptor binding properties in BTBR and C57 mice, and the effects of single dose acute drug treatments targeting those sites on BTBR performance in tests of sociability and compulsive behavior. A major finding was that differences in SERT expression and 5-HT1A function occur among BTBR and C57 strains. These might contribute to the social behavior impairments of BTBR mice via differential regulation of 5-HT neurotransmission.
Binding properties of the BTBR SERT and behavioral effects of its blockade
Various genetic polymorphisms affecting SERT structure and function have been linked to or are associated with autism susceptibility (Sutcliffe et al. 2005; Brune et al. 2006; Raznahan et al. 2010; Veenstra-Vanderweele et al. 2009). Assuming that one or more SERT polymorphisms might also occur in BTBR mice, we measured SERT density in various regions of the brain by quantitative autoradiography using [3H] cyanoimipramine, and in hippocampal membrane homogenates with [3H] citalopram, and found it to be about 20–40% lower throughout the brain than the SERT of C57 mice. The observed relative reduction in SERT binding in BTBR mice may be due in part to differential affinity of SERTs among these strains for the SSRI citalopram. The affinity of the BTBR SERT for [3H] citalopram (KD = 2 ± 0.3 nM) was roughly half as strong as that of the C57BL/6J mouse SERT (KD = 1.1 + 0.2 nM), since drug-ligand affinities are inversely proportional to their dissociation constant (KD) values. While differential affinity may account for some share of the lower SERT density found in BTBR mice, our findings also suggest that BTBR SERT density is also lower than the SERT density in C57 mouse brain. This is because at radioligand concentrations producing maximal SERT binding in both strains, the lower density of BTBR SERT expression relative to C57 mice remains. Our [3H] citalopram homogenate saturation binding data also show that hippocampal SERT Bmax in BTBR is roughly 40% lower than in C57BL/6J mice. Since lower SERT density in BTBR vs. C57 brains occurred with use of two different SERT-specific radioligands and binding techniques, we believe that it is not an artifact. Western blot or mRNA analysis might be used to compare SERT expression between these strains in future studies.
Differences in hormonal regulation of SERT expression could contribute to the lower SERT binding found in BTBR mice relative to C57 strains. BTBR mice have elevated baseline serum corticosterone levels, and stress induces an exaggerated increase in the level of this steroid hormone above baseline as compared to C57BL/6 mice (Benno et al. 2009). Elevated glucocorticoids have been shown to produce age and duration dependent effects on SERT expression, since acute exposure to dexamethasone in neonatal rats increased SERT expression, but sub-chronic exposure 20 month old rats decreased SERT expression as measured by [3H] paroxetine (McGrath et al. 1997; Slotkin et al. 1997). In 3-month old adrenalectomized rats, high subdermal levels of corticosterone administered sub-chronically also reduced the density of SERT and 5-HT1A binding sites (Maines et al. 1999). However, sub-chronic oral administration of corticosterone failed to reduce hippocampal [3H] 5-HT uptake at 10 nM in rats, or alter the potency of citalopram to block it (Fernandez et al. 2001). Likewise, the modest difference in SERT expression alone is unlikely to account for the social behavior impairments of BTBR mice, which were also improved by acute exposure to the SSRI fluoxetine. In addition to higher corticosterone, plasma progesterone and its metabolite 5α-pregnan-3α-ol-20-one are elevated in BTBR serum and lower in the cerebellum as compared to C57BL/6 mice (Frye and Llaneza, 2010). Hence the modest relative reductions in BTBR SERT density may result from inherently high corticosterone levels, along with other hormones that may regulate SERT.
Our findings of lower BTBR SERT expression and SSRI affinity can also be related to a pair of concurrent SNP haplotypes for the SERT gene (Glu39→Gly plus Arg152→Lys, called ER (native form) and GK (mutant)) that differ among C57 and other strains including BTBR and 129S, wherein GK impairs C57 SERT capacity to take up 5-HT (Carneiro et al. 2009). In that study, there was a non-significant trend toward C57BL/10 mice (GK) having slightly higher midbrain [3H] paroxetine binding than ER strains. Prefrontal cortex and striatal tissue 5-HT content in BTBR and C57BL/6 mice is not significantly different (Onaivi et al. 2010). Hence we postulated that SERT expression might be elevated in C57 mice to accommodate its impaired functionality relative to the more efficient ER SERT of BTBR mice. Studies are underway to compare BTBR and C57 SERT capacity for 5-HT uptake in vivo and in vitro.
Despite potentially reduced SERT density and its lower affinity for SSRIs, SERT blockade appears to improve social behavior in BTBR mice at 10 mg/kg, as seen in the present study and in Chadman (2010). In tail suspension tests BTBR mice were also more responsive to SSRI treatments than C57BL/6 mice (Crowley et al. 2006). Use of SSRIs has been modestly effective for treatment of repetitive and compulsive behaviors in some autism patients, but they do not generally improve sociability, and are ineffective in patients with impaired SERT function (Kirsch et al. 2008; Henry et al. 2009; West et al. 2009). SERT knock out (−/−) mice, like BTBR, 129S and a few other strains, also exhibit impaired social interaction behavior relative to SERT wild-type (+/+) mice (Moy et al. 2009). However, most human carriers of common polymorphisms impairing SERT function are heterozygous, so SERT heterozygous (+/−) mice are more realistic models of human SERT abnormalities, yet SERT +/− mice are relatively social, with similar baseline behavior to SERT +/+ mice (Moy et al. 2009). However stressed SERT +/− mice, and adult SERT +/+ and SERT +/− pups of SERT +/− dams stressed during pregnancy exhibit significant reductions in subsequent sociability tests (Bartolomucci et al. 2010; Jones et al. 2010). It would be of interest to see if stressed SERT +/− mouse social behavior would also be improved by fluoxetine. Such an outcome seems unlikely, given that human carriers of SERT polymorphisms tend also to be nonresponsive to SSRI treatments (Henry et al. 2009). Given the 40–60% prevalence of common SERT polymorphisms in human populations (Lesch et al. 1996; Gelernter et al. 1997; Noshkova et al. 2008), the increased susceptibility of 5-HTTLPR short allele carriers toward depression following stressful life events (Caspi et al. 2003), and their association with autism susceptibility (Sutcliffe et al. 2005; Brune et al. 2006; Raznahan et al. 2010; Veenstra-Vanderweele et al. 2009), clearly alternative drug targets to the SERT are needed for this sizable subpopulation of psychiatric patients.
The BTBR 5-HT2A receptor, ligand binding and antagonist effects on behavior
The 5-HT2 receptor has also been identified as a potential candidate gene for autism in some populations (Veenstra-VanderWeele et al. 2002; Cho et al. 2007). The density of this receptor did not differ among BTBR and C57BL/10J brains among the brain regions measured in this study. Risperidone, a D2/5-HT2 antagonist which is commonly used to control aggression and self-injury in ASD, is not particularly effective at improving sociability in autistic patients (West et al. 2009). The present data are in agreement with this, as evidenced by failure of risperidone at 0.1 mg/kg to increase time spent by BTBR mice in the proximity of a stranger mouse in the present study. It is notable that Silverman et al. (2010) and Chadman (2010) also found that risperidone failed to improve social interaction at all doses tested, and both labs found that this drug suppressed locomotor activity in BTBR mice at higher doses.
Risperidone treatment (0.1 mg/kg) significantly reduced marble burying, while neither 2 mg/kg buspirone or 10 mg/kg fluoxetine treatments altered this parameter in BTBR mice. Mouse marble burying behavior is thought to be indicative of drug efficacy for management of obsessive compulsive behavior, and is reduced by 5-HT2A antagonists such as risperidone and haloperidol, and by 5-HT1A full agonists such as 8-OH-DPAT (Matsushita et al. 2005; Bruins-Slot et al. 2008; Thomas et al. 2009). Functional interactions between these 5-HT receptor subtypes may modulate stereotyped behaviors, as evidenced by the observation that increased 5-HT availability potentiates 5-HT2A receptor-mediated head twitch through presynaptic 5-HT1A autoreceptor blockade (Fox et al. 2010). While we found no difference in 5-HT2A receptor expression among strains, we cannot rule out the possibility that functional 5-HT2A receptor alterations may influence this behavior.
G-protein coupling to the BTBR 5-HT1A receptor, and behavioral effects of buspirone
Consistent with observations in brain tissue from young adults with ASDs (Blatt et al. 2001), we found no difference in serotonin 5-HT1A receptor density between BTBR and C57BL/10J mice. However, our finding of enhanced agonist-stimulated 5-HT1A [35S] GTPγS binding to G-proteins in CA1 of hippocampus, but not in the dorsal raphe of BTBR mice indicates a heightened potential for postsynaptic responsiveness, with no apparent effect on autoreceptor functionality. Given that BTBR mice have elevated baseline corticosterone levels, we anticipated reduced G-protein coupling capacity in the dorsal raphe, since chronic administration of corticosterone reduced 5-HT1A agonist stimulated [35S] GTPγS binding in dorsal raphe of adult wild-type littermates of brain derived neurotrophic factor (BDNF) knockout mice (Hensler et al. 2007). However, in that same study, corticosterone-treated BDNF knock-out mice did not exhibit a reduction in agonist-stimulated [35S] GTPγS binding in the raphe. Hence the effects of corticosterone on 5-HT1A receptor functional capacity may depend on levels of BDNF expression, and timing and duration of exposure, among other factors.
There is still little known about factors mediating a relationship between 5-HT1A receptors in the CA1 of hippocampus and social interaction behavior, particularly in the 3 chambered mouse social interaction tests, in which anxiety state must play some role (Crawley 2007). The dorsal hippocampus appears to be involved in this behavior, since agonism of benzodiazepine, 5-HT1A or 5-HT2C receptors in this region affects anxiety and alters social interaction in open arena rat social interaction tests (File and Seth 2003). We found that the atypical anxiolytic buspirone, at a dose of 2 mg/kg, significantly improved sociability in BTBR mice. Buspirone acts as a partial agonist at 5-HT1A receptors, and its actions at 2 mg/kg are likely to be mediated through partial blockade of postsynaptic receptors and full agonist activity at presynaptic 5-HT1A autoreceptors (Yocca 1990; File and Seth 2003). While buspirone has not been extensively studied as a treatment for autism symptoms, it reduced hyperactivity and stereotyped behaviors in 3 autistic children and reduced aggression in one autistic woman with no adverse effects (Realmuto et al. 1989; Brahm et al. 2008). Perhaps buspirone should be tested further for its therapeutic potential to improve social interaction behavior in autism.
Sociability behavioral test components and C57 mice as normative controls
In the present study BTBR social interaction behavior was improved by fluoxetine and buspirone treatments, but not by risperidone. However all drugs administered in this study reduced BTBR’s preference for social novelty in the test, which immediately followed the social interaction test. Whether this outcome is indicative of a property conveying therapeutic benefit remains unclear. Preference of C57BL/6J mice for social novelty is abolished if locations of the old and new strangers are switched (Pearson et al. 2010). We did not explore alternative stranger locations in our BTBR social novelty tests, the original strangers were in the same location from the prior social interaction test, as is standard procedure per Moy et al. (2004; 2007; 2009).
Because we observed little difference among C57BL/6J and C57BL/10J mice in social interaction in a visual burrow system (Benno et al. 2008), we expected their behavior in the three-chambered social interaction test to be similar. For some components of the test this was true, for example we found that the time engaged in social sniff of stranger mice did not differ among the C57 strains in the social interaction test, and their behavior in the social novelty test was similar. However, we found that C57BL/10J mice tended to spend more time in the middle chamber of the arena, and made significantly fewer chamber entries than the C57BL/6J mice, because they were generally less ambulatory in the social interaction test. In this respect, C57BL/10J mice (47 ± 4 box entries) and BTBR mice (37 ± 4 box entries) are both slower and less exploratory than C57BL/6J mice (68 ± 4 box entries) in the social interaction arena. Based on this parameter, C57BL/10J mice might be considered better controls for sociability than BTBR mice. However, we also found that C57BL/10J failed to exhibit significant preference for sociability (chambers with novel mice over empty cages) in social interaction tests, whereas C57BL/6 mice did. However, the significant “preference for sociability” displayed by the C57BL/6 mice in our hands was modest, although it was dramatically different from the apparent preference for the empty cage chamber exhibited by our saline treated BTBR mice. It has been suggested that other strains, such as the FVB strain may be better standard controls for sociability than C57BL/6J mice (Moy et al. 2007; Bolivar et al. 2007).
Other possible influential factors for stereotypical BTBR behavior
BTBR mice lack a corpus callosum (Wahlsten et al. 2003), and comparisons of the impact of cross hemispheric connectivity (or lack thereof) on mouse social behavior in different strains has yielded inconsistent results (Fairless et al. 2008; Yang et al. 2009). These abnormalities in forebrain development in mice are controlled by genes in two areas on the X chromosome and occur in all BTBR mice (Kusek et al. 2006; MacPherson et al. 2008). C57BL/10J mice perform differently in cognition, nest construction and motor function tests as compared C57BL/6J mice, and they exhibit a range of disruptions in corpus callosum structure, albeit none as severe as those observed in BTBR mice (Deacon et al. 2007; Whalsten et al. 2003). In the BTBR mice activity-dependent reversal of long term potentiation occurs rapidly, and contextual fear memory is impaired, but object recognition remains normal or is enhanced as compared to C57BL/6 mice (MacPherson et al. 2008). Studies of people congenitally lacking a corpus callosum commonly report social immaturity, social incompetence, literal-mindedness and limited empathy (Paul et al. 2004; Symington et al. 2010). It is not clear how this level of sophistication in communication can be assessed in the context of the three chambered social interaction test for mice. However, the absent corpus callosum and reduced hippocampal commisure may contribute to the typical performance of BTBR mice in sociability tests.
In the present study we have shown that BTBR mice exhibit neurochemical and behavioral properties indicative of altered serotonergic neurotransmission that might contribute to their impaired social behavior. Of course, other factors we have not explored herein may also be involved. For example, excess or mistimed serotonin exposure during brain development may contribute to or interact with previously described neuroanatomical abnormalities in the BTBR brain to contribute to its behavioral deficiencies (Whitaker-Azmitia 2005; Borue et al. 2007; Boylan et al. 2007; Pascucci et al. 2008, Whalsten et al. 2003; MacPherson et al. 2008). BTBR mice and all 129S-dervied strains have a 25-bp deletion in the Disrupted In Schizophrenia 1 (Disc1) gene with potential effects on neurogenesis and dendritic spine growth in the hippocampus and other limbic areas relevant to social behavior (Clapcote and Roder 2006; 2007; Jackson 2008; Duan et al, 2007; Chubb et al. 2008; Ahyan et al. 2010). Hence some of the reduced SERT density we observe in BTBR vs. C57 mice may be due to a different organization of the serotonergic neuron outgrowths or network in the brain.
Other genetic factors and/or interactions could also alter the protein expression and/or ligand-binding properties of other monoamine transporters or receptors, key metabolic enzymes or peptide hormones (e.g. Mortensen et al. 2001; Murphy et al. 2003; Wang and Lewis 2009). Indeed, additional functional coding SNPs that differ among BTBR and C57BL/6 mice have been found, including one altering the enzyme kynurine 3-hydroxylase, two affecting flavin adenine nucleotide (FAD) binding, and one affecting the mitochondrial transmembrane region (McFarlane et al. 2008). Since C57 and BTBR mice are products of over 70 years of differential recombination and inbreeding, other mutations or combinations of fixed alleles affecting this locus or its gene expression could have accumulated, as such lines are likely to differ by several hundred SNPs (Witmer et al. 2003; Petkov et al. 2004). Some, but clearly not all, of these factors may warrant further attention.
In summary, BTBR mice are among several possible translational research tools with potential utility for examining the neuropathology of disorders wherein sociability impairment is prevalent, and in which novel therapeutic targets for the improved treatment of such disorders can be tested. Taken together our findings suggest that altered serotonin system function is evident in BTBR mice and may contribute to its unusual behavioral repertoire.
Acknowledgments
This research was supported by a research grant from the San Antonio Area Foundation (GGG), a NIOSH T42-OH008421-05 Pilot Project sub award from SWCOEH at UT Houston (GGG), NIH MH64489 (LCD), MH52369 (JGH), MH071488 (JGH, LCD), and funding from Dean Sandra DeYoung, College of Science and Health, William Paterson University (RHB, ESO). We thank Norman Schanz (William Paterson University) and Steven Alvarado (University of Texas Health Science Center at San Antonio (UTHSCSA)) for their outstanding assistance with the mouse colonies. We are grateful to Alan Frazer and David Morilak in the Dept. of Pharmacology, UTHSCSA for provision of ligand and permitting our use of their lab equipment, and Stephen T. Schultz, Commander Naval Medical Research Unit, San Antonio TX for his critical review of this manuscript.
Abbreviations used
- 5-HT
serotonin
- SERT
serotonin transporter
- ASDs
autism spectrum disorders
Footnotes
The authors have no conflicts of interest.
References
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.144. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann N Y Acad Sci. 1990;600:331–40. doi: 10.1111/j.1749-6632.1990.tb16893.x. [DOI] [PubMed] [Google Scholar]
- Bartlett CW, Gharani N, Millonig JH, Brzustowicz LM. Three autism candidate genes: a synthesis of human genetic analysis with other disciplines. Int J Dev Neurosci. 2005;23:221–34. doi: 10.1016/j.ijdevneu.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Carola V, Pascucci T, Puglisi-Allegra S, Cabib S, Lesch KP, Parmigiani S, Palanza P, Gross C. Increased vulnerability to psychosocial stress in heterozygous serotonin transporter knockout mice. Dis Model Mech. 2010;3:459–70. doi: 10.1242/dmm.004614. [DOI] [PubMed] [Google Scholar]
- Benno RH, Liggett A, Sagato F, Schanz N. The potential role of stress as a mechanism in the production of autism spectrum disorders in the BTBR T+tf/J mouse. Soc Neurosci Abstr. 2008:446.5. [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–5. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31:537–43. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borue X, Chen J, Condron BG. Developmental effects of SSRIs: lessons learned from animal studies. Int J Dev Neurosci. 2007;25:341–7. doi: 10.1016/j.ijdevneu.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan CB, Blue ME, Hohmann CF. Modeling early cortical serotonergic deficits in autism. Behav Brain Res. 2007;176:94–108. doi: 10.1016/j.bbr.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahm NC, Fast GA, Brown RC. Buspirone for autistic disorder in a woman with an intellectual disability. Ann Pharmacother. 2008;42:131–7. doi: 10.1345/aph.1K427. [DOI] [PubMed] [Google Scholar]
- Bruins-Slot LA, Bardin L, Auclair AL, Depoortere R, Newman-Tancredi A. Effects of antipsychotics and reference monoaminergic ligands on marble burying behavior in mice. Behav Pharmacol. 2008;19:145–52. doi: 10.1097/FBP.0b013e3282f62cb2. [DOI] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH. 5-HTTLPR Genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163:2148–56. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, Erikson KM, Blakely RD. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci. 2009;106:2047–52. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman K. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.09.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chandana SR, Behen ME, Juhász C, Muzik O, Rothermel RD, Mangner TJ, Chakraborty PK, Chugani HT, Chugani DC. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–82. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cho IH, Yoo HJ, Park M, Lee YS, Kim SA. Family-based association study of 5-HTTLPR and the 5-HT2A receptor gene polymorphisms with autism spectrum disorder in Korean trios. Brain Res. 2007;1139:34–41. doi: 10.1016/j.brainres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Chugani DC. Role of altered brain serotonin mechanisms in autism. Mol Psych. 2002;7:S16–S17. doi: 10.1038/sj.mp.4001167. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Roder JC. Deletion polymorphism of Disc1 is common to all 129 mouse substrains: implications for gene-targeting studies of brain function. Genetics. 2006;173:2407–10. doi: 10.1534/genetics.106.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Roder JC. Inbred mouse strains 101/RI, BTBR T tf/J and LP/J have a deletion in Disc1. MGI Direct Data Submission. 2007 Ref ID J:118317. [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–59. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology. 2006;183:257–64. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Largent BL, Snowman AM, Snyder SH. Selective labeling of serotonin uptake sites in rat brain by [3H] citalopram contrasted to labeling of multiple sites by [3H] imipramine. J Pharmacol Exp Ther. 1987;242:364–71. [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, Thomas CL, Rawlins JN, Morley BJ. A comparison of the behavior of C57BL/6 and C57BL/10 mice. Behav Brain Res. 2007;179:239–47. doi: 10.1016/j.bbr.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Devlin B, Cook E, Coon H, Dawson G, Grigorenko E, McMahon W, Minshew N, Pauls D, Smith M, Spence M, Rodier P, Stodgell C, Network CG, Schellenberg G. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10:1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Dolzan V, Serretti A, Mandelli L, Koprivsek J, Kastelic M, Plesnicar BK. Acute antipyschotic efficacy and side effects in schizophrenia: association with serotonin transporter promoter genotypes. Prog Neuropsychopharmacol Biol Psych. 2008;32:1562–6. doi: 10.1016/j.pnpbp.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–30. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–7. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Coomans V, Mormede P, Chaouloff F. Effects of corticosterone ingestion on hippocampal [(3)H]serotonin reuptake in inbred rat strains. Endocr Regul. 2001;35:119–26. [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fox MA, Stein AR, French HT, Murphy DL. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT) Br J Pharmacol. 2010;159:879–87. doi: 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–7. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary WA, Toga AW, Wooten GF. Quantitative film autoradiography for tritium: methodological considerations. Brain Res. 1985;337:99–108. doi: 10.1016/0006-8993(85)91613-0. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) alleleand haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Henry CA, Shervin D, Neumeyer A, Steingard R, Spybrook J, Choueiri R, Bauman M. Retrial of selective serotonin reuptake inhibitors in children with pervasive developmental disorders: a retrospective chart review. J Child Adolesc Psychopharmacol. 2009;19:111–7. doi: 10.1089/cap.2008.037. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Advani T, Monteggia LM. Regulation of serotonin-1A receptor function in inducible brain-derived neurotrophic factor knockout mice after administration of corticosterone. Biol Psychiatry. 2007;62:521–9. doi: 10.1016/j.biopsych.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharm. 2002;27:914–23. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Jackson Laboratory. JAX Mice Database Data Sheet 002282 BTBR T+ tf/J. 2008 http://jaxmice.jax.org/strain/002282.html.
- Jansen F, Heiming RS, Lewejohann L, Touma C, Palme R, Schmitt A, Lesch KP, Sachser N. Modulation of behavioural profile and stress response by 5-HTT genotype and social experience in adulthood. Behav Brain Res. 2010 Feb 11;207(1):21–9. doi: 10.1016/j.bbr.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith RM, Edwards KS, Givens B, Tilley MR, Beversdorf DQ. Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction in mice. Int J Dev Neurosci. 2010;28:529–36. doi: 10.1016/j.ijdevneu.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick DJ, Frazer A. Quantitative autoradiography of serotonin uptake sites in rat brain using [3H] cyanoimipramine. Brain Res. 1988;454:78–88. doi: 10.1016/0006-8993(88)90805-0. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusek GK, Wahlsten D, Herron BJ, Bolivar VJ, Flaherty L. Localization of two new X-linked quantitative trait loci controlling corpus callosum size in the mouse. Genes Brain Behav. 2007;6:359–63. doi: 10.1111/j.1601-183X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil. 2006;27:254–89. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem. 2003;84:1256–1265. doi: 10.1046/j.1471-4159.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- Maines LW, Keck BJ, Smith JE, Lakoski JM. Corticosterone regulation of serotonin transporter and 5-HT1A receptor expression in the aging brain. Synapse. 1999;32:58–66. doi: 10.1002/(SICI)1098-2396(199904)32:1<58::AID-SYN8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–7. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Res. 2008;1210:179–88. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharm. 2003;28:402–12. doi: 10.1038/sj.npp.1300057. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Egashira N, Harada S, Okuno R, Mishima K, Iwasaki K, Nishimura R, Fujiwara M. Perospirone, a novel antipsychotic drug, inhibits marble-burying behavior via 5-HT1A receptor in mice: implications for obsessive-compulsive disorder. J Pharmacol Sci. 2005;99:154–9. doi: 10.1254/jphs.fp0050144. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McGrath KE, Seidler FJ, Slotkin TA. Convergent control of serotonin transporter expression by glucocorticoids and cocaine in fetal and neonatal rat brain. Brain Res Dev Brain Res. 1997;104:209–13. doi: 10.1016/s0165-3806(97)00144-2. [DOI] [PubMed] [Google Scholar]
- Montañez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2002;86:210–9. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Kristensen AS, Wiborg O. Species-scanning mutagenesis of the serotonin transporter reveals residues essential in selective, high-affinity recognition of antidepressants. J Neurochem. 2001;79:237–47. doi: 10.1046/j.1471-4159.2001.00587.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorders: the challenge for behavioral genetics. Am J Med Genet. 2006;142C:40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–42. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Uhl GR, Holmes A, Ren-Patterson R, Hall FS, Sora I, Detera-Wadleigh S, Lesch KP. Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders. Genes Brain Behav. 2003;2:350–64. doi: 10.1046/j.1601-1848.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–36. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Matsuzaki H, Suda S, Sugiyama T, Takei N, Mori N. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Noskova T, Pivac N, Nedic G, Kazantseva A, Gaysina D, Faskhutdinova G, Gareeva A, Khalilova Z, Khusnutdinova E, Kovacic DK, Kovacic Z, Jokic M, Seler DM. Ethnic differences in the serotonin transporter polymorphism (5-HTTLPR) in several European populations. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1735–9. doi: 10.1016/j.pnpbp.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Benno R, Halpern T, Mehanovic M, Schanz N, Sanders C, Yan X, Ishiguro H, Liu QR, Berzal AL, Viveros MP, Ali SF. Consequences of Cannabinoid and Monoaminergic System Disruption in a Mouse Model of Autism Spectrum Disorders. Current Neuropharmacology. 2010 doi: 10.2174/157015911795017047. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orabona GM, Griesi-Oliveira K, Vadasz E, Bulcão VL, Takahashi VN, Moreira ES, Furia-Silva M, Ros-Melo AM, Dourado F, Matioli R, Otto P, Passos-Bueno MR. HTR1B and HTR2C in autism spectrum disorders in Brazilian families. Brain Res. 2009;1250:14–9. doi: 10.1016/j.brainres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–47. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Andolina D, Ventura R, Puglisi-Allegra S, Cabib S. Reduced availability of brain amines during critical phases of postnatal development in a genetic mouse model of cognitive delay. Brain Res. 2008;1217:232–8. doi: 10.1016/j.brainres.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Paul LK, Schieffer B, Brown WS. Social processing deficits in primary agenesis of the corpus callosum: Narratives from the Thematic Apperception Test. Archives of Clinical Neuropsychology. 2004;19:215–225. doi: 10.1016/S0887-6177(03)00024-6. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Defensor EB, Blanchard DC, Blanchard RJ. C57BL/6J mice fail to exhibit preference for social novelty in the three-chamber apparatus. Behav Brain Res. 2010;213:189–94. doi: 10.1016/j.bbr.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–11. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Pugliese L, Barker GJ, Daly E, Powell J, Bolton PF, Murphy DG. Serotonin transporter genotype and neuroanatomy in autism spectrum disorders. Psychiatr Genet. 2009;19:147–50. doi: 10.1097/YPG.0b013e32832a505a. [DOI] [PubMed] [Google Scholar]
- Realmuto G, August G, Garfinkel B. Clinical effect of buspirone in autistic children. J Clin Psychopharmacol. 1989;9:122–5. doi: 10.1097/00004714-198904000-00009. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DV, Burke TF, McCasland M, Hensler JG. Serotonin-1A receptor function in the dorsal raphe nucleus following chronic administration of the selective serotonin reuptake inhibitor sertraline. J Neurochem. 2008;105:1091–9. doi: 10.1111/j.1471-4159.2007.05201.x. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–88. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo SL, Tsatsanis K. What is known about autism: genes, brain, and behavior. Am J Pharmacogenomics. 2005;5:71–92. doi: 10.2165/00129785-200505020-00001. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–89. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, McCook EC, Ritchie JC, Carroll BJ, Seidler FJ. Serotonin transporter expression in rat brain regions and blood platelets: aging and glucocorticoid effects. Biol Psychiatry. 1997;41:172–83. doi: 10.1016/S0006-3223(96)00215-6. [DOI] [PubMed] [Google Scholar]
- Symington SH, Paul LK, Symington MF, Ono M, Brown WS. Social cognition in individuals with agenesis of the corpus callosum. Soc Neurosci. 2010;5:296–308. doi: 10.1080/17470910903462419. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–79. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–73. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez M, Burke TF, Hensler JG. Selective heterologous regulation of 5-HT1A receptor-stimulated 35S GTPgammaS binding in the anterior cingulate cortex as a result of 5-HT2 receptor activation. Brain Res. 2002;957:174–82. doi: 10.1016/s0006-8993(02)03637-5. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Jessen TN, Thompson BJ, Carter M, Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Modeling rare gene variation to gain insight into the oldest biomarker in autism: construction of the serotonin transporter Gly56Ala knock-in mouse. J Neurodev Disord. 2009;1:158–171. doi: 10.1007/s11689-009-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Kim SJ, Lord C, Courchesne R, Akshoomoff N, Leventhal BL, Courchesne E, Cook EH., Jr Transmission disequilibrium studies of the serotonin 5-HT2A receptor gene (HTR2A) in autism. Am J Med Genet. 2002;114:277–83. doi: 10.1002/ajmg.10192. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, Nabeshima T. Synergistic effect of galantamine with risperidone on impairment of social interaction in phencyclidine-treated mice as a schizophrenic animal model. Neuropharmacology. 2007;52:1179–87. doi: 10.1016/j.neuropharm.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Wang CI, Lewis RJ. Emerging structure-function relationships defining monoamine NSS transporter substrate and ligand affinity. Biochem Pharmacol. 2009;79:1083–1091. doi: 10.1016/j.bcp.2009.11.019. [DOI] [PubMed] [Google Scholar]
- West L, Waldrop J, Brunssen S. Pharmacologic treatment for the core deficits and associated symptoms of autism in children. J Pediatr Health Care. 2009;23:75–89. doi: 10.1016/j.pedhc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Witmer PD, Doheny KF, Adams MK, Boehm CD, Dizon JS, Goldstein JL, Templeton TM, Wheaton AM, Dong PN, Pugh EW, Nussbaum RL, Hunter K, Kelmenson JA, Rowe LB, Brownstein MJ. The development of a highly informative mouse Simple Sequence Length Polymorphism (SSLP) marker set and construction of a mouse family tree using parsimony analysis. Genome Res. 2003;13:485–491. doi: 10.1101/gr.717903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007a;25:515–21. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007b;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–77. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocca FD. Neurochemistry and neurophysiology of buspirone and gepirone: interactions at presynaptic and postsynaptic 5-HT1A receptors. J Clin Psychopharmacol. 1990;10:6S–12S. doi: 10.1097/00004714-199006001-00003. [DOI] [PubMed] [Google Scholar]