Abstract
RNA interference (RNAi), a gene-silencing phenomenon whereby double-stranded RNA (dsRNA) triggers the sequence-specific degradation of homologous mRNA. RNAi has been quickly and widely applied to discover gene functions and holds great potential to provide a new class of therapeutic agents. However, new chemistry and delivery approaches are greatly needed to silence disease-causing genes without toxic effects. We reasoned that conjugation of the cholesterol moiety to cationic lipids would enhance RNAi efficiencies and lower the toxic effects of lipid-mediated RNAi delivery. Here, we report the first design and synthesis of new cholesterol-conjugated cationic lipids for RNAi delivery using microwave-assisted quaternization (MAQ) of tertiary amines. This strategy can be employed to develop new classes of non-viral gene delivery agents under safe and fast reaction conditions.
Keywords: RNAi, cholesterol-conjugated lipids, siRNA delivery, chemically modified siRNA, therapeutic silencing
RNA interference (RNAi), a gene-silencing phenomenon whereby double-stranded RNA (dsRNA) triggers the sequence-specific degradation of homologous mRNA (1). RNAi has been quickly and widely applied to discover gene functions and holds great potential to provide a new class of therapeutic agents (2). During RNAi, long dsRNA is processed by Dicer into short-interfering RNAs (siRNAs), and incorporated into the RNA-induced silencing complex (RISC) (3), a multiturnover enzyme complex that cleaves the target mRNA (4). Endogenously produced small RNAs, called miRNAs, inhibits translation by binding imperfectly matched sequences in the 3’ untranslated region (3’UTR) of target mRNA (5, 6). The RNAi machinery can also be programmed in cells by introducing duplexes of siRNAs (7, 8) that are assembled into siRISC containing Dicer, Argonautes and other proteins (reviewed in (4)). Therefore, new siRNA-based therapeutic agents could be designed to lower concentrations of specific disease-causing gene products.
The potential advantage of RNAi in medical applications is that it may provide a cure for diseases that cannot be treated by conventional small molecular medicines. By introducing siRNA to the cell, specific genes can be silenced, resulting in either decreased translational product of the silenced gene, or increased protein levels of a gene that is downregulated by the silenced sequence. However, in vivo delivery of siRNA has been a challenge due to the instability of siRNA in blood (in the case of systemic delivery), its relatively large molecular size, and its highly negative charge. Recent advances in understanding the rules for chemically modifying siRNA sequences without compromising their gene-silencing efficiency (9–11) have allowed the design and synthesis of therapeutically effective siRNA molecules that can silence target genes in vivo (12, 13). Furthermore, siRNAs have recently been delivered in vivo to successfully inhibit various gene functions. This delivery has been facilitated by conjugating cholesterol to siRNA (13) or to oligonucleotide inhibitors of miRNA (14), by forming stable nucleic acid-lipid particles (SNALP) of siRNA (12, 15), and by assembling lipid-siRNA complexes (16, 17). In addition, a protamine-antibody fusion protein has been used to deliver siRNAs to HIV-infected cells (18). Recently, the design and creation of interfering nanoparticles (iNOPs) as new systemic gene-silencing agents has been reported (19). iNOPs have two subunits: (i) a well-defined functionalized lipid nanoparticle as a delivery agent and (ii) a chemically modified siRNA for sustained silencing in vivo. iNOPs containing only 1–5 mg kg(−1) siRNA into mice, an endogenous gene for apolipoprotein B (apoB) was silenced in liver, plasma levels of apoB decreased, and total plasma cholesterol was lowered. iNOP treatment was nontoxic and did not induce an immune response (19).
Despite this progress, new chemistry and delivery approaches are greatly needed to silence disease-causing genes without toxic effects. We reasoned that conjugation of the cholesterol moiety to cationic lipids would enhance RNAi efficiencies and lower the toxic effects of lipid-mediated RNAi delivery. Cationic vectors have been extensively employed to deliver nucleic acids in cells and in animals (reviewed in (20)). Chemistry of quaternization of cationic lipids is quite challenging and requires chemically harsh and potentially hazardous conditions (21, 22). Microwave-assisted organic synthesis reactions have been an important tool in combinatorial approaches to generate a variety of compounds (23, 24). Substantial reductions in reaction times and improved yields can be achieved for a wide selection of organic reactions (25, 26). Here, we report the design and synthesis of new cholesterol-conjugated cationic lipids for RNAi delivery using microwave-assisted quaternization (MAQ) of tertiary amines. This strategy can be employed to develop new classes of non-viral gene delivery agents under safe and fast reaction conditions.
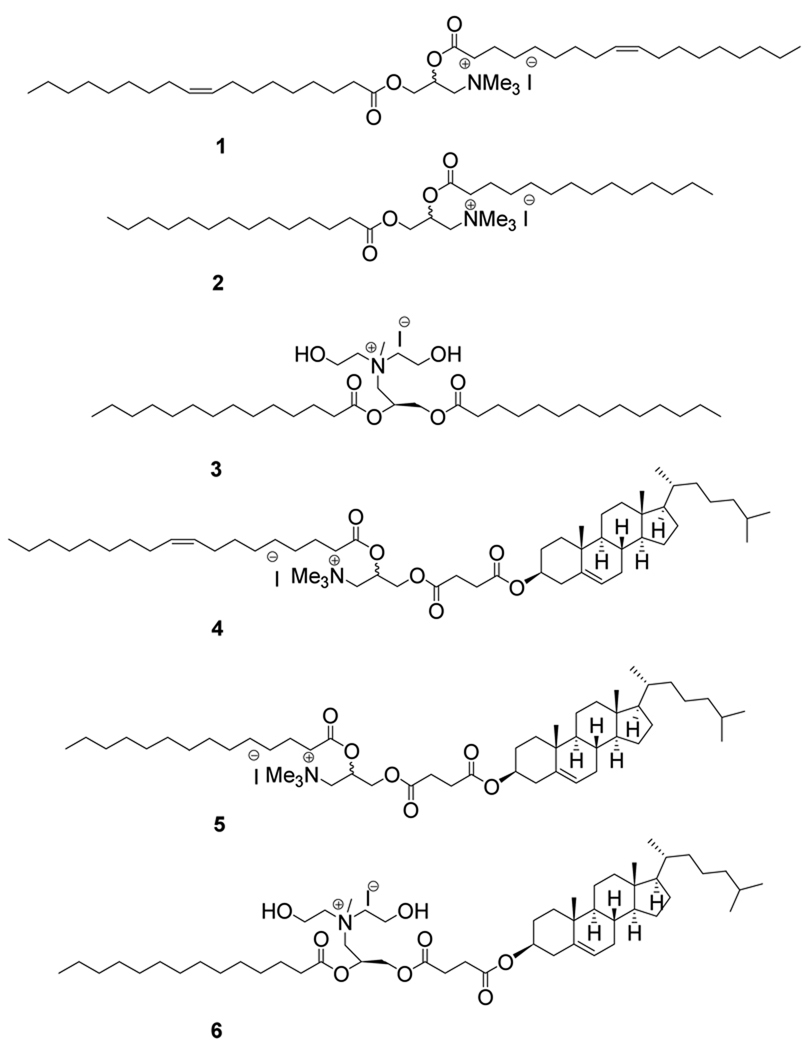
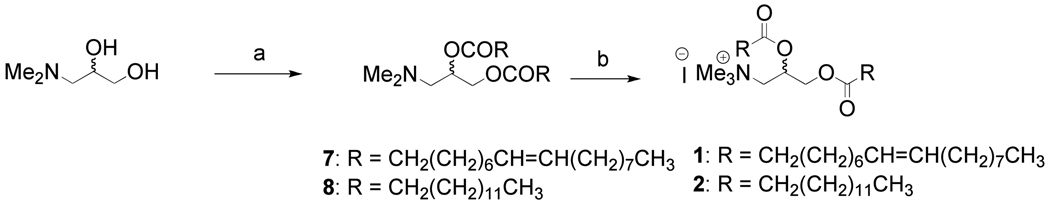
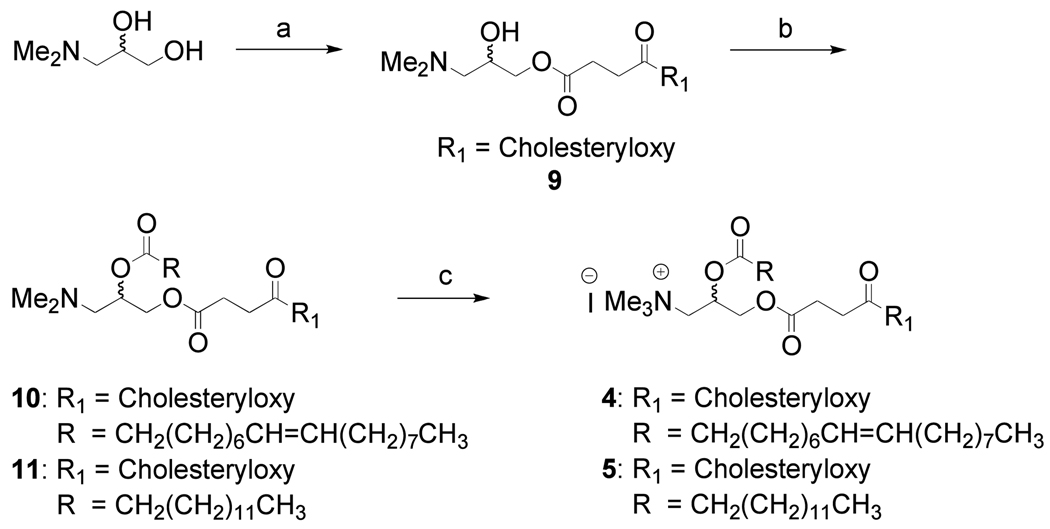
Lipids 4–6 were designed to improve RNAi delivery and to reduce related toxic effects on cells (Figure 1). The key difference in molecular structure is that one lipid chain of the commercially available transfection reagents (1–3) has been replaced by cholesteryl hemisuccinane moity in lipids 4–6. Scheme 1 outlines the synthetic procedure for lipids 1 & 2. The hydroxyl groups of the starting material 3-(dimethylamino)-1,2-propanediol was acylated with RCOCl using pyridine as base following a reported procedure (21). The mixture of intermediate tertiary amine (7 or 8) and MeI in CHCl3-DMSO (1:1) solution was subjected to 150W microwave irradiation at 70 °C for 1 h to give the target lipids 1&2 in very high yield. Microwave assisted quaternization of tertiary amines required the lesser quantity of reagent (MeI) and shortened the reaction period giving very high yield. To the best of our literature knowledge, this is the first report on microwave-irradiated quaternization (MAQ) of tertiary amine for the synthesis of cationic lipids. The synthesis strategy for cholesterol based cationic lipids is shown in Scheme 2. The primary hydroxyl group of the starting material 3-(dimethylamino)-1,2-propanediol was selectively coupled with cholesteryl hemisuccinate using DCC as coupling reagent to give 9 in 34% yield. The free hydroxyl group of the intermediate 9 was acylated with RCOCl using pyridine as base to give tertiary amine intermediates 10 & 11. The tertiary amine intermediates thus obtained was subjected to micwowave-assisted quaternization as described above to give the cholesterol based cationic lipids 4 & 5 as diastereomeric mixture in very high yield.
Figure 1.
Structures of commercially available cationic lipids (1–3) and cholesterol-conjugated cationic lipids (4–6) are shown. Commonly used names of commercially available lipids are: 1, DOTAP; 2, DMTAP; and 3, Transfast. Cholesterol conjugated lipids are denoted as: 4, Dotap_chol; 5, Dmtap_chol; and 6, Transfast_chol.
Scheme 1.
Synthesis of lipids 1 & 2
Reagents and condotions: (a) RCOCl, pyridine, DMAP, CH2Cl2, 6 h; (b) microwwave irradiation, 150W, 70 °C, 1 h, 90%
Scheme 2.
Synthesis of lipids 4 & 5
Reagents and conditions: (a) cholesteryl hemisuccinate, DCC, 0 °C 5 min. then rt 6 h, 34%; (b) RCOCl, pyridine, DMAP, CH2Cl2, 6 h, 87%; (c) microwave irradiation, 150W, 70 °C, CHCl3-DMSO (1:1), 1 h, 90%.
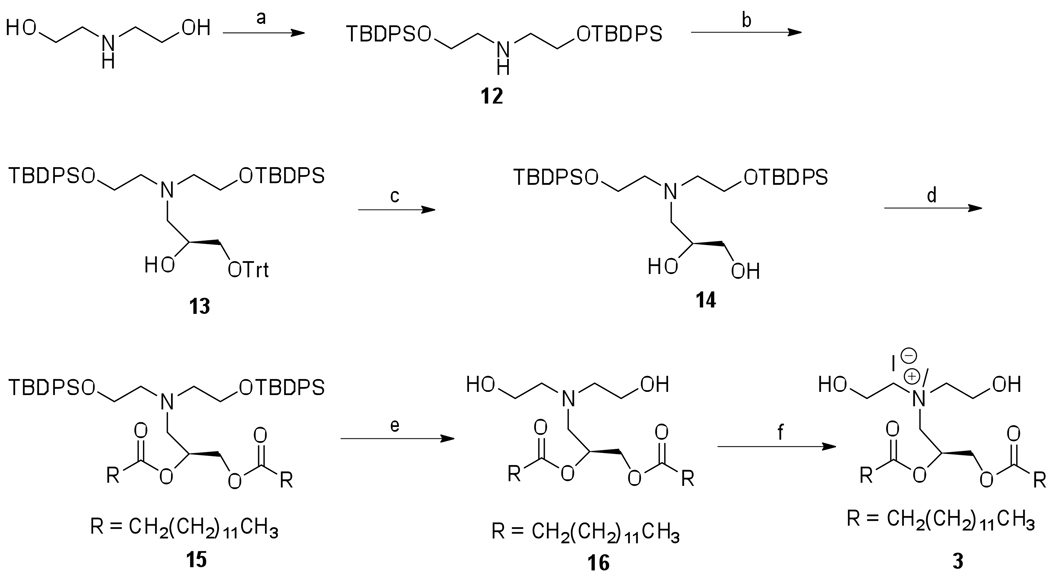
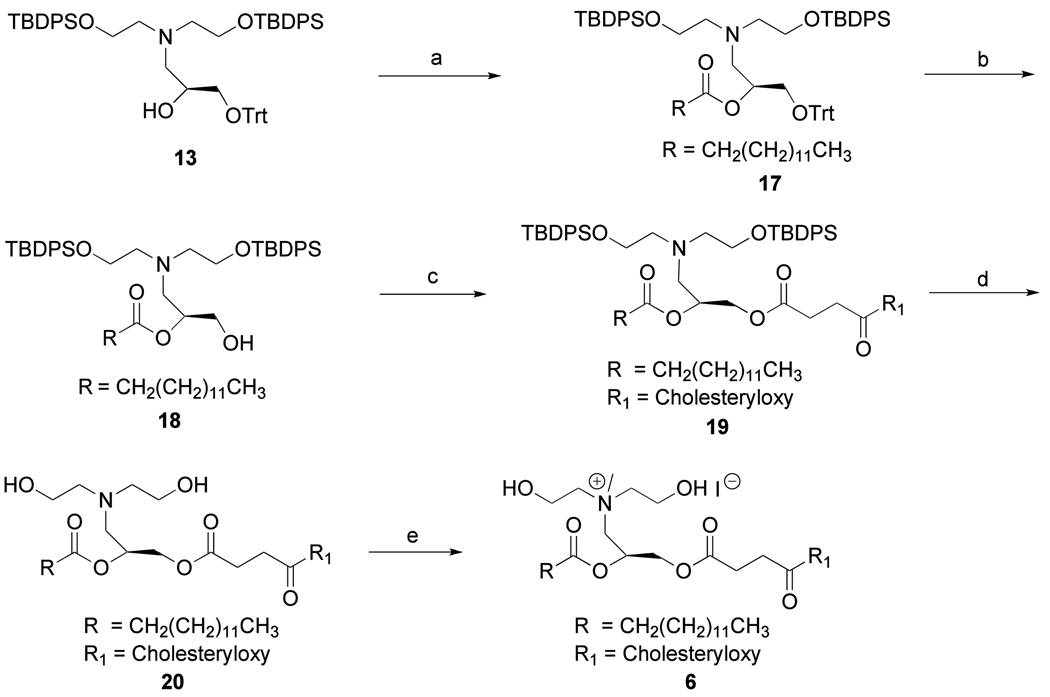
The synthesis of lipid 3 was carried out following the reported procedure, (22) except the microwave-assisted quaternization of tertiary amine in the final step (Scheme 3). The synthesis strategy for lipid 6 is shown in Scheme 4. The free hydroxyl group of the intermediate 13 was acylated with myristoyl chloride using Et3N as base in presence of catalytic amount of DMAP to give 17. Trityl deprotection of 17 was achieved by treating with 85% HCO2H at room temperature to give the intermediate 18. The hydroxyl group of 18 was coupled with the free carboxyl group of cholesteryl hemisuccinate in DMF solution using DCC as coupling agent to give 19 (79%), which was next treated with Bu4NF·3H2O to give the desilylated intermediate 20. Quaternization of the tertiary amine 20 was performed under 150W microwave irradiation at 80 °C for 3 h to give the cholesterol based cationic lipid 6 in 33% yield.
Scheme 3.
Synthesis of lipid 3
Reagents: (a) Et3N, DMAP, TBDPSCl, CH2Cl2, 0 °C to rt 20 h, 90%; (b) LiClO4, (R)-(+)-Trityl glycidyl ether, EtOH, 28 h, 65 °C, 93%; (c) 85% HCO2H, Et2O, rt, 20 h, 67%;(d) Et3N, DMAP, CH3(CH2)12COCl, 0 °C to rt, 5 h, 47%; (e) Bu4NF.3H2O, THF, 5.5 h, 0 °C to rt, 72%; (f) Mel, microwave irradiation (150W, 80 °C, 3.5 h), 81%.
Scheme 4.
Synthesis of lipid 6
Reagents: (a) Et3N, DMAP, CH3(CH2)12COCl, CH2Cl2, 0 °C to rt, 24 h, 81%; (b) 85% HCO2H, Et2O, 20 h, rt, 64%;(c) cholesteryl hemisuccinate, DCC, DMF, rt, 6 h, 79%; (d) Bu4NF.3H2O, THF, 0 °C to rt, overnight, 69%; (e) Mel, microwwave irradiation (150W, 80 °C, 3.5 h), 33%.
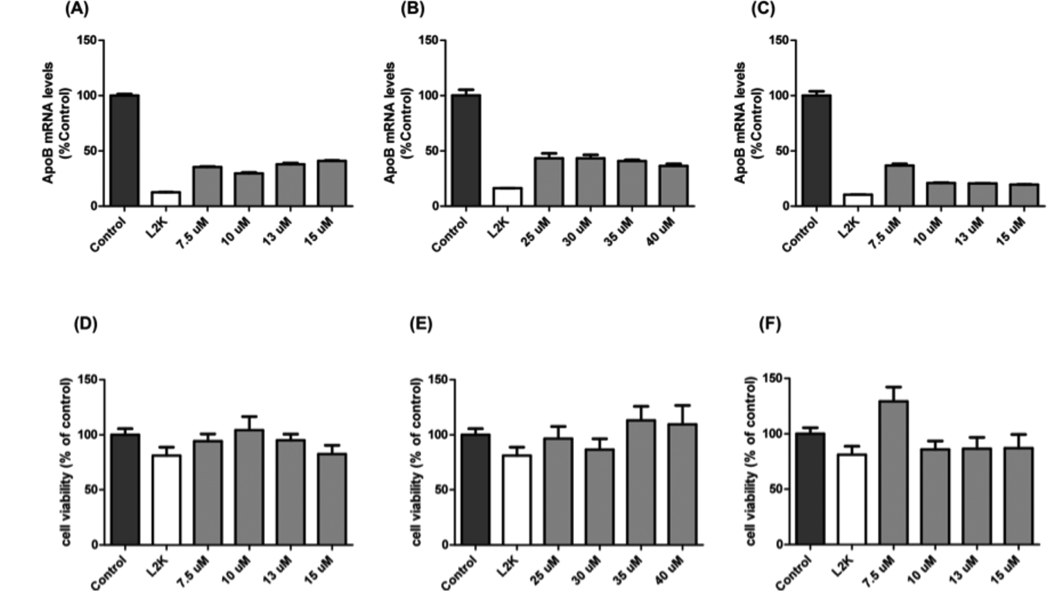
To determine whether the new lipids (4–6), Dotap_cholesterol, Dmtap_cholesterol or Transfast_cholesterol, could deliver active siRNA to its target and silence apo B mRNA in FL83B cells, we complexed siRNA using these lipids and analyzed gene silencing in cells by quantitative RT-PCR. Unmodified apo B siRNA complexed to the above lipids was able to silence apo B mRNA expression (~70%) for both Dotap_cholesterol and Dmtap_cholesterol, while Transfast_cholesterol was able to silence Apo B mRNA levels by 80% in FL83B cells when compared to controls (Figure 2A). Notably, the level of apo B mRNA silencing using Dotap_chol, Dmtap_chol or Transfast_chol as an RNA transporter was similar to that of cells when siRNA was delivered by commonly used transfection agents such as Lipofectamine 2000 (19).
Figure 2. In vitro silencing of apo B by RNAi delivered by cholesterol modified lipids.
Representative graphs show apo B mRNA levels in FL83B cells treated with different concentrations of lipids (A, Dotap_chol; B, Dmtap_chol; C, Transfast_chol) and corresponding cell viability after 24 hours of siRNA-lipid treatments (D, Dotap_chol; E, Dmtap_chol; F, Transfast_chol). Apo B mRNA levels are expressed as a % of control transfection. Each value was derived from the mean ± SEM of duplicate cultures and is representative of at least two separate experiments. Cell toxicity levels are expressed as a % of control transfection. Each value was derived from the mean ± SEM of duplicate cultures and is representative of at least two separate experiments.
We next analyzed the toxicity profiles of these lipid-RNA complexes. We confirmed that the observed reduction in apo B mRNA levels in FL83B cells was not due to cell toxicity of the lipids complexed to the siRNA by using a modified MTS cell toxicity assay (Figure 2B). Taken together, these results show that new cholesterol-conjugated lipids are non-toxic and can deliver siRNA for efficient gene silencing.
EXPERIMENTAL PROCEDURES
General Methods
1H NMR and 13C NMR spectra were recorded at 400 MHz and 100 MHz, respectively. ESI-MS were recorded with Microwmass ZQ. All the reagents were purchased from commercial source. When required, reactions were carried out under argon atmosphere with standard techniques for the exclusion of air and moisture. All solvents were dried before use. TLC was performed using fluorescent 60F254 coated plates.
(±)-N,N-Dimethyl-N-[2,3-bis(9-(Z)-octadecanoyloxy)-propyl]amine (7)
To a solution of 3-(dimethylamino)-1,2-propandiol (0.2 mL, 1.68 mmol), pyridine (0.55 mL, 6.72 mmol) and DMAP (20 mg, 0.17 mmol), in CH2Cl2 (10 mL) at 0 °C was added drop wise oleoyl chloride (1.38 mL, 4.2 mmol). The reaction mixture was allowed to warm up slowly to room temperature. It was stirred for 6 h at room temperature, diluted with CH2Cl2 (50 mL), washed sequentially with 5% aqueous sodium bicarbonate (15 mL), water (15 mL), and saturated aqueous NaCl (15 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 0.2% MeOH in CHCl3) furnished compound 7 (0.782 g, 72%) as oily liquid.1H NMR (CDCl3): δ 5.47-5.32 (m, 4H), 5.28–5.18 (m, 1H), 4.40 (dd, J = 11.8, 3.0 Hz, 1H), 4.13 (dd, J = 12.0, 6.8 Hz, 1H), 2.56–2.42 (m, 2H), 2.35 (dt, J = 7.6, 2.8 Hz, 4H), 2.30 (s, 6H), 2.12–1.97 (m, 8H), 1.71–1.58 (m, 4H), 1.44–1.23 (m, 40H), 0.92 (t, J = 7.0 Hz, 6H).
(±)-N,N-Dimethyl-N-[2,3-bis(tetradecanoyloxy)-propyl]amine (8)
1H NMR (CDCl3): δ 5.24–5.15 (m, 1H), 4.35 (dd, J = 12.0, 3.2 Hz, 1H), 4.08 (dd, J = 11.6, 6.0 Hz, 1H), 2.52–2.38 (m, 2H), 2.30 (dt, J = 7.2, 3.6 Hz, 4H), 2.61 (s, 6H), 1.67–1.53 (m, 4H), 1.36–1.17 (m, 40H), 0.87 (t, J = 6.4 Hz, 6H).
(±)-N,N,N-Trimethyl-N-[2,3-bis(9(Z)-octadecanoyloxy)-propyl]ammonium iodide (1)
A solution of 7 (190 mg, 0.29 mmol) and methyl iodide (0.022 mL, 0.35 mmol) in CHCl3 (1 mL) and DMSO (1 mL) was taken in a 10 mL microwave vial covered with a plastic cap. The reaction mixture was subjected to 150W microwave irradiation at 70 °C for 1 h. The reaction mixture was concentrated to dryness under high vacuum. Purification of this crude product by column chromatography (silica gel, elution with 8% MeOH in CHCl3) furnished 1 (208 mg, 90%) as a white solid.1H NMR (CDCl3): δ 5.64–5.56 (m, 1H), 5.39–5.28 (m, 4H), 4.58–4.47 (m, 2H), 4.14 (dd, J = 12.0, 5.6 Hz, 1H), 3.86 (dd, J = 14.4, 8.8 Hz, 1H), 3.53 (s, 9H), 2.35 (t, J = 2.6 Hz, 4H), 2.07–1.92 (m, 8H), 1.69–1.53 (m, 4H), 1.39–1.18 (m, 40H), 0.87 (t, J = 7.2 Hz, 6H); 13C NMR (CDCl3) δ 173.42, 172.96, 130.30, 130.26, 129.91, 128.85, 66.44, 65.88, 63.08, 55.02, 34.41, 34.19, 32.13, 29.99, 29.97, 29.93, 29.76, 29.55, 29.48, 29.40, 29.39, 29.33, 29.31, 29.29, 27.45, 27.42, 27.39, 24.97, 24.84, 22.91, 14.36; MS (ESI) m/z calculated: 663.1 [M+H]+, found: 663.3 [M+H]+.
(±)-N,N,N-Trimethyl-N-[2,3-bis(tetradecanoyloxy)-propyl]ammonium iodide (2)
1H NMR (CDCl3): δ 5.59–5.51 (m, 1H), 4.45 (dd, J = 12.0, 3.6 Hz, 1H), 4.18 (d, J = 14.0 Hz, 1H), 4.07 (dd, J = 12.0, 5.6 Hz, 1H), 3.76 (dd, J = 14.8, 8.8 Hz, 1H) 3.35 (s, 9H), 2.31 (dt, J = 13.6, 2.0 Hz, 4H), 1.63-1.51 (m, 4H), 1.33-1.14 (m, 40H), 0.84 (t, J = 7.2 Hz, 6H); 13C NMR (CDCl3) δ 173.63, 173.11, 66.54, 65.83, 63.21, 54.84, 34.35, 34.10, 32.10, 29.86, 29.83, 29.80, 29.68, 29.64, 29.54, 29.49, 29.42, 29.29, 29.26, 24.91, 24.80, 22.87, 14.27; MS (ESI) m/z calculated: 554.9 [M+H]+, found: 555.1 [M+H]+.
N,N-Dimethyl-N-[2-hydroxy, 3-succinyloxy(4-cholesteryloxy)-propyl]amine (9)
To a ice cooled solution of cholesteryl hemisuccinate (4.09 g, 8.42 mmol) in DMF (35 mL) 3-(dimethylamino)-1,2-propandiol (1.0 mL, 8.42 mmol) followed by N,N’-dicyclohexylcarbodiimide (3.47 g, 16.84 mmol) was added. After 5 min., the ice bath was removed and the reaction mixture was stirred for an additional 6 h at room temperature. The resulting precipitation of dicyclohexylurea was removed by filtration. The filtrate was transferred to a 100 mL round bottom flask and concentrated to dryness under vacuum. The pasty mass was dissolved in CHCl3 (200 mL) and washed with H2O (30 mL), saturated aqueous NaCl (30 mL), dried (Na2SO4) and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 5% MeOH in CHCl3) furnished 9 (1.68 g, 34%) as wax. 1H NMR (CDCl3): δ 5.36 (d, J = 4.0 Hz, 1H), 4.67–4.56 (m, 1H), 4.19 (dd, J = 11.6, 3.6 Hz, 1H), 4.04 (dd, J = 11.6, 6.0 Hz, 1H), 3.97–3.89 (m, 1H), 2.70–2.56 (m, 4H), 2.44 (t, J = 10.0 Hz, 1H), 2.36–2.23 (m, 9H), 2.05–1.75 (m, 5H), 1.66–0.80 (m, 33H), 0.67 (s, 3H).
N,N-Dimethyl-N-[2-(9(Z)-octadecanoyloxy), 3-succinyloxy(4-cholesteryloxy)-propyl]amine (10)
To a solution of 9 (178 mg, 0.3 mmol), Pyridine (0.036 mL, 0.45 mmol) and DMAP (6 mg, 0.04 mmol), in CH2Cl2 (4 mL) at 0 °C was added drop wise oleoyl chloride (0.12 mL, 0.36 mmol). The reaction mixture was allowed to warm up slowly to room temperature. It was stirred for 6 h at room temperature, diluted with CH2Cl2 (20 mL), washed sequentially with 5% aqueous sodium bicarbonate (10 mL), water (10 mL), and saturated aqueous NaCl (10 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 0.2% MeOH in CHCl3) furnished 10 (0.222 g, 87%) as a wax. 1H NMR (CDCl3): δ 5.39–5.28 (m, 3H), 5.23–5.14 (m, 1H), 4.66–4.55 (m, 1H), 4.40–4.34 (m, 1H), 4.11 (dd, J = 12.0, 6.4 Hz, 1H), 2.66–2.54 (m, 4H), 2.51–2.38 (m, 2H), 2.35–2.27 (m, 4H), 2.25 (s, 6H), 2.07–1.76 (m, 9H), 1.69–0.80 (m, 58H), 0.67 (s, 3H).
N,N-Dimethyl-N-[2-(tetradecanoyloxy), 3-succinyloxy(4-cholesteryloxy)-propyl]amine (11)
1H NMR (CDCl3): δ 5.36 (d, J = 4.8 Hz, 1H), 5.22–5.14 (m, 1H), 4.66–4.56 (m, 1H), 4.37 (dd, J = 12.0, 3.2 Hz, 1H), 4.11 (dd, J = 11.6, 6.0 Hz, 1H), 2.67–2.54 (m, 4H), 2.43(dt, J = 14.0, 7.2 Hz, 2H), 2.35-2.27 (m, 4H), 2.25 (s, 6H), 2.03–1.90 (m, 2H), 1.89-1.66 (m, 3H), 1.68-0.82 (m, 58H), 0.67 (s, 3H).
N,N,N-Trimethyl-N-[2-(9(Z)-octadecanoyloxy), 3-succinyloxy(4-cholesteryloxy)-propyl]ammonium iodide (4)
A solution of 10 (175 mg, 0.2 mmol) and methyl iodide (0.0152 mL, 0.246 mmol) in CHCl3 (1 mL) and DMSO (1 mL) was taken in a 10 mL microwave vial covered with a plastic cap. The reaction mixture was subjected to 150W microwave irradiation at 70 °C for 1 h. The reaction mixture was concentrated to dryness under high vacuum. Purification of this crude product by column chromatography (silica gel, elution with 8% MeOH in CHCl3) furnished 4 (183 mg, 90%) as a yellowish solid. 1H NMR (CDCl3): δ 5.62–5.54 (m, 1H), 5.39–5.27 (m, 3H), 4.60–4.52 (m, 1H), 4.48 (dd, J = 12.0, 4.0 Hz, 1H), 4.39 (d, J = 13.2 Hz, 1H), 4.22 (dd, J = 12.0, 4.4 Hz, 1H), 3.96 (dd, J =14.4, 9.2 Hz, 1H), 3.53 (s, 9H), 2.71–2.57 (m, 4H), 2.36 (dt, J = 7.2, 3.6 Hz, 2H), 2.33–2.22 (m, 2H), 2.08–1.75 (m, 9H), 1.66–0.82 (m, 58H), 0.66 (s, 3H). 13C NMR (CDCl3) δ 172.89, 172.23, 172.13, 139.68, 130.28, 129.88, 123.11, 74.87, 66.17, 65.89, 62.77, 56.87, 56.34, 54.95, 50.13, 42.52, 39.90, 39.72, 38.32, 38.29, 37.12, 36.81, 36.39, 36.02, 34.38, 32.13, 32.11, 32.05, 29.99, 29.96, 29.77, 29.55, 29.42, 29.36, 29.30, 28.45, 28.23, 27.98, 27.46, 27.42, 24.80, 24.50, 24.o7, 23.05, 22.91, 22.78, 21.24, 19.52, 18.93, 14.37, 12.07; MS (ESI) m/z calculated: 867.4 [M+H]+, found: 867.6 [M+H]+.
N,N,N-Trimethyl-N-[2-(tetradecanoyloxy), 3-succinyloxy(4-cholesteryloxy)-propyl]ammonium iodide (5)
1H NMR (CDCl3): δ 5.62–5.52 (m, 1H), 5.37–5.29 (m, 1H), 4.60–4.50 (m, 1H), 4.48 (dd, J = 12.4, 4.4 Hz, 1H), 4.41 (d, J = 13.6 Hz, 1H), 4.21 (dd, J = 12.4, 4.8 Hz, 1H), 3.96 (dd, J = 16.0, 9.2 Hz, 1H), 3.52 (s, 9H), 2.70–2.56 (m, 4H), 2.35 (dt, J = 7.6, 3.6 Hz, 2H), 2.32–2.22 (m, 2H), 2.04–1.75 (m, 5H), 1.64–0.78 (m, 58H), 0.65 (s, 3H); 13C NMR (CDCl3) δ 172.93, 172.22, 172.07, 139.69, 123.07, 74.82, 66.12, 65.92, 62.89, 56.87, 56.34, 54.96, 50.14, 42.51, 39.90, 39.71, 38.30, 38.28, 37.12, 36.80, 36.39, 36.01, 34.40, 32.16, 32.09, 32.04, 29.96, 29.93, 29.91, 29.76, 29.61, 29.53, 29.35, 29.28, 28.45, 28.23, 27.98, 27.96, 24.81, 24.50, 24.07, 23.05, 22.93, 22.78, 21.23, 19.52, 18.92, 14.37, 12.06; MS (ESI) m/z calculated: 813.3 [M+H]+, found: 813.5 [M+H]+.
N,N-[Bis(2-tert-butyldiphenylsilyloxyethyl)]amine (12)
To a solution of diethanol amine (1.0 mL, 10.42 mmol), Et3N (3.56 mL, 25.52 mmol) and DMAP (127 mg, 1.04 mmol) in CH2Cl2 at 0 °C was added tert-butyldiphenylchlorosilane (6.75 mL, 26.05 mmol) drop wise. The reaction mixture was allowed to warm to room temperature slowly. It was stirred for 20 h at room temperature, diluted with CH2Cl2 (50 mL), washed sequentially with 5% NaHCO3 (30 mL), water (30 mL) and saturated aqueous NaCl (30 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 2% methanol in CHCl3) furnished 12 (5.45 g, 90%) as oily liquid. 1H NMR (CDCl3): δ 7.74–7.67 (m, 8H), 7.48–7.34 (m, 12H), 3.81 (t, J = 5.2 Hz, 4H), 2.81 (t, J = 5.2 Hz, 4H), 1.065 (s, 18H).
3-[N,N-bis(2-tert butyldiphenylsilyloxyethyl)amino]-1-(Triphenylmethoxy)-2-propanol (13)
To a solution of 12 (4.52 g, 7.78 mmol) and lithium perchlorate (2.27g, 21.34 mmol) in ethanol (30 mL) was added (R)-(+)-trityl glycidyl ether (2.95 g, 9.33 mmol) at room temperature. The reaction mixture was stirred at 65 °C for 28 h, cooled to room temperature, diluted with CH2Cl2 (100 mL) and washed with 5% NaHCO3 (40 mL) followed by saturated aqueous NaCl (40 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 0.5% methanol in CHCl3) furnished 13 (6.48 g, 93%) as oily liquid. 1H NMR (CDCl3): δ 7.64–7.12 (m, 35H), 3.83–3.62 (m, 5H), 3.16–3.08 (m, 2H), 3.01 (dd, J = 10.0, 5.2 Hz, 2H), 2.90–2.78 (m, 4H), 0.98 (s, 18H).
3-[N,N-bis(2-tert butyldiphenylsilyloxyethyl)amino]-1,2-propanol(14)
To a solution of 13 (2.0 g, 2.23 mmol) in diethyl ether (3 mL) was added 85% formic acid (8.2 mL) at room temperature. The reaction mixture was stirred for 20 h, cooled at 0 °C and solid NaHCO3 was added in portion to neutralize the acidic solution. It was then diluted with diethyl ether (80 mL) and washed with water (25 mL) followed by saturated aqueous NaCl (25 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 4% MeOH in CHCl3) furnished 14 (0.97g, 67%) as oily liquid.1H NMR (CDCl3): δ 7.70–7.61 (m, 8H), 7.49–7.31 (m, 12H), 3.73-3.55 (m, 6H), 4.397 (dd, J = 11.6, 4.4 Hz, 1H), 2.78–2.54 (m, 5H), 2.57 (d, J = 6.8 Hz, 1H) 1.02 (s, 18H).
3-[N,N-bis(2-tert butyldiphenylsilyloxyethyl)amino]-1,2-bis(tetradecanoyloxy)propane (15)
To a solution of 14 (0.65 g, 0.99 mmol), Et3N (0.35 mL, 2.48 mmol) and DMAP (12 mg, 0.1 mmol), in CH2Cl2 (5 mL) at 0 °C was added drop wise myristoyl chloride (0.65 mL, 2.43 mmol). The reaction mixture was allowed to warm up slowly to room temperature. It was stirred for 5 h at room temperature, diluted with CH2Cl2 (50 mL), washed sequentially with 5% aqueous sodium bicarbonate (15 mL), water (15 mL), and saturated aqueous NaCl (15 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 2.5% EtOAc in Hexane) furnished 15 (0.50 g, 47%) as oily liquid. 1H NMR (CDCl3): δ 7.67–7.23 (m, 20H), 5.04–4.95 (m, 1H), 4.25 (dd, J = 12.0, 2.8 Hz, 1H), 4.03 (dd, J = 11.6, 5.6 Hz, 1H), 3.61 (t, J = 8.0 Hz, 4H), 2.75–2.58 (m, 6H), 2.24–2.33 (m, 4H), 1.60–1.50(m, 4H), 1.35–1.18(m, 40H), 1.01 (s, 18H), 0.87 (t, J = 6.4 Hz, 6H).
3-[N,N-bis(2-hydroxyethyl)amino]-1,2-bis(tetradecanoyloxy)propane (16)
Tetrabutylammonium fluoride trihydrate (0.50 g, 1.60 mmol) was added to an ice cooled solution of 15 (0.43 g, 0.40 mmol) in THF (3 mL) under N2 atmosphere. The reaction mixture was allowed to warm up to room temperature. It was stirred overnight at room temperature, diluted with CH2Cl2 (30 mL) and washed sequentially with 5% NaHCO3 (10 mL), water (10 mL) and saturated aqueous NaCl (10 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 2% methanol in CHCl3) furnished 16 (172 mg, 72%,) as a wax. 1H NMR (CDCl3): δ 5.24–5.14 (m, 1H), 4.35 (dd, J = 12.0, 3.2 Hz, 1H), 4.10 (dd, J = 12.4, 6.4 Hz, 1H), 3.60 (t, J = 5.2 Hz, 4H), 2.77-2.66 (m, 6H), 2.38-2.26 (m, 4H), 1.66–1.54(m, 4H), 1.36–1.18(m, 40H), 0.87 (t, J = 6.0 Hz, 6H).
N,N-[Bis(2-hydroxyethyl)]-N-methyl-N[2,3-bis(tetradecanoyloxy)propyl]ammonium iodide (3)
A mixture of 16 (122 mg, 0.2 mmol) and methyl iodide (1.5 mL) was taken in a 10 mL microwave vial covered with a plastic cap. The reaction mixture was then subjected to 150W microwave irradiation at 80 °C for 3.5 h. The reaction mixture was concentrated to dryness under high vacuum. Purification of this crude product by column chromatography (silica gel, elution with 8% MeOH in CHCl3) furnished 3 (120 mg, 81%) as a yellowish solid.1H NMR (CDCl3): δ 5.73–5.64 (m, 1H), 4.47 (dd, J = 12.0, 3.6 Hz, 1H), 4.32–3.74 (m, 11H), 3.37 (s, 3H), 2.42–2.30 (m, 4H), 1.66–1.53(m, 4H), 1.37–1.18(m, 40H), 0.87 (t, J = 6.8 Hz, 6H); 13C NMR (CDCl3) δ 173.60, 173.10, 65.84, 65.72, 65.18, 64.48, 63.54, 56.08, 56.03, 51.51, 34.52, 34.25, 32.16, 29.95, 29.91, 29.78, 29.75, 29.61, 29.54, 29.38, 29.34, 24.99, 24.91, 22.93, 14.37; MS (ESI) m/z calculated: 615.0 [M+H]+, found: 615.2 [M+H]+.
3-[N,N-bis(2-tert-butyldiphenylsilyloxyethyl)amino]-1-(Triphenylmethoxy)-2-(tetradecanoyloxy)propane (17)
To a solution of 13 (1.60 g, 1.78 mmol), Et3N (0.50 mL, 3.56 mmol) and DMAP (22 mg, 0.178 mmol), in CH2Cl2 (12 mL) at 0 °C was added drop wise myristoyl chloride (0.53 mL, 1.96 mmol). The reaction mixture was allowed to warm up slowly to room temperature. It was stirred for 24 h at room temperature, diluted with CH2Cl2 (50 mL), washed sequentially with 5% aqueous sodium bicarbonate (15 mL), water (15 mL), and saturated aqueous NaCl (15 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 0.2% MeOH in CHCl3) furnished 17 (1.60 g, 81%) as oily liquid. 1H NMR (CDCl3): δ 7.34–7.05 (m, 35H), 5.05–4.96 (m, 1H), 3.53–3.42 (m, 4H), 3.08 (dd, J = 10.0, 3.2 Hz, 1H), 2.99 (dd, J = 10.0, 6.0 Hz, 1H), 2.68-2.54 (m, 6H), 2.25 (t, J = 8.0 Hz, 2H), 1.64–1.55 (m, 2H), 1.35–1.14 (m, 20H), 0.98 (s, 18H), 0.88 (t, J = 6.8 Hz, 3H).
3-[N,N-bis(2-tert-butyldiphenylsilyloxyethyl)amino]-2-(tetradecanoyloxy)-1-propanol (18)
To a solution of 17 (1.55 g, 1.40 mmol) in diethyl ether (2 mL) was added 85% formic acid (5.2 mL) at room temperature. The reaction mixture was stirred for 20 h at room temperature, cooled at 0 °C and solid NaHCO3 was added in portion to neutralize the acidic solution. The reaction mixture was then diluted with diethyl ether (70 mL) and washed with water (20 mL) and saturated aqueous NaCl (20 mL). The organic layer was dried (Na2SO4), filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 1% MeOH in CHCl3) furnished 18 (0.77g, 64%) as a oily liquid. 1H NMR (CDCl3): δ 7.65–7.24 (m, 20H), 4.08 (dd, J = 11.2, 3.6 Hz, 1H), 3.95 (dd, J = 11.2, 5.6 Hz, 1H), 3.84–3.56 (m, 5H), 2.83–2.58 (m, 6H), 2.31 (t, J = 8.0 Hz, 2H), 1.68–1.50 (m, 2H), 1.36-1.16 (m, 20H), 1.02 (s, 18H), 0.88 (t, J = 7.0 Hz, 3H).
3-[N,N-bis(2-tert-butyldiphenylsilyloxyethyl)amino]-2-(tetradecanoyloxy)-1-[succinyloxy(4-cholesteryloxy)]propane (19)
To a ice cooled solution of 18 (0.52 g, 0.60 mmol) and cholesteryl hemisuccinate (0.44 g, 0.90 mmol) in DMF (5 mL) was added N,N’-dicyclohexylcarbodiimide (0.31 g, 1.5 mmol). After 5 min., the ice bath was removed and the reaction mixture was stirred for 6 h at room temperature. The resulting precipitation of dicyclohexylurea was removed by filtration. The filtrate was diluted with dichloromethane (30 mL) and washed with H2O (10 mL), saturated aqueous NaCl (10 mL), dried (Na2SO4) and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 5% MeOH in CHCl3) furnished 19 (0.632 g, 79%) as wax. . 1H NMR (CDCl3): δ 7.68–7.28 (m, 20H), 5.40–5.31 (m, 1H), 5.04–4.93 (m, 1H), 4.65– 4.51 (m, 1H), 4.26 (dd, J = 11.6, 2.4 Hz, 1H), 4.03 (dd, J = 12.0, 6.0 Hz, 1H), 3.61 (t, J = 6.0 Hz, 4H), 2.75-2.42 (m, 10H), 2.31 (d, J = 7.2 Hz, 2H), 2.18 (t, J = 7.6 Hz, 2H), 2.08–1.75 (m, 5H), 1.64–0.78 (m, 76H), 0.67 (s, 3H).
3-[N,N-bis(2-hydroxyethyl)amino]-2-(tetradecanoyloxy)-1-[succinyloxy(4-cholesteryloxy)]propane (20)
Tetrabutylammonium fluoride trihydrate(0.49 g, 1.55 mmol) was added to an ice cooled solution of 19 (0.52 g, 0.39 mmol) in THF (3 mL) under N2 atmosphere. The reaction mixture was allowed to warm up to room temperature. It was stirred at 0 °C for 5.5 h, then diluted with CH2Cl2 (20 mL) and washed with water (10 mL) followed by saturated aqueous NaCl (10 mL). The organic layer was dried, filtered and concentrated in vacuo. Purification by column chromatography (silica gel, elution with 2% methanol in CHCl3) furnished 20 (231 mg, 69%) as a wax. . 1H NMR (CDCl3): δ 5.40–5.33 (m, 1H), 5.27–5.18 (m, 1H), 4.66–4.54 (m, 1H), 4.30 (dd, J = 12.0, 3.6 Hz, 1H), 4.08 (dd, J = 12.0, 5.6 Hz, 1H), 3.68–3.50 (m, 4H), 2.90 (brs, 2H), 2.81–2.48 (m, 10H), 2.42–2.22 (m, 4H), 2.04–1.75 (m, 5H), 1.70–0.77 (m, 58H), 0.66 (s, 3H).
N,N-[Bis(2-hydroxyethyl)]-N-methyl-N-[2-(tetradecanoyloxy)-1-[succinyloxy(4-cholesteryloxy)]ammonium iodide (6)
A mixture of 20 (167 mg, 0.19 mmol) and methyl iodide (2 mL) was taken in a 10 mL microwave vial covered with a plastic cap. The reaction mixture was then subjected to 150W microwave irradiation at 80 °C for 3.5 h. The reaction mixture was concentrated to dryness under high vacuum. Purification of this crude product by column chromatography (silica gel, elution with 8% MeOH in CHCl3) furnished 6 (62 mg, 33%) as yellowish solid. 1H NMR (CDCl3): δ 5.77–5.70 (m, 1H), 5.36 (d, J = 5.2 Hz, 1H), 4.60–4.50 (m, 1H), 4.47 (dd, J = 12.0, 3.6 Hz, 1H), 4.33–3.67 (m, 11H), 3.40 (s, 3H), 2.82–2.47 (m, 4H), 2.35 (t, J = 7.6 Hz 2H), 2.31–2.22 (m, 2H), 2.06-1.92 (m, 2H), 1.90–1.76 (m, 3H), 1.64–0.82 (m, 58H) 0.67 (s, 3H); 13C NMR (CDCl3) δ 173.64, 172.30, 172.05, 139.54, 123.20, 75.19, 66.28, 65.77, 65.48, 64.30, 63.33, 56.88, 56.34, 56.18, 56.12, 51.47, 50.15, 42.53, 39.93, 39.73, 38.25, 37.10, 36.80, 36.40, 36.02, 34.22, 32.17, 32.12, 32.06, 29.96, 29.92, 29.79, 29.61, 29.59, 29.36, 29.26, 28.45, 28.24, 27.98, 24.97, 24.51, 24.07, 23.05, 22.93, 22.79, 21.24, 19.51, 18.93, 14.37, 12.08; MS (ESI) m/z calculated: 873.3 [M+H]+, found: 873.6 [M+H]+.
Preparations of siRNA
All siRNAs used in these studies were chemically synthesized by Dharmacon (USA) and received as desalted, deprotected oligonucleotides. Duplexes were annealed by standard procedures as described previously (10, 11).
In vitro RNAi activity with chol-lipids
FL83B (mouse hepatocytes) cells were maintained at 37 °C with 5% CO2 in F12 khangians modified culture medium (ATCC, USA) supplemented with 10% fetal bovine serum (FBS), 100U/mL penicillin and 100µg/mL streptomycin. Cells were regularly passaged and plated in 96-well and 6 well-culture plates 16 hours prior to transfection at 70% confluency. Lipids were suspended in a HEPES buffered saline and different concentrations of poly(ethylene glycol) methyl ether (Mn ~2000, Sigma) were added to lipid suspensions, followed by sonicating for 8 min at 25 °C. The molar ratios of lipids to PEG were as follows: Dotap_chol: PEG, 4:1, Dmtap_chol: PEG, 3: 1, Transfect_chol: PEG, 5: 2. Complexes of 100nM unmodified apo B siRNA (sense 5′-GUCAUCACACUGAAUACCAAU-3′, antisense: 5′-AUUGGUAUUCAGUGUGAUGACAC-3′) and different concentrations of Dotap_cholesterol or Dmtap_cholesterol, or Transfast_cholesterol were prepared by incubation for 20 minutes at room temperature in Opti-MEM culture medium (Invitrogen). Cells were transfected with 1ml of the complex per well for 6 hours at 37 °C. Medium was removed after 6 hours and replaced with full growth medium without antibiotics and incubated for an additional 24 hours. Cell viability was assessed using a CellTiter 96® AQueous One Solution cell proliferation assay according to the manufacture’s instructions (Promega, USA). Total RNA was extracted using RNeasy mini spin columns and DNase I treated before quanatation (Qiagen, USA). cDNA was prepared by random priming using Superscript II reverse transcriptase (Invitrogen, USA). Real time quantitative PCR (qPCR) was performed using SYBR Green (Qiagen USA). ApoB mRNA levels were assessed using the following primers apo B: forward: 5′-TTCCAGCCATGGGCAACTTTACCT-3′ and reverse: apo B 5′-TACTGCAGGGCGTCAGTGACAAAT-3′. apo B mRNA levels were then normalized against the housekeeping gene GAPDH: forward: 5′-ATCAAGAAGGTGGTGAAGCAGGCA-3′ and reverse: 5′-TGGAAGAGTGGGAGTTGCTGTTGA-3′.
Acknowledgments
We thank Craig Mello, Greg Hannon, and members of the Rana lab for helpful discussions and critical reading of the manuscript. This work was supported in part by grants from the NIH to T.M.R.
References
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- 3.Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 4.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 5.Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4:e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu CY, Rana TM. Small RNAs: regulators and guardians of the genome. J Cell Physiol. 2007;213:412–419. doi: 10.1002/jcp.21230. [DOI] [PubMed] [Google Scholar]
- 7.Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci U S A. 2001;98:9742–9747. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 9.Amarzguioui M, Holen T, Babaie E, Prydz H. Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Res. 2003;31:589–595. doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. Rna. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu YL, Rana TM. RNAi in human cells: basic structural and functional features of small interfering RNA. Mol Cell. 2002;10:549–561. doi: 10.1016/s1097-2765(02)00652-4. [DOI] [PubMed] [Google Scholar]
- 12.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 13.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 14.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 16.Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 17.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006 doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 18.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 19.Baigude H, McCarroll J, Yang CS, Swain PM, Rana TM. Design and creation of new nanomaterials for therapeutic RNAi. ACS Chem Biol. 2007;2:237–241. doi: 10.1021/cb7000582. [DOI] [PubMed] [Google Scholar]
- 20.Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Bennett MJ, Aberle AM, Balasubramaniam RP, Malone JG, Malone RW, Nantz MH. Cationic lipid-mediated gene delivery to murine lung: correlation of lipid hydration with in vivo transfection activity. J Med Chem. 1997;40:4069–4078. doi: 10.1021/jm970155q. [DOI] [PubMed] [Google Scholar]
- 22.Nantz MH, Bennett MJ, Balasubramaniam RP. The Regents of the University of California. Oakland, CA, USA: 1999. [Google Scholar]
- 23.Lee HK, Cao H, Rana TM. Design, microwave-assisted synthesis, and photophysical properties of small molecule organic antennas for luminescence resonance energy transfer. J Comb Chem. 2005;7:279–284. doi: 10.1021/cc0498480. [DOI] [PubMed] [Google Scholar]
- 24.Lee HK, Rana TM. Microwave-assisted parallel synthesis of a 4,6-diamino-2,2-dimethyl-1,2-dihydro-1-phenyl-s-triazine library. J Comb Chem. 2004;6:504–508. doi: 10.1021/cc049950x. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Ortiz A, del la Hoz A. Microwave assisted high throughput synthesis. Comb Chem High Throughput Screen. 2007;10:733–734. doi: 10.2174/138620707783018522. [DOI] [PubMed] [Google Scholar]
- 26.Roberts BA, Strauss CR. Toward rapid, "green", predictable microwave-assisted synthesis. Acc Chem Res. 2005;38:653–661. doi: 10.1021/ar040278m. [DOI] [PubMed] [Google Scholar]