Abstract
New treatment approaches are needed for patients with asthma. Apolipoprotein A–I (apoA-I), the major structural protein of high density lipoproteins, mediates reverse cholesterol transport and also has atheroprotective and anti-inflammatory effects. Here, we hypothesized that an apolipoprotein A–I mimetic peptide might be effective at inhibiting asthmatic airway inflammation. A 5A peptide, which is a synthetic, bi-helical apoA-I mimetic, was administered to wild-type A/J mice via osmotic mini-pump prior to the induction of house dust mite (HDM)-induced asthma. HDM-challenged mice that received the 5A apoA-I mimetic peptide had significant reductions in the number of bronchoalveolar lavage fluid eosinophils, lymphocytes and neutrophils, as well as in histopathological evidence of airway inflammation. The reduction in airway inflammation was mediated by a reduction in expression of Th2- and Th17-type cytokines, as well as in chemokines that promote T cell and eosinophil chemotaxis, including CCL7, CCL17, CCL11 and CCL24. Furthermore, the 5A apoA-I mimetic peptide inhibited the alternative activation of pulmonary macrophages in the lungs of HDM-challenged mice. The 5A apoA-I mimetic peptide also abrogated the development of airway hyperreactivity and reduced several key features of airway remodeling, including goblet cell hyperplasia and the expression of collagen genes (Col1a1 and Col3a1). Our results demonstrate that the 5A apoA-I mimetic peptide attenuates the development of airway inflammation and airway hyperreactivity in an experimental murine model of house dust mite-induced asthma. These data support the conclusion that strategies utilizing apoA-I mimetic peptides, such as 5A, might be developed further as a possible new treatment approach for asthma.
Introduction
Apolipoproteins play a key role in the pathogenesis and prevention of atherosclerosis. Apolipoprotein A–I (apoA-I) is the major structural protein of high density lipoproteins (HDL), which have important atheroprotective properties(1–4). Mice over-expressing the human apolipoprotein A–I (APOAI) gene have increased levels of HDL and reduced atherosclerosis, while administration of HDL to cholesterol-fed rabbits induces the regression of atherosclerotic lesions(2, 4). The mechanism by which apoA-I attenuates atherosclerosis is via reverse cholesterol transport from macrophages residing in atherosclerotic plaques, which is then transported to the liver and excreted into the gastrointestinal tract(3). This process is mediated by binding of apoA-I to the ATP-binding cassette (ABC) transporter A1 (ABCA1), which facilitates cholesterol efflux from cells(3, 5–7). The function of HDL is not limited to reverse cholesterol transport. HDL has anti-oxidant properties, improves endothelial function, and mediates anti-thrombotic effects(8). HDL also has multiple anti-inflammatory properties(3, 8, 9). HDL inhibits the expression of inflammatory adhesion molecules and platelet-activating factor production by endothelial cells(10–12). HDL interrupts pro-inflammatory signal transduction cascades, such as sphingosine kinase and ERK (extracellular signal-regulated kinase) signaling pathways, as well as NF-κB activation(13). HDL also attenuates the generation of reactive oxygen species, inhibits proteasome activation, and reduces matrix metalloprotease expression(14, 15).
Based upon its atheroprotective effects, there has been considerable interest in developing apoA-I-based therapies as a mechanism to increase HDL levels and thereby prevent the development of cardiovascular disease(3). Furthermore, acute HDL therapy has been proposed as an approach for the treatment of patients with acute coronary syndrome or recent myocardial infarction via its ability to decrease the size of atherosclerotic plaques(3, 16). One approach to apoA-I-based therapies has been the development of apoA-I mimetic peptides(1, 3, 17). ApoA-I mimetic peptides have an amphipathic α-helical structure that recapitulates the secondary structure of the native apoA-I protein, which contains 10 amphipathic α-helices(17). Amphipathic α helices, which are defined as having opposing polar and nonpolar faces oriented along the long axis of the helix, are an important structural motif of apolipoproteins that facilitate interactions with lipids(18). Similar to the full-length protein, ApoA-I mimetic peptides that contain amphipathic helices promote cholesterol efflux from cells by both ABCA-1-dependent and –independent pathways(1, 3, 5, 9, 19). ApoA-I mimetic peptides have also been shown to have cardioprotective, anti-oxidant, and anti-inflammatory properties(1, 9, 20, 21). Consequently, ApoA-I mimetic peptides are being developed as a treatment for myocardial infarction and atherosclerosis based upon their ability to mediate reverse cholesterol transport and attenuate inflammation, such as the inhibition of pro-inflammatory cytokine generation and macrophage activation(3, 9).
Since airway inflammation is a key pathogenic manifestation of asthma, we hypothesized that apoA-I mimetic peptides might be effective as a novel therapeutic approach for asthma. Here, we show that administration of a 5A peptide, which is a synthetic, bi-helical apoA-I mimetic, attenuates the key manifestations of house dust mite-induced asthma, such as airway inflammation and airway hyperreactivity(3, 5).
Materials and Methods
House Dust Mite-induced Asthma
Six to eight week old female A/J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Asthma was induced by nasal inhalation of house dust mite (HDM) (Dermatophagoides pteronyssinus) extract (Greer, Lenoir NC), 25 µg of protein in 10 µl of saline, for 5 days each week for 4 weeks(22). The HDM extract contained 0.05 units per µl of endotoxin. Osmotic mini-pumps (Model 2004, Alzet, Cupertino, CA), which administered either the 5A apoA-I mimetic peptide (1 mg/kg/day) or a control peptide (1 mg/kg/day), were implanted 3 days prior to the initial nasal HDM challenge in order to give the animals sufficient time to recover from surgery prior to the induction of asthma. The control peptide, which is known to be functionally inactive, corresponded to the scrambled sequence of the apolipoprotein E low density lipoprotein receptor binding domain(23). The 5A apoA-I mimetic peptide was synthesized as previously described, while the control peptide was synthesized by Genescript (Piscataway, NJ)(5). All experimental protocols were approved by the Animal Care and Use Committee of the National Heart, Lung and Blood Institute.
Bronchoalveolar Lavage and Lung Histopathologic Examination
Bronchoalveolar lavage was performed using three instillations of 0.5 ml PBS. Red blood cells were lysed with ACK buffer for 2 min at 4°C and cells were re-suspended in 0.3 ml RPMI-1640 containing 10% FBS. Total cells were counted using a hemocytometer and Diff-Quik-stained cytospin slides (Siemens, Switzerland) were utilized for differential cell counts. Lungs were inflated to a pressure of 25 cm H20 prior to fixation in 10% formalin for 24 h, dehydrated through gradient ethanol and embedded in paraffin prior to cutting of sagittal sections at a thickness of 5 µm. Sections were then stained with hematoxylin and eosin or periodic acid Schiff (PAS).
Analysis of lung histology revealed intra- and inter-animal heterogeneity regarding the presence of goblet cell hyperplasia within individual airways. To quantify goblet cell hyperplasia throughout the entire lung of each animal, all the airways present (large (conducting), medium (central), and small (distal)) within representative lung sections were analyzed and the number of airways containing PAS-positive cells were recorded. Goblet cell hyperplasia is presented as the percentage of airways containing PAS-positive cells. The number of airways inspected in each animal is also presented. The quantification of goblet cell hyperplasia was performed by one of the investigators who was blinded to the identity of the animals.
Quantitative RT-PCR
Lungs were minced into 1 mm pieces, placed in RNAlater (Ambion, Austin, TX) and stored at −80°C until total RNA was isolated using the mirVana kit (Ambion). RNA was treated with 10 units of DNase I per 20 µg of RNA, reverse transcribed using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) and amplified using TaqMan Universal PCR Master Mix, FAM dye-labeled Taqman MGB probes and a 7500 Real Time PCR System running Sequence Detector version 2.1 software. Gene expression was quantified relative to expression of 18S rRNA using the control sample as calibrator to calculate the difference in Ct values (ΔΔCt) and presented as relative mRNA expression.
Flow Cytometry
Lung cells were isolated and flow cytometric analysis of alternatively activated macrophages was performed using modifications of methods described by Lewkowich (24) and Lee (25). Briefly, lungs were rinsed with ice-cold PBS, minced into small pieces and incubated at 37°C for 30 min in RPMI-1640 containing 26 units of Liberase TH (Roche Diagnostics) and 0.5 mg/ml of DNase I (Sigma-Aldrich). Lungs were disrupted by passage through a 100-µm cell strainer, followed by lysis of red blood cells using ACK lysis buffer (Biosource International). Lung cells were washed with RPMI-1640 containing 10% FBS, pelleted by centrifugation and re-suspended at a concentration of 1 × 106 cells/ml. Staining reactions were performed at 4°C using anti-CD11b-PerCP (eBioscience), F4/80-PE (eBioscience), and CD206-AlexaFluor647 (Biolegend). Flow cytometry was performed using a LSR-II SORP flow cytometer (Becton Dickinson, San Jose, CA). After excluding cellular debris using a FSC/SSC scatter plot, a macrophage gate was determined based upon light scatter properties. Alveolar macrophages with high autoflourescence were identified and the number of CD11b+/F4/80+/CD206+ cells were counted.
Airway Hyperreactivity
Airway resistance was measured in anesthetized mice using an Elan RC Fine Pointe system (Buxco, North Carolina), which contains a fully automated built-in ventilator and an in-line aerosol controller. Following anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg), a midline incision was made in the skin extending from the point of the jaw to just above the thoracic inlet. The skin was reflected laterally to show the underlying sternohyoideus and sternothyroideus muscles. Using blunt dissection and continuing on the midline, these two muscle groups were separated laterally to expose the underlying trachea. Following tracheal cannulation with a 19 gauge beveled metal catheter, mice were mechanically ventilated with a constant inspiratory flow and a second dose of ketamine (100 mg/kg) was given prior to nebulization of PBS or increasing doses of methacholine, which included 0.1875, 0.375, 0.75 and 1.5 mg/ml. Airway resistance was recorded at 10 second intervals for 3 min and average values are presented as cm H2O/ml/s.
Measurement of Serum IgE
Total serum IgE was measured with an OptEIA™ (BD Biosciences Pharmingen, San Diego, CA).
Statistics
Results are presented as mean ± SEM. A one-way ANOVA with a Bonferroni’s multiple comparison test or a two-way ANOVA with a Bonferroni post-test test (GraphPad Prism version 5.0a) were used and a P value less than 0.05 was considered significant.
Results
The 5A apoA-I Mimetic Peptide Inhibits Airway Inflammation in a Murine Model of HDM-induced Asthma
Intranasal administration of house dust mite for 5 days per week for 4 weeks induced airway inflammation characterized by an increase in the total number of inflammatory cells present in bronchoalveolar lavage fluid (BALF), as well as in the number of eosinophils, lymphocytes and neutrophils (Figure 1). Systemic administration of the 5A apoA-I mimetic peptide by osmotic mini-pump attenuated the total number of BALF inflammatory cells in HDM-challenged mice as compared to those that received the control peptide (Figure 1A). Furthermore, the numbers of BALF eosinophils, lymphocytes and neutrophils were significantly reduced in HDM-challenged mice that received the 5A peptide (Figure 1B). Histopathologic examination of lung sections confirmed that airway inflammation was markedly attenuated in HDM-challenged mice that received the 5A peptide, but not in those that received the control peptide (Figure 2A).
Figure 1. The 5A apoA-I mimetic peptide inhibits the induction of airway inflammation in a murine model of house dust mite-induced asthma.
An osmotic mini-pump containing either the 5A apoA-I or a control peptide was implanted prior to the induction of asthma in wild-type A/J mice by nasal administration of house dust mite (HDM) or saline, 5 days per week for 4 consecutive weeks. Numbers of total cells (n = 10 mice, * P < 0.0001) (Panel A) and inflammatory cell types (Panel B) in bronchoalveolar lavage fluid (BALF) are shown (n = 10 mice, * P < 0.05, HDM vs. HDM + 5A). A representative result from three independent experiments is shown.
Figure 2. Effect of the 5A apoA-I mimetic peptide on lung histology and airway hyperreactivity in a murine model of house dust mite-induced asthma.
A. Histologic sections of lung were stained with hematoxylin and eosin (H & E) or periodic acid-Schiff (PAS) stains and images obtained at 200× or 1000×. The calibration bar indicates 100 µm for the 200× images and 25 µm for the 1,000× images. A representative image is shown. B. Airway resistance (cm H20/ml/s) was measured following nebulization of increasing doses of methacholine. (n = 8 – 10 mice, * P < 0.05 vs. saline; ** P < 0.001 HDM + 5A vs. HDM). A representative result from three independent experiments is shown.
The 5A apoA-I Mimetic Peptide Inhibits Airway Hyperreactivity in HDM-induced Asthma
Administration of the 5A peptide to HDM-challenged mice also completely inhibited the induction of airway hyperreactivity. As shown in Figure 2B, levels of airway resistance in HDM-challenged mice that received the 5A peptide were similar to that of saline-challenged mice. In contrast, levels of airway resistance in HDM-challenged mice that received the control peptide, were elevated to levels similar to HDM-challenged mice. This demonstrates that the 5A apoA-I mimetic peptide inhibits the induction of airway hyperreactivity in house dust mite-induced asthma.
The 5A apoA-I Mimetic Peptide Attenuates Manifestations of Airway Remodeling in HDM-induced Asthma
Having shown that the 5A apoA-I mimetic peptide inhibited airway inflammation and airway hyperreactivity, we assessed its effect on airway remodeling responses, such as mucin gene expression and goblet cell hyperplasia. As shown in Figure 3, HDM-challenged mice that received the 5A peptide had reductions in mRNA encoding the MUC5AC mucin gene and Clca3, a calcium-activated chloride channel that is associated with goblet cell hyperplasia, as compared to HDM-challenged mice(26). Similarly, the goblet cell hyperplasia was reduced in HDM-challenged mice that received the 5A peptide as compared to those that did not (Figure 2 and Figure 3). The effect of the 5A peptide on expression of collagen genes that contribute to sub-epithelial collagen deposition was also assessed(27). As shown in Figure 3, the 5A peptide significantly reduced the expression of the genes encoding type I (Col1a1) and type III (Col3a1) collagens. These data demonstrate that the 5A apoA-I mimetic peptide reduced several key manifestations of airway remodeling in house dust mite-induced asthma, such as goblet cell hyperplasia, as well as the expression of genes encoding airway mucins and collagens.
Figure 3. The 5A apoA-I mimetic peptide reduces mucin gene expression and goblet cell hyperplasia in a murine model of house dust mite-induced asthma.
A, B. Quantification of lung mRNA levels for MUC5AC and Clca3 (n = 6 mice, * P < 0.001; HDM vs. Saline; ** P < 0.05, HDM vs. HDM + 5A). A representative result from three independent experiments is shown. C. Goblet cell hyperplasia presented as the percentage of airways containing PAS-positive cells (n = 10 mice, * P < 0.001 vs. HDM vs. Saline; ** P < 0.001, HDM vs. HDM + 5A). 32.9 ± 1.6 airways were inspected in each mouse. Pooled data from two independent experiments are shown. D, E. Quantification of lung mRNA levels for Col1a1 and Col3a1 (n = 6 mice, * P < 0.001; HDM vs. Saline; ** P < 0.001, HDM vs. HDM + 5A). A representative result from three independent experiments is shown.
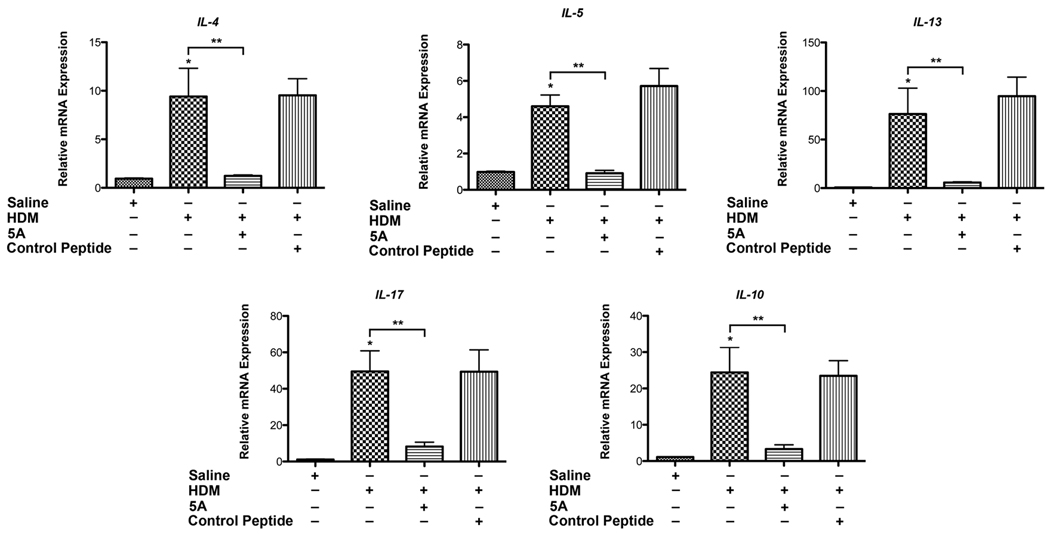
Administration of the 5A apoA-I Mimetic Peptide Inhibits the Expression of Th2 and Th17 Cytokines in HDM-induced Asthma
We next investigated the mechanisms by which the 5A apoA-I mimetic peptide attenuates HDM-induced airway inflammation. Th2 CD4+ T cells are increased in asthmatic airways and produce canonical Th2 cytokines, such as IL-4 and IL-13(28). IL-4 promotes the differentiation and proliferation of Th2 cells and IgE production, whereas IL-13 is an effector cytokine that mediates mucin production and airway hyperreactivity (AHR) in asthma(29). IL-17A, a product of Th17 cells, is required during the induction of allergic asthma and can also mediate neutrophil-mediated inflammation(28, 30). As shown in Figure 4, mRNA levels of IL-4, IL-5, IL-13 and IL-17A were reduced in lung homogenates from HDM-challenged mice that had been treated with the 5A apoA-I mimetic peptide. Administration of the 5A apoA-I mimetic peptide also inhibited HDM-induced increases in IL-10 mRNA expression. This result is consistent with the conclusion that the 5A apoA-I mimetic peptide inhibits the induction of inflammatory responses mediated by Th2 and Th17 cytokines in HDM-mediated asthma.
Figure 4. The 5A apoA-I mimetic peptide inhibits the expression of Th2 and Th17 cytokines in the lungs of house dust mite-challenged asthma.
Quantification of lung mRNA levels for IL-4, IL-5, IL-13, IL17A and IL-10 by qRT-PCR presented as relative mRNA expression (n = 6 mice, * P < 0.05 HDM vs. Saline; ** P < 0.05, HDM vs. HDM + 5A). A representative result from three independent experiments is shown.
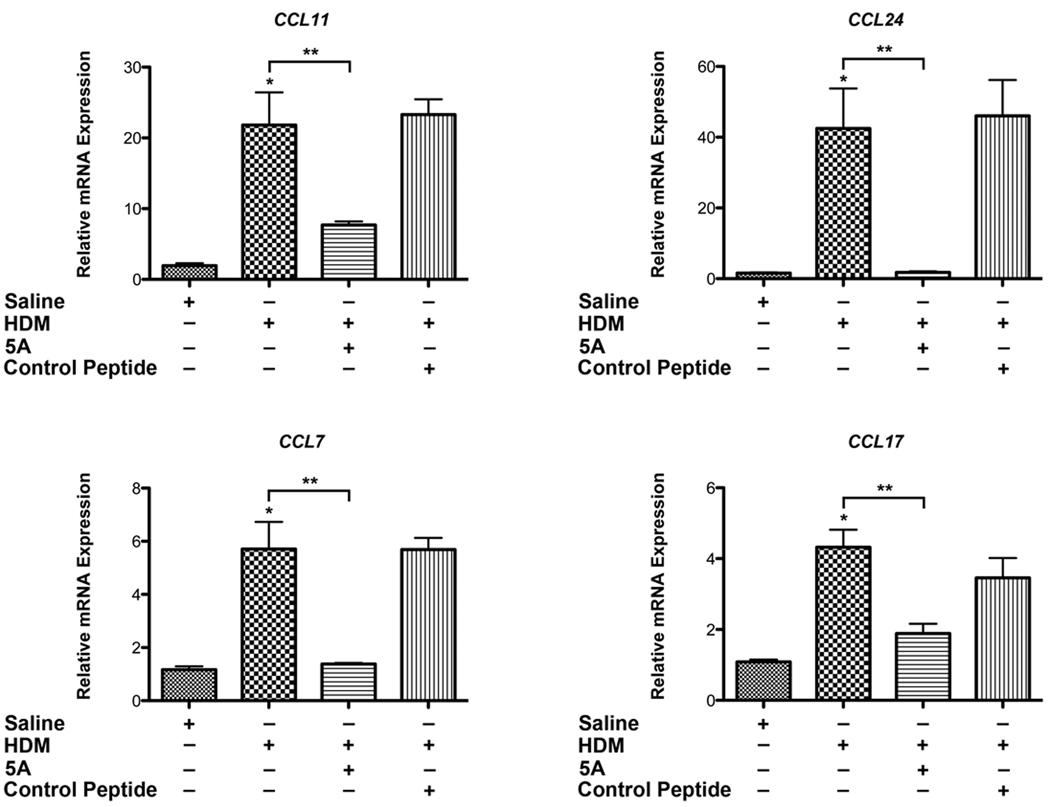
The 5A apoA-I Mimetic Peptide Inhibits Lung C-C Chemokine Expression in HDM-induced Asthma
Since the recruitment of inflammatory cells to the lungs of HDM-challenged mice was significantly reduced by the 5A apoA-I mimetic peptide, we investigated whether this effect might be mediated by a reduction in chemokine expression. CCL11 (eotaxin-1) and CCL24 (eotaxin-2) are important chemotactic factors for eosinophils and basophils, whereas CCL7 (MCP-3) has chemotactic activity towards eosinophils, basophils and monocytes via binding to CCR3(28, 31). CCL11 (eotaxin-1), CCL24 (eotaxin-2) and CCL7 (MCP-3) also mediate T cell recruitment to the lung in the setting of asthma(32). CCL17 (TARC), which is produced by dendritic cells and airway epithelial cells, also mediates the chemotaxis of Th2 T cells to the lung during allergic inflammation via CCR4(28, 33). As shown in Figure 5, mice that had been treated with the 5A apoA-I mimetic peptide had significantly reduced expression of mRNA encoding CCL7, CCL11, CCL17 and CCL24. This shows that a mechanism by which the 5A apoA-I mimetic peptide attenuates HDM-mediated increases in T cells and eosinophils is via the suppression of key C-C chemokines.
Figure 5. The 5A apoA-I mimetic peptide inhibits the expression of C-C chemokines in a murine model of house dust mite-induced asthma.
Quantification of lung mRNA levels for CCL7, CCL11, CCL17 and CCL24 by qRT-PCR presented as relative mRNA expression (n = 6 mice, * P < 0.01, HDM vs. Saline; ** P < 0.01, HDM vs. HDM + 5A). A representative result from three independent experiments is shown.
Administration of the 5A apoA-I Mimetic Peptide Does Not Inhibit Serum IgE
Since treatment with the 5A peptide was initiated prior to sensitization with HDM, we measured total serum IgE levels to assess whether the ability of 5A to attenuate airway inflammation in HDM-induced asthma was a consequence of inhibition of the allergic sensitization process. As shown in Figure 6, administration of the 5A peptide did not reduce total serum IgE levels. This suggests that the mechanism by which the 5A peptide attenuates airway inflammation in asthma does not involve impaired allergic sensitization to house dust mite antigens.
Figure 6. The 5A apoA-I mimetic peptide does not inhibit HDM-induced increases in serum IgE.
Quantification of serum IgE levels (n = 8, P = NS, HDM vs. HDM + 5A). A representative result from three independent experiments is shown.
The 5A apoA-I Mimetic Peptide Attenuates Alternative Macrophage Activation in HDM-challenged Mice
In contrast to the classical pathway of macrophage activation by IFN-γ, macrophages can undergo alternative activation by the Th2 cytokines, IL-4 and IL-13, in the setting of allergic and parasitic inflammation(34, 35). Consistent with this, alternatively activated macrophages (AAM) have been implicated in the pathogenesis of allergic asthma and mediate the recruitment of eosinophils to sites of parasitic infection(25, 34). Alternatively activated macrophages express the macrophage mannose receptor (Mrc1, CD206), as well as Ym1/2 (Chi3L3 and Chi3L4), arginase 1, and resistin-like-α (Fizz1/RELM-α)(36). The macrophage mannose receptor is a C-type lectin that binds pathogenic viruses, bacteria and fungi(37). Chi3L3 (Ym1, eosinophil chemotactic factor L) and the closely related Chi3L4 (Ym2), are enzymatically inactive chitinases that function as eosinophil chemotactic factors in asthma(35, 38, 39). Arginase 1 converts arginine to ornithine and thereby reduces available substrate for inducible nitric oxide synthase(40). Fizz1 (resistin-like α̣) is up-regulated in parasitic pulmonary inflammation and functions as an endogenous negative regulator of Th2 inflammatory responses(41). Here, we show that treatment with the 5A apoA-I mimetic peptide attenuates the HDM-mediated increases in the total number of CD11b+/F4-80+/ CD206+ alveolar macrophages (Figure 7). Similarly, expression of mRNA encoding arginase 1, Chi3L3, Chi3L4 (data not shown), and Fizz1 were reduced in the lungs of HDM-challenged mice that were treated with the 5A apoA-I mimetic peptide. These data are consistent with the conclusion that the 5A apoA-I mimetic peptide inhibits the alternative activation of macrophages in house dust mite-challenged mice.
Figure 7. The 5A apoA-I mimetic peptide inhibits alternative macrophage activation in a murine model of house dust mite-induced asthma.
A. Quantification of the total number of CD11b+/F4-80+/CD206+ macrophages present in whole lung digests (n = 10, * P < 0.05 HDM vs. Saline; ** P < 0.05 HDM vs. HDM + 5A). Pooled data from two independent experiments are shown. B – D. Quantification of lung mRNA levels for arginase 1 (Arg1), Chi3L3, and Fizz1 (n = 6 mice, * P < 0.001, HDM vs. Saline; ** P < 0.001, HDM vs. HDM + 5A). A representative result from three independent experiments is shown.
Discussion
More than 22 million individuals in the United States have asthma(42). Of these, approximately 5% to 10% have severe disease that is difficult to control despite treatment with high doses of inhaled corticosteroids plus long-acting β2-agonists or oral corticosteroids(43, 44). Limited alternative treatment options exist for these individuals who are refractory to standard therapies. Additional controller medications that can be utilized as add-on therapy are limited to anti-IgE monoclonal antibodies and leukotriene modifiers, such as leukotriene receptor antagonists and 5-lipoxygenase inhibitors(42). Therefore, new treatment options are needed for asthmatics, especially for those with severe disease who experience significant morbidity and have high health care-related costs.
Apolipoprotein A–I, a major constituent of high density lipoproteins, can prevent and reverse atherosclerosis by mediating cholesterol efflux from lipid-laden macrophages(1, 3). ApoA-I also attenuates inflammation in atherosclerosis by removing pro-inflammatory oxidized phospholipids from low density lipoproteins and arterial cell walls(1, 45). ApoA-I has also been shown to have anti-inflammatory effects on a variety of cell types that play an important role in the pathogenesis of asthma, such as dendritic cells, T cells, neutrophils and macrophages. For example, apoA-I prevents dendritic cell maturation, reduces T lymphyocyte and neutrophil activation, suppresses macrophage cytokine production, and blocks T cell-monocyte interactions(46–51). Taken together, these findings suggest that the anti-inflammatory effects of apolipoprotein A–I might be utilized in a therapeutic fashion to attenuate airway inflammation in asthma.
The expense and difficulty in preparing sufficient quantities of pure, pharmaceutical quality apoA-I protein have limited the development of apoA-I as a therapeutic agent(3). To address this problem, several apoA-I mimetic peptides that retain the beneficial effects of apoA-I and HDL on cholesterol efflux and atherosclerosis have been developed(1, 3). Consistent with this, administration of apoA-I mimetic peptides have been shown to have anti-inflammatory effects in models of atherosclerosis and cardiac ischemia-reperfusion injury, as well as to attenuate endothelial dysfunction(52–56). Furthermore, apoA-I mimetic peptides have demonstrated anti-inflammatory properties in murine models of viral infection and collagen-induced arthritis(57, 58).
Since airway inflammation plays a major role in the pathogenesis of asthma, we assessed whether administration of an apoA-I mimetic peptide could suppress inflammatory and immune responses in a HDM-challenge model of asthma. We utilized the 5A apoA-I mimetic peptide, which is a bihelical amphipathic peptide that mediates cholesterol efflux and reduces atherosclerosis via the ABCA1 transporter(3, 5, 59). Each helix is comprised of 18 amino acids linked by a proline(5). In contrast to other apoA-I mimetic peptides that are cytotoxic based upon their ability to insert into cell membranes and disrupt the lipid bilayer, the 5A peptide does not induce hemolysis of red blood cells(5). Here, we demonstrate that the 5A apoA-I mimetic peptide dramatically inhibits the induction of many of the key pathologic features of house dust mite-induced asthma, including airway inflammation and airway hyperreactivity. The 5A apoA-I mimetic peptide also reduced the severity of several key manifestations of airway remodeling, such as goblet cell hyperplasia and expression of the MUC5AC mucin gene expression and genes encoding type I and type III collagens. The ability of the 5A apoA-I mimetic peptide to inhibit airway inflammation was mediated by multiple mechanisms that included both the attenuated expression of Th2- and Th17-type cytokines, as well as the reduced expression of chemokines that promote the chemotaxis of T cells, dendritic cells, and eosinophils. Furthermore, the 5A apoA-I mimetic peptide inhibited the recruitment of alternatively activated macrophages to the lungs of HDM-challenged mice. In contrast, the 5A apoA-I mimetic peptide did not inhibit HDM-induced increases in serum IgE levels, which is consistent with the conclusion that the mechanism by which 5A attenuates asthma is not a consequence of impaired allergic sensitization.
The mechanism by which the 5A peptide mediates its inhibitory effects on the induction of asthma may be mediated by its interaction with the ATP-binding cassette (ABC) transporter A1 (ABCA1). Consistent with this, the 5A peptide has been shown to mediate enhanced lipid efflux from HeLa cells, as well as inhibit TNF-mediated NF-κB activation in vascular endothelial cells, in an ABCA1-dependent fashion(5, 62). ABCA1 is expressed by several cell types in the lung, including airway smooth muscle cells, type I and type II pneumocytes, and pulmonary macrophages(63–68). ABCA1 plays an important role in the maintenance of normal lung lipid composition, structure and function, as evidenced by a phenotype of cholesterol accumulation and alveolar proteinosis in ABCA1 knockout mice(64). An alternative mechanism by which the 5A apoA-I mimetic peptide may mediate its effects is via binding to pro-inflammatory proteins and lipids. For example, apoA-I can associate with lipopolysaccharide binding protein (LBP) and thereby allow HDL to neutralize bacterial lipopolysaccharides(69). An additional possibility is that the 5A peptide may interact with other apolipoprotein receptors that recognize apolipoprotein ligands containing amphipathic helical structures.
It is important to address several points regarding our study. First, the effects of the 5A peptide were accomplished at a dose of 1 mg/kg/day, which is significantly lower than the 30 mg/kg dose that has been utilized to promote reverse cholesterol transport in a murine model of atherosclerosis(59). Second, our model utilized a 4 week period of exposure to house dust mite to assess the effect of the 5A peptide on several key manifestations of airway remodeling. Additional experiments using models with longer periods of exposure to house dust mite could also be utilized to characterize the effects of the 5A peptide on additional manifestations of airway remodeling, such as angiogenesis(60). Third, we utilized an invasive measurement of airway resistance to determine the effects of the 5A peptide on airway hyperreactivity, rather than a non-invasive method, such as unrestrained plethysmography, which may not directly correlate with changes in airway resistance(61). Lastly, although our study was not designed to assess toxicity related to administration of the 5A peptide, no untoward effects were noted.
In summary, we have shown that administration of a 5A apoA-I mimetic peptide attenuates the induction of many of the key pathogenic features of house dust mite-induced asthma, including airway inflammation and airway hyperreactivity. These results identify apoA-I mimetic peptides, such as 5A, as a novel therapeutic strategy that could be developed to treat asthmatic patients who do not respond to standard therapies, such as those with severe asthma.
Acknowledgements
We are extremely appreciative of the staff of the NHLBI Laboratory of Animal Medicine and Surgery, whose commitment, professional advice and excellent technical support made this study possible. We are also very appreciative of the Pathology Core Facility, NHLBI for their assistance with the histopathological analyses. We are most appreciative of Drs. Joel Moss and Martha Vaughan for their helpful discussions.
Funding: Division of Intramural Research, NHLBI, NIH
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Navab M, Anantharamaiah GM, Reddy ST, Fogelman AM. Apolipoprotein A–I mimetic peptides and their role in atherosclerosis prevention. Nat Clin Pract Cardiovasc Med. 2006;3:540–547. doi: 10.1038/ncpcardio0661. [DOI] [PubMed] [Google Scholar]
- 2.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A–I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi AA, Amar M, Shamburek RD, Remaley AT. Apolipoprotein AI mimetic peptides: possible new agents for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2007;8:201–212. [PubMed] [Google Scholar]
- 4.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D'Souza W, Sviridov D, Remaley AT. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem. 2008;283:32273–32282. doi: 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Nofer JR, Remaley AT, Feuerborn R, Wolinnska I, Engel T, von Eckardstein A, Assmann G. Apolipoprotein A–I activates Cdc42 signaling through the ABCA1 transporter. J Lipid Res. 2006;47:794–803. doi: 10.1194/jlr.M500502-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Florentin M, Liberopoulos EN, Wierzbicki AS, Mikhailidis DP. Multiple actions of high-density lipoprotein. Curr Opin Cardiol. 2008;23:370–378. doi: 10.1097/HCO.0b013e3283043806. [DOI] [PubMed] [Google Scholar]
- 9.Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc Med. 2005;15:158–161. doi: 10.1016/j.tcm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Wadham C, Albanese N, Roberts J, Wang L, Bagley CJ, Gamble JR, Rye KA, Barter PJ, Vadas MA, Xia P. High-density lipoproteins neutralize C-reactive protein proinflammatory activity. Circulation. 2004;109:2116–2122. doi: 10.1161/01.CIR.0000127419.45975.26. [DOI] [PubMed] [Google Scholar]
- 11.Baker PW, Rye KA, Gamble JR, Vadas MA, Barter PJ. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J Lipid Res. 1999;40:345–353. [PubMed] [Google Scholar]
- 12.Sugatani J, Miwa M, Komiyama Y, Ito S. High-density lipoprotein inhibits the synthesis of platelet-activating factor in human vascular endothelial cells. J Lipid Mediat Cell Signal. 1996;13:73–88. doi: 10.1016/0929-7855(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 13.Xia P, Vadas MA, Rye KA, Barter PJ, Gamble JR. High density lipoproteins (HDL) interrupt the sphingosine kinase signaling pathway. A possible mechanism for protection against atherosclerosis by HDL. J Biol Chem. 1999;274:33143–33147. doi: 10.1074/jbc.274.46.33143. [DOI] [PubMed] [Google Scholar]
- 14.Robbesyn F, Auge N, Vindis C, Cantero AV, Barbaras R, Negre-Salvayre A, Salvayre R. High-density lipoproteins prevent the oxidized low-density lipoprotein-induced epidermal [corrected] growth factor receptor activation and subsequent matrix metalloproteinase-2 upregulation. Arterioscler Thromb Vasc Biol. 2005;25:1206–1212. doi: 10.1161/01.ATV.0000164805.73558.80. [DOI] [PubMed] [Google Scholar]
- 15.Robbesyn F, Garcia V, Auge N, Vieira O, Frisach MF, Salvayre R, Negre-Salvayre A. HDL counterbalance the proinflammatory effect of oxidized LDL by inhibiting intracellular reactive oxygen species rise, proteasome activation, and subsequent NF-kappaB activation in smooth muscle cells. FASEB J. 2003;17:743–745. doi: 10.1096/fj.02-0240fje. [DOI] [PubMed] [Google Scholar]
- 16.Brewer HB, Jr, Remaley AT, Neufeld EB, Basso F, Joyce C. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1755–1760. doi: 10.1161/01.ATV.0000142804.27420.5b. [DOI] [PubMed] [Google Scholar]
- 17.Getz GS, Wool GD, Reardon CA. Apoprotein A–I mimetic peptides and their potential anti-atherogenic mechanisms of action. Curr Opin Lipidol. 2009;20:171–175. doi: 10.1097/MOL.0b013e32832ac051. [DOI] [PubMed] [Google Scholar]
- 18.Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- 19.Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL, Santamarina-Fojo S, Brewer HB. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res. 2003;44:828–836. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Gomaraschi M, Calabresi L, Rossoni G, Iametti S, Franceschini G, Stonik JA, Remaley AT. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A–I mimetic peptides. J Pharmacol Exp Ther. 2008;324:776–783. doi: 10.1124/jpet.107.129411. [DOI] [PubMed] [Google Scholar]
- 21.Anantharamaiah GM, Mishra VK, Garber DW, Datta G, Handattu SP, Palgunachari MN, Chaddha M, Navab M, Reddy ST, Segrest JP, Fogelman AM. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A–I mimetic peptides. J Lipid Res. 2007;48:1915–1923. doi: 10.1194/jlr.R700010-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 23.Croy JE, Brandon T, Komives EA. Two apolipoprotein E mimetic peptides, ApoE(130–149) and ApoE(141–155)2, bind to LRP1. Biochemistry. 2004;43:7328–7335. doi: 10.1021/bi036208p. [DOI] [PubMed] [Google Scholar]
- 24.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A, Kearley J, Coyle A, Chupp G, Reed J, Flavell RA, Elias JA. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci U S A. 2001;98:5175–5180. doi: 10.1073/pnas.081510898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu HW, Halliday JL, Martin RJ, Leung DY, Szefler SJ, Wenzel SE. Collagen deposition in large airways may not differentiate severe asthma from milder forms of the disease. Am J Respir Crit Care Med. 1998;158:1936–1944. doi: 10.1164/ajrccm.158.6.9712073. [DOI] [PubMed] [Google Scholar]
- 28.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ying S, Robinson DS, Meng Q, Barata LT, McEuen AR, Buckley MG, Walls AF, Askenase PW, Kay AB. C-C chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-hour eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-hour tissue eosinophilia, and relationship to basophils and other C-C chemokines (monocyte chemoattractant protein-3 and RANTES) J Immunol. 1999;163:3976–3984. [PubMed] [Google Scholar]
- 32.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J. 2004;23:876–884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- 34.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 37.Kim SJ, Ruiz N, Bezouska K, Drickamer K. Organization of the gene encoding the human macrophage mannose receptor (MRC1) Genomics. 1992;14:721–727. doi: 10.1016/s0888-7543(05)80174-0. [DOI] [PubMed] [Google Scholar]
- 38.Greenlee KJ, Corry DB, Engler DA, Matsunami RK, Tessier P, Cook RG, Werb Z, Kheradmand F. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J Immunol. 2006;177:7312–7321. doi: 10.4049/jimmunol.177.10.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwashita H, Morita S, Sagiya Y, Nakanishi A. Role of eosinophil chemotactic factor by T lymphocytes on airway hyperresponsiveness in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 2006;35:103–109. doi: 10.1165/rcmb.2005-0134OC. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.N. A. E. P. National Heart, Lung, and Blood Institute, National Institutes of Health; Expert Panel Report 3: Guidelines for the Diagnosis and Managment of Asthma. 2007 U.S. Department of Health and Human Services.
- 43.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 44.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, Reddy ST, Sevanian A, Fonarow GC, Fogelman AM. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–1508. [PubMed] [Google Scholar]
- 46.Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, 3rd, Roux-Lombard P, Burger D. Apolipoprotein A–I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 47.Kim KD, Lim HY, Lee HG, Yoon DY, Choe YK, Choi I, Paik SG, Kim YS, Yang Y, Lim JS. Apolipoprotein A–I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem Biophys Res Commun. 2005;338:1126–1136. doi: 10.1016/j.bbrc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 48.Liao XL, Lou B, Ma J, Wu MP. Neutrophils activation can be diminished by apolipoprotein A–I. Life Sci. 2005;77:325–335. doi: 10.1016/j.lfs.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 49.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A–I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler Thromb Vasc Biol. 2009;29:843–849. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blackburn WD, Jr, Dohlman JG, Venkatachalapathi YV, Pillion DJ, Koopman WJ, Segrest JP, Anantharamaiah GM. Apolipoprotein A–I decreases neutrophil degranulation and superoxide production. J Lipid Res. 1991;32:1911–1918. [PubMed] [Google Scholar]
- 52.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buga GM, Frank JS, Mottino GA, Hakhamian A, Narasimha A, Watson AD, Yekta B, Navab M, Reddy ST, Anantharamaiah GM, Fogelman AM. D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a Western diet. J Lipid Res. 2008;49:192–205. doi: 10.1194/jlr.M700433-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Buga GM, Frank JS, Mottino GA, Hendizadeh M, Hakhamian A, Tillisch JH, Reddy ST, Navab M, Anantharamaiah GM, Ignarro LJ, Fogelman AM. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J Lipid Res. 2006;47:2148–2160. doi: 10.1194/jlr.M600214-JLR200. [DOI] [PubMed] [Google Scholar]
- 55.Van Lenten BJ, Wagner AC, Jung CL, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49:2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaziri ND, Moradi H, Pahl MV, Fogelman AM, Navab M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 2009;76:437–444. doi: 10.1038/ki.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charles-Schoeman C, Banquerigo ML, Hama S, Navab M, Park GS, Van Lenten BJ, Wagner AC, Fogelman AM, Brahn E. Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin Immunol. 2008;127:234–244. doi: 10.1016/j.clim.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 58.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM. D-4F, an apolipoprotein A–I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 59.Amar MJ, D'Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Remaley AT. 5A Apolipoprotein Mimetic Peptide Promotes Cholesterol Efflux and Reduces Atherosclerosis in Mice. J Pharmacol Exp Ther. 2010;334:634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee SY, Cho JY, Miller M, McElwain K, McElwain S, Sriramarao P, Raz E, Broide DH. Immunostimulatory DNA inhibits allergen-induced peribronchial angiogenesis in mice. J Allergy Clin Immunol. 2006;117:597–603. doi: 10.1016/j.jaci.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Bates J, Irvin C, Brusasco V, Drazen J, Fredberg J, Loring S, Eidelman D, Ludwig M, Macklem P, Martin J, Milic-Emili J, Hantos Z, Hyatt R, Lai-Fook S, Leff A, Solway J, Lutchen K, Suki B, Mitzner W, Pare P, Pride N, Sly P. The use and misuse of Penh in animal models of lung disease. Am J Respir Cell Mol Biol. 2004;31:373–374. doi: 10.1165/ajrcmb.31.3.1. [DOI] [PubMed] [Google Scholar]
- 62.Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, Barter PJ, Rye KA, Lambert G. The 5A apolipoprotein A–I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol. 30:246–252. doi: 10.1161/ATVBAHA.109.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, Rothblat GH, Bates SR. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L869–L878. doi: 10.1152/ajplung.00077.2003. [DOI] [PubMed] [Google Scholar]
- 64.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L980–L989. doi: 10.1152/ajplung.00234.2005. [DOI] [PubMed] [Google Scholar]
- 65.Bates SR, Tao JQ, Yu KJ, Borok Z, Crandall ED, Collins HL, Rothblat GH. Expression and biological activity of ABCA1 in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2008;38:283–292. doi: 10.1165/rcmb.2007-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawn RM, Wade DP, Couse TL, Wilcox JN. Localization of human ATP-binding cassette transporter 1 (ABC1) in normal and atherosclerotic tissues. Arterioscler Thromb Vasc Biol. 2001;21:378–385. doi: 10.1161/01.atv.21.3.378. [DOI] [PubMed] [Google Scholar]
- 67.Delvecchio CJ, Bilan P, Nair P, Capone JP. LXR-induced reverse cholesterol transport in human airway smooth muscle is mediated exclusively by ABCA1. Am J Physiol Lung Cell Mol Physiol. 2008;295:L949–L957. doi: 10.1152/ajplung.90394.2008. [DOI] [PubMed] [Google Scholar]
- 68.Delvecchio CJ, Bilan P, Radford K, Stephen J, Trigatti BL, Cox G, Parameswaran K, Capone JP. Liver X receptor stimulates cholesterol efflux and inhibits expression of proinflammatory mediators in human airway smooth muscle cells. Mol Endocrinol. 2007;21:1324–1334. doi: 10.1210/me.2007-0017. [DOI] [PubMed] [Google Scholar]
- 69.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]