Abstract
Transforming Growth Factor β (TGF-β) is crucial for valve development and homeostasis. The long form of Latent TGF-β binding protein 1 (LTBP1L) covalently binds all TGF-β isoforms and regulates their bioavailability. Ltbp1L expression analysis during valvulogenesis revealed two patterns of Ltbp1L production: an early one (E9.5–11.5) associated with endothelial to mesenchymal transformation (EMT); and a late one (E12.5-birth) contemporaneous with valve remodeling. Similarly, histological analysis of Ltbp1L−/− developing valves identified two different pathologies: generation of hypoplastic endocardial cushions in early valvulogenesis, followed by development of hyperplastic valves in late valvulogenesis. Ltbp1L promotes valve EMT, as Ltbp1L absence yields hypoplastic endocardial cushions in vivo and attenuated EMT in vitro. Ltbp1L−/− valve hyperplasia in late valvuogenesis represents a consequence of prolonged EMT. We demonstrate that Ltbp1L is a major regulator of Tgf-β activity during valvulogenesis since its absence results in a perturbed Tgf-β pathway that causes all Ltbp1L−/− valvular defects.
Keywords: LTBP1, TGF-β, EMT, valves
Introduction
Cardiac valvulogenesis is an intricate process, reflected in the high incidence of congenital malformations involving valve development and maturation (Supino et al., 2004; Pierpont et al., 2007). The onset of heart valve development is defined by the formation of endocardial cushions in the atrioventricular canal (AVC) and the outflow tract (OFT) of the primitive heart (Person et al., 2005). Endocardial cushions represent swellings composed primarily of extracellular matrix (ECM), produced by the myocardium and lined by the endocardium. Cushion development is initiated by myocardial signaling to the endocardium, resulting in endocardial cells undergoing an endothelial to mesenchymal transformation (EMT) and migrating into the intervening cardiac jelly (Armstrong and Bischoff, 2004). In mouse cardiac development, EMT in the AVC occurs around E9.5, whereas EMT in the OFT starts around E10.5 and is greatly reduced by E12.5 in both cardiac canals (Camenisch et al., 2002). Two endocardial cushions (superior and inferior) form within the AVC; they subsequently fuse, yielding mitral and tricuspid septal valves. The mural leaflets of the AV valves derive from mesenchymal cushions that arise laterally in the AVC after inferior and superior cushion fusion (de Lange et al., 2004). The semilunar valves, however, result from a complex arrangement of proximal and distal cushions of the OFT. In early valve development, undifferentiated mesenchymal cells migrate and rapidly proliferate in the cardiac jelly, but, as the cushions remodel into mature valve leaflets, the valvular mesenchymal cells proliferate less and become more differentiated and compartmentalized into discrete regions of the leaflet (Lincoln et al., 2004; Hinton et al., 2006). Proper cushion formation and maturation are important for other events in heart development, such as septation and chamber alignment. Also, even at an early stage of development, the cushion mesenchyme provides physical barriers that prevent blood regurgitation (Schroeder et al., 2003; Nomura-Kitabayashi et al., 2009). Hence, abnormalities in cushion development and function result in embryonic lethality and/or a variety of congenital heart diseases (CHD), including the misalignment between atria and ventricles, as well as ventricular and/or atrial septal defects (Mjaatvedt et al., 1998; Camenisch et al., 2000; Jiao et al., 2006). Thus, there is significant interest in understanding the mechanisms that underlie the complex process of valvulogenesis.
TGF-βs (TGFB1-3) were among the first cytokines described to be involved in valvulogenesis, especially in initiation and cessation of endocardial cushion EMT (Potts and Runyan, 1989; Boyer et al., 1999; Camenisch et al., 2002; Yang et al., 2008; Azhar et al., 2009). Crucial functions for TGF-β during EMT were first suggested by in vitro experiments using AV explants cultured in a 3D collagen matrix. In these assays, addition of antibodies blocking specific TGF-β isoforms or receptors showed that TGF-β signaling is essential for AV cushion EMT (Brown et al., 1996; Boyer et al., 1999; Camenisch et al., 2002). TGF-β2 and -3 isoforms are important for chick AV explant EMT, whereas only Tgf-β2 is crucial in the mouse system (Potts and Runyan, 1989; Camenisch et al., 2002). However, an essential role for Tgf-β in cardiac EMT was not confirmed in vivo, given that AV and OFT EMT occur in all single Tgfb isoform-specific knockout mouse strains (Azhar et al., 2003). Further investigation of in vivo participation of TGF-β ligands in cardiac cushion EMT revealed a role for Tgf-β2 in both initiation and cessation of EMT (Azhar et al., 2009). Tgf-β2 promotes EMT initiation, thus, in the earliest phase of AV cushion development, the number of mesenchymal cells in Tgfb2 nulls is decreased. However, in the late stages of AV cushion development, Tgf-β2 is important for EMT cessation; absence of Tgf-β2 in vivo causes prolonged EMT followed by development of enlarged cushions and hyperplastic valves (Bartram et al., 2001; Azhar et al., 2009).
TGF-βs are usually secreted as a tri-molecular complex, called the large latent complex (LLC). The LLC is composed of the mature TGF-β dimer, the TGF-β propeptide dimer (LAP; latency associated peptide) and a molecule of the latent TGF-β binding protein (LTBP) (Rifkin, 2005; Todorovic et al., 2005). LTBP1, 3, and 4 covalently bind latent TGF-β, regulating the cytokine’s secretion, ECM deposition and activation (Dabovic et al., 2002; Sterner-Kock et al., 2002; Annes et al., 2003; Todorovic et al., 2007). Deposition into the ECM is believed to be modulated primarily via the interaction of LTBPs and the microfibrillar protein fibrillin-1 (Hynes, 2009; Ramirez and Rifkin, 2009). LTBPs display structural and functional variability due to usage of different promoters, alternative splicing and post-translational modifications (Todorovic and Rifkin, 2008). LTBP1 exists in two major forms: long (-L) and short (-S), transcribed from independent promoters (Koski et al., 1999). In early mouse development only Ltbp1L is detected by Northern blotting, whereas Ltbp1S expression initiates in mid-gestation and becomes the predominant form in adult animals (Weiskirchen et al., 2003; Todorovic et al., 2007). Ltbp1 is important in mouse cardiac EMT as addition of Ltbp1-blocking antibodies to mouse AVC explants inhibited EMT in 3D collagen culture assays (Nakajima et al., 1997). The crucial role of Ltbp1 in heart development was confirmed when the long form of mouse Ltbp1 was inactivated by gene-targeting (Todorovic et al., 2007). Mice lacking Ltbp1L display cardiac developmental malformations, comprised of defective outflow tract (OFT), ventricular and atrial septation and faulty remodeling of pharyngeal arch arteries. These abnormalities present as persistent truncus arteriosus (PTA) and interrupted aortic arch (IAA) and are the underlying cause of post-natal morbidity of Ltbp1L−/− mice. However, 40% of Ltbp1L nulls die in utero, which cannot be accounted for by PTA and IAA defects. Ltbp1L expression in AV and OFT endocardial cushions prompted us to investigate the role of Ltbp1L in valvulogenesis.
Here we show that lack of Ltbp1L during embryonic development leads to abnormal AV cushion development, evident as hypoplastic endocardial cushions due to retarded EMT in early valvulogenesis, and as hyperplastic mitral and tricuspid valves, due to prolonged EMT in late valvulogenesis. Abnormal valve development in Ltbp1L nulls is accompanied by decreased Tgf-β activity in the remodeling mesenchyme, revealing a critical in vivo role for Ltbp1L as an extracellular regulator of Tgf-β activity during valvulogenesis.
Results
Ltbp1L Expression During Valvulogenesis
A promotorless β-galactosidase (lac Z) cassette was fused in-frame with the upstream coding sequence of Ltbp1L during gene targeting, allowing us to monitor reporter gene expression in an Ltbp1L-dependent fashion (Todorovic et al., 2007).
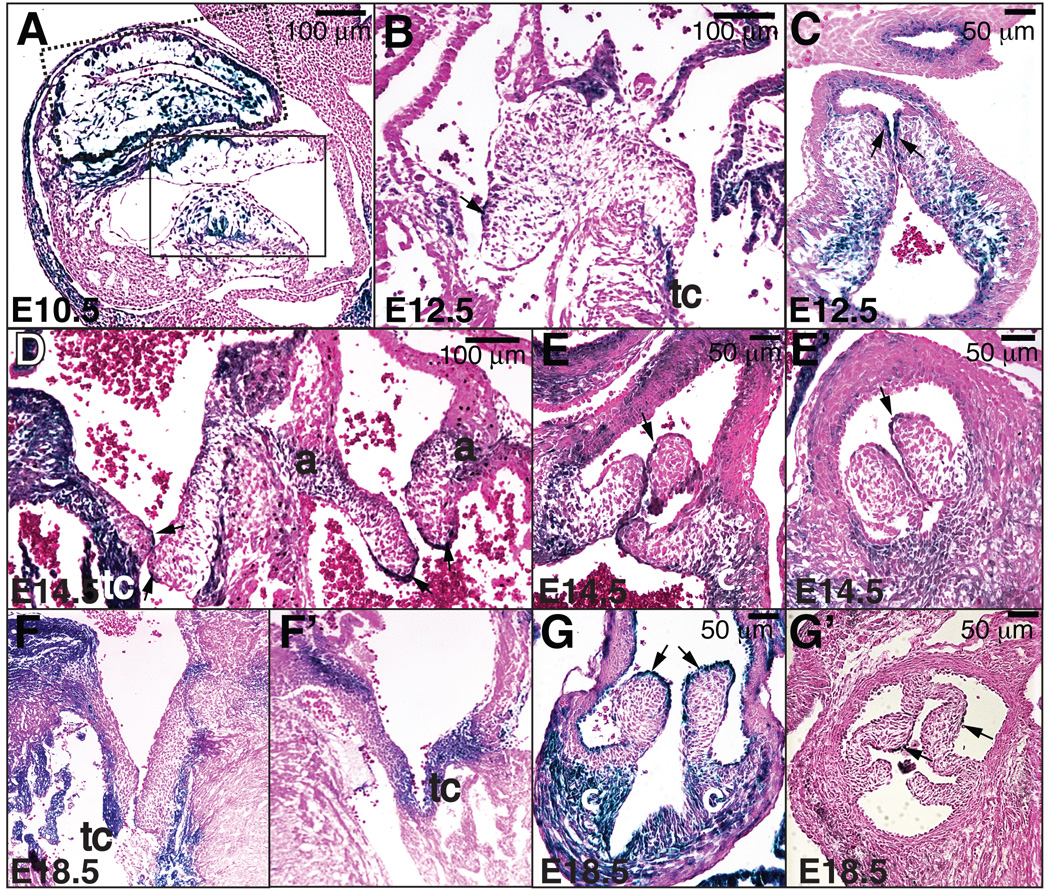
Ltbp1L cardiac expression was first detected at E9.5 and localized to a few myocardial cells underlying the AV and OFT cushions (Todorovic et al., 2007). From E10.5 through E11.5 Ltbp1L expression expanded concomitantly with the progression of EMT, gradually including all of the myocardium underlying endocardial cushions, cushion mesenchymal cells and some endocardial cells lining the cushions both in AVC (solid box area in Fig. 1A) and OFT (dotted box area in Fig. 1A). Mesenchymal cells that populate OFT endocardial cushions derive either from OFT endothelium or cardiac neural crest cells (CNCCs). Our expression analysis showed that all mesenchymal cells within the OFT endocardial cushions, regardless of their origin, express Ltbp1L. However, from E12.5 onwards the Ltbp1L expression pattern changed considerably, becoming reduced in AV and OFT mesenchymal cells (Fig. 1B-G’) and more pronounced in the valve support apparatus: e.g. the annulus (a), the tendinous cords (tc), and the connective tissue (c) supporting the semilunar valves. Ltbp1L expression in valve endothelial cells became more restricted and mostly localized to the tips of the remodeling valves (arrows in Fig. 1B-E’, 1G-G’). In summary, the Ltbp1L expression pattern during embryonic and fetal development suggested Ltbp1L involvement in cardiac EMT and valve remodeling.
Figure 1. Ltbp1L expression in the developing cardiac valves.
Lateral (A) and transverse sections (B-G’) of E10.5 (A), E12.5 (B-C), E14.5 (D-E’) and E18.5 (F-G’) Ltbp1L heterozygous hearts, stained with X-gal and Eosin. In E10.5 OFT (dotted box) and AV cushions (solid box), Ltbp1L is expressed by the myocardium underlying the cushions, mesenchymal cells within the cushions and some endocardial cells (A). From E12.5 onwards (B-G’), Ltbp1L expression within valvular mesenchyme decreases but becomes pronounced in the valve support apparatus: tendinous cords (tc), annulus (a), and connective tissue (c) supporting the semilunar valves. Arrows mark valvular endothelium expressing Ltbp1L.
Abnormal Valve Development in Ltbp1L−/− Mice
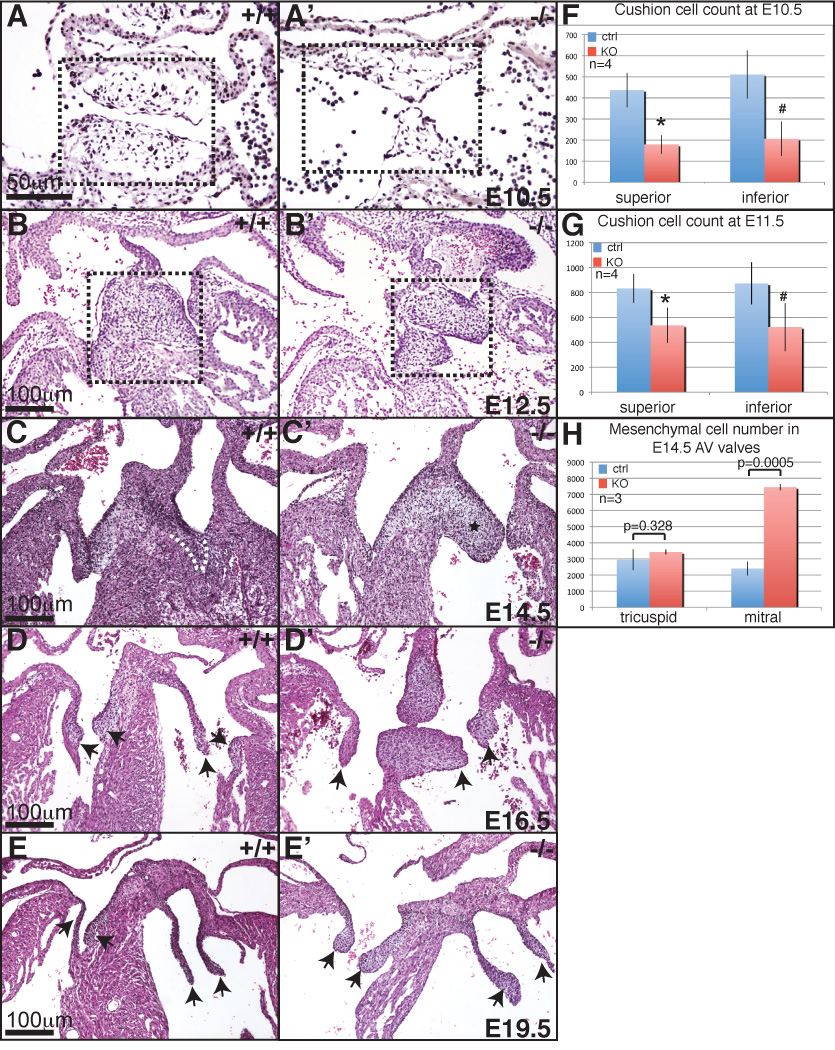
At 10.5 dpc Ltbp1L nulls were grossly indistinguishable from their control littermates (Todorovic et al., 2007) and their frequency was in accordance with a Mendelian distribution (see Table 1). However, detailed histological analysis revealed that all 10.5 dpc Ltbp1L−/− embryos analyzed (n=11) displayed decreased cellularity of AV endocardial cushions (compare Fig. 2A and 2A’, boxed areas). Quantification of mesenchymal cells during the EMT in early valvulogenesis (E10.5–11.5) in four pairs of age-matched control and mutant embryos confirmed that mesenchymal cell number in both inferior and superior AVC endocardial cushions in Ltbp1L mutants was reduced compared to control littermates (Fig. 2F-G).
Table 1.
Distribution and survival of Ltbp1L+/− X Ltbp1L+/− progeny during development
| Age | wt | het | ko | Total No. |
|---|---|---|---|---|
| E10.5 | 60 live + 2 dead (24.5%) |
120 live + 7 dead (50.2%) |
59 live+5 dead (25.3%) |
239 (100%) |
| E14.5 | 113 live (26.8%) |
228 live + 2 dead (54.7%) |
70 live +8 dead (18.5%) |
421 (100%) |
| Newborn | 137 (30.45%) |
245 (54.45%) |
68 (15.1%) |
450 (100%) |
Figure 2. Abnormal valve development in Ltbp1L−/− mice.
H-E stained lateral sections of E10.5 control (A) and mutant (A’) AV cushions. Note fewer mesenchymal cells in mutant AV cushions (box areas in A and A’). H-E stained transverse sections of E12.5, E14.5, E16.5 and E19.5 control (B, C, D, E) and Ltbp1L−/− (B’, C’, D’, E’) hearts. Box areas in B and B’ delineate AV cushions. In panels C-E’ mitral valves are on the right, tricuspid on the left of each section. E14.5 mutant mitral valve is hyperplastic (asterix in C’; control mitral valve is outlined in C). In older mutant embryos both mitral and tricuspid valves are hyperplastic (D’, E’). Arrows indicate valve leaflets. F-H. Quantification of mesenchymal cells in AV valves at 10.5 dpc (F), 11.5 dpc (G) and 14.5 dpc (H).
At E12.5 AV EMT is completed, the inferior and superior AV cushions fuse and valve remodeling initiates. However, as the process of AV EMT gradually subsides, the cellularity of Ltbp1L−/− AV cushions becomes comparable to that of the controls (Figure 2B and B’, boxed areas). Further advancement of valvulogenesis resulted in yet another valvular defect in Ltbp1L mutants. Histological analysis of E14.5 AV control and mutant hearts showed that nascent mitral, but not tricuspid valves in Ltbp1L nulls were thick and hyperplastic (compare Figure 2C and C’: the arterial mitral leaflet is outlined in 2C and labeled by an asterix in 2C’), which was confirmed by counts of the cushion cells in mitral and tricuspid valves (Figure 2H). The extent of mitral valve hyperplasia was variable among mutants; 18.2% of Ltbp1L nulls (4 out of 22) did not display any obvious pathology in AV valves whereas the remaining 81.8% (18 out of 22) of Ltbp1L−/− 14.5 dpc embryos displayed hyperplastic mitral valves with up to a 3 fold increase in mesenchymal cell number. A number of Ltbp1L−/− hearts surviving past E14.5 displayed hyperplastic mitral and tricuspid valves (Figure 2D-D’, E-E’, arrows indicate valve leaflets).
In summary, our histological analysis of developing AV valves in Ltbp1L nulls showed that the absence of Ltbp1L in AVC affects both initial and late phase of valve development, resulting in formation of hypoplastic endocardial cushions in early valvulogenesis followed by gradual development of hyperplastic mitral and, to some extent, tricuspid valves in late valve development.
Attenuated Initiation of EMT in AVC of Ltbp1L Mutants
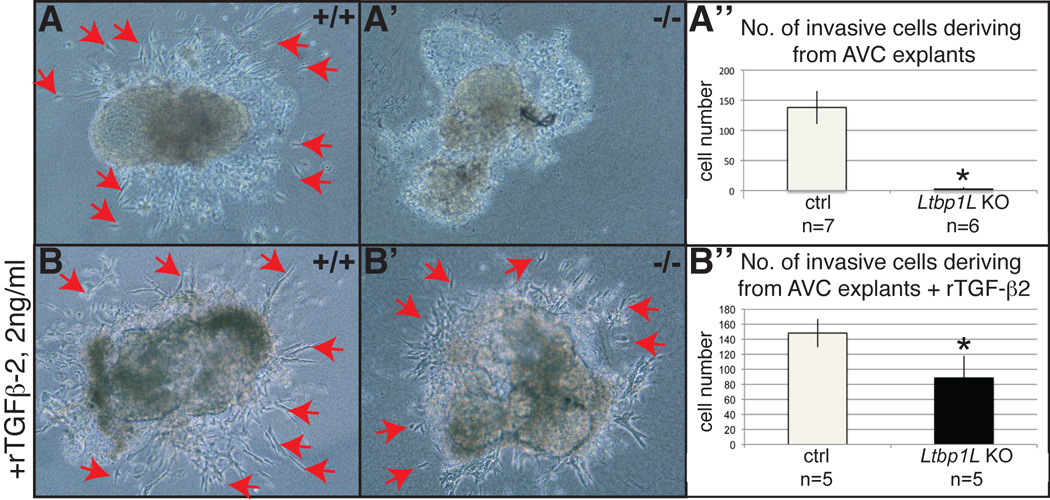
Early valvulogenesis in Ltbp1L nulls is characterized by a decreased mesenchymal cell number in AV endocardial cushions (compare outlined areas in Figure 2A and A’). Decreased cellularity of AV endocardial cushions can result from: a) decreased proliferation; b) increased apoptosis; c) slower EMT rate. To distinguish mechanistically amongst these possibilities, we first determined the number of proliferating Ltbp1L mutant and control mesenchymal cells by staining with antibody to phospho-histone 3 in E10.5 AVC. This analysis yielded equivalent values for mutant and control embryos (data not shown). Next, we stained E10.5 sections at the AVC level with antibody to active caspase-3, which visualizes apoptotic cells. We observed very few apoptotic cells in AVC in both control and mutant embryos (data not shown). Finally, we examined whether Ltbp1L was required for the initiation of EMT in AVC. We used an ex vivo assay with AVC explants isolated from E9.5 hearts, before the onset of EMT, cultured on a three-dimensional collagen gel lattice for 48 hours. Control explants (n=16) underwent EMT and produced a number of invasive mesenchymal cells (Fig. 3A, red arrows indicate mesenchymal cells invading 3D collagen). In contrast, endothelial cells in Ltbp1L−/− AVC explants (n=6) expanded forming a sheet of cobble stone-shaped cells, but failed to transform into invasive mesenchymal cells (Fig. 3A’). To determine whether Ltbp1L is required for ex vivo EMT as a regulator of TGF-β bioavailability or as an important component of the extracellular scaffold for mesenchymal cell migration, we performed a rescue experiment by adding 2 ng/ml of recombinant TGF-β2 to the explant cultures. Exogenous TGF-β restored the capacity of Ltbp1L−/− AVC explants (n=5) to undergo EMT (compare Figure 3B and B’). The average number of resulting invasive mesenchymal cells was increased to 60% of the controls (Figure 3B”).
Figure 3. Ltbp1L is necessary for EMT initiation ex vivo.
A-B’. Ex vivo AVC collagen gel assay of control (A, B) and Ltbp1L null (A’, B’) 9.5 dpc embryos, after 48 hours of explant culturing without (A-A’) or with (B-B’) recombinant TGF-β2 (2ng/ml). Control explants undergo EMT both in the absence or presence of rTGF-β2 in culture medium (A and B, red arrows indicate invading mesenchymal cells). Ltbp1L−/− AVC explants fail to spontaneously undergo EMT (A’) and addition of rTGF-β2 significantly restores their EMT potential (B’). Invasive mesenchymal cells deriving from AVC explants are quantified in A”, B”.
This result recapitulates the previously described finding where wild-type AV explants treated with anti-LTBP1 antiserum failed to undergo EMT and the addition of mature TGF-β reversed the inhibitory effect of anti-LTBP1 antibody (Nakajima et al., 1997), confirming that Ltbp1L exerts an important role in EMT initiation ex vivo, probably through its regulation of TGF-β activity.
Altogether, our results suggest that lack of Ltbp1L in vivo results in attenuated EMT initiation and, consequently, reduced cellularity of endocardial cushions in early valve development.
Hyperplastic Mitral Valves in Ltbp1L Nulls at Mid-gestation
At E14.5, 81.2 % of Ltbp1L nulls display thick and hyperplastic mitral valves (compare Figure 2C and C’, quantified in 2H), whereas the cellularity of tricuspid valves is similar to those of control littermates. Ltbp1L−/− embryos that did not show any obvious pathology of AV valves at mid-gestation were not included in our further studies.
Development of hyperplastic mitral valves at E14.5 can result from: a) increased proliferation of valve mesenchymal cells; b) decreased apoptosis of valvular mesenchymal cells or c) prolonged transformation of valve endothelial cells into valve mesenchymal cells (EMT). We estimated the proliferation index in AVC in three pairs of age-matched control and Ltbp1L−/− embryos. Two out of three Ltbp1L nulls showed slightly higher proliferation index in both mitral and tricuspid valves (Supplemental Fig. 1A); however, this increase in proliferation was not statistically significant suggesting that Ltbp1L−/− mitral valve hyperplasia does not stem from increased proliferation. Staining of nascent AV valves with an anti-active caspase-3 antibody visualized comparable numbers of apoptotic cells in mutant and control valves (Supplemental Fig. S1B), suggesting that decreased apoptosis does not represent the cause of Ltbp1L−/− mitral valve hyperplasia.
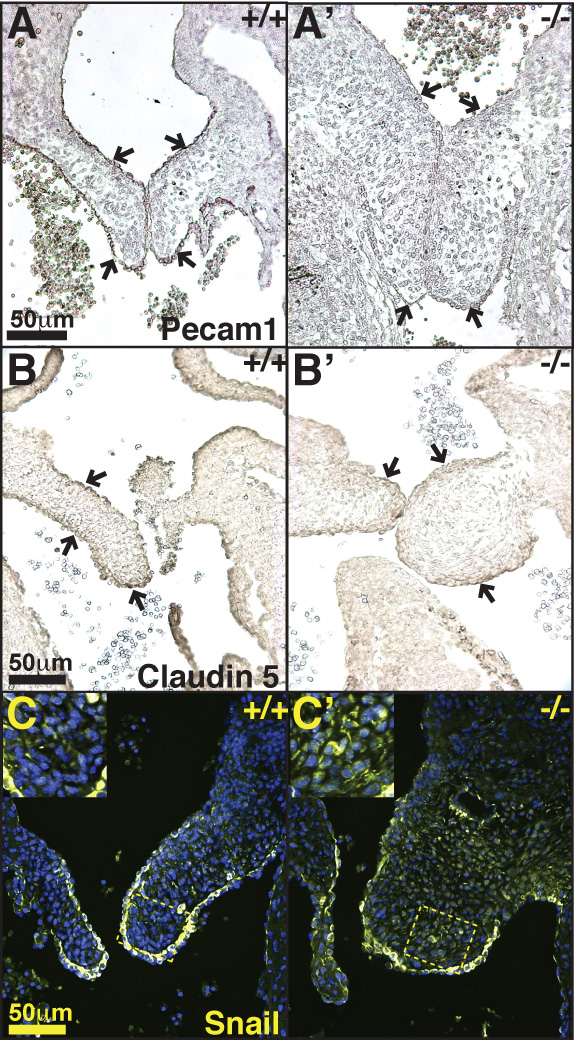
Vascular endothelial cells express various markers, including Pecam1 and claudin 5 (Cldn5) (Kokudo et al., 2008; Azhar et al., 2009). During heart EMT, Pecam1 and claudin 5 expression in AV cushion endothelium is reduced (Kokudo et al., 2008). However, upon cessation of endocardial EMT (E12.5–13.5), the pre-EMT level of endothelial marker expression within the valvular endothelium is re-established. We visualized the distribution of Pecam1 and claudin 5 in E14.5 control and Ltbp1L−/− heart sections (Figure 4A-A’, B-B’). The control hearts displayed strong staining of valve endothelium (Figure 4A, B), in agreement with cessation of cardiac EMT and re-establishment of endothelial expression program. However, both endothelial markers were poorly expressed in Ltbp1L−/− AV valves (compare Figure 4A and A’, B and B’; arrows indicate mitral valves’ endothelium), suggesting that Ltbp1L−/− AV valve endothelium might still be progressing through the EMT. Of note, on the same tissue sections Pecam1 and claudin 5 immunostaining easily visualized Ltbp1L−/− endothelial cells lining the dorsal aorta (Supplemental Figure S2A-A’) and the atria (not shown), indicating that the lack of Ltbp1L had a localized effect on AV valve endothelium gene expression pattern. Furthermore, endothelial marker expression by valvular endothelium in Ltbp1L nulls at later stages of development resembles that of the controls, as revealed by claudin 5 immunostaining at E16.5 (Supplemental Figure S2B-B’). This result implies that the endothelial gene expression program in Ltbp1L−/− valves recovers to the normal level over a longer period of time.
Figure 4. Ltbp1L is necessary for EMT cessation in mid-gestation.
A-B’. Distribution of endothelial markers Pecam1 (A, A’) and claudin 5 (B, B’) in E14.5 AV valves. Arrows indicate valvular endothelium. Note decreased expression of endothelial markers in Ltbp1L mutants (A’, B’). C-C’. Distribution of EMT marker transcription factor Snail in control (C) and mutant (C’) E14.5 mitral valves. Note increased Snail staining in mutant mitral valves.
Transcription factor Snail expression is associated with EMT (Batlle et al., 2000; Cano et al., 2000). We analyzed Snail distribution in E14.5 wild type and Ltbp1L−/− AV valves. In control mitral valves Snail was visualized mostly in valvular endothelium (Fig. 4C), whereas in Ltbp1L−/− hyperplastic mitral valves Snail was abundantly detected in mesenchymal cells in addition to the endothelium (Figure 4C’).
Taken all together, our results suggest that mitral valve hyperplasia in Ltbp1L nulls at mid-gestation is a consequence of prolonged EMT rather than disregulation of proliferation and/or apoptosis of valve mesenchymal cells.
Tgf-β Signaling During Valve Development of Ltbp1L Nulls
LTBP1L binds all three isoforms of latent TGF-β and regulates their bioavailability (Todorovic et al., 2005). We previously demonstrated that Ltbp1L deficit during mouse development causes attenuation of Tgf-β signaling in cardiac neural crest cells (CNCC), which adversely affects CNCC function and leads to aberrant septation of the cardiac OFT and remodeling of the associated vessels (Todorovic et al., 2007). Hence, we predicted that lack of Ltbp1L during murine valvulogenesis would affect Tgf-β activity within the forming valvular apparatus.
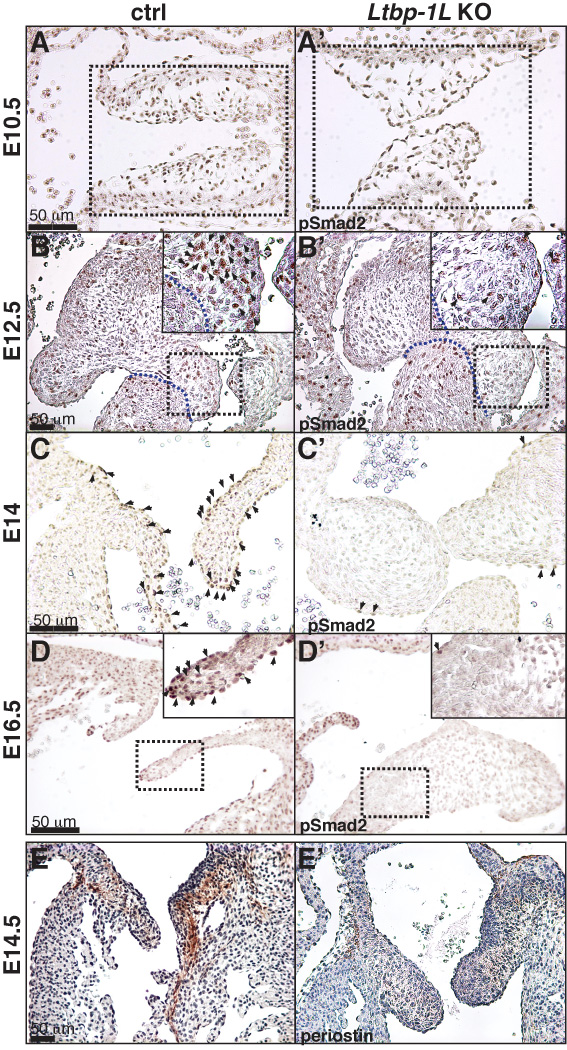
We examined Tgf-β activity in forming valves in Ltbp1L−/− mice by following the localization of phosphorylated Smad2 (pSmad2) and the distribution of periostin (Postn), whose expression is positively regulated by Tgf-β (Horiuchi et al., 1999; Li et al., 2006; Cohn et al., 2007). Smad2 is a key molecule involved in propagation of Tgf-β signals. Upon Tgf-β binding to its receptors, Smad2 is phosphorylated and chaperoned to the nucleus, where it participates in transcriptional regulation (ten Dijke and Hill, 2004). During valve formation and modeling, pSmad2 is easily visualized in many nuclei within the forming valvular apparatus and surrounding myocardium (Figure 5A-D’). In early valvulogenesis, during EMT, the canonical Tgf-β signaling pathway is unaffected in Ltbp1L nulls, as no difference in pSmad2 staining is evident despite clear histological difference of AVC cushions between 10.5 dpc control and Ltbp1L−/− embryos (Figure 5A, A’). However, from E12.5 onwards, a decrease in pSmad2 immunostaining was detected in Ltbp1L−/− forming valves but not in the surrounding myocardium (Figure 5B-D’), suggesting a localized attenuation of Tgf-β signaling within Ltbp1L−/− endocardial cushions and remodeling valves.
Figure 5. Tgf-β signaling during valve development of Ltbp1L nulls.
Distribution of pSmad2 in E10.5 (A, A’), E12.5 (B, B’), E14 (C, C’) and E16.5 (D, D’) control (A, B, C, D) and Ltbp1L−/− (A’, B’, C’, D’) AVC. Box areas in A and A’ represent AV cushions (superior upper, inferior lower). Box areas in B, B’, D and D’ are magnified to show mesenchymal cell-specific attenuation of Smad2 phosphorilation. Arrows indicate nuclear accumulation of pSmad2. E-E’. Immunolocalization of periostin in control (E) and Ltbp1L−/− (E’) E14.5 mitral valves.
TGF-β increases periostin expression in many cell types (Horiuchi et al., 1999; Li et al., 2006; Cohn et al., 2007). In the developing murine heart, periostin expression is first observed in the AVC and OFT cushions and, after the EMT has been completed, strong periostin expression is detected within developing leaflets and valvular support apparatus (Norris et al., 2008; Snider et al., 2008). We visualized the distribution of periostin by immunohistochemistry in E14.5 developing hearts and found a marked reduction in periostin staining in Ltbp1L−/− valvular apparatus (Figure 5E, E’). This result reinforces our observation of decreased Tgf-β signaling within the forming AV valves in Ltbp1L−/− embryos.
Expression and Distribution of Tgf-β in Mid-gestational Hearts
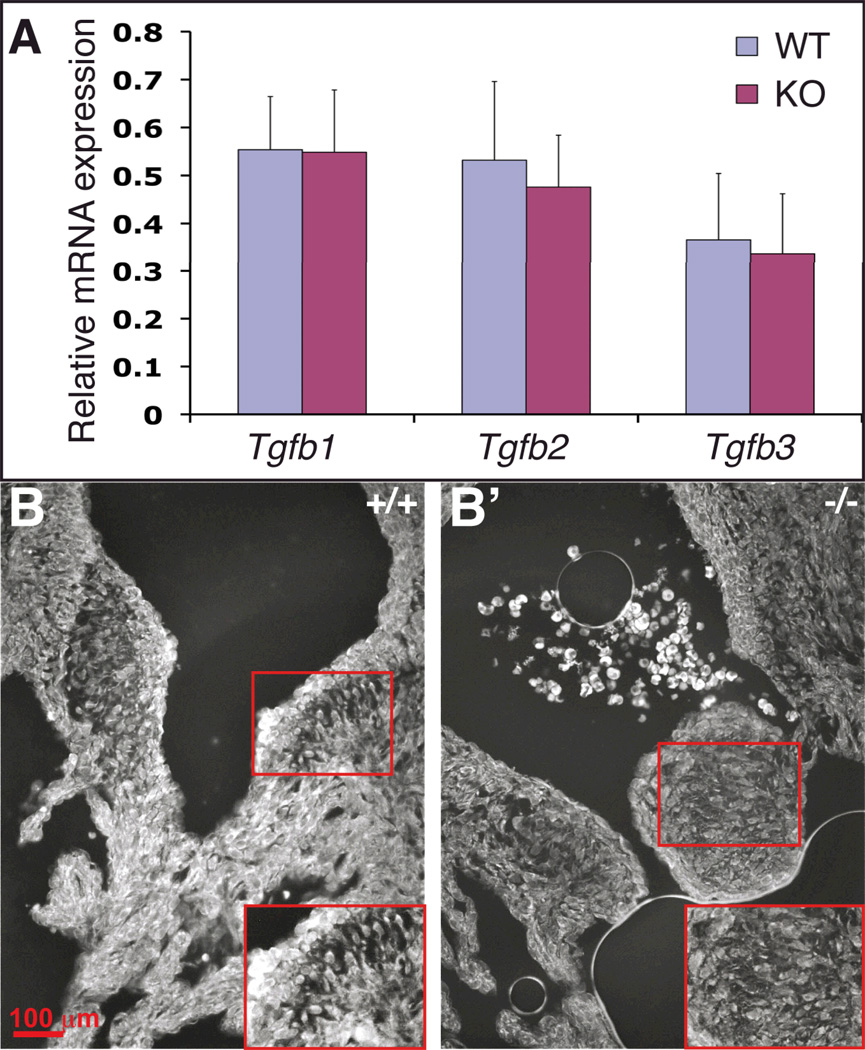
Decreased Tgf-β signaling during late valvulogenesis in Ltbp1L nulls can result from decreased Tgf-β production, secretion and/or activation. First, we analyzed the expression of Tgfb isoforms in E14.5 control and mutant hearts by Q-RT-PCR. The expression levels of Tgfb isoforms in mutant and control hearts were comparable (Figure 6A). However, as the source of cDNA for the Tgfb expression study was E14.5 whole hearts, our analysis might not have detected subtle changes in expression in localized regions of the heart (i.e. the valves). Hence, we studied Tgf-β2/3 distribution in E14.5 control and Ltbp1L−/− valves by immunofluorescence. The assay revealed a moderate decrease in Tgf-β2/3 distribution in E14.5 Ltbp1L−/− valves (compare Figure 6B and B’). This result is in agreement with the previously observed decrease in canonical Tgf-β signaling in late valvulogenesis.
Figure 6. Tgfb expression and distribution in E14.5 wild type and Ltbp1L−/− hearts.
(A) Quantification of Tgfb1, Tgfb2 and Tgfb3 expression by Q-RT-PCR, using cDNA isolated from 4 sets of E14.5 control (blue) and KO (burgundy) hearts.
(B-B’) Immunofluorescent staining of E14.5 AV valves using an antibody that preferentially recognizes Tgf-β2 and cross-reacts with Tgf-β3 isoform (image fields outlined by red squares are expanded to visualize mesenchymal cells in control (B) and Ltbp-1L null (B’)).
Ltbp1L−/− Semilunar Valves are Thick and Hyperplastic
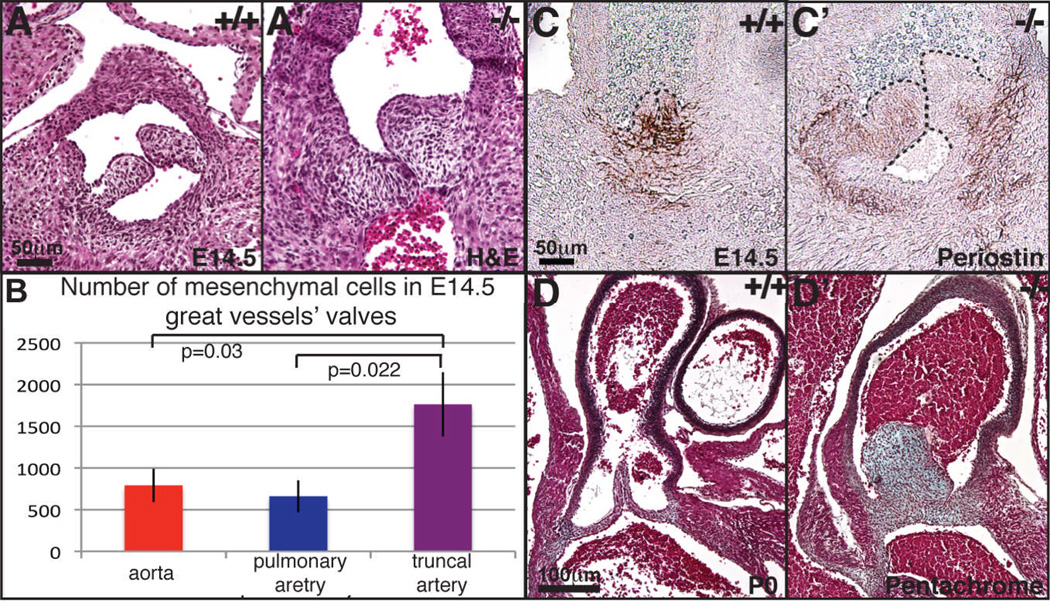
Ltbp1L−/− embryos develop one instead of two great vessels at the arterial pole of the heart, yielding PTA (Todorovic et al., 2007). This unique outflow vessel possesses a valvular apparatus comprised of three semilunar leaflets (data not shown). In the early stages of semilunar valve development, during EMT and CNCC invasion of the OFT, the number of mesenchymal cells in the OFT of Ltbp1L nulls is comparable to that of control embryos (Todorovic et al., 2007). However, at midgestation, Ltbp1L nulls develop thick and hyperplastic semilunar valves (Figure 7A-A’, C-C’). We quantified E14.5 semilunar mesenchymal cells in serial sections of nascent aorta and pulmonary artery in control embryos and truncus arteriosus of Ltbp1L nulls. Our analysis showed that truncal semilunar valves contain at least two times more mesenchymal cells than aortic or pulmonary semilunar valves in control embryos (Figure 7B). The higher number of cells in mid-gestational Ltbp1L−/− semilunar valves is accompanied by attenuated Tgf-β signaling, as judged by expression of Tgf-β responsive gene periostin (Figure 7C-C’). Thick and hyperplastic semilunar valves persist until birth (Figure 7D, D’).
Figure 7. Ltbp1L nulls develop hyperplastic semilunar valves.
H-E staining of E14.5 transverse sections of control aortic (A) and Ltbp1L−/− truncal (A’) semilunar valves. Mesenchymal cells within the semilunar valves in nascent aorta and pulmonary artery in control and truncus arteriosus in Ltbp1L nulls are quantified in serial sections of three pairs of control and mutant littermates (B). Transverse E14.5 sections of control (C) and Ltbp1L−/− (C’) semilunar valves stained for periostin (Postn), a TGF-β response gene. Pentachrome staining of newborn semilunar valves of pulmonary aorta (D) and truncus arteriosus (D’) revealed thicker semilunar valves with an excess of valvular ECM in Ltbp1L−/− nulls.
Discussion
This study describes a novel and complex role for Ltbp1L in cardiac valvulogenesis, based on developmental analysis of Ltbp1L-deficient mice. Ltbp1L expression studies showed that Ltbp1L is expressed within the nascent valvular apparatus throughout valvulogenesis. We observed two distinct patterns of expression, an early one (from E9.5 to E11.5) concomitant with endocardial EMT and involving all components of endocardial cushions: myocardium, endocardium and nascent mesenchymal cells, and a late one (from E12.5 to birth) associated with valve remodeling including valve support apparatus and specific regions of valve endothelium. Histological analysis of developing valves in Ltbp1L nulls demonstrated a similar two-phase sequence of pathologies, starting with formation of hypoplastic AV endocardial cushions in early valvulogenesis, during EMT, and continuing with gradual development of hyperplastic valves in late valvulogenesis. We demonstrate that Ltbp1L promotes AVC EMT because its absence leads to hypoplastic endocardial cushions in vivo and attenuated EMT in vitro, with no apparent effect on cellular proliferation or apoptosis. Conversely, in mid-gestation, Ltbp1L participates in controlling EMT cessation, as its loss yields hyperplastic mitral valves.
Only 60% of Ltbp1L nulls are recovered at birth, suggesting embryonic lethality. Our survival analysis during development revealed that 40% of mutant embryos die in late embryonic and fetal development (Table 1). Given the severity of nascent valve defects in some Ltbp1L mutants we analyzed, we speculate that valve dysfunction and, consequently, heart failure might be the cause of prenatal mortality in Ltbp1L nulls.
Since Ltbp1L covalently interacts with latent Tgf-β and regulates its ECM localization and activation (Ramirez and Rifkin, 2009), we predicted that defective valvulogenesis arising in the absence of Ltbp1L is due to anomalous Tgf-β activity. Indeed, we demonstrate that addition of active TGF-β2 to Ltbp1L−/− AVC explant cultures significantly restores the explants’ EMT potential, suggesting that requirement for Ltbp1L in AVC EMT is TGF-β-mediated at least in part. Next, we show decreased phosphorylation of Smad2, a key transmitter of Tgf-β signaling, and decreased amounts of periostin, a protein whose expression is positively regulated by TGF-β, in remodeling valves from 12.5 dpc onward. We show that Ltbp1L represents a crucial component of valve ECM that orchestrates Tgf-β activity during valvulogenesis.
Numerous reports have emphasized the role of TGF-β signaling in valvulogenesis. TGF-βs transduce their signals from the membrane to the nucleus by bringing together two dimers composed of type I (ALK5 or TGFBR1) and type II (TGFBR2) receptors. Upon ligand binding, TGFBR2 phosphorylates and activates the type I receptor which, in turn, phosphorylates SMAD2 and/or SMAD3. PSMAD2/3 are chaperoned to the nucleus by SMAD4, where they interact with other transcription factors and regulate gene expression. In most cell types, TGF-β binds to a tetrameric receptor complex composed of a TGFBR2 dimer and an ALK5 dimer. However, in endothelial cells, TGF-β can signal through two different Type I receptors: TGFBR1 and ALK1 (ACVRL1)(Oh et al., 2000; Goumans et al., 2002; Goumans et al., 2003). Moreover, TGF-β ligation to ALK5/ALK1/TGFBR2 complex elicits phosphorylation of two different SMAD pathways: SMAD2/3 and SMAD1/5 (Goumans et al., 2003). Endothelial-specific deletion of different TGF-β receptors indicated their importance in endocardial EMT, but also created some confusion in the field. Endocardial cells lacking the Alk5 receptor do not go through EMT both in vivo and in vitro (Wang et al., 2005; Mercado-Pimentel et al., 2007; Sridurongrit et al., 2008), suggesting a crucial role for Alk5 in endocardial EMT. Defect in AVC EMT in Acvrl1 nulls has not been described (Oh et al., 2000), indicating that the Alk1 receptor does not play a role in endocardial EMT. However, endothelium-specific attenuation of TgfbrII expression inhibits EMT only in vitro, as no effect on endocardial EMT was observed in vivo (Jiao et al., 2006). Jiao and colleagues offered two scenarios to explain the discrepancy between in vivo and in vitro studies: 1) Tgf-β1-3 may act through another type II receptor, though so far only one TgfbrII has been identified; or 2) Tgf-β signaling during endocardial EMT can be compensated for by other members of Tgf-β superfamily, such as Bmp2, though such compensation mechanism has not been seen in endothelium-specific Alk5 nulls. Growing evidence suggests that Alk2 (Acvr1) might represent a good candidate for Tgf-β-binding in endocardial cushions for several reasons: 1) ALK2 can mediate TGF-β signals in some cell types (Miettinen et al., 1994; Lai et al., 2000); 2) deletion of Alk2 receptor from endothelial cells results in abrogation of endocardial EMT both in vivo and in vitro (Wang et al., 2005), and 3) Alk2-deficient endocardial cells display attenuated phosphorylation of both Bmp Smads 1/5/8 and Tgf-β Smads 2/3 (Wang et al., 2005).
Interestingly, like TgfbrII endothelium-specific nulls and Tgfb2 knockouts, Ltbp1L−/− embryos undergo endocardial EMT (though with retarded initiation), but show no EMT in AVC explant assays. Addition of recombinant TGF-β2 to Ltbp1L−/− explant cultures significantly rescues the process of EMT. Similarly, LTBP1-blocking antibodies inhibit endocardial EMT in vitro, and addition of recombinant TGF-β to AVC explants treated with anti-LTBP1 antibodies restores EMT (Nakajima et al., 1997). These results suggest that Ltbp1L exerts an important role in regulation of Tgf-β activity during endocardial EMT in vitro. Intriguingly, we observed that during AVC EMT Ltbp1L mutants develop hypoplastic endocardial cushions, a pathology shared also with Tgfb2 nulls and to some extent with Tgfbr2 Tie2-Cre deficient embryos, which develop hypoplastic inferior but not superior endocardial cushion (Jiao et al., 2006). An apparent discrepancy between in vitro and in vivo results in respect to initiation of endocardial EMT in Tgfbr2 Tie2-Cre, Tgfb2 and Ltbp1L null embryos might be due to somehow artificial dependence on Tgf-β activity in ex vivo system.
Formation of hypoplastic endocardial cushions in Ltbp1L nulls was not accompanied by obvious dysregulation of canonical Tgf-β signaling within the cushions. We investigated if the lack of Ltbp1L affected non-canonical Tgf-β signaling pathways involved in EMT, such as the MAP kinase pathway (Chaudhury and Howe, 2009). Immuno-fluorescent stainings for phospho-p38 and phospho-ERK in E10.5 AVC did not reveal any significant difference in the distribution of these signaling molecules between Ltbp1L−/− and control endocardial cushions (data not shown).
Our results and the results of others raise an interesting hypothesis that during endocardial EMT, Tgf-β signaling pathway might be unorthodox, involving receptors such as Alk5 and Alk2, and possibly a Type II receptor other than Tgfbr2. These receptor complexes might bind both Tgf-β and Bmp ligands and somehow balance between Bmp-specific pSmad1/5/8 and Tgf-β-specific pSmad2/3 signaling. It appears that initiation of endocardial EMT is more sensitive to a decrease in Bmp rather than in Tgf-β signals, since myocardium-specific inactivation of Bmp2 inhibits AVC EMT during mouse heart development (Ma et al., 2005; Sugi et al., 2004). However, a decrease in Tgf-β activity caused by lack of Tgf-β2 or Ltbp1L results in retarded initiation of endocardial EMT. The fact that this histopathological change is not accompanied by attenuation of Smad2/3 phosphorylation in Ltbp1L nulls raises an interesting idea that altered Smad1/5/8 signaling or other elements of Bmp signaling pathway might contribute to the phenotype of Ltbp1L−/− endocardial cushions. We intend to investigate this idea further in our future research.
All three Tgf-β isoforms are expressed in heart AVC and OFT during valvulogenesis, and, yet, only Tgfb2 nulls display abnormalities in valve development (Bartram et al., 2001; Azhar et al., 2009). Recent report by Azhar and colleagues (Azhar et al., 2009) demonstrated a role for Tgf-β2 in 1) promoting early-stage EMT and 2) down-regulation of EMT later in development, both in vitro and in vivo. Similar to Ltbp1L nulls, Tgf-β2-deficient embryos develop hypoplastic endocardial cushions in early valvulogenesis but in late valve development, develop hyperplastic mitral and tricuspid valves. We show that Tgfb2 expression in E14.5 Ltbp1L−/− hearts is normal but that Tgf-β2/β3 protein level is slightly decreased. Moreover, our pSmad2 stainings reveal that canonical Tgf-β signaling is significantly down-regulated from E12.5 onwards, thus suggesting that Ltbp1L’s role in regulation of Tgf-β2 availability and, possibly, the bioavailability of other Tgf-β isoforms, is mainly through its control of the secretion or/and activation process of latent Tgf-β.
An understanding of molecular mechanisms governing the regulation of TGF-β activity within the valvular apparatus is crucial to understanding of many heart valve diseases associated with dysregulation of TGF-β signaling, such as Marfan syndrome. Marfan syndrome (MFS) is an autosomal-dominant, systemic disorder of connective tissue, caused by mutations in fibrillin-1 (Fbn1) gene. Clinical manifestations of MFS include cardiac complications, such as aortic dissection and mitral valve prolapse and dysfunction (Judge and Dietz, 2008). Mice with different mutations in Fbn1 gene recapitulate all the clinical features of MFS, including the development of thick, hyperplastic and myxomatous mitral valves. These valves showed increased Tgf-β signaling and Tgf-β antagonism in vivo rescued the valve phenotype, suggesting a cause and effect relationship (Ng et al., 2004). We show that a decrease in Tgf-β signaling during heart development can also result in thick and hyperplastic valves. It is possible that both decrease and increase in Tgf-β signaling within cardiac valves can have a similar outcome, as it was demonstrated in other organs, such as the lungs (Neptune et al., 2003; Bonniaud et al., 2004; Dabovic et al., 2009). However, it is of note that thick mitral valves in Ltbp1L nulls are observed already at midgestation whereas in MFS mouse models this pathology is obvious only after birth. The different developmental stages at which Ltbp1L and Fbn1 mutants acquire thick mitral valves might be a consequence of decreased versus increased Tgf-β signaling, once again emphasizing the importance of precise, tight and sophisticated regulation of extracellular Tgf-β activity.
Experimental Procedures
Generation of Ltbp-1L−/− mice was previously described (Todorovic et al., 2007). Mice used in this study were of mixed background (SvEv129/C57Bl6/F1). Mice were used in accordance with Institutional Animal Care Use Committee Policies and Procedures (http://www.med.nyu.edu/dlar/policies/) at NYU School of Medicine.
X-gal staining
E10.5–18.5 embryos or embryonic hearts were stained with X-gal as described (Deckelbaum et al., 2006). In short, freshly dissected embryos were fixed in glutaraldehyde fixative (5 mmol/L EGTA, 2 mmol/L MgCl2, 0.1 mol/L NaPi pH 7.3, 0.1% (v/v) glutaraldehyde, 1.5% formaldehyde (v/v)) for 20 minutes to 2 hours. After three washes in PBS/0.02% NP-40, whole embryos or hearts were stained ON in freshly prepared staining solution (5 mmol/L K3Fe(CN)6, 5 mmol/L K4Fe(CN)6, 2 mmol/L MgCl2, 0.01% Na Deoxycolate, 0.02% NP-40, 1 mg/ml X-gal in PBS). Stained embryos were post-fixed in 4% PFA in PBS ON/+4°C and processed for paraffin embedding.
Histology and Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde for 12–16 h, dehydrated and embedded in paraffin. Sections (6–8 µm) were stained with Hematoxylin and Eosin or subjected to an immunostaining. The following primary antibodies were used for immunostainings: rabbit polyclonal to phospho–histone 3 (1:200), Cell Signaling; rabbit monoclonal to cleaved caspase 3 (1:200), Millipore; rabbit polyclonal to phospho-SMAD2 (1:300), Chemicon; rabbit polyclonal to periostin (1:300), Abcam; rat anti-mouse monoclonal to Pecam1 (CD31) (1:200), BD Pharmingen; rabbit polyclonal to Claudin 5 (1:100), Abcam; rabbit polyclonal to p-ERK1/2 (1:50), Santa Cruz Biotechnology; rabbit monoclonal to phospho-p38 MAPK (1:200), Cell Signaling; rabbit polyclonal to Snail (1:200), Abcam; and rabbit polyclonal to TGF-β2 (1:10), Santa Cruz. For phospho-histone 3, cleaved caspase 3, p-ERK1/2, phospho-p38 MAPK, Snail and periostin immunostanings antigen retrieval from paraffin sections was done in DakoCytomation Target Retrieval Solution, 20 minutes @95°C. For pSmad2 immunostainings antigen retrieval was performed in Na-citrate buffer pH6, heated 1 minute/full power and 3 minutes/10% power in a microwave oven. For Pecam1 and claudin 5 immunostainings antigen retrieval was performed by a short trypsine digestion of de-paraffinized tissue sections (0.1% trypsin in PBS, 1 min @37°C). Immunofluorescent stainings for Tgf-β2 were performed without antigen retrieval but in the presence of biotin-labelled Tyramide Signal Amplification reagent (Perkin Elmer), followed by streptavidin-Alexa Fluor incubation. The protocol for immunofluorescent staining with anti-Snail, -p-ERK1/2 and -phospho-p38 MAPK antibodies included a 15 min treatment with 100 mM CuSO4 to quench auto-fluorescence prior to a blocking step.
Quantification of mesenchymal cells in the AVC
The number of mesenchymal cells in the AVC of control and mutant E10.5, E11.5 and E14.5 littermates was determined in transverse and lateral sections every 12–16 µm. Four pairs of E10.5 and E11.5 and three pairs of E14.5 control and mutant littermates were used and the average numbers of these quantification were plotted. Quantifications of phospho-histone 3- and cleaved caspase 3- positive cells was performed identically.
AVC explant cultures
3D collagen gels (1mg/ml, type I rat tail collagen from BD) were prepared in OptiMEM supplemented with 1% fetal bovine serum (FBS, Atlanta Biologicals), 1xITS (insulin, transferrin and selenium, BRL-Gibco) and penicillin/streptomycin (1x, Invitrogen) as previously described (Sridurongrit et al., 2008). AV canals were dissected from E9.5 embryos, cut longitudinally to expose the lumen and placed on collagen gels. Additional media (DMEM + 1% FCS, with or without 2 ng/ml of rTGF-β2, R&D Systems) was added to the cultures 2 h later and incubation was continued under standard tissue culture conditions (37°C, 100% humidity, 8% CO2). The media was changed each day of culturing. Explants were photographed after 48–60 h of culturing, using Nikon digital sight DS-U1 camera and Nikon eclipse TS100 microscope.
cDNA Synthesis and Quantitative Real-Time RT-PCR Analysis
RNA was extracted from 4 pairs of E14.5 wild type and KO hearts using the RNeasy Protect Mini kit (Qiagen) with DNase treatment included. Reverse transcription was performed using 50 ng of RNA and the Sensiscript Reverse Transcriptase (Qiagen). The resulting cDNA was used for semi-quantitative and quantitative real-time PCR (Q-RT-PCR) analysis. Q-RT-PCRs were performed using specific primers and QuantiFast SYBR Green PCR Kit (Qiagen) on an iCycler Thermal Cycler (Bio-Rad). The amount of each gene was calculated using CT value and corresponding standard curve. Each target transcript expression was quantified relative to the b-actin gene. Primers used: Actβ sense AGCCTTCCTTCTTGGGTATGG, antisense GCCACCGATCCACACAGAGTA; TGF-β1 sense CAACAATTCCTGGCGTTACC, antisense AGCCCTGTATTCCGTCTCCT; TGF-β2 sense TGGCTTCACCACAAAGACAG, antisense GTGCCATCAATACCTGCAAA; TGF-β3 sense TGAATGGCTGTCTTTCGATG, antisense ACATGGACAGTGGATGCTGA. Annealing temperature was 60°C.
Supplementary Material
A) Proliferation index in E14.5 atrio-ventricular valves. Y axes represents the number of proliferating (phospho-histone 3-positive) mesenchymal cells per 1000 cells within AV valves. Horizontal black bars represent the average number of proliferating mesenchymal cells for each valve and genotype. B) Apoptotic index in E14.5 mitral valves. Y axes represents the number of apoptotic (activated caspase 3-positive) mesenchymal cells per 1000 cells within the mitral valves. Horizontal black bars represent the average number of apoptotic mesenchymal cells.
Immunostainings for endothelial markers Pecam1 (A, A’) and claudin 5 (B, B’) of the endothelium lining dorsal aorta in E14.5 control (A) and Ltbp1L null (A’) embryos and E16.5 control (B) and Ltbp1L mutant (B’) mitral valves. Arrows indicate endothelium.
Acknowledgments
We thank NYU Langone Medical Center Histology Core, which is supported in part by CCSG P30CA016087, Adam Chubak and John Ponessa for technical assistance, and Branka Dabovic and Joseph Ambrogio for critical reading of the manuscript.
Sources of Funding: NIH grants R01CA34282 and P01AR049698 to DBR.
References
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn. 2009;238:431–442. doi: 10.1002/dvdy.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Schultz Jel J, Grupp I, Dorn GW, 2nd, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003;14:391–407. doi: 10.1016/s1359-6101(03)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the Type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol. 1996;174:248–257. doi: 10.1006/dbio.1996.0070. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Chaudhury A, Howe PH. The tale of transforming growth factor-beta (TGFbeta) signaling: a soigne enigma. IUBMB Life. 2009;61:929–939. doi: 10.1002/iub.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol. 2009;219:14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Colarossi C, Obata H, Zambuto L, Perle MA, Rifkin DB. Bone abnormalities in latent TGF-[beta] binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-[beta] bioavailability. J Cell Biol. 2002;156:227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- Deckelbaum RA, Majithia A, Booker T, Henderson JE, Loomis CA. The homeoprotein engrailed 1 has pleiotropic functions in calvarial intramembranous bone formation and remodeling. Development. 2006;133:63–74. doi: 10.1242/dev.02171. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development. 2006;133:4585–4593. doi: 10.1242/dev.02597. [DOI] [PubMed] [Google Scholar]
- Judge DP, Dietz HC. Therapy of Marfan syndrome. Annu Rev Med. 2008;59:43–59. doi: 10.1146/annurev.med.59.103106.103801. [DOI] [PubMed] [Google Scholar]
- Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- Koski C, Saharinen J, Keski-Oja J. Independent promoters regulate the expression of two amino terminally distinct forms of latent transforming growth factor-beta binding protein-1 (LTBP-1) in a cell type-specific manner. J Biol Chem. 1999;274:32619–32630. doi: 10.1074/jbc.274.46.32619. [DOI] [PubMed] [Google Scholar]
- Lai YT, Beason KB, Brames GP, Desgrosellier JS, Cleggett MC, Shaw MV, Brown CB, Barnett JV. Activin receptor-like kinase 2 can mediate atrioventricular cushion transformation. Dev Biol. 2000;222:1–11. doi: 10.1006/dbio.2000.9698. [DOI] [PubMed] [Google Scholar]
- Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB, Conway SJ, McNamara CA, Sarembock IJ. Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis. 2006;188:292–300. doi: 10.1016/j.atherosclerosis.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Hubbard AD, Runyan RB. Endoglin and Alk5 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2007;304:420–432. doi: 10.1016/j.ydbio.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Miyazono K, Kato M, Takase M, Yamagishi T, Nakamura H. Extracellular fibrillar structure of latent TGF beta binding protein-1: role in TGF beta-dependent endothelial-mesenchymal transformation during endocardial cushion tissue formation in mouse embryonic heart. J Cell Biol. 1997;136:193–204. doi: 10.1083/jcb.136.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura-Kitabayashi A, Phoon CK, Kishigami S, Rosenthal J, Yamauchi Y, Abe K, Yamamura KI, Samtani R, Lo CW, Mishina Y. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, Potts JD, Goodwin RL, Markwald RR. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008;316:200–213. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989;134:392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–403. doi: 10.1007/s00109-003-0456-5. [DOI] [PubMed] [Google Scholar]
- Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev Biol. 2008;322:208–218. doi: 10.1016/j.ydbio.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Supino PG, Borer JS, Yin A, Dillingham E, McClymont W. The epidemiology of valvular heart diseases: the problem is growing. Adv Cardiol. 2004;41:9–15. doi: 10.1159/000079779. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Frendewey D, Gutstein DE, Chen Y, Freyer L, Finnegan E, Liu F, Murphy A, Valenzuela D, Yancopoulos G, Rifkin DB. Long form of latent TGF-beta binding protein 1 (Ltbp1L) is essential for cardiac outflow tract septation and remodeling. Development. 2007;134:3723–3732. doi: 10.1242/dev.008599. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Jurukovski V, Chen Y, Fontana L, Dabovic B, Rifkin DB. Latent TGF-beta binding proteins. Int J Biochem Cell Biol. 2005;37:38–41. doi: 10.1016/j.biocel.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Rifkin DB. TGF-beta Availability: Latent TGF- .beta and Latent TGF- beta Binding Proteins. Totowa, NJ, United States: Humana Press; 2008. p. 735. [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskirchen R, Moser M, Gunther K, Weiskirchen S, Gressner AM. The murine latent transforming growth factor-beta binding protein (Ltbp-1) is alternatively spliced, and maps to a region syntenic to human chromosome 2p21-22. Gene. 2003;308:43–52. doi: 10.1016/s0378-1119(03)00464-5. [DOI] [PubMed] [Google Scholar]
- Yang JH, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem Biophys Res Commun. 2008;374:512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Proliferation index in E14.5 atrio-ventricular valves. Y axes represents the number of proliferating (phospho-histone 3-positive) mesenchymal cells per 1000 cells within AV valves. Horizontal black bars represent the average number of proliferating mesenchymal cells for each valve and genotype. B) Apoptotic index in E14.5 mitral valves. Y axes represents the number of apoptotic (activated caspase 3-positive) mesenchymal cells per 1000 cells within the mitral valves. Horizontal black bars represent the average number of apoptotic mesenchymal cells.
Immunostainings for endothelial markers Pecam1 (A, A’) and claudin 5 (B, B’) of the endothelium lining dorsal aorta in E14.5 control (A) and Ltbp1L null (A’) embryos and E16.5 control (B) and Ltbp1L mutant (B’) mitral valves. Arrows indicate endothelium.