SUMMARY
UDP-galactofuranose (UDP-Galf) is a substrate for two types of enzymes, UDP-galactopyranose mutase and galactofuranosyltransferases, which are present in many pathogenic organisms but absent from mammals. In particular, these enzymes are involved in the biosynthesis of cell wall galactan, a polymer essential for the survival of the causative agent of tuberculosis, Mycobacterium tuberculosis. We describe here the synthesis of derivatives of UDP-Galf modified at C-5 and C-6 using a chemoenzymatic route. In cell-free assays, these compounds prevented the formation of mycobacterial galactan, via the production of short “dead-end” intermediates resulting from their incorporation into the growing oligosaccharide chain. Modified UDP-furanoses thus constitute novel probes for the study of the two classes of enzymes involved in mycobacterial galactan assembly, and studies with these compounds may ultimately facilitate the future development of new therapeutic agents against tuberculosis.
HIGHLIGHTS.
Novel UDP-furanoses, modified at C-5 or C-6, were chemoenzymatically prepared
UDP-furanose derivatives inhibit galactan assembly at concentrations as low as 9 μM
Inhibition of galactan synthesis is due to the formation of “dead-end” intermediates
These UDP-furanoses are new tools for probing mycobacterial cell wall assembly
INTRODUCTION
The development of new drugs against tuberculosis (TB) still presents a major challenge (Balganesh et al., 2008) due to the widespread distribution of the disease, the occurrence of multidrug and extensively drug-resistant strains of Mycobacterium tuberculosis (Chan and Iseman, 2008) and the deadly combination of HIV and TB infections (Corbett et al., 2003). In this area, a major target for drug development is the mycobacterial cell wall, and its biosynthesis has received increasing study in the past several years (Barry et al., 2007). At the core of this intricate structure is the mycolyl–arabinogalactan–peptidoglycan (mAGP) complex, which is composed of covalently linked peptides, heteropolymeric carbohydrates (peptidoglycan and arabinogalactan) and highly hydrophobic mycolic acids. The mAGP is a well validated target for drug development and some of the most effective antituberculotics prevent the formation of this component of the M. tuberculosis cell wall (Barry et al., 2007). The extremely impermeable cell wall efficiently protects pathogenic mycobacteria against multiple stress factors faced during the course of infection; at the same time, it constitutes a rather vulnerable structure, a true Achilles heel of the bacterium.
Among the four first-line anti-TB drugs, two impinge on the assembly of distinct structures of the mAGP. Isoniazid inhibits the production of mycolic acids (Takayama et al., 1972; Winder et al., 1971) and ethambutol blocks the generation of a complete arabinan domain (Mikusova et al. 1995; Takayama and Kilburn, 1989). The galactan portion of the mAGP represents yet another, and so far underexplored, potential target for anti-TB drug development due to its essential role for the viability of mycobacteria (Pan et al., 2001) and xenobiotic status of its building block – galactofuranose (Galf) – in the human host (Pedersen and Turco, 2003).
In a series of investigations performed by Brennan, McNeil and colleagues, the structure of the mAGP galactan has been established as a linear chain of about 30 alternating (1→5)- and (1→6)-linked β-D-Galf residues (Besra et al., 1995; Daffe et al., 1993; McNeil et al., 1987). The galactan region is linked to C-6 of N-glycolyl/N-acetyl muramic acid residues (Mahapatra et al., 2005; Mahapatra et al., 2005) of peptidoglycan via a linker disaccharide unit, α-L-Rhap-(1→3)-D-GlcpNAc-1-P (Daffe et al., 1990; McNeil et al., 1990). The arabinan portions of the mAGP are, in turn, attached to O-5 of the (1→6)-linked Galf residues close to the reducing end of the galactofuran (Alderwick et al., 2005) in the form of three highly branched chains, each consisting of 31 Araf residues (Bhamidi et al., 2008).
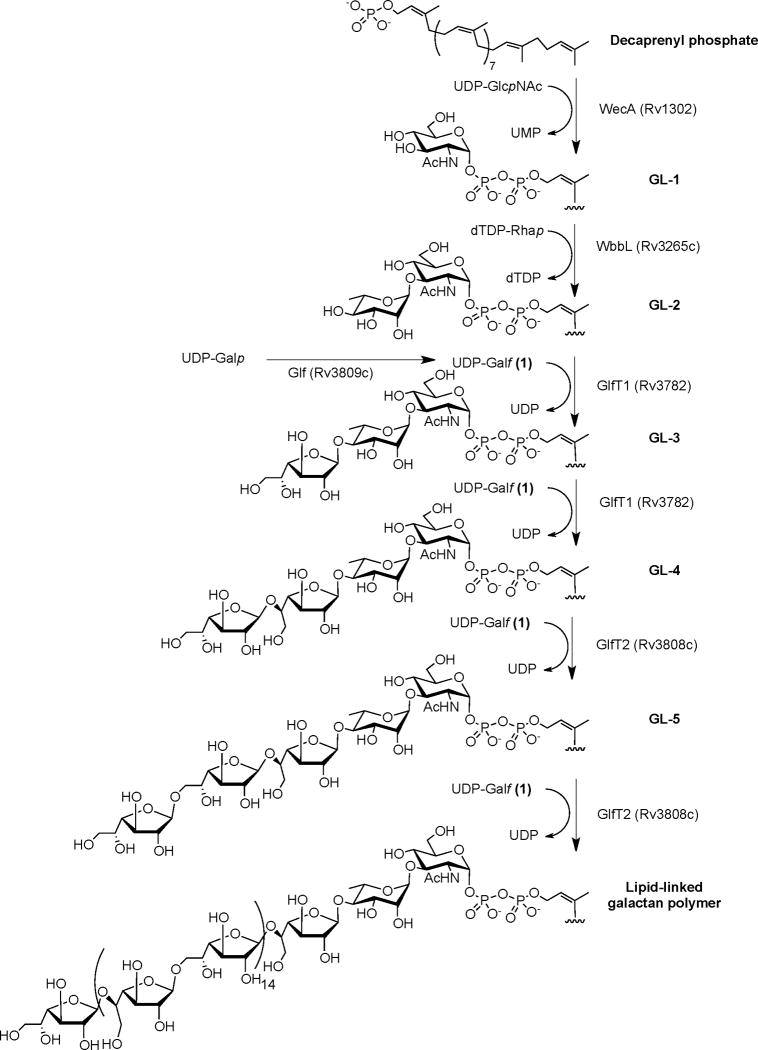
The biosynthesis of the galactan component of the mAGP has, to a large extent, been elucidated (Belanova et al., 2008). As illustrated in Figure 1, its assembly begins on decaprenyl-P-P-GlcpNAc-Rhap (GL-2), which is formed by the sequential action of two enzymes, a GlcNAc-1-phosphate-transferase (Rv1302) and a rhamnosyltransferase (Rv3265c) (Mikusova et al., 1996; Mills et al., 2004). We have shown that only two galactofuranosyltransferases, GlfT1 (Rv3782) and GlfT2 (Rv3808c), appear to account for full galactan synthesis (Belanova et al., 2008). The bifunctional enzyme GlfT1 attaches the first two Galf residues to the GL-2 intermediate forming both Galf-β-(1→4)-Rhap and Galf-β-(1→5)-Galf glycosidic bonds (Belanova et al., 2008; Mikusova et al., 2006). The product of this reaction, decaprenyl-P-P-GlcpNAc-Rhap-Galf-Galf (GL-4) serves as the direct substrate for the full galactan polymerization catalyzed by GlfT2, another dual acting enzyme with both β-(1→5) and β-(1→6) activity (Kremer et al., 2001; Mikusova et al., 2000; Rose et al., 2006). Galactofuranose residues for transferase reactions are donated by UDP-α-D-Galf (1, Figure 2), which is produced from UDP-α-D-Galp by the action of UDP-galactopyranose mutase (Glf, Rv3809c) (Weston et al., 1997; Soltero-Higgin et al., 2004).
Figure 1.
Metabolic pathway for biosynthesis of mycobacterial galactan. The pathway can be followed by in vitro reaction using mycobacterial enzyme fractions and UDP-[14C] Galp as a tracer. Glycolipids GL-1 to GL-5 are extracted into CHCl3–CH3OH (2:1), and lipid-linked galactan polymer into more polar solvents.
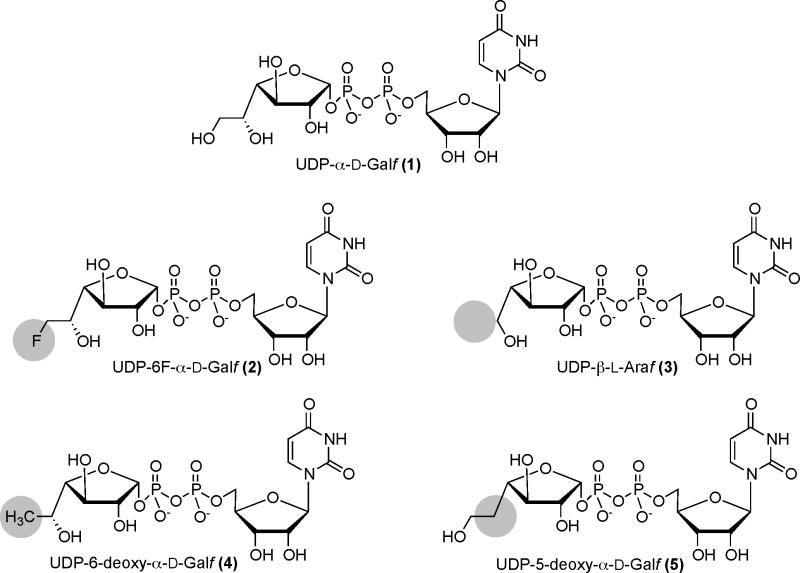
Figure 2.
Structure of UDP-Galf (1) and analogs 2–5 modified at C-5 or C-6
Despite the information presented above, questions remain about the specificity of GlfT1 and GlfT2 and more detailed investigations of these enzymes has been hampered by the lack of access to their donor and acceptor substrates. However, recently we have published procedures for the preparation of a panel of synthetic analogs that correspond to GL-2 and GL-3, the natural acceptor substrates for GlfT1, as well as analogs of GL-4 and GL-5, substrates for GlfT2 (Completo and Lowary, 2008). In addition, recent advances in the preparation of the donor substrate, UDP-α-D-Galf (Peltier et al., 2007; Timmons et al., 2008; Rose et al., 2008), have paved the way for further examination of these galactofuranosyltransferases, which will lead to better understanding of these intriguing bifunctional enzymes.
Synthetic substrate analogs, particularly deoxygenated and fluorinated compounds (Pongdee and Liu, 2004), are valuable tools for probing the mechanism of enzyme-catalyzed reactions. Many are good enzyme inhibitors, some of which have been developed into clinically useful chemotherapeutic agents (Pongdee and Liu, 2004). Recently, we have prepared a series of synthetic fluorinated and deoxygenated analogues of UDP-α-D-Galf (1) that are subtly modified in the two-carbon exocyclic side chain (Fig. 2) (Peltier et al., 2007; Timmons et al., 2008). In place of the hydroxymethyl group at the C-5 position of (1), UDP-6F-α-D-Galf (2) bears a fluoromethyl function; the fluorine atom is nearly isosteric to a hydroxyl group but acts only as hydrogen bond acceptor, rather than as both a hydrogen-bond donor and acceptor. UDP-β-L-Araf (3) lacks the C-5 hydroxymethyl altogether. UDP-6-deoxy-α-D-Galf (4) carries a hydrophobic methyl group in place of the hydroxymethyl substituent and UDP-5-deoxy-α-D-Galf (UDP-5-deoxy-β-L-arabino-hexofuranose, 5) lacks the polar hydroxyl group at C-5; both are expected to have altered hydrogen-bonding capabilities compared to the parent substrate. Moreover, as depicted in Fig. 1, these analogs, may be able to interfere with the elongation of the lipid-linked galactan polymer, through their incorporation into the growing chain by GlfT1 and GlfT2. In the present study, we investigated the impact of UDP-Galf analogs 2–5 on the biosynthesis of mycobacterial galactan. A better understanding of this process could lead to targeting of this crucial structure of the mycobacterial cell wall for the development of new drugs against tuberculosis.
RESULTS
Chemical synthesis of UDP-Galf analogs modified at C-5 or C-6 (2–5)
The 6-deoxy-6-fluoro-UDP-Galf derivative 2 and UDP-L-Araf 3 were prepared as previously described (Peltier et al., 2007; Peltier et al., 2008).
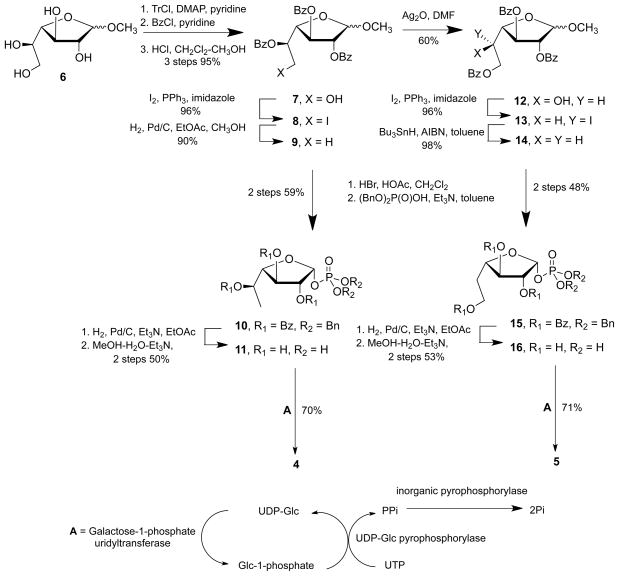
The synthesis of 4 (Fig. 3) started from the known methyl glycoside 6 (Completo and Lowary, 2008; Pathak et al., 1999), which was converted in three steps and excellent overall yield into alcohol 7. Next, treatment of 7 with triphenylphosphine and iodine at room temperature in the presence of imidazole (Garegg and Samuelsson, 1980) gave primary iodide 8 in 96% yield. Subsequent cleavage of the carbon–iodine bond by catalytic hydrogenation at elevated pressure (15 psi) afforded a 90% yield of 9. Treatment of 9 with 44% HBr in HOAc led to the corresponding glycosyl bromide, which, following its formation, was immediately reacted with dibenzyl phosphate thus yielding 10 as the pure α-isomer in 59% yield. The modest yield of the reaction is ascribed to the hydrolysis of the less stable β-anomer upon chromatography, which has been reported in the preparation of α-galactofuranosyl phosphate (de Lederkremer et al., 1994). The two benzyl groups in 10 were cleaved by catalytic hydrogenation and the three benzoyl groups were then removed by treatment with 5:2:1 methanol–triethylamine–water to give 6-deoxy-α-D-galactofuranosyl phosphate 11 in 50% overall yield from 10.
Figure 3.
Synthesis of 4 and 5.
With 11 in hand, it was converted to the corresponding sugar nucleotide 4 using an enzymatic approach, as has been previously reported for the preparation of UDP-Galf by Field and coworkers (Errey et al., 2004). Thus, incubation of 11 in with UDP-glucose and UTP in the presence of three enzymes: a promiscuous galactose-1-phosphate uridyltranserase, UDP-glucose pyrophorylase and inorganic pyrophosphatase, gave 4 in 70% overall yield (Rose et al., 2008). The anomeric proton in the 1H NMR spectrum of 4 appeared as a doublet of doublets (3JH1,H2 = 5.9 Hz and 3JH1,Pα = 4.2 Hz), and in the 1H-decoupled 31P NMR spectrum, two doublets (J = 20.7 Hz) were present at δP = −10.2 and −10.6 ppm, arising from the two coupled phosphorous atoms of the sugar nucleotide moiety. These data are in good agreement with those reported previously for the parent sugar nucleotide, UDP-Galf (Koplin et al., 1997).
The preparation of 5 (Fig. 3) was achieved starting from primary alcohol 7 by treatment with silver oxide in DMF, which induced a benzoyl migration reaction that gave secondary alcohol 12 in 60% yield. Reaction of 12 with triphenylphosphine and iodine, as was done for the preparation of 8, provided the secondary iodide 13 in excellent (96%) yield. Although in the formation of 8, the reaction proceeded easily at room temperature, in the case of 13, it was necessary to heat the reaction mixture at 90 °C. To reduce 13, catalytic hydrogenation was investigated. Using conditions that had worked well in the synthesis of the 6-deoxy derivative none of the anticipated product was obtained. Even increasing the hydrogen pressure to 45 psi (3 atm), was unsuccessful. Radical deoxygenation was therefore employed to cleave the carbon–iodine bond (Patroni et al., 1986). Thus, 13 was heated at reflux with tin hydride and AIBN to afford 14 in 98% yield. As described for the preparation of 10, deoxy-glycoside 14 was converted into a glycosyl bromide by reaction with 44% HBr in HOAc, and this product treated with dibenzyl phosphate and triethylamine to afford 5-deoxy-β-L-arabino-hexofuranoside phosphate 15 in 48% overall yield. The product was deprotected in two steps to yield 16 in 53% yield. The same enzymatic synthesis employed in the preparation of 4 was used to convert 16 into the target compound 5 in 71% yield. Proof of the structure was obtained from NMR spectroscopy: H-1 appears as a doublet of doublet (3JH1,H2 = 5.9 Hz and 3JH1,Pα = 4.2 Hz) in the 1H NMR spectrum and the proton-decoupled 13P NMR spectrum shows two doublets (−7.6 and −9.1 ppm, 3JP,P = 20.7 Hz).
Mycobacterial galactan biogenesis is severely inhibited by UDP-Galf analogs 2–5 modified at C-5, or C-6
Previously, we have shown that mycobacterial galactan build-up can be monitored in a cell-free reaction containing mycobacterial membrane and cell wall fractions, and UDP-[14C]Galp (Mikusova et al., 2000). The lipid carrier for galactan assembly, decaprenyl phosphate, was supplied by the crude, enzymatically-active membranes and cell wall. The reaction mixture was supplemented with UDP-GlcpNAc and dTDP-Rhap for in situ formation of the GlfT1 substrate, decaprenyl-P-P-GlcpNAc-Rhap (GL-2), and occasionally by recombinant Glf for more efficient conversion of UDP-Galp into UDP-Galf. In this experiment, production of three distinct glycolipids, GL-3, GL-4, and GL-5 is observed, which can be extracted with CHCl3–CH3OH (2:1). Further extraction of the reaction mixture with more polar solvents (Mikusova et al., 2000) results in the isolation of a heterogeneous population of metabolic intermediates, termed lipid-linked galactan polymer. We have shown that these compounds contain GlcNAc, Rha, and the expected (1→5)- and (1→6)-linked Galf residues (Mikusova et al., 2000).
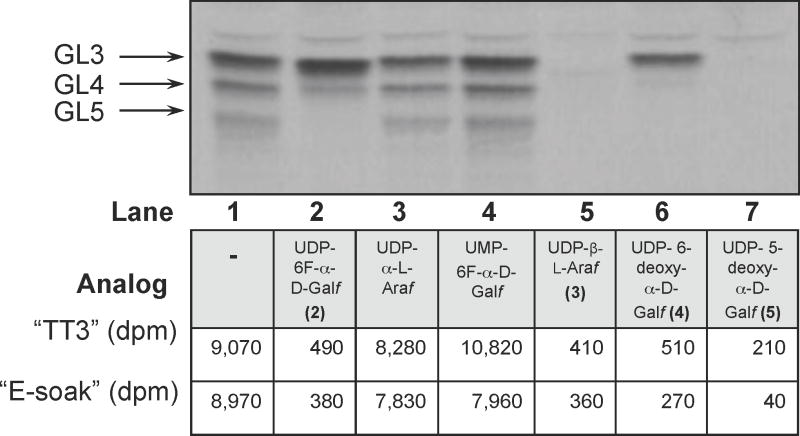
We have tested the effect of analogs 2–5 (Fig. 2) on galactan build-up in the above-described system, along with UMP-6F-α-D-Galf and UDP-α-L-Araf (the 1,2-trans isomer of 3) (Peltier et al., 2008), which we expected would serve as negative controls (Fig. 4). Each compound was added to the reaction mixtures at concentration of 100 μg/mL and, after incubation, the products were extracted as described in the Experimental Procedures. TLC analysis of the CHCl3–CH3OH (2:1) fraction revealed that the addition of UDP-β-L-Araf (3) and UDP-5-deoxy-α-D-Galf (5) resulted in the complete inhibition of galactose-containing glycolipids, while significant reduction of galactolipid production, with particular absence of GL5, was observed in case of UDP-6F-α-D-Galf (2) and UDP-6-deoxy-α-D-Galf (4). The synthesis of the lipid-linked galactan polymer, obtained by extraction of the incubation mixture with CHCl3–CH3OH–H2O; 10:10:3 and “E-soak”, was almost completely inhibited in the presence of the tested analogs 2–5. As expected, addition of UMP-6F-α-D-Galf and UDP-α-L-Araf did not have any effect on elongation of the galactan chain.
Figure 4.
The effects of UDP-Galf analogs on the cell-free production of GL-3 to GL-5 and lipid-linked galactan polymer. TLC profile of CHCl3–CH3OH (2:1) fraction; bands were visualized by autoradiography. Quantification of the lipid-linked galactan polymer was performed by scintillation counting.
UDP-Galf analogs 2–5 serve as substrates of GlfT1
Such a severe inhibition of galactan build-up by the UDP-Galf analogs could be explained in two ways. One possibility is that the compounds are inhibiting one or more of the three enzymes involved in galactan assembly: GlfT1, GlfT2 or Glf. The second possibility is that the compounds are serving as efficient substrates for these enzymes resulting in competition between the natural substrate and the synthetic analog. Incorporation of non-radioactive substrates into the galactan products would appear as a decrease in radioactivity in the bands corresponding to the individual galactolipids.
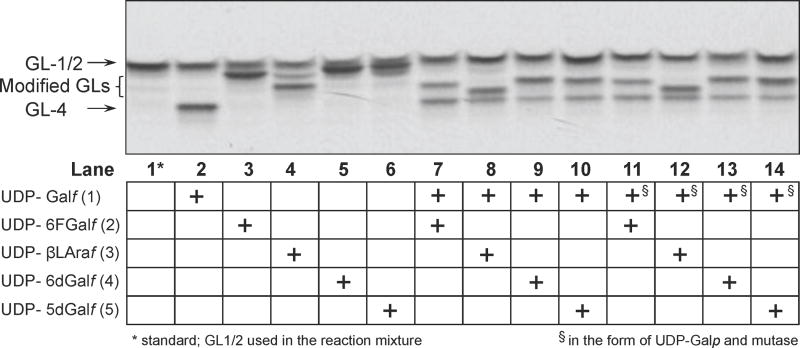
We examined these possibilities by direct investigation of the effects of 2–5 on GlfT1. These compounds were included in the assay for examination of GlfT1 activity, as recently described (Belanova et al., 2008). In our previous work, we exploited UDP-Galp and Glf as a source of UDP-Galf (1) for the reaction catalyzed by GlfT1. However, synthetic 1 can also be used. Thus, the reaction mixture contained the crude lysate of E. coli cells expressing recombinant GlfT1 from M. smegmatis, radiolabelled GL-1/2 mixture, in which GL-2 served as a natural acceptor substrate, as well as the natural donor substrate UDP-Galf (1) and/or its synthetic analogs. Reaction products were analyzed by TLC (Fig. 5).
Figure 5.
The effects of 2–5 on the activity of recombinant GlfT1. TLC profile of CHCl3–CH3OH (2:1) fraction; the bands were visualized by autoradiography. Natural substrate and synthetic analogs were used in the same 200 μM concentration.
From these experiments it is clear that analogs 2–5 serve as substrates for GlfT1, giving rise to glycolipids migrating on TLC slightly higher than GL-3 or GL-4 due to the more hydrophobic nature of the incorporated analogs (Fig. 5, lanes 3–6). It appears that GlfT1 incorporates two residues from UDP-β-L-Araf (3) (Fig. 5, lane 4), and only one residue from other three tested compounds. It can be expected that particularly in case of the 5-deoxy analog (5), chain termination should occur at the first GlfT1 reaction. Transfer of the 5-deoxy-Galf residue to GL-2 produces a modified GL-3 analog, lacking the requisite hydroxyl group for chain extension by the second transferase activity of GlfT1.
When the tested compounds were included in the reaction mixture containing an equimolar concentration of the natural donor substrate 1, we observed synthesis of the natural product of the reaction, GL-4, as well as another compound, which we propose corresponds to modified GL-4, containing both Galf and its modified counterpart (Fig. 5, lanes 7–14). These products were formed regardless of the manner in which UDP-Galf was provided to the enzyme, either directly in the form of 1 (Fig. 5, lanes 7–10), or indirectly from UDP-Galp and Glf (Fig. 5, lanes 11–14). These results indicate that none of the compounds 2–5 appreciably inhibit the mutase enzyme that produces UDP-Galf.
Incorporation of UDP-Galf analogs into GL-4 results in the production of “dead-end” intermediates
The finding that 3 and 5 are GlfT1 substrates came as rather surprising, because lack of radiolabelled galactolipid products in the initial experiments gave the impression of the efficient inhibition of the metabolic utilization of the radioactive UDP-Galp by these analogs (Fig. 4, lanes 5 and 7). However, as mentioned above we could rule out inhibition of Glf, because when UDP-Galp and Glf were used as source of UDP-Galf (1), the production of natural GL-4 and modified GL-4 was observed in the presence of the analog, confirming efficient in situ production of UDP-Galf (Fig. 5, lanes 11–14).
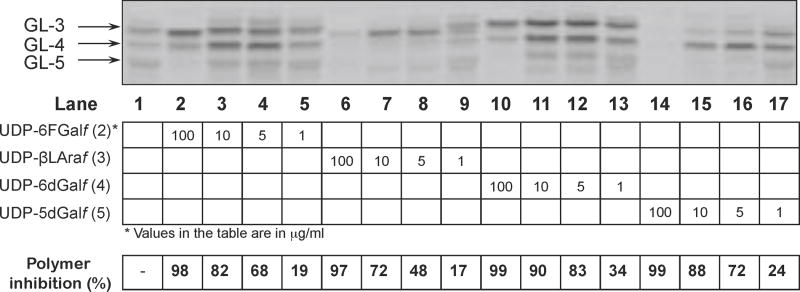
One explanation for this apparent contradiction would be if 3 and 5 had a rather high affinity for GlfT1 resulting in the preferential production of modified glycolipids GL-3 and/or GL-4, containing solely the modified residues, which would not be radiolabelled in the experiment using UDP-[14C]Galp. To test this hypothesis, we used the above assay for a competition study with varying concentrations of all four compounds 2–5 (Fig. 6). It is clear from the TLC profile of CHCl3–CH3OH (2:1) extracts that, indeed, a ten-fold lowering of the concentrations of UDP-β-L-Araf (3) or UDP-5-deoxy-α-D-Galf (5) in the reaction mixtures from the originally used 100 μg/mL led to less efficient competition of the natural substrate UDP-Galf (1) with the analogs, resulting in the synthesis of radiolabeled compounds (Fig. 6, lane 7 and 15).
Figure 6.
Dose response for the addition of 2–5 into the in vitro reactions for mycobacterial galactan build-up. TLC of the [14C]Galf – containing glycolipids produced in the reaction mixtures with decreasing concentration of 2–5. Inhibition of galactan polymer production was evaluated by scintillation counting.
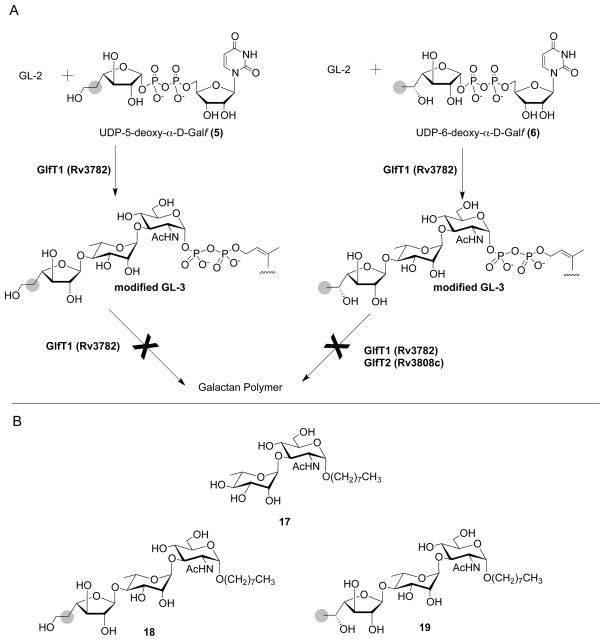
Of all the compounds, the UDP-5-deoxy-α-D-Galf (5) was the most potent competitor with 1 in the reaction catalyzed by GlfT1, and UDP-6-deoxy-α-D-Galf (4) was comparable to UDP-6F-α-D-Galf (2). In the presence of about 15-fold higher amount of the latter two analogs in the reaction mixtures compared to the radioactive natural substrate, efficient production of radiolabeled modified glycolipids is still observed confirming incorporation of much less abundant radioactive natural substrate in the reaction mixture to the products (Fig. 6, lanes 2 and 10). This is in contrast with the results for 5 (Fig. 6, lane 14), which appears to be used preferentially as a substrate by GlfT1 under these conditions. In the dose response experiment performed with compounds 2–5 less than equimolar concentration of the analogs (9 μM) compared to the radioactive natural substrate (11 μM) was sufficient to achieve about 50%–80% inhibition of galactan synthesis, respectively (Fig. 6, lanes 4, 8, 12 and 16). We thus propose that in the presence of 2–5 modified “dead-end” glycolipid intermediates are formed (Fig. 7A) leading to truncation of galactan polymerization.
Figure 7.
A. “Dead-end” galactolipid intermediates inhibit further elongation of mycobacterial galactofuran. B. Synthetic GL-2 analog 17 and the reaction products produced upon its reaction with either 4 or 5 in the presence of GlfT1.
To test this hypothesis, we made use of a synthetic GL-2 analog, octyl disaccharide 17 (Completo and Lowary, 2008) (Fig. 7B), which had previously been shown to be a GlfT1 substrate (Belanova et al., 2008). Disaccharide 17 and 5 were first incubated in the presence of GlfT1 and then the reaction mixture was analyzed by LC-MS. As predicted, a product corresponding to trisaccharide 18 (Calculated m/z = 648.3202 [M + Na+], Found m/z =648.3191) was formed (data not shown). Similar reaction of 17 with the 6-deoxy analog 4 gave a trisaccharide product 19 (Calculated 648.3202 [M + Na+], Found m/z =648.3194).
Effects of UDP-Galf analogs 2–5 on GlfT2
Although the work outlined above suggests that 2–5 exert their effect on galactan polymerization via GlfT1, the overall inhibition of galactan synthesis in the presence of these compounds could be further strengthened by inhibition of GlfT2. We have therefore examined the effect of these compounds on recombinant GlfT2 using a recently-described spectrophotometric assay (Rose et al., 2008). Using a trisaccharide acceptor substrate, and a 2 mM concentration of the synthetic analogs and 0.5 mM concentration of UDP-Galf, we have observed 25% inhibition of GlfT2 activity for both UDP-6F-α-D-Galf (2) and UDP-5-deoxy-α-D-Galf (5), and 32% inhibition in case of UDP-6-deoxy-α-D-Galf (4). The 6-deoxy-derivative also serves as a substrate for GlfT2 achieving 50% of the activity of the natural substrate at 2 mM. UDP-β-L-Araf (3) served as a very weak substrate of the enzyme (~3% of the natural substrate; it did not act as an inhibitor of GlfT2.
DISCUSSION
The method described here for the preparation of 4 and 5 makes use of a chemoenzymatic approach that has previously been employed for the preparation of UDP-Galf (Rose et al., 2008; Errey et al., 2004). Advantage of this approach over a purely chemical approach is that the formation of the sugar nucleotide from the corresponding Galf-1-phosphate derivative (i.e., 11 and 16) proceeds more efficiently and in higher yield. An obvious limitation of this approach is that the enzyme may not recognize all modified Galf-1-phosphate analogs of interest. Nevertheless, the successful preparation of 4 and 5, as well as the preparation of 2 and 3 reported earlier (Peltier et al., 2008), demonstrates that the key uridyl transferase will tolerate modification of the exocyclic diol moiety, albeit with varying degree of efficiency.
The straightforward availability of UDP-Galf synthetic analogs has prompted us to test their effects on mycobacterial galactan biosynthesis. Our data confirm that 2–5 in a concentration 100 μg/mL almost completely abolish cell-free production of the galactan polymer. To the best of our knowledge this is the first report of compounds that so severely inhibit galactan biosynthesis.
Three enzymes are involved in mycobacterial galactan assembly, Glf and the glycosyltransferases GlfT1 and GlfT2. Glf has received the most attention to date (Eppe et al., 2009; Yuan et al., 2008; Barlow and Blanchard, 2000; Zhang and Liu, 2001; Richards and Lowary, 2009) and a number of inhibitors of this enzyme have been identified (Kovensky et al., 1999; Liautard et al., 2008; Itoh et al., 2007). Most notably are a family of 2-aminothiazole derivatives, some which have also been demonstrated to inhibit mycobacterial growth in the same range as the clinically-used anti-tuberculosis agents ethambutol and rifampicin (Dykhuizen et al., 2008).
Over the past 10 years several reports on the preparation of compounds that could serve as probes and potential inhibitors of GlfT1 and GlfT2 have been published, but the focus has been primarily on acceptor analogs. Among them, β-D-Galf-(1→4)-α-L-Rhap and β-(1→5)- and β-(1→6)-linked octyl Galf disaccharides were shown to serve as substrates for these enzymes, and showed moderate antimycobacterial effects (Pathak et al., 1999; Pathak et al., 2001). Modest inhibition of galactosyltransferase activity has also been reported for analogs of the arabinogalactan linker disaccharide α-L-Rhap-(1→3)-α-D-GlcpNAc (Wen et al., 2003). More recent investigations have focused on the synthesis and use of Galf oligomers for probing the specificity of GlfT1 and GlfT2 in vitro (Belanova et al., 2008; Completo and Lowary, 2008; Gandolfi-Donadio et al., 2003).
Despite the focus on acceptor derivatives, some work has addressed the preparation of donor analogs as probes of GlfT1 and GlfT2. For example, Fleet and coworkers studied the effects of Galf iminosugar analogues on galactan biosynthesis and found about 60% inhibition at a concentration of 200 μg/mL, but this was attributed to inhibition of Glf, which was needed in the assay to generate the donor species (Lee et al., 1997). In other studies, Thomas and coworkers extended the investigation of iminosugars to develop inhibitors based on a proposed model of the transition state of the transferase reaction. These investigations led to the identification of the first donor-based inhibitor of galactosyltransferase activity (presumably GlfT2) in M. smegmatis with an IC50 value of 4.8 mM (Cren et al., 2004). Finally, a recent investigation by Bugg and coworkers described the synthesis and evaluation of uridine-linked transition state mimics of the GlfT2 transfer reaction, which inhibited the enzyme, although the level of inhibition was not quantified (Trunkfield et al., 2010).
In the present report we show that selected UDP-Galf analogs modified at C-5 or C-6 inhibit galactan assembly in an M. smegmatis cell-free assay by 50% or more at only 9 μM (5 μg/mL) concentration. The incorporation of these analogs into the growing galactan chain by GlfT1, leading to modified dead-end glycolipid intermediates appears to be the predominant mechanism by which polymer production is halted. However, at the same time, UDP-6-deoxy-α-D-Galf (4) showed activity as the substrate of GlfT2 and thus this enzyme may also introduce 6-deoxy-Galf residues into the polymer chain leading to similar “dead-end” intermediates. In addition, compounds 2, 4 and 5 served also as weak inhibitors of GlfT2. Earlier work has shown that UDP-6F-α-D-Galf (2) and UDP-β-L-Araf (3) serve as substrates for Glf from E. coli (Eppe et al., 2009; Zhang and Liu, 2001) and thus, in principle these compounds can compete with the natural substrate in turn inhibiting the efficiency of galactan polymerization. However, again this pathway also appears to be comparatively minor as experiments studying the effect of 3 and 5 on GL-4 formation showed that comparable results were obtained either when synthetic UDP-Galf was used in the reaction, or when this intermediate was produced in situ using UDP-Galp and Glf.
Although sugar nucleotides such as 2–5 are expected to have limited potential as lead drug candidates, due to the their poor cell penetration, these species nevertheless have the potential to be very useful tools in probing galactan assembly in mycobacteria. For example, because GlfT1 catalyzes two sequential glycosyl transfer reactions, the product of the first glycosylation (GL-3) is a substrate for the second transferase activity leading to the formation of GL-4. As described above, GlfT1 uses the 5-deoxy-UDP-Galf derivative 5 to generate a GL-3 derivative that is incapable of being further glycosylated. Hence, this derivative could prove useful in studies in which it is desirable to dissect the two different transferase activities of the enzyme. Analogous investigations with GlfT2 can be envisioned. These compounds will also be useful in studying the formation of galactofuranose-containing glycoconjugates in other organisms (Richards and Lowary, 2009; Peltier et al., 2008). In addition to various Glfs and galactofuranosyltransferases from a host of organisms, such investigations could be extended to a recently identified protein that transfers UDP-Galf across the Golgi membrane in Aspergillus fumigatus (Engel et al., 2009).
SIGNIFICANCE
In this paper, we report that derivatives of the sugar nucleotide UDP-Galf prevent the formation of mycobacterial galactan, an important cell wall component in the organism that causes the disease TB, M. tuberculosis. Four UDP-Galf derivatives, modified at C-5 or C-6 by replacement of a single hydroxyl group with either hydrogen or fluorine or through removal of C-6 and the associated hydroxyl group, were synthesized using a chemoenzymatic route, which has significant advantages over the chemical synthesis of these compounds. When evaluated in a cell-free assay at 100 μg/mL, all four compounds abolished the formation of the full-length galactan polymer. Instead, short “dead-end” intermediates, resulting from incorporation of the modified carbohydrate residues into the growing carbohydrate chain, are produced. Thus, these compounds have potential as chain terminators in studies of galactan biosynthesis in mycobacteria and as probes of Galf metabolism in other microorganisms.
EXPERIMENTAL PROCEDURES
Synthesis of UDP-Galf analogs modified at C-5 or C-6
See Supporting Information.
Preparation of Enzymatically Active Membranes and Cell Envelope from M. smegmatis
Enzymatically active membranes and cell envelope (wall and membrane) were prepared essentially as described from M. smegmatis mc2155 grown in Nutrient Broth (Mikusova et al., 1996). Briefly, cells (10 g) were suspended in about 40 mL of 50 mM MOPS buffer, pH 7.9, containing 5 mM 2-mercaptoethanol and 10 mM MgCl2 (buffer A), subjected to probe sonication and centrifuged at 23,000 × g for 20 min at 4 °C. The pellet was resuspended in buffer A, and Percoll (Amersham Pharmacia Biotech) was added to achieve a 60% suspension, which was centrifuged at 23,000 × g for 60 min at 4 °C. The white upper band was isolated, and Percoll was removed by repeated suspension in buffer A and centrifugation. The fraction (cell envelope) was resuspended in buffer A to a protein concentration of 10–20 mg/mL for use. Membranes were obtained by centrifugation of the 23,000 × g supernatant at 100,000 × g for 2 h at 4 °C and suspended in buffer A to give a protein concentration of 20–30 mg/mL.
Preparation of the E. coli cell lysate containing recombinant GlfT1 for the in vitro assays
100 mL culture of the overproducing strain E. coli BL21(DE3)/pET28a-MSMEG_6367 (Belanova et al., 2008) was grown to OD600 0.6 and placed on ice for 1 h. The culture was induced with IPTG at a final concentration of 0.2 mM, overnight at 16 °C. The cells were harvested, disintegrated by probe sonication (15 × 10 s pulses with 30 s cooling intervals between pulses) in 5-fold excess of Buffer A. The cell lysate was cleared by centrifugation for 20 min at 20,000 × g at 4 °C; and the supernatant was used as a source of GlfT1.
Preparation of dTDP-Rha
The synthesis of dTDP-Rha relied on the presence of the full array of the Rha synthetic enzymes and endogenous cofactors in M. smegmatis and was prepared from dTDP-Glc using the 100,000 × g supernatant of disrupted M. smegmatis as an enzyme source, as described (Mikusova et al., 1996).
Preparation of UDP-Galp Mutase
Histidine tagged mutase from Klebsiella pneumoniae was purified essentially as described (Beis et al., 2005). The recombinant plasmid for the preparation of Glf was kindly provided by Prof. David A. R. Sanders from the University of Saskatchewan, Saskatoon, Canada.
Reaction Mixtures, Fractionation and Analysis of Reaction Products
For the initial establishment of the effects of the synthetic analogs of UDP-Galf on galactan build-up we prepared reaction mixtures in which mycobacterial membrane (0.7 mg of protein) and cell envelope (1 mg of protein) fractions served as enzyme sources and UDP-[U-14C]Galp (NEN; 278 mCi/mmol, 0.25 μCi) was used to monitor formation of the galactofuran biosynthetic intermediates. NADH was added to the reaction mixtures in 2.5 mM concentration to promote the activity of the endogenous mutase and cold sugar nucleotides for in situ production of GL-2 - UDP-GlcNAc and TDP-Rha were added in 20 μM concentration. The reactions were supplemented with the UDP-Galf analogs in concentrations between 1–100 μg/mL. Volumes of the reactions were adjusted to 80 μL with buffer A.
After incubation of the reaction mixtures for 1 h at 37 °C, CHCl3–CH3OH (2:1; 1.5 mL) was added, which was left rocking at room temperature for 10 min and centrifuged (3,000 × g). The CHCl3-CH3OH phase was removed from the pellet and treated as described before (Mikusova et al., 2000). To remove residual radiolabel from the pellet, 50% CH3OH in H2O containing 0.9% NaCl (0.5 mL) was added, and the mixture was briefly bath-sonicated and centrifuged at 3,000 × g. The supernatant was discarded, and the pellet was further extracted with 50% CH3OH in H2O (0.5 mL) and 100% CH3OH (0.5 mL), which were also discarded. The washed pellet was extracted with 0.5 mL of the solvent “TT3” (CHCl3–CH3OH–H2O; 10:10:3) (Rush et al., 1993) to remove more polar products (the lipid-linked galactofuran polymer) and finally with 0.5 mL “E-soak” (water–ethanol–diethyl ether–pyridine–concentrated ammonium hydroxide; 15:15:5:1:0.017) (Angus and Lester, 1972) to obtain [14C]Gal-labeled lipid-linked products of greater polarity To the CHCl3–CH3OH (2:1) extract, 170 μL of buffer A was added to achieve a biphasic mixture. The upper aqueous phase was removed and discarded, and the bottom phase was backwashed with CHCl3–CH3OH–H2O (3:47:48) (Folch et al., 1957). The backwashed bottom phase was dried under a stream of N2 at room temperature, redissolved in 50 μL of CHCl3–CH3OH–H2O–NH4OH (65:25:3.6:0.5). Amounts of the radiolabelled products in the individual extracts were quantified by scintillation counting. Qualitative analysis of CHCl3–CH3OH (2:1) extracts was performed by TLC on Silica Gel plates (Merck) in CHCl3–CH3OH–NH4OH–1 M ammonium acetate–H2O (180:140:9:9:23) and the radiolabeled lipid bands were visualized by autoradiography.
In order to examine direct effects of the studied UDP-Galf analogs on the activity of GlfT1, we have prepared crude GL-1/2 mixture in vitro and used it in the reaction. Reaction mixture for GL-1/2 production contained 9 mg of membranes from M. smegmatis mc2155, 2 μCi of UDP-[U-14C]GlcNAc (NEN, 288 mCi/mmol), 20 μM TDP-Rha and buffer A in a final volume of 640 μL. After 1 h incubation at 37 °C [14C] GlcNAc radiolabeled glycolipids were extracted with CHCl3–CH3OH (2:1) and subjected to biphasic Folch wash as described above (Folch et al., 1957). In vitro reactions using radioactive GL-1/2, as the galactose acceptors were performed as follows: 3000 dpm of glycolipid preparation were dried in the stream of N2. This was followed by addition of the enzyme source [crude lysate of E. coli BL21(DE3)/pET28a-MSMEG_6367 producing recombinant GlfT1 (Belanova et al., 2008); ~ 0.3 mg of protein] and UDP-Galf and/or the studied analogs in 200 μM concentration. In cases where UDP-Galp was used as the source of UDP-Galf, the reaction mixture was supplemented with purified Glf and 2.5 mM freshly prepared NADH. Volume of the reaction was adjusted with Buffer A to the final volume 80 μL. The whole mixture was subsequently briefly bath sonicated. Incubation was carried out for 1 h at 37 °C. Extraction of the reaction products and their TLC analysis was performed as described above.
For LC-MS analysis of the GlfT1 reaction products mixtures containing 3.2 mM GL-2 acceptor analog 17 (Completo and Lowary, 2008), 150 μM donor analogs 4 or 5, and 1 mg of crude GlfT1 enzyme (as above) and buffer A in a final volume of 320 μL were incubated in triplicate for 2 h at 37 °C. The reactions were stopped by addition of 6 mL of CHCl3–CH3OH (2:1), followed by addition of 680 μL of water to a achieve biphasic Folch wash (Folch et al., 1957). The water phase was backwashed twice with 4 mL of CHCl3 and 1 mL of CH3OH. Organic phases from the triplicates were combined and dried under the stream of N2. The extract was subjected to mild acid hydrolysis in 300 μL of 1-propanol and 600 μL of 20 mM HCl at 60 °C for 30 min. After cooling, the mixture was neutralized with 30 μL of 0.2 mM NaOH, dried and subjected to n-butanol–water partitioning, as follows: 1.5 mL n-butanol saturated with water and 1.5 mL water were added to the dried samples, mixed for 20 min, centrifuged and the upper n-butanol phase was kept. The water phase was extracted two more times with n-butanol and the combined n-butanol fractions were dried under N2. This sample was further hydrolysed in mild alkali conditions with 500 μL of CHCl3–CH3OH (2:1) and 500 μL 0.2 M NaOH in CH3OH at 37 °C for 20 min. Following neutralization with 2 μL of glacial acetic acid, the reaction products were again extracted by n-butanol–water partitioning as described above and then dried. Samples were analyzed on Waters Q-TOF Premier LC-MS system in positive ion mode.
Spectrophotometric assay for monitoring GlfT2 activity
Recombinant GlfT2 was prepared as described previously (Rose et al., 2006). Spectrophotometric GlfT2 assays were performed in 384-array microtiter plate wells, as described (Rose et al., 2008). The reaction buffer contained 50 mM MOPS, pH 7.9, 50 mM KCl, 20 mM MgCl2, 1.1 mM NADH, 3.5 mM PEP, 7.5 U pyruvate kinase (PK, EC 2.7.1.40), and 16.8 U lactate dehydrogenase (LDH, EC 1.1.1.27). A standard assay reaction contained UDP-Galf (1) at a final concentration of 0.5 mM and a trisaccharide acceptor substrate, octyl β-D-galactofuranosyl-(1→5)-β-D-galactofuranosyl-(1→6)-β-D-galactofuranoside (Rose et al., 2008) at 2 mM. Each reaction was initiated by the addition of 0.75 μg GlfT2 to the assay mixture. The final assay volume was 40 μL. Reactions were monitored at 37 °C using a Spectra Max 340PC microplate reader controlled with SOFTmax® PRO software (Molecular Devices, Sunnyvale, CA) in the kinetics read mode, as described (Rose et al., 2008).
Acknowledgments
This work was supported by the Slovak Research and Development Agency under the contract No. RPEU-0012-06, by the Research & Development Operational Programme funded by the ERDF (“Centre of Excellence for Exploitation of Informational Biomacromolecules in the Disease Prevention and Improvement of Quality of Life”), by European Commission under contract LSHP-CT-2005-018923 ,, NM4TB“ (KM), grant NIH, NIAID AIDS-FIRCA TW 006487 (PJB and KM), and by the Alberta Ingenuity Centre for Carbohydrate Science and The Natural Sciences and Engineering Research Council of Canada (TLL). The authors also want to acknowledge l’Agence Nationale de la Recherche ANR JCJC06_140075 (VF) and Rennes Métropoles (PP) for financial support. Mr. M. Poulin is thanked for technical assistance. We thank Dr. Randy Whittal and Mr. Bela Reiz in the Mass Spectrometry Laboratory at the University of Alberta for carrying out the LC-MS analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderwick LJ, Radmacher E, Seidel M, Gande R, Hitchen PG, Morris HR, Dell A, Sahm H, Eggeling L, Besra GS. Deletion of Cg-emb in corynebacterianeae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J Biol Chem. 2005;280:32362–32371. doi: 10.1074/jbc.M506339200. [DOI] [PubMed] [Google Scholar]
- Angus WW, Lester RL. Turnover of inositol and phosphorus containing lipids in Saccharomyces cerevisiae; extracellular accumulation of glycerophosphorylinositol derived from phosphatidylinositol. Arch Biochem Biophys. 1972;151:483–495. doi: 10.1016/0003-9861(72)90525-5. [DOI] [PubMed] [Google Scholar]
- Balganesh TS, Alzari PM, Cole ST. Rising standards for tuberculosis drug development. Trends Pharmacol Sci. 2008;29:576–581. doi: 10.1016/j.tips.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Barlow JN, Blanchard JS. Enzymatic synthesis of UDP-(3-deoxy-3-fluoro)-D-galactose and UDP-(2-deoxy-2-fluoro)-D-galactose and substrate activity with UDP-galactopyranose mutase. Carbohydr Res. 2000;328:473–480. doi: 10.1016/s0008-6215(00)00135-x. [DOI] [PubMed] [Google Scholar]
- Barry CE, Crick DC, McNeil MR. Targeting the formation of the cell wall core of M. tuberculosis. Infect Disord Drug Targets. 2007;7:182–202. doi: 10.2174/187152607781001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanova M, Dianiskova P, Brennan PJ, Completo GC, Rose NL, Lowary TL, Mikusova K. Galactosyl transferases in mycobacterial cell wall synthesis. J Bacteriol. 2008;190:1141–1145. doi: 10.1128/JB.01326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis K, Srikannathasan V, Liu H, Fullerton SW, Bamford VA, Sanders DA, Whitfield C, McNeil MR, Naismith JH. Crystal structures of Mycobacteria tuberculosis and Klebsiella pneumoniae UDP-galactopyranose mutase in the oxidised state and Klebsiella pneumoniae UDP-galactopyranose mutase in the (active) reduced state. J Mol Biol. 2005;348:971–982. doi: 10.1016/j.jmb.2005.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besra GS, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. A new interpretation of the structure of the mycolyl-arabinogalactan complex of Mycobacterium tuberculosis as revealed through characterization of oligoglycosylalditol fragments by fast-atom bombardment mass spectrometry and 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1995;34:4257–4266. doi: 10.1021/bi00013a015. [DOI] [PubMed] [Google Scholar]
- Bhamidi S, Scherman MS, Rithner CD, Prenni JE, Chatterjee D, Khoo KH, McNeil MR. The identification and location of succinyl residues and the characterization of the interior arabinan region allow for a model of the complete primary structure of Mycobacterium tuberculosis mycolyl arabinogalactan. J Biol Chem. 2008;283:12992–13000. doi: 10.1074/jbc.M800222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ED, Iseman MD. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis. 2008;21:587–595. doi: 10.1097/QCO.0b013e328319bce6. [DOI] [PubMed] [Google Scholar]
- Completo GC, Lowary TL. Synthesis of galactofuranose-containing acceptor substrates for mycobacterial galactofuranosyltransferases. J Org Chem. 2008;73:4513–4525. doi: 10.1021/jo800457j. [DOI] [PubMed] [Google Scholar]
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- Cren S, Gurcha SS, Blake AJ, Besra GS, Thomas NR. Synthesis and biological evaluation of new inhibitors of UDP-Galf transferase–a key enzyme in M. tuberculosis cell wall biosynthesis. Org Biomol Chem. 2004;2:2418–2420. doi: 10.1039/B411554F. [DOI] [PubMed] [Google Scholar]
- Daffe M, Brennan PJ, McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J Biol Chem. 1990;265:6734–6743. [PubMed] [Google Scholar]
- Daffe M, McNeil M, Brennan PJ. Major structural features of the cell wall arabinogalactans of Mycobacterium, Rhodococcus, and Nocardia spp. Carbohydr Res. 1993;249:383–398. doi: 10.1016/0008-6215(93)84102-c. [DOI] [PubMed] [Google Scholar]
- Dykhuizen EC, May JF, Tongpenyai A, Kiessling LL. Inhibitors of UDP-galactopyranose mutase thwart mycobacterial growth. J Am Chem Soc. 2008;130:6706–6707. doi: 10.1021/ja8018687. [DOI] [PubMed] [Google Scholar]
- Engel J, Schmalhorst PS, Dork-Bousset T, Ferrieres V, Routier FH. A single UDP-galactofuranose transporter is required for galactofuranosylation in Aspergillus fumigatus. J Biol Chem. 2009;284:33859–33868. doi: 10.1074/jbc.M109.070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppe G, Peltier P, Daniellou R, Nugier-Chauvin C, Ferrieres V, Vincent SP. Probing UDP-galactopyranose mutase binding pocket: a dramatic effect on substitution of the 6-position of UDP-galactofuranose. Bioorg Med Chem Lett. 2009;19:814–816. doi: 10.1016/j.bmcl.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Errey JC, Mukhopadhyay B, Kartha KP, Field RA. Flexible enzymatic and chemo-enzymatic approaches to a broad range of uridine-diphospho-sugars. Chem Commun. 2004:2706–2707. doi: 10.1039/b410184g. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gandolfi-Donadio L, Gallo-Rodriguez C, de Lederkremer RM. Syntheses of β-D-Galf-(1→6)-β-D-Galf-(1→5)-D-Galf and β-D-Galf-(1→5)-β-D-Galf-(1→6)-D-Galf, trisaccharide units in the galactan of Mycobacterium tuberculosis. J Org Chem. 2003;68:6928–6934. doi: 10.1021/jo034365o. [DOI] [PubMed] [Google Scholar]
- Garegg PJ, Samuelsson B. Novel reagent system for converting a hydroxy-group into an iodo-group in carbohydrates with inversion of configuration. Part 2. J Chem Soc, Perkin Trans. 1980;1:2866–2869. [Google Scholar]
- Itoh K, Huang Z, Liu HW. Synthesis and analysis of substrate analogues for UDP-galactopyranose mutase: implication for an oxocarbenium ion intermediate in the catalytic mechanism. Org Lett. 2007;9:879–882. doi: 10.1021/ol0631408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplin R, Brisson JR, Whitfield C. UDP-galactofuranose precursor required for formation of the lipopolysaccharide O antigen of Klebsiella pneumoniae serotype O1 is synthesized by the product of the rfbDKPO1 gene. J Biol Chem. 1997;272:4121–4128. doi: 10.1074/jbc.272.7.4121. [DOI] [PubMed] [Google Scholar]
- Kovensky J, McNeil MR, Sinaÿ P. D-Galactofuranosyl phosphonates. First synthesis of UDP-C-D-galactofuranose. J Org Chem. 1999;64:6202–6205. [Google Scholar]
- Kremer L, Dover LG, Morehouse C, Hitchin P, Everett M, Morris HR, Dell A, Brennan PJ, McNeil MR, Flaherty C, Duncan K, Besra GS. Galactan biosynthesis in Mycobacterium tuberculosis. Identification of a bifunctional UDP-galactofuranosyltransferase. J Biol Chem. 2001;276:26430–26440. doi: 10.1074/jbc.M102022200. [DOI] [PubMed] [Google Scholar]
- de Lederkremer RM, Nahmad VB, Varela O. Synthesis of α-D-galactofuranosyl phosphate. J Org Chem. 1994;59:690–692. [Google Scholar]
- Lee RE, Smith MD, Nash RJ, Griffiths RC, McNeil M, Grewal RK, Yan W, Besra GS, Brennan PJ, Fleet GWJ. Inhibition of UDP-Gal mutase and mycobacterial galactan biosynthesis by pyrrolidine analogues of galactofuranose. Tetrahedron Lett. 1997;38:6733–6736. [Google Scholar]
- Liautard V, Desvergnes V, Itoh K, Liu HW, Martin OR. Convergent and stereoselective synthesis of iminosugar-containing Galf and UDP-Galf mimicks: evaluation as inhibitors of UDP-Gal mutase. J Org Chem. 2008;73:3103–3115. doi: 10.1021/jo8001134. [DOI] [PubMed] [Google Scholar]
- Mahapatra S, Scherman H, Brennan PJ, Crick DC. N Glycolylation of the nucleotide precursors of peptidoglycan biosynthesis of Mycobacterium spp. is altered by drug treatment. J Bacteriol. 2005;187:2341–2347. doi: 10.1128/JB.187.7.2341-2347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra S, Yagi T, Belisle JT, Espinosa BJ, Hill PJ, McNeil MR, Brennan PJ, Crick DC. Mycobacterial lipid II is composed of a complex mixture of modified muramyl and peptide moieties linked to decaprenyl phosphate. J Bacteriol. 2005;187:2747–2757. doi: 10.1128/JB.187.8.2747-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M, Wallner SJ, Hunter SW, Brennan PJ. Demonstration that the galactosyl and arabinosyl residues in the cell-wall arabinogalactan of Mycobacterium leprae and Mycobacterium tuberculosis are furanoid. Carbohydr Res. 1987;166:299–308. doi: 10.1016/0008-6215(87)80065-4. [DOI] [PubMed] [Google Scholar]
- McNeil M, Daffe M, Brennan PJ. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J Biol Chem. 1990;265:18200–18206. [PubMed] [Google Scholar]
- Mikusova K, Slayden RA, Besra GS, Brennan PJ. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob Agents Chemother. 1995;39:2484–2489. doi: 10.1128/aac.39.11.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikusova K, Mikus M, Besra GS, Hancock I, Brennan PJ. Biosynthesis of the linkage region of the mycobacterial cell wall. J Biol Chem. 1996;271:7820–7828. doi: 10.1074/jbc.271.13.7820. [DOI] [PubMed] [Google Scholar]
- Mikusova K, Yagi T, Stern R, McNeil MR, Besra GS, Crick DC, Brennan PJ. Biosynthesis of the galactan component of the mycobacterial cell wall. J Biol Chem. 2000;275:33890–33897. doi: 10.1074/jbc.M006875200. [DOI] [PubMed] [Google Scholar]
- Mikusova K, Belanova M, Kordulakova J, Honda K, McNeil MR, Mahapatra S, Crick DC, Brennan PJ. Identification of a novel galactosyl transferase involved in biosynthesis of the mycobacterial cell wall. J Bacteriol. 2006;188:6592–6598. doi: 10.1128/JB.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JA, Motichka K, Jucker M, Wu HP, Uhlik BC, Stern RJ, Scherman MS, Vissa VD, Pan F, Kundu M, Ma YF, McNeil M. Inactivation of the mycobacterial rhamnosyltransferase, which is needed for the formation of the arabinogalactan-peptidoglycan linker, leads to irreversible loss of viability. J Biol Chem. 2004;279:43540–43546. doi: 10.1074/jbc.M407782200. [DOI] [PubMed] [Google Scholar]
- Pan F, Jackson M, Ma Y, McNeil M. Cell wall core galactofuran synthesis is essential for growth of mycobacteria. J Bacteriol. 2001;183:3991–3998. doi: 10.1128/JB.183.13.3991-3998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak AK, Besra GS, Crick D, Maddry JA, Morehouse CB, Suling WJ, Reynolds RC. Studies on β-D-Galf-(1→4)-α-L-Rhap octyl analogues as substrates for mycobacterial galactosyl transferase activity. Bioorg Med Chem. 1999;7:2407–2413. doi: 10.1016/s0968-0896(99)00199-6. [DOI] [PubMed] [Google Scholar]
- Pathak AK, Pathak V, Seitz L, Maddry JA, Gurcha SS, Besra GS, Suling WJ, Reynolds RC. Studies on β-(1→5) and β-(1→6) linked octyl Galf disaccharides as substrates for mycobacterial galactosyltransferase activity. Bioorg Med Chem. 2001;9:3129–3143. doi: 10.1016/s0968-0896(01)00179-1. [DOI] [PubMed] [Google Scholar]
- Patroni JJ, Stick RV, Engelhardt LM, White AH. The Deoxygenation of some carbohydrate diols via the derived cyclic thiocarbonate. Aus J Chem. 1986;39:699–711. [Google Scholar]
- Pedersen LL, Turco SJ. Galactofuranose metabolism: a potential target for antimicrobial chemotherapy. Cell Mol Life Sci. 2003;60:259–266. doi: 10.1007/s000180300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier P, Daniellou R, Nugier-Chauvin C, Ferrieres V. Versatile synthesis of rare nucleotide furanoses. Org Lett. 2007;9:5227–5230. doi: 10.1021/ol702392x. [DOI] [PubMed] [Google Scholar]
- Peltier P, Euzen R, Daniellou R, Nugier-Chauvin C, Ferrieres V. Recent knowledge and innovations related to hexofuranosides: structure, synthesis and applications. Carbohydr Res. 2008;343:1897–1923. doi: 10.1016/j.carres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Peltier P, Guégan JP, Daniellou R, Nugier-Chauvin C, Ferrières V. Stereoselective Chemoenzymatic Synthesis of UDP-1,2-cis-furanoses from α,β-Furanosyl 1-Phosphates. Eur J Org Chem. 2008;2008:5988–5994. [Google Scholar]
- Pongdee R, Liu HW. Elucidation of enzyme mechanisms using fluorinated substrate analogues. Bioorg Chem. 2004;32:393–437. doi: 10.1016/j.bioorg.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Richards MR, Lowary TL. Chemistry and biology of galactofuranose-containing polysaccharides. ChemBioChem. 2009;10:1920–1938. doi: 10.1002/cbic.200900208. [DOI] [PubMed] [Google Scholar]
- Rose NL, Completo GC, Lin SJ, McNeil M, Palcic MM, Lowary TL. Expression, purification, and characterization of a galactofuranosyltransferase involved in Mycobacterium tuberculosis arabinogalactan biosynthesis. J Am Chem Soc. 2006;128:6721–6729. doi: 10.1021/ja058254d. [DOI] [PubMed] [Google Scholar]
- Rose NL, Zheng RB, Pearcey J, Zhou R, Completo GC, Lowary TL. Development of a coupled spectrophotometric assay for GlfT2, a bifunctional mycobacterial galactofuranosyltransferase. Carbohydr Res. 2008;343:2130–2139. doi: 10.1016/j.carres.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Rush JS, Shelling JG, Zingg NS, Ray PH, Waechter CJ. Mannosylphosphoryldolichol-mediated reactions in oligosaccharide-P-P-dolichol biosynthesis. Recognition of the saturated α-isoprene unit of the mannosyl donor by pig brain mannosyltransferases. J Biol Chem. 1993;268:13110–13117. [PubMed] [Google Scholar]
- Soltero-Higgin M, Carlson EE, Gruber TD, Kiessling LL. A unique catalytic mechanism for UDP-galactopyranose mutase. Nat Struct Mol Biol. 2004;11:539–543. doi: 10.1038/nsmb772. [DOI] [PubMed] [Google Scholar]
- Takayama K, Wang L, David HL. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1972;2:29–35. doi: 10.1128/aac.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Kilburn JO. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989;33:1493–1499. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons SC, Hui JP, Pearson JL, Peltier P, Daniellou R, Nugier-Chauvin C, Soo EC, Syvitski RT, Ferrieres V, Jakeman DL. Enzyme-catalyzed synthesis of furanosyl nucleotides. Org Lett. 2008;10:161–163. doi: 10.1021/ol7023949. [DOI] [PubMed] [Google Scholar]
- Trunkfield AE, Gurcha SS, Besra GS, Bugg TDH. Inhibition of Escherichia coli glycosyltransferase MurG and Mycobacterium tuberculosis Gal transferase by uridine-linked transition state mimics. Bioorg Med Chem. 2010;18:2651–2663. doi: 10.1016/j.bmc.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Crick DC, Brennan PJ, Hultin PG. Analogues of the mycobacterial arabinogalactan linkage disaccharide as cell wall biosynthesis inhibitors. Bioorg Med Chem. 2003;11:3579–3587. doi: 10.1016/s0968-0896(03)00366-3. [DOI] [PubMed] [Google Scholar]
- Weston A, Stern RJ, Lee RE, Nassau PM, Monsey D, Martin SL, Scherman MS, Besra GS, Duncan K, McNeil MR. Biosynthetic origin of mycobacterial cell wall galactofuranosyl residues. Tuber Lung Dis. 1997;78:123–131. doi: 10.1016/s0962-8479(98)80005-1. [DOI] [PubMed] [Google Scholar]
- Winder FG, Collins PB, Whelan D. Effects of ethionamide and isoxyl on mycolic acid synthesis in Mycobacterium tuberculosis BCG. J Gen Microbiol. 1971;66:379–380. doi: 10.1099/00221287-66-3-379. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Bleile DW, Wen X, Sanders DA, Itoh K, Liu HW, Pinto BM. Investigation of binding of UDP-Galf and UDP-[3-F]Galf to UDP-galactopyranose mutase by STD-NMR spectroscopy, molecular dynamics, and CORCEMA-ST calculations. J Am Chem Soc. 2008;130:3157–3168. doi: 10.1021/ja7104152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu H. Mechanistic investigation of UDP-galactopyranose mutase from Escherichia coli using 2- and 3-fluorinated UDP-galactofuranose as probes. J Am Chem Soc. 2001;123:6756–6766. doi: 10.1021/ja010473l. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu HW. Chemical synthesis of UDP-β-L-arabinofuranose and its turnover to UDP-β-L-arabinopyranose by UDP-galactopyranose mutase. Bioorg Med Chem Lett. 2001;11:145–149. doi: 10.1016/s0960-894x(00)00616-8. [DOI] [PubMed] [Google Scholar]