Abstract
Day 3 thymectomy (D3Tx) results in a loss of peripheral tolerance mediated by natural T regulatory cells (nTR) and development of autoimmune ovarian dysgenesis (AOD) and dacryoadenitis (ADA) in A/J and (C57BL/6J × A/J) F1 hybrids (B6A) but not in C57BL/6J (B6) mice. Previously, using quantitative trait locus (QTL) linkage analysis, we showed that D3Tx-AOD is controlled by five unlinked QTL (Aod1-Aod5) and H2. In the present study, using D3Tx B6-ChrA/J/NaJ chromosome substitution strains, we confirm that QTL on chromosome (Chr) 16 (Aod1a/Aod1b), Chr3 (Aod2), Chr1 (Aod3), Chr2 (Aod4), Chr7 (Aod5), and Chr17 (H2) control D3Tx-AOD susceptibility. Additionally, we present the first data mapping QTL controlling D3Tx-ADA to Chr17 (Ada1/H2), Chr1 (Ada2), and Chr3 (Ada3). Importantly, B6-ChrXA/J mice were as resistant to D3Tx-AOD and D3Tx-ADA as B6 mice thereby excluding Foxp3 as a susceptibility gene in these models. Moreover, we report quantitative differences in the frequency of nTR cells in the lymph nodes (LNs), but not spleen or thymus, of AOD/ADA-resistant B6 and AOD/ADA-susceptible A/J, B6A, and B6-Chr17A/J mice. Similar results correlating with experimental allergic encephalomyelitis and orchitis susceptibility were seen with B10.S and SJL/J mice. Using H2-congenic mice we show that the observed difference in frequency of LN nTR cells is controlled by H2. These data support the existence of a LN-specific, H2-controlled mechanism regulating the prevalence of nTR cells in autoimmune disease susceptibility.
Introduction
T regulatory (TR) cells are a central component of immune suppression and are critical in establishing and maintaining self-tolerance (1, 2). Deficiencies in number, localization, or function of TR cells have been implicated in autoimmunity (1–3). Investigations into the interplay between TR cells and other immune cells, such as T effector (TE) cells, may provide greater insight into the pathogenesis of autoimmune disease. Natural TR (nTR) cells develop in the thymus as a functionally distinct and mature subpopulation of T cells that persist in the periphery with stable function (1, 2). During their generation, nTR cells are positively selected by high affinity TCR interactions with self-peptides expressed on thymic stromal and/or cortical cells (4–7). In the periphery, the interaction with APCs expressing MHC molecules on their surface is necessary for the maintenance and expansion of nTR cells (8–10). A fundamental feature of nTR cells is the expression of the forkhead/winged-helix transcription factor, Foxp3/Foxp3, which is essential for their function (1, 2). Foxp3 mutations in humans and mice impair the development and function of nTR cells and result in autoimmune inflammatory diseases (3).
Autoimmune disease is initiated by the loss of tolerance to self-Ag, with the concomitant expansion of autoreactive T cells. The LN environment is crucial for the priming of TE cells in response to auto-Ag (11–13). Under normal circumstances, autoreactive T cells are actively suppressed in the periphery by nTR cells (2). Importantly, nTR cells present in the regional LN continuously control organ-specific autoimmune diseases (e.g. AOD, type I diabetes) and inflammatory processes (e.g. allergy) (14–19), underscoring the importance of the LN environment in regulating immune responses.
D3Tx leads to a variety of organ-specific autoimmune diseases in genetically susceptible strains of mice (20, 21). In susceptible A/J and B6A F1 hybrid female mice, AOD and ADA are the predominant autoimmune diseases observed, whereas B6 mice are resistant (16, 20). Previously, we mapped QTL controlling D3Tx-AOD susceptibility to Chrs 1, 2, 3, 7, 16 and 17 (22, 23). In this study, we utilized B6-ChrA/J/NaJ chromosome substitution strains (also known as consomic strains) (24) to confirm the linkage of these QTL to their respective Chrs. Moreover, we show that QTL on Chr1 (Ada2), Chr3 (Ada3), and Chr17 (Ada1/H2), also control susceptibility to D3Tx-ADA. These data provide evidence for the existence of shared and disease-specific QTL in the genetic control of D3Tx-AOD and D3Tx-ADA. Reduction of a complex disease state into subphenotypes that represent one aspect of disease pathogenesis can facilitate analysis. In particular, monogenic subphenotypes permit refinement of the candidate region to an interval sufficiently small to allow identification of the causative gene by classical positional candidate gene cloning techniques (25). Given the role of nTR cells in D3Tx-induced autoimmune disease, we hypothesized that the AOD/ADA QTL may control nTR cell development and function. Indeed, our data reveal the existence of a LN-specific, H2-controlled mechanism regulating the prevalence of nTR cells in autoimmune disease susceptibility.
Materials and Methods
Animals and D3Tx
A/J, C57BL/6J (B6), (B6 x AJ)F1 (B6A), C57BL/10J (B10), B6-Chr1A/J (CSS1), B6-Chr2A/J (CSS2), B6-Chr3A/J (CSS3), B6-Chr4A/J (CSS4), B6-Chr5A/J (CSS5), B6-Chr7A/J (CSS7), B6-Chr16A/J (CSS16), B6-Chr17A/J (CSS17), B6-ChrXA/J (CSSX), B6.AK-H2k/FlaEgJ (B6.AK), B6.C-H2d/bByJ (B6.C), B10.BR-H2k H2-T18a/SgSnJ (B10.BR), B10.PL-H2u H2-T18a/(73NS)SnJ (B10.PL), B10.D2-Hc1 H2d H2-T18c/nSnJ (B10.D2n), B10.S-H2s/SgMcdJ (B10.S), and B10.A-H2a H2-T18a/SgSnJ (B10.A) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). D3Tx was conducted by suction under hypothermia (26). At 120 days of age, the mice were euthanized, tissues collected, and processed for histopathology and immune profiling. Animals with residual thymus were excluded. Mice were housed at 25°C with 12/12-h light-dark and 40–60% humidity. Naïve, age-matched female mice were used throughout. The experimental procedures performed in this study were under the guidelines of the Animal Care and Use Committees of the University of Vermont (Burlington, VT) and the University of Virginia (Charlottesville, VA).
Analysis of AOD and ADA phenotypes
Ovaries were fixed in Bouin’s fixative, embedded in paraffin, and 5 μm sections were stained with H&E. Multiple-step sections were evaluated in a double-blind manner and the ovaries scored for oophoritis and atrophy as previously described (22). Severity of AOD was scored from 0 to 4 (Figure 1A & B), with 4 being the most severe involving significant ovarian atrophy, with or without hypertrophy of the interstitial cells. Briefly, grade 1 is focal monocytic inflammation usually in the ovarian interstitium including the hilar region; grade 3 is severe inflammation that also involves the ovarian follicles with oocyte depletion; and grade 2 has an intermediate degree of inflammation. Severity of ADA was graded in a similar manner (Figure 1C & D). Severe ADA was associated with loss of glandular epithelia and the presence of tertiary lymphoid structures.
Figure 1. Ovarian and lachrymal gland histopathology in D3Tx mice.
(A) Normal ovary with normal and atretic follicles. (B) Severe oophoritis with mononuclear cell inflammation and loss of oocytes. (C) Normal lacrimal gland dominated by glandular structure. (D) Heavy mononuclear cell inflammation in lacrimal gland with total destruction of glandular structure, while the ducts remain intact. All sections are stained with H&E, ×200.
Antibodies and flow cytometric analysis
The inguinal, axillary, and brachial LNs (pooled), spleen, and thymus were excised and dissociated into single cell suspensions. For the identification and phenotypic analysis of nTR cells (CD4+CD8−TCRβ+Foxp3+) the following surface anti-mouse monoclonal antibodies were used: TexRed-conjugated anti-CD4 (clone RM4–5; Caltag, Bulingame, CA); Alexa647-conjugated anti-CD8 (clone 3B5), and PECy7-conjugated anti-CD357 (GITR, clone DTA-1; BD Pharmingen, Franklin Lakes, NJ); APCCy7-conjugated anti-CD25 (clone PC61, BioLegend, San Diego, CA); FITC-conjugated anti-TCRβ (clone H57-597), FITC-conjugated anti-CD103 (clone 2E7), APC-conjugated ani-CCR7 (clone 4B12), PECy5.5-conjugated anti-CD62L (clone MEL-14), and PE-conjugated anti-Foxp3 (clone FJK-16s; eBioscience, San Diego, CA). Intracellular Foxp3 was stained with the mouse/rat Foxp3 Staining set (eBioscience), according to the manufacturer’s instructions. Viable cells were selected for flow cytometric analysis (LSR II, BD, San Jose, CA) based on forward and side scatter light properties. The analysis was performed with FlowJo software (TreeStar Software, Inc, Portland, OR).
Suppression assay
CD4+ cells from LN and spleen were purified by using CD4 cell-enrichment column (R&D), labeled with PE-anti-CD25 Ab, incubated with anti-PE beads (Miltenyi Biotech, Auburn, CA). CD4+CD25+ T cell purity was consistently >90%. CD4+CD25− TE cells were cultured for 3 days with irradiated spleen cells as APC (1×105/well), with or without CD4+CD25+ TR cells at 0.5:1 (TR:TE) cell ratio. The cell cultures were pulsed with 0.5 μCi [3H] thymidine for the last 18 hrs. TE cell proliferation without TR cells was set at 100% for each strain. Percentage of inhibition in the presence of TR cells was calculated.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 4 software (GraphPad software Inc, San Diego, CA). Significance of differences was determined using Fisher’s exact test, Kruskal-Wallis and Dunnett’s tests, two way ANOVA and Bonferroni’s test, or Mann-Whitney test. For all analyses p ≤ 0.05 was considered significant.
Results
D3Tx-AOD and D3Tx-ADA in B6-ChrA/J chromosome substitution strains
Previous genetic studies mapped QTL controlling D3Tx-AOD to Chr1, 2, 3, 7, 16 and 17 (22, 23). In this study we utilized D3Tx chromosome substitution strains harboring each of the AOD-QTL to confirm these linkages and assess their inheritance in F1 hybrids. Significant differences in susceptibility to D3Tx-AOD and disease severity were seen with consomic lines 1, 2, 3, 7, 16, and 17 but not with consomic lines 4, 5, and X (Table I). These results confirm the linkage of D3Tx-AOD to Aod1, Aod2, Aod3, Aod4, Aod5 and H2 on Chr16, 3, 1, 2, 7 and 17, respectively (22, 23). Susceptibility to D3Tx-ADA co-segregates with D3Tx-AOD in A/J, B6 and B6A hybrid mice (16, 20). To determine whether the Chrs harboring QTL controlling D3Tx-AOD also influence susceptibility to ADA, we studied D3Tx-ADA in the same chromosome substitutions strains (Table I). Consomic lines 1, 3, and 17 were concordant for susceptibility to AOD and ADA whereas consomic lines 2, 7, and 16 were discordant. Consomic lines 4, 5, and X mice did not exhibit significant ADA. Similar results are obtained for each consomic line when the disease incidence and severity data for the consomic lines and F1 hybrids are pooled, i.e., incidence and disease severity are not significantly different, before testing the significance of the observed differences between a CSS and the resistant B6 background strain. The exception being consomic line 16 where the combined incidence and severity of ADA is significantly greater than the incidence (45.9%, n=37 vs. 80%, n=80; p=0.009) and disease severity (0.8 ± 0.2 vs. 0.3 ±0.1; p=0.004) seen in resistant B6 mice with pooled CSS and F1 hybrid data. In this case B6-Chr16A/J mice are concordant for AOD and ADA rather than discordant. These are the first data physically mapping QTL controlling susceptibility to D3Tx-ADA, and we have designated these as Ada1 (Chr17), which by convention is H2, Ada2 (Chr1), and Ada3 (Chr3).
Table I.
Susceptibility to autoimmune oophoritis (AOD) and dacryoadenitis (ADA) in D3Tx B6-ChrA/J chromosome substitution strains.
| Incidence |
Lesion severity |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lesion | Strain | Aff | Unaff | % Aff | p-value | Mean PI | p-value | Locus |
| AOD | A/J | 22 | 4 | 84.6 | 2.0 ± 0.2 | |||
| C57BL/6J | 10 | 101 | 9.0 | < 0.0001a | 0.1 ± 0.03 | < 0.0001c | ||
| C57BL/6J | 10 | 101 | 9.0 | < 0.0001b | 0.1 ± 0.03 | < 0.0001d | ||
| B6-Chr1A/J | 12 | 4 | 75.0 | < 0.0001 | 1.4 ± 0.3 | *** | Aod3 | |
| B6-Chr2A/J | 7 | 10 | 41.2 | 0.002 | 0.6 ± 0.2 | Aod4 | ||
| B6-Chr3A/J | 8 | 8 | 50.0 | 0.0002 | 0.8 ± 0.2 | ** | Aod2 | |
| B6-Chr4A/J | 3 | 14 | 17.6 | 0.3 ± 0.2 | ||||
| B6-Chr5A/J | 1 | 6 | 14.3 | 0.1 ± 0.1 | ||||
| B6-Chr7A/J | 10 | 8 | 55.6 | < 0.0001 | 1.0 ± 0.2 | *** | Aod5 | |
| B6-Chr16A/J | 5 | 11 | 31.3 | 0.02 | 0.5 ± 0.2 | Aod1a, Aod1b | ||
| B6-Chr17A/J | 7 | 12 | 36.8 | 0.004 | 0.6 ± 0.2 | * | H2 | |
| B6-ChrXA/J | 2 | 11 | 15.4 | 0.3 ± 0.2 | ||||
| ADA | A/J | 9 | 3 | 75.0 | 2.0 ± 0.2 | |||
| C57BL/6J | 17 | 63 | 21.3 | 0.0004a | 0.1 ± 0.03 | 0.0006c | ||
| C57BL/6J | 17 | 63 | 21.3 | < 0.0001b | 0.1 ± 0.03 | < 0.0001d | ||
| B6-Chr1A/J | 11 | 5 | 68.8 | 0.0004 | 1.3 ± 0.3 | *** | Ada2 | |
| B6-Chr2A/J | 3 | 11 | 17.6 | 0.4 ± 0.2 | ||||
| B6-Chr3A/J | 13 | 3 | 81.3 | < 0.0001 | 1.6 ± 0.3 | *** | Ada3 | |
| B6-Chr4A/J | 2 | 13 | 13.3 | 0.2 ±0.1 | ||||
| B6-Chr5A/J | 0 | 7 | 0 | 0 | ||||
| B6-Chr7A/J | 5 | 13 | 27.8 | 0.8 ± 0.3 | ||||
| B6-Chr16A/J | 6 | 10 | 37.5 | 0.7 ± 0.3 | ||||
| B6-Chr17A/J | 15 | 5 | 75.0 | < 0.0001 | 1.6 ± 0.3 | *** | Ada1/H2 | |
| B6-ChrXA/J | 5 | 8 | 38.5 | 0.6 ±0.2 | ||||
The significance of the observed differences in the incidence of AOD and ADA between B6 and A/J mice was determined using the Fisher’s exact test.
The significance of the observed differences in the incidences of AOD and ADA among B6 and the consomic strains was determined by Chi-square analysis using a 2×10 contingency table. Post-hoc significance of differences between B6 and each consomic strain was determined by subdividing the contingency table and for each comparison the significance of observed differences determined using the Fisher’s exact test.
The significance of the observed differences in the pathology indexes (PI) between B6 and A/J mice was determined using the Mann-Whitney test.
The significance of the observed differences in pathology indices observed among B6 and the consomic strains was determined using the Kruskal-Wallis test with post-hoc multiple comparison between B6 and each consomic strain carried out using Dunn’s Multiple Comparison test
p<0.05
p<0.01
p<0.001
For each AOD (Table II) and ADA (Table III) QTL, disease susceptibility was inherited in a dominant fashion when analyzed as either as qualitative or quantitative traits, with the exception of Aod3 and Ada2 on Chr1. When analyzed as a qualitative trait, i.e., incidence, susceptibility controlled by both loci was dominant. In contrast, when analyzed as quantitative traits both exhibited a gene dose effect in that F1 hybrids exhibited an intermediate phenotype.
Table II.
| Incidence |
Lesion severity |
|||||||
|---|---|---|---|---|---|---|---|---|
| Locus | Strain | Aff | Unaff | % Aff | p-value | Mean PI | p-value | Inheritancec |
| C57BL/6J | 10 | 101 | 9.0 | 0.1 ± 0.03 | ||||
| Aod3 | B6-Chr1A/J | 12 | 4 | 75.0 | 1.4 ± 0.3 | |||
| F1 hybrid | 5 | 8 | 38.5 | 0.6 ± 0.3 | ||||
| Overall | < 0.0001a | < 0.0001b | ||||||
| B6 vs. CSS | < 0.0001 | *** | ||||||
| B6 vs. F1 | 0.01 | * | ||||||
| CSS vs. F1 | 0.07 | * | ||||||
| B6<F1=CSS | B6<F1<CSS | Dom-GD | ||||||
| Aod4 | B6-Chr2A/J | 7 | 10 | 41.2 | 0.6 ± 0.2 | |||
| F1 hybrid | 7 | 10 | 41.2 | 0.7 ± 0.2 | ||||
| Overall | < 0.0001 | < 0.0001 | ||||||
| B6 vs. CSS | 0.002 | ** | ||||||
| B6 vs. F1 | 0.002 | ** | ||||||
| CSS vs. F1 | 1.0 | ns | ||||||
| B6<F1=CSS | B6<F1=CSS | Dom | ||||||
| Aod2 | B6-Chr3A/J | 8 | 8 | 50.0 | 0.8 ± 0.2 | |||
| F1 hybrid | 6 | 5 | 54.5 | 0.7 ± 0.2 | ||||
| Overall | < 0.0001 | < 0.0001 | ||||||
| B6 vs. CSS | 0.0002 | *** | ||||||
| B6 vs. F1 | 0.0006 | *** | ||||||
| CSS vs. F1 | 1.0 | ns | ||||||
| B6<F1=CSS | B6<F1=CSS | Dom | ||||||
| Aod5 | B6-Chr7A/J | 10 | 8 | 55.6 | 1.0 ± 0.2 | |||
| F1 hybrid | 7 | 13 | 35.0 | 0.6 ± 0.2 | ||||
| Overall | < 0.0001 | < 0.0001 | ||||||
| B6 vs. CSS | < 0.0001 | *** | ||||||
| B6 vs. F1 | 0.005 | *** | ||||||
| CSS vs. F1 | 0.3 | ns | ||||||
| B6<F1=CSS | B6<F1=CSS | Dom | ||||||
| Aod1a, Aod1b | B6-Chr16A/J | 5 | 11 | 31.3 | 0.5 ± 0.2 | |||
| F1 hybrid | 10 | 12 | 45.5 | 0.7 ± 0.2 | ||||
| Overall | < 0.0001 | < 0.0001 | ||||||
| B6 vs. CSS | 0.02 | * | ||||||
| B6 vs. F1 | 0.0001 | *** | ||||||
| CSS vs. F1 | 0.5 | ns | ||||||
| B6<F1=CSS | B6<F1=CSS | Dom | ||||||
| H2 | B6-Chr17A/J | 7 | 12 | 36.8 | 0.6 ± 0.2 | |||
| F1 hybrid | 6 | 18 | 25.0 | 0.4 ± 0.2 | ||||
| Overall | 0.002 | 0.001 | ||||||
| B6 vs. CSS | 0.004 | *** | ||||||
| B6 vs. F1 | 0.03 | * | ||||||
| CSS vs. F1 | 0.5 | ns | ||||||
| B6<F1=CSS | B6<F1=CSS | Dom | ||||||
The significance of the differences in incidences observed among B6, consomic strain, and (B6 × parental consomic strain) F1 hybrids was determined by Chi-square using a 2×3 contingency table. Post-hoc significance of differences between strains was determined by subdividing the contingency table and for each comparison the significance of differences determined using the Fisher’s exact test.
The significance of the differences in pathology indices observed among B6, consomic strain and (B6 × parental consomic strain) F1 hybrids was determined using the Kruskal-Wallis test with post-hoc multiple comparisons carried out using Dunn's Multiple Comparison test
p<0.05
p<0.01
p<0.001
Dom-dominant; GD-gene dosage.
Table III.
Susceptibility to D3Tx-ADA in (B6 × B6-ChrA/J) F1 hybrid mice.
| Incidence |
Lesion severity |
|||||||
|---|---|---|---|---|---|---|---|---|
| Locus | Strain | Aff | Unaff | % Aff | p-value | Mean PI | p-value | Inheritancec |
| C57BL/6J | 17 | 63 | 21.3 | 0.3 ± 0.1 | ||||
| Ada2 | B6-Chr1A/J | 11 | 5 | 68.8 | 1.3 ± 0.3 | |||
| F1 hybrid | 6 | 7 | 46.1 | 0.9 ± 0.3 | ||||
| Overall | 0.0004a | < 0.0001b | ||||||
| B6 vs. CSS | 0.0004 | *** | ||||||
| B6 vs. F1 | 0.05 | ns | ||||||
| CSS vs. F1 | 0.3 | ns | ||||||
| B6<F1=CSS | B6=F1=CSS | Dom-GD | ||||||
| B6<CSS | ||||||||
| Ada3 | B6-Chr3A/J | 13 | 3 | 81.3 | 1.6 ± 0.3 | |||
| F1 hybrid | 6 | 5 | 54.5 | 1.1 ± 0.4 | ||||
| Overall | < 0.0001 | < 0.0001 | ||||||
| B6 vs. CSS | < 0.0001 | *** | ||||||
| B6 vs. F1 | 0.03 | * | ||||||
| CSS vs. F1 | 0.2 | ns | ||||||
| B6<F1=CSS | B6<F1=CSS | Dom | ||||||
| Ada1 | B6-Chr17A/J | 15 | 5 | 75.0 | 1.6 ± 0.3 | |||
| H2 | F1 hybrid | 14 | 10 | 58.3 | 1.1 ± 0.2 | |||
| Overall | < 0.0001 | < 0.0001 | ||||||
| B6 vs. CSS | < 0.0001 | *** | ||||||
| B6 vs. F1 | 0.0009 | ** | ||||||
| CSS vs. F1 | 0.3 | ns | ||||||
| B6<F1=CS | B6<F1=CSS | Dom | ||||||
The significance of the differences in incidences observed among B6, consomic strain, and (B6 × parental consomic strain) F1 hybrids was determined by Chi-square using a 2×3 contingency table. Post-hoc significance of differences between strains was determined by subdividing the contingency table and for each comparison the significance of differences determined using the Fisher’s exact test.
The significance of the differences in pathology indices observed among B6, consomic strain, and (B6 × parental consomic strain) F1 hybrids was determined using the Kruskal-Wallis test with post-hoc multiple comparisons carried out using Dunn's Multiple Comparison test
p<0.05
p<0.01
p<0.001
Dom-dominant; GD-gene dose.
nTR cell frequency in the LN correlates with D3Tx-AOD and D3Tx-ADA susceptibility
The cellular basis of autoimmune disease elicited by D3Tx has not been defined, although it is likely dependent on lymphopenia and a relative paucity of nTR cells and, therefore, a TR:TE cell imbalance (16, 27, 28). Depletion of TR cells during the first 3 weeks after D3Tx enhances AOD severity (29).
The frequency of nTR cells in the thymus has been reported to be genetically controlled (30–32). To determine whether or not such differences segregate with AOD and ADA susceptibility, we compared the proportion of nTR cells among untreated young adult B6, A/J, and B6A mice. The frequency of Foxp3+ nTR cells among the single positive CD4 thymocytes and CD4+TCR+ splenocytes of B6, A/J and B6A mice was not significantly different (Figure 2A). In contrast, the frequency of nTR cells in the LN of A/J and B6A was significantly lower compared to that seen in B6 mice (p<0.0001) (Figure 2A). Importantly, the total number of LN cells did not differ significantly among the strains (Figure 2B).
Figure 2. Frequency of nTR cells in the LN is genetically controlled and segregates with AOD and ADA susceptibility in B6, A/J and B6A F1 hybrid mice.
(A) Flow cytometric analysis of CD4+CD8−TCRβ+Foxp3+ nTR cell frequency in different lymphoid tissues of B6, A/J and B6A mice. (B) Total LN cells of B6, A/J and B6A mice. Statistical significance was determined using the Kruskal-Wallis test and post hoc multiple comparisons performed using Dunnett’s test (**p<0.01). Flow cytometric data represent the mean ± s.d. of ≥ 6 individual mice. (C) B6 or A/J CD4+CD25− responder cells were cultured with B6A irradiated spleen cells and co-cultured at a 0.5:1 (TR:TE) ratio with A/J or B6 CD4+CD25+ cells, respectively. Inhibition of TE cell proliferation in the presence of TR cells is shown. Each bar represents the mean ± s.d. of 3 independent experiments; the significance of differences was determined using the Mann-Whitney test (*p<0.05).
A two-factor design comparing the expression of CD25, CCR7, CD62Lhigh, CD103, and CD357 was used to determine if the molecular signatures that characterize Foxp3+ nTR cells differ between LN and spleen by strain, and segregate with AOD/ADA-susceptibility (Suppl. Figure 1). Neither a significant effect of strain nor strain-by-tissue interaction was observed for CD25, CCR7, CD62Lhigh, and CD357. However, a significant effect of tissue was seen for all four markers with CD25, CCR7, and CD62Lhigh expression being greater in the LN compared to the spleen while CD357 expression was greater in the spleen than in the LN (Suppl. Figure 1a–d). CD103 expression did not exhibit a significant tissue effect; however, a significant effect of strain and strain-by-tissue interaction was detected with B6/J < B6A < A/J (Suppl. Figure 1e). Importantly, only the overall combined frequency of Foxp3+ nTR cells within the LN segregated with D3Tx-AOD/ADA susceptibility suggesting the presence of unique genetically determined factors in the LN environment that influence nTR cells and autoimmune disease susceptibility.
To investigate whether differences in the suppressive activity of B6 and A/J nTR cells exist, we performed in vitro TR cell-suppression assays. A significant difference in the inhibitory effects of nTR cells from B6 and A/J mice on polyclonal responder TE cells was not observed (Figure 2C). These results show differences in LN nTR cell frequency, but not suppressive activity, co-segregates with disease susceptibility in B6A mice. AOD/ADA-resistant B6 mice exhibit a higher frequency of nTR cells in the LN compared to AOD/ADA-susceptible A/J and B6A mice.
EAE and EAO resistance correlates with a higher frequency of nTR cells in the LN
To investigate whether the frequency of nTR cells in the LN is also associated with resistance to another autoimmune disease influenced by TR cells, we compared the frequency of nTR cells in young adult B10.S with SJL/J mice, which are experimental allergic encephalomyelitis (EAE)-and experimental autoimmune orchitis (EAO) -resistant and EAE- and EAO-susceptible, respectively (33, 34). The frequency of nTR cells in the LN of B10.S mice was higher than that of SJL/J mice (p=0.0004) (Figure 3), correlating with susceptibility and resistance to EAE and EAO.
Figure 3. The frequency of nTR cells in the LN correlates with EAE and EAO susceptibility.
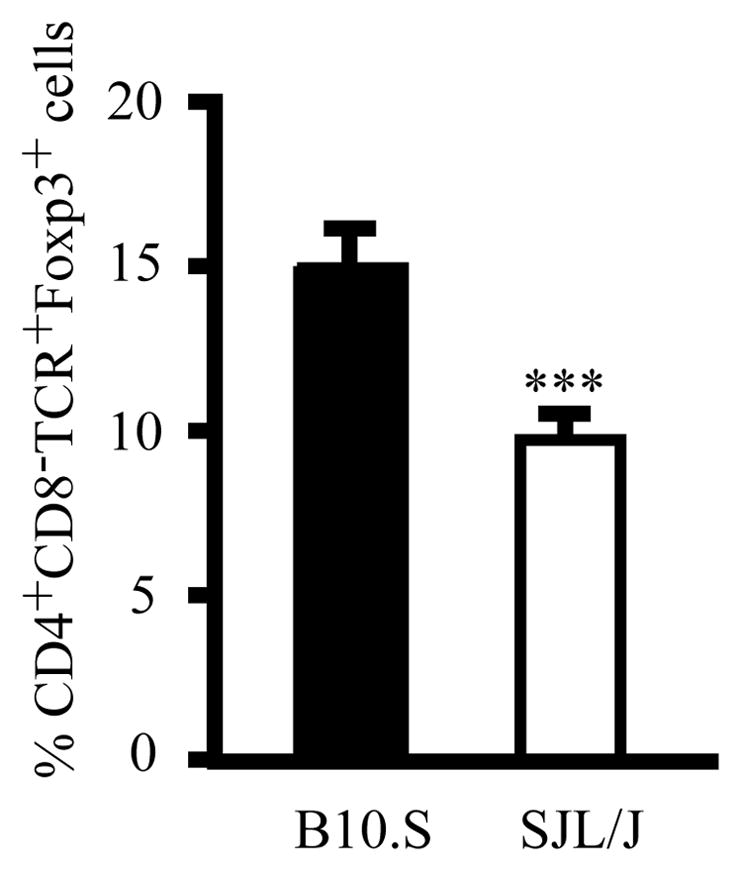
Flow cytometric analysis of CD4+CD8−TCRβ+Foxp3+ nTR cell frequency in the LN of young adult B10.S and SJL/J mice. Each bar represents the mean ± s.d. of ≥ 4 individual mice; the significance of differences was determined using the Mann-Whitney test (***p<0.001).
Chr17 QTL determine the frequency of nTR cells in the LN
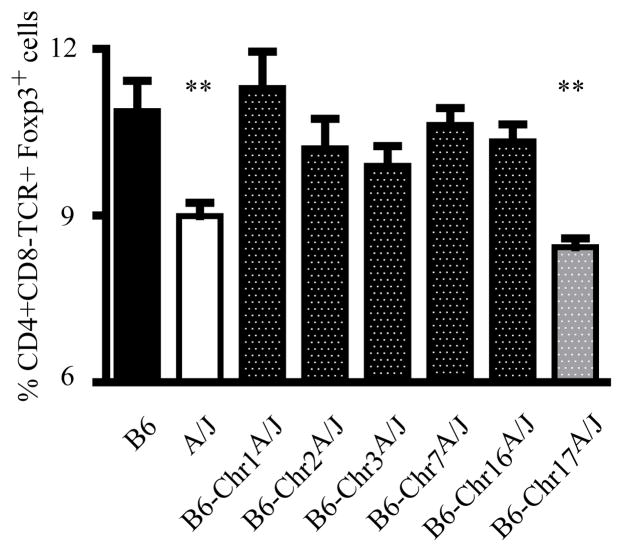
Given our findings, we hypothesized that the QTL controlling susceptibility to D3Tx-AOD and D3Tx-ADA may determine disease susceptibility by regulating the development and/or function of nTR cells within the LN. Therefore, we examined the frequency of nTR cells in the LNs of the chromosome substitution strains harboring AOD/ADA QTL (Table I). The frequency of nTR cells in the LNs differed significantly among the strains studied (p<0.0001) with A/J and consomic line 17 being lower than B6 and the other chromosome substitution strains, but not different from each other (Figure 4). With the exception of consomic line 2 having the lowest frequency of splenic nTR cells, significant differences in the frequency of nTR cells in the thymus or spleen were not detected among the chromosome substitution strains (data not shown). These data demonstrate that the QTL controlling differential LN nTR frequency segregating among A/J, B6, and B6A mice reside on Chr17.
Figure 4. QTL on Chr17 determine the number of LN nTR cells.
Flow cytometric analysis of CD4+CD8−TCRβ+Foxp3+ nTR cell frequency in the LNs of chromosome substitution strains and B6 mice. Each bar represents the mean ± s.d. of 8 individual mice; statistical significance was determined using the Kruskal-Wallis test and post hoc multiple comparison performed using Dunnett’s test (**p<0.01).
H2 determines the prevalence of nTR cells in the LN
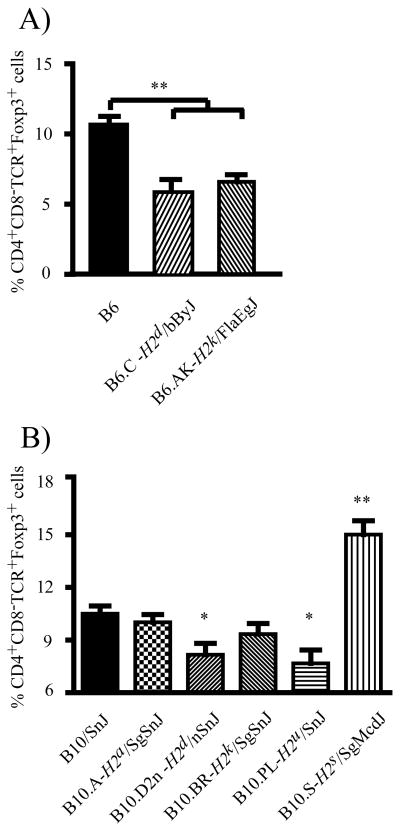
Since the development and maintenance of nTR cells is dependent on their interaction with MHC molecules (5, 8–10, 35) and H2 resides on Chr17, we utilized H2-congenic mice on the B6 and B10 backgrounds to test the hypothesis that the nTR cell frequency in the LN is controlled by H2. The frequency of LN nTR cells among B6 H2-congenic mice was significantly different (p<0.001), with B6.C (H2d) and B6.AK (H2k) mice exhibiting fewer LN nTR cells compared to B6 (H2b) (Figure 5A). Similarly, B10.D2/n (H2d) and B10.PL (H2u) mice had fewer LN nTR cells compared to B10 (H2b), B10.BR (H2k), and B10.A (H2a) congenic mice; and B10, B10.BR, and B10.A mice are resistant to D3Tx-AOD (20). In contrast, the frequency of nTR cells in the LN of B10.S mice was higher than that observed in all other B10 H2-congenic lines (Figure 5B). Taken together, these findings demonstrate that H2 significantly contributes to differences in the frequency of LN nTR cells.
Figure 5. H2 determines the frequency of nTR cells in the LN.
Flow cytometric analysis of CD4+CD8−TCRβ+Foxp3+ nTR cell frequency in the LN of (A) B6 H2-congenic mice, and (B) B10 H2-congenic mice. Each bar represents the mean ± s.d. of 10 individual mice; statistical significance was determined using the Kruskal-Wallis test and post hoc multiple comparisons performed using Dunnett’s test (*p<0.05; **p<0.01).
Discussion
D3Tx-induce a variety of organ-specific autoimmune diseases in inbred mice (20, 21). In susceptible A/J and B6A female mice, AOD and ADA are the predominant autoimmune diseases observed, whereas B6 mice are resistant (16, 20). These observations suggest that there may be shared-genetic mechanisms controlling susceptibility to these D3Tx-autoimmune diseases. The molecular and cellular mechanisms involved in resistance and/or susceptibility to D3Tx-AOD/ADA are only partially understood, but strongly suggest the involvement of TR cells in controlling disease (16, 27, 29).
The most important genetic factor controlling thymic TR cell development and function is the transcription factor Foxp3/Foxp3 (1, 2). The data presented in this paper demonstrate a genetic mechanism, controlled by H2, which regulates the prevalence of nTR cells in the LN, independent of the thymic output and/or function of nTR cells. Moreover, the frequency of LN TR cells is a subphenotype phenotype correlating with H2 control of susceptibility to D3Tx-AOD/ADA.
In this report we confirmed the linkage of AOD-controlling QTLs to Chrs 1 (Aod3), 2 (Aod4), 3 (Aod2), 7 (Aod5), 16 (Aod1) and 17 (H2). As previously mentioned, susceptibility to D3Tx-ADA co-segregates with D3Tx-AOD in A/J, B6A, and B6 mice (20, 21). It was notable to find that consomic lines 1, 3, and 17 were concordant for susceptibility to ADA with the same inheritance patterns, and consomic lines 2, 7, and 16 were discordant. These data highlight the possible existence of shared- and disease-specific QTL in the genetic control of these two D3Tx-induced autoimmune diseases. Additionally, the absence of D3Tx-AOD and D3Tx-ADA in consomic lines 4, 5, and X confirms that susceptibility to AOD and ADA is genetically controlled (22, 23). Moreover, the data obtained with the X chromosome consomic mice excludes Foxp3 as a susceptibility gene in these two nTR dependent models of autoimmune disease.
Comprehensive studies have been done to understand how Foxp3 is regulating TR cell development and function. Although development and suppressive activity of TR cells are key factors in restraining autoimmune responses, it is possible that some other factors, such as frequency and localization of TR cells, are also critical. The significance of these other components is substantiated by the evidence that development of autoimmune disease has been associated with decreased numbers of TR cells-independently of Foxp3 mutations (36–38), and the enrichment of Ag-specific TR cells in the draining LN relevant to the target tissue (39). We have shown that the frequency of peripheral nTR cells is not solely determined by thymic production (30) and support the existence of a LN specific, genetically controlled mechanism regulating the prevalence of nTR cells. Our data are in agreement with a study published by Feuerer et al (30) which found that the nTR cell frequency is similar in the thymus and spleen of a number of inbred strains. In the LN, however, we found a significant difference in the nTR cell frequency with a distinct pattern of CD25, CCR7, CD62Lhigh, and CD357 expression. Thus, our data clearly demonstrate that genetically controlled mechanisms in the LNs can regulate the proportion of nTR cells independently of thymic output, and this proportion may impact autoimmune disease susceptibility. As a result, we show that the frequency of LN nTR cells and not their suppressive capacity segregates with D3Tx-AOD/ADA susceptibility among B6/J, A/J and B6A mice. Likewise, a lower frequency of LN nTR cells was detected in EAE-susceptible SJL/J mice when compared with EAE-resistant B10.S, even though the frequency of thymic nTR cells was higher in SJL/J mice (data not shown). Studies published by Reddy et al (40) show that when PLP139–151-specific nTR cells were compared between these two strains, higher frequency of tetramer-positive nTR cells in the LN of B10.S mice were detected compared to SJL/J. In addition, comparable suppressor activity between B10.S and SJL/J PLP139–151-specific nTR cells were detected when assayed on polyclonal activated TE cells. These results indicate that the frequency of LN nTR cells, but not their suppressive activity, correlates with susceptibility and resistance to EAE and EAO, two classical tissue adjuvant models of autoimmune disease. Altogether, these data indicated that, in addition to development of nTR cells, the frequency and localization (i.e., LN) of nTR cells is an important factor for predicting D3Tx-induced autoimmune disease susceptibility.
By using B6/J and B10 congenic strains of mice which carry different H2 loci on a B6/J or B10 background, respectively, we demonstrate that H2 significantly contributes to different proportion of LN nTR cells; however, we acknowledge the importance of H2 in the development of thymic nTR cell development as reported by Tellier et al (32). Moreover, the fact that the frequency of LN nTR cells is significantly different between B6 (H2b) and B6.AK (H2k) (10.7% vs. 6.6%; p < 0.0001) but not different between B10 (H2b) and B10.BR (H2k) mice (10.51% vs. 9.41%; p = 0.23), supports the concept that epistatic interactions between H2 and polymorphic genes that distinguish B6 and B10 mice selectively influence nTR cell numbers within the LN. In this regard, there are at the current level of resequencing 453 non-synonymous coding region single nucleotide polymorphisms (SNPs) and 419 SNPs in potential regulatory regions (mRNA-UTR) that distinguish B6 and B10 mice http://www.informatics.jax.org/. While we have shown that H2 plays a role in quantitative localization of polyclonal nTR cells in the LN, other genes acting within a specific LN may contribute to selectively controlling differences in Ag-specific nTR cell function (20, 39, 41, 42).
We recently reported that the regional LNs of naïve animals are enriched in nTR cells with the capacity to suppress autoimmune responses specific to and maintained by the corresponding auto-Ag present in the relevant target-tissue; in contrast, TE cells do not share this trait and are widely distributed throughout the body (39). Studies examining the TCR-VDJ sequences of individual T cells revealed a distinct TCR repertoire between TR and TE cells with the TR cell-TCR repertoire varying as a function of regional distribution (42). In addition, during the course of type I diabetes in NOD mice, there are changes in gene expression specific to the pancreatic LN microenvironment, even before insulitis is present (41); these changes may dictate the fate of the immune response mediated by nTR cells. Moreover, removal of the pancreatic LN prevents diabetes in NOD mice (43). Our findings have identified an unequal and selective distribution of nTR cells in the LN, and support the concept of immune regulation by TR cells based on anatomical distribution. Thus genes influencing disease-specific nTR cells will contribute more directly to susceptibility and resistance to organ-specific autoimmune disease when acting in concert at the level of the primary LN draining a given target organ than when acting systemically.
Supplementary Material
Acknowledgments
We thank Rajkumar Noubade, Mercedes Rincon, Oliver Dienz, Sean A. Diehl, Laure K. Case, and Emma Wall for helpful discussion.
These studies were supported by National Institutes of Health Grants RO1 AI041747.
Abbreviations used in this paper
- D3Tx
day 3 thymectomy
- AOD
autoimmune ovarian dysgenesis
- QTL
quantitative trait locus
- ADA
autoimmune dacryoadenitis
- B6
C57BL/6J
- B6A
(C57BL/6J × A/J) F1 hybrid
- TR
regulatory T cell
- nTR
thymic-derived or natural TR cell
- LN
lymph node
- Chr
chromosome
- TE
effector T cell
- EAE
experimental allergic encephalomyelitis
- EAO
experimental allergic orchitis
References
- 1.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler SF. FOXP3: of mice and men. Annual review of immunology. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 4.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. The Journal of experimental medicine. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature immunology. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 6.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribot J, Enault G, Pilipenko S, Huchenq A, Calise M, Hudrisier D, Romagnoli P, van Meerwijk JP. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection. J Immunol. 2007;179:6741–6748. doi: 10.4049/jimmunol.179.10.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira C, Singh Y, Furmanski AL, Wong FS, Garden OA, Dyson J. Non-obese diabetic mice select a low-diversity repertoire of natural regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8320–8325. doi: 10.1073/pnas.0808493106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joetham A, Takeda K, Miyahara N, Matsubara S, Ohnishi H, Koya T, Dakhama A, Gelfand EW. Activation of naturally occurring lung CD4(+)CD25(+) regulatory T cells requires CD8 and MHC I interaction. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15057–15062. doi: 10.1073/pnas.0706765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westendorf AM, Fleissner D, Groebe L, Jung S, Gruber AD, Hansen W, Buer J. CD4+Foxp3+ regulatory T cell expansion induced by antigen-driven interaction with intestinal epithelial cells independent of local dendritic cells. Gut. 2009;58:211–219. doi: 10.1136/gut.2008.151720. [DOI] [PubMed] [Google Scholar]
- 11.Mandik-Nayak L, Wipke BT, Shih FF, Unanue ER, Allen PM. Despite ubiquitous autoantigen expression, arthritogenic autoantibody response initiates in the local lymph node. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14368–14373. doi: 10.1073/pnas.182549099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. The Journal of experimental medicine. 2002;196:1079–1090. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tung KS, Setiady YY, Samy ET, Lewis J, Teuscher C. Autoimmune ovarian disease in day 3-thymectomized mice: the neonatal time window, antigen specificity of disease suppression, and genetic control. Current topics in microbiology and immunology. 2005;293:209–247. doi: 10.1007/3-540-27702-1_10. [DOI] [PubMed] [Google Scholar]
- 14.Carson WFt, Guernsey LA, Singh A, Vella AT, Schramm CM, Thrall RS. Accumulation of regulatory T cells in local draining lymph nodes of the lung correlates with spontaneous resolution of chronic asthma in a murine model. International archives of allergy and immunology. 2008;145:231–243. doi: 10.1159/000109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 16.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. The Journal of experimental medicine. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 18.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nature genetics. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 21.Nishizuka Y, Sakakura T. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology. 1971;89:886–893. doi: 10.1210/endo-89-3-886. [DOI] [PubMed] [Google Scholar]
- 22.Roper RJ, Ma RZ, Biggins JE, Butterfield RJ, Michael SD, Tung KS, Doerge RW, Teuscher C. Interacting quantitative trait loci control loss of peripheral tolerance and susceptibility to autoimmune ovarian dysgenesis after day 3 thymectomy in mice. J Immunol. 2002;169:1640–1646. doi: 10.4049/jimmunol.169.3.1640. [DOI] [PubMed] [Google Scholar]
- 23.Wardell BB, Michael SD, Tung KS, Todd JA, Blankenhorn EP, McEntee K, Sudweeks JD, Hansen WK, Meeker ND, Griffith JS, et al. Aod1, the immunoregulatory locus controlling abrogation of tolerance in neonatal thymectomy-induced autoimmune ovarian dysgenesis, maps to mouse chromosome 16. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4758–4762. doi: 10.1073/pnas.92.11.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- 25.Avner P. Complex traits and polygenic inheritance in the mouse. Methods. 1998;14:191–198. doi: 10.1006/meth.1997.0577. [DOI] [PubMed] [Google Scholar]
- 26.Alard P, Thompson C, Agersborg SS, Thatte J, Setiady Y, Samy E, Tung KS. Endogenous oocyte antigens are required for rapid induction and progression of autoimmune ovarian disease following day-3 thymectomy. J Immunol. 2001;166:4363–4369. doi: 10.4049/jimmunol.166.7.4363. [DOI] [PubMed] [Google Scholar]
- 27.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. The Journal of experimental medicine. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith H, I, Chen M, Kubo R, Tung KS. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989;245:749–752. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- 29.Samy ET, Wheeler KM, Roper RJ, Teuscher C, Tung KS. Cutting edge: Autoimmune disease in day 3 thymectomized mice is actively controlled by endogenous disease-specific regulatory T cells. J Immunol. 2008;180:4366–4370. doi: 10.4049/jimmunol.180.7.4366. [DOI] [PubMed] [Google Scholar]
- 30.Feuerer M, Jiang W, Holler PD, Satpathy A, Campbell C, Bogue M, Mathis D, Benoist C. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romagnoli P, Tellier J, van Meerwijk JP. Genetic control of thymic development of CD4+CD25+FoxP3+ regulatory T lymphocytes. European journal of immunology. 2005;35:3525–3532. doi: 10.1002/eji.200535225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tellier J, van Meerwijk JP, Romagnoli P. An MHC-linked locus modulates thymic differentiation of CD4+CD25+Foxp3+ regulatory T lymphocytes. International immunology. 2006;18:1509–1519. doi: 10.1093/intimm/dxl084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C. New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 34.Roper RJ, Doerge RW, Call SB, Tung KS, Hickey WF, Teuscher C. Autoimmune orchitis, epididymitis, and vasitis are immunogenetically distinct lesions. The American journal of pathology. 1998;152:1337–1345. [PMC free article] [PubMed] [Google Scholar]
- 35.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. Journal of clinical immunology. 30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 37.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. The Journal of experimental medicine. 2005;201:1333–1346. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi M, Nakamura K, Honda K, Kitamura Y, Mizutani T, Araki Y, Kabemura T, Chijiiwa Y, Harada N, Nawata H. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Digestive diseases and sciences. 2006;51:677–686. doi: 10.1007/s10620-006-3191-2. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler KM, Samy ET, Tung KS. Cutting edge: normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J Immunol. 2009;183:7635–7638. doi: 10.4049/jimmunol.0804251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodama K, Butte AJ, Creusot RJ, Su L, Sheng D, Hartnett M, Iwai H, Soares LR, Fathman CG. Tissue- and age-specific changes in gene expression during disease induction and progression in NOD mice. Clinical immunology. 2008;129:195–201. doi: 10.1016/j.clim.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. The Journal of experimental medicine. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. The Journal of experimental medicine. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.