Abstract
When rats were pretreated with ethanol (3.0 g/kg, IP), subsequent microinjection of thyrotropin-releasing hormone (TRH) (500 or 1000 ng) into the medial septum, 30 minutes later, significantly shortened the time necessary for the rats to regain their righting reflex. Conversely, microinjection of TRH into the nucleus accumbens (1000 ng/side) or the area of the raphe obscurus (1000 ng) had no effect on ethanol-induced depression, although both of these structures mediate specific TRH effects in the CNS. In order to determine if this antagonism was due to a specific TRH interaction, TRH Fab fragments were microinjected into the medial septum just prior to the microinjection of TRH. Under these conditions, TRH did not alter ethanol’s depressant actions. Finally, this TRH antagonism of ethanol-induced depression appears attributable to a net increase in neuronal activity, because electrical stimulation (160 μA, 120 Hz, 1.5 msec duration) of the medial septum antagonized ethanol’s impairment of the righting reflex. These results are discussed in relationship to a potential CNS site for the action of ethanol.
Keywords: Thyrotropin-releasing hormone (TRH), Ethanol-induced depression, Medial septum, Nucleus accumbens, Raphe obscurus, Righting reflex
Ethanol produces many effects on the central nervous system (CNS), most of which are depressant in nature. It has been proposed that this generalized depression of neuronal function results from ethanol’s ability to increase the fluidity of neuronal membranes [3,6]. Thus, as the membrane fluidity increases, the ability of the neuron to properly function should decrease. One possible translation of this theory to ethanol’s diverse behavioral effects begins with the supposition that specific anatomical structures in the CNS mediate specific behaviors. Therefore, when ethanol reaches a concentration sufficient to impair neurons that are necessary for normal function in a specific CNS structure, behaviors mediated through that structure become impaired. However, the vast heterogeneity of the CNS creates the major obstacle in testing this hypothesis: identification of a CNS structure which appears directly linked to a behavior that ethanol modifies.
The CNS peptide, thyrotropin-releasing hormone (TRH), has been shown to antagonize certain ethanol-induced changes in behavior. Ethanol-induced loss of righting reflex, sleep time, hypothermia and decrease in locomotor activity [1, 2, 4] all are reversed by the central administration of TRH. Furthermore, this analeptic action of TRH occurs most potently in the medial septum, when the TRH was tested against pentobarbital-induced sedation [7]. Given this background, the following studies were initiated to evaluate the potential site specificty of TRH’s antagonism of ethanol-induced loss of righting reflex. Three CNS sites, medial septum, nucleus accumbens and the area of the raphe obscurus, were chosen where TRH microinjection has been shown to alter behavior. For example, Myamoto et al. [12] found TRH to increase locomotor activity when placed into the nucleus accumbens, and TRH microinjections into the area of the raphe obscurus has been shown to alter respiratory timing [11]. Additionally, we sought to characterize the nature of the TRH/ethanol interaction.
METHOD
Animals
All of the animals used in the experiments were naive, male rats (Sprague-Dawley (Crl:Cd(Sd)Br), weighing between 250 and 350 grams) purchased from Charles River Breeding Laboratories. The rats had free access to food and water and were maintained on a 12 hour light-dark cycle. All manipulations were performed during the light phase of the cycle (7:00 a.m. to 7:00 p.m.).
Implantation
Initially, the rats were anesthetized with 40 mg/kg pentobarbital and then placed into a stereotaxic frame. A 12 mm long, 26 gauge stainless steel tube was lowered to a position 1.5 mm dorsal to the intended microinjection site. This dorsal placement to the site of microinjection minimizes damage to the area and the possibility of back diffusion up the cannula guide during microinjection. The stereotaxic coordinates for each microinjection site according to the atlas of Paxinos and Watson [13] were as follows: medial septum—interaural line 9.2 mm, lateral 0.0, vertical 6.0; raphe obscurus area—interaural line −4.8, lateral 0.0, vertical 9.5; nucleus accumbens—interaural line 10.7, lateral 1.5, vertical 7.0. Guide cannula were implanted bilaterally for the nucleus accumbens. For the electrode placement, two, twisted, stainless steel wires, (0.01 inches diameter, insulated except for the cross sectional tip) were implanted into the medial septum using the same coordinates as above. The tip spread averaged 500 microns. All of the implants were anchored with cranioplastic cement to screws placed in the skull. For the guide cannulae, a 32 gauge stylet was inserted to prevent blockage. The animals were allowed at least five days for recovery from the surgery.
TRH Fab Fragment Preparation
The TRH antiserum was generated as previously described [17]. A purified fraction of IgG was prepared by affinity chromatography over a column of protein-A Sepha-rose. After equilibrium of the protein-A column with a buffer containing 0.2 M NaPO4, 0.13 M NaCl, 0.1 mM phenylmethylsulfonylfluoride (PMSF) and 0.2% sodium azide (pH 7.4), the antiserum was added. The column was washed with the same buffer until the protein background was negligible. Purified IgG was eluted with a buffer containing 0.1 M glycine, 0.1 mM PMSF, 0.2% sodium axide, pH 3. Following elution, the purified IgG was dialyzed against a buffer containing 0.2 M NaHCO3, 0.01 M Na2HCO3 and 0.15 M NaCl, pH 9.3. Subsequent testing of the immunological activity of the purified IgG showed that it retained full immunological potency. For preparation of the TRH fragmented antibody (Fab) fragments, a purified gammaglobulin was dialyzed against a solution of 10 mM phosphate. 0.15 m NaCl, 1 mM EDTA, and 25 mM mercaptoethanol (pH 7.3). After dialysis, the IgG in phosphobuffered saline was pressure concentrated to approximately 10 mg/ml. For proteolysis, approximately 100 μg of crystalline papain (Worthington Biochemical Corp, Freehold, NJ), is added for each 10 mg of purified IgG and the mixture incubated at 37°C. Separation of the purified TRH Fab fragments was carried out by affinity chromatography over a column of protein sepharose. The papain digest was dialyzed against a phosphate, saline buffer and applied to the protein-A column. Under these conditions the purified TRH Fab fragments eluted with the starting buffer and residual fractions were retained on the column. Verification of the elution position of the TRH Fab fragments was accomplished by the addition of the putative TRH Fab fragments to a radioimmunoassay and observing the inhibition of the precipitation of radiolabelled TRH in the standard immunoassay. The TRH Fab fragments were pressure concentrated to 10 μg protein/μl.
Procedure
The animals in each group first received an IP injection of ethanol (3.0 g/kg) at time zero. A 10% solution was used to minimize peritoneal irritation [15]. The animals then received a microinjection of saline, or TRH (100, 500 or 1000 ng) 30 minutes after the ethanol pretreatment (no difference was found between saline microinjection and sham injector placement). The microinjections were made by placing a 13.5 mm long 33 gauge stainless steel tube into the cannula guide. Then 0.5 μ1 of the solution was infused over a five minute period, using an infusion pump. The injector was left in place for one minute post injection in order to allow diffusion of the solution from the microinjection site. The injector was then removed and the stylet was replaced. In the experiment where the TRH Fab fragments were microinjected prior to the TRH microinjection, the animals first received an IP injection of 3.5 g/kg ethanol. This increased dose of ethanol was used because in the previous experiments, a 500 ng dose of TRH immediately reversed ethanol’s depression of the righting reflex. Therefore, the dose of ethanol was increased in order to be able to observe any potentiation which might occur between TRH and the TRH Fab fragments. The TRH Fab fragments (10 μg/ml) was microinjected 24 minutes after the ethanol pretreatment in a 1 μl volume. This increased volume was used because the TRH Fab fragments were not expected to diffuse as readily as the TRH, so it was important to make sure that the area of TRH influence contained the TRH Fab fragments. Finally, for the stimulation study, the animals received electrical stimulation (160 μA, 120 HZ, 1.5 msec duration) 30 minutes after the ethanol administration. The stimulation was continued until the animals regained their righting reflex, or until 10 minutes transpired (4 of the 5 animals regained their righting reflex prior to the ten minute period).
The righting reflex was determined by placing the animals on their backs following the ethanol administration. Most of the animals lost their righting reflex within 5 minutes after the ethanol injection. When an animal had righted himself three times within a one minute period, the time was recorded as that when the animal had regained his righting reflex.
Histological Verifications
Following the microinjection experiments, animals were anesthetized with 400 mg/kg chloral hydrate and 0.5 μ1 of Luxol blue was microinjected 1.5 mm below the cannulae guide using a 33 gauge injector. After decapitation, the animals’ brains were frozen onto a mounting platform. The brain was sliced in a cryostat, and the placement of the injector tip was plotted according to the atlas of Paxinos and Watson [13]. The electrode placements were verified similarly.
Statistics
The treatment groups were compared to the appropriate control groups by a Student’s t-test. A Bonferoni t-test for multiple comparisons was used for the medial septum comparisons.
RESULTS
Effects of TRH Microinjection Into Various Brain Sites
When 500 or 1000 ng of TRH was microinjected into the medial septum (see Fig. 1), the time necessary for the animals to regain their righting reflex was significantly shortened in a dose-related manner (see Table 1), while saline had no effect. In many instances, the animals regained their righting reflex within a 5 minute period after the TRH microinjection. In contrast, the microinjection of large doses of TRH into the nucleus accumbens (1000 ng/side) or the raphe obscurus area (1000 ng) did not alter ethanol’s impairment of the righting reflex (see Figs. 2, 3, and Table 1).
FIG. 1.
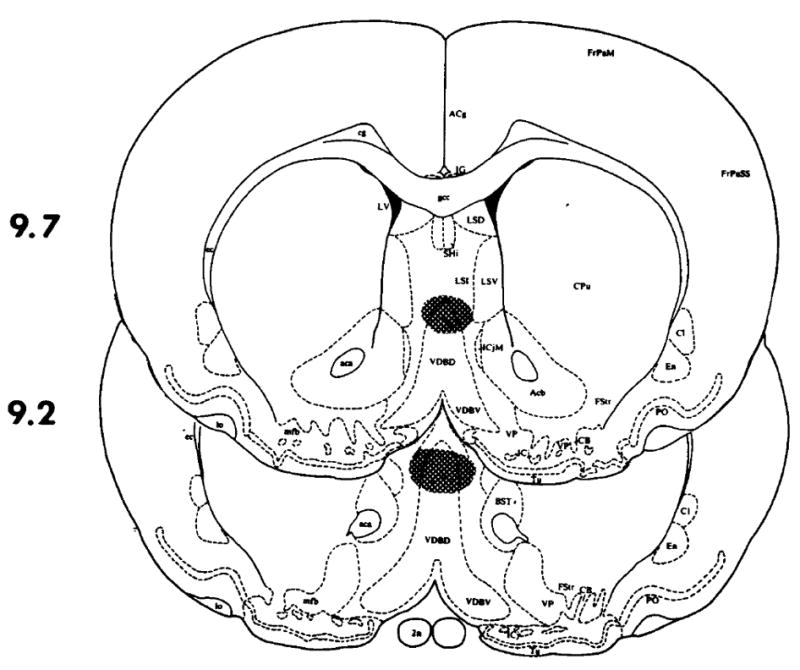
The shaded area represents the extent of microinjection sites where TRH significantly antagonized ethanol’s impairment of the righting reflex in rats. Placements outside this shaded area were generally not as effective, and were not included. The drawings are from the atlas of Paxinos and Watson [13] showing the coordinate from the interaural line.
TABLE 1.
THE EFFECTS OF TRH MICROINJECTION INTO SPECIFIC BRAIN SITES ON ETHANOL-INDUCED LOSS OF RIGHTING REFLEX*
| Brain Area | Saline | Sleep Time (Min ± SEM) | ||
|---|---|---|---|---|
| 100 ng TRH | 500 ng TRH | 1000 ng TRH | ||
| Medial Septum | 77 ± 4 | 79 ± 5 | 49 ± 3† | 41 ± 2† |
| Nucleus Accumbens | 80 ± 9 | — | — | 74 ± 16 |
| Raphe Obscurus | 81 ± 3 | — | — | 76 ± 7 |
All animals (N≥5 per group) received 3.0 g/kg ETOH (IP) 30 min prior to microinjection of saline or TRH, and the time to regain the righting reflex was determined (see the Method section for further details).
p<0.01, Bonferoni t-test.
FIG. 2.
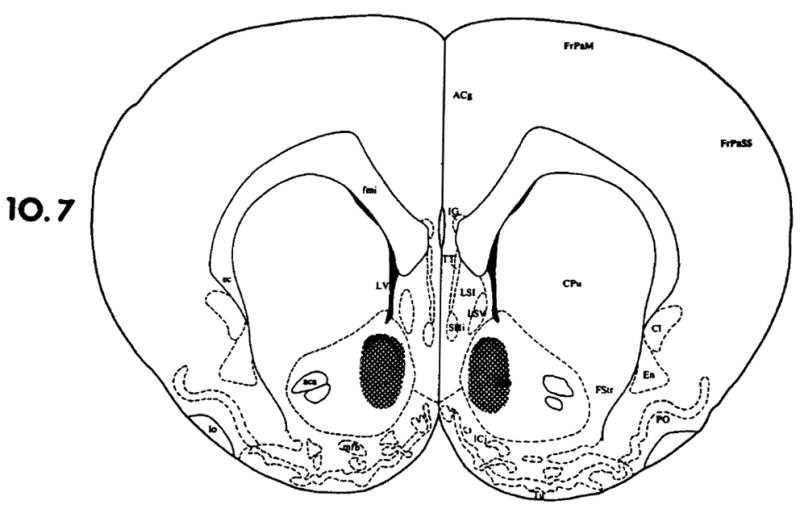
The shaded area represents the extent of TRH microinjection sites that were acceptable for the nucleus accumbens. A dose of 1000 ng TRH per side had no effect on ethanol’s impairment of the righting reflex in rats. The drawings are from the atlas of Paxinos and Watson [13] showing the coordinates from the interaural line.
FIG. 3.
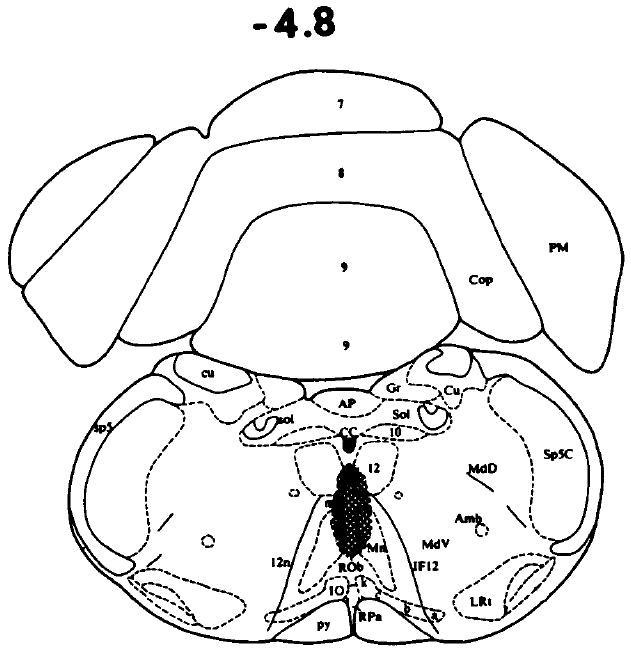
The shaded area represents the extent of acceptable TRH microinjection sites in the area of the raphe obscurus. A dose of 1000 ng TRH had no effect on ethanol’s impairment of the righting reflex in rats. The drawings are from the atlas of Paxinos and Watson [13] showing the coordinate from the interaural line.
Effects of TRH Fab Fragments on the Analeptic Action of TRH
It is possible that the microinjection of TRH into the medial septum caused a non-specific irritation of the neurons, resulting in increased neuronal firing. When TRH Fab fragments were microinjected prior to the TRH in the medial seputm, the TRH microinjection no longer altered ethanol’s impairment of the righting reflex (see Table 2). This blockade of TRH’s analeptic action appeared specific for the TRH Fab fragment, because microinjection of Fab fragments of similar size to the TRH Fab fragments, but with no TRH immunoreactivity, did not prevent TRH’s reversal of ethanol-induced depression (Table 2, Controls). Also, the TRH Fab fragments did not have any effect by themselves. Subsequent microinjection of TRH into the medial septum of animals, that had previously received the TRH Fab fragments/TRH microinjection, significantly shortened ethanol’s impairment of the righting reflex.
TABLE 2.
THE EFFECTS OF A TRH ANTIBODY FRAGMENT ON THE REVERSAL OF ETHANOL-INDUCED LOSS OF RIGHTING REFLEX BY MICROINJECTION OF TRH INTO THE MEDIAL SEPTUM*
| Treatment | Sleep Time (Min ± SEM) |
|---|---|
| Control† | 143 ± 8 |
| Anti-TRH | 121 ± 10 |
| Anti-TRH + TRH (500 ng) | 134 ± 16 |
| TRH (500 ng) | 77 ± 12‡ |
A11 animals (N≥4 per group) received 3.5 g/kg ethanol (IP) 24 min prior to the microinjection of the TRH Fab fragments and 30 min prior to the TRH microinjections (see the Method section for further details).
The controls received 3.5 g/kg ethanol (IP) and 24 min later microinjection of inactive Fab fragments, or saline. No differences between the two treatments were found.
p<0.01, two-tailed t-test.
Effects of Electrical Stimulation of the Medial Septum on Ethanol-Induced Loss of Righting Reflex
Explanations for the TRH antagonism of ethanol-induced loss of righting reflex could involve a net increase, or decrease in the neuronal output from the medial septum, both of which have experimental precident [5, 8, 16]. When the medial septum was electrically stimulated (120 Hz, 160 μA, 1.5 msec duration), the animals immediately regained their righting reflex, but the microinjection of procaine (70 μg) only tended to prolong the depressant effects of ethanol (see Table 3). This reversal of ethanol-induced loss of righting reflex by electrical stimulation resembled that found after the microinjection of 1000 ng of TRH.
TABLE 3.
THE EFFECTS OF ELECTRICAL STIMULATION OR PROCAINE IN THE MEDIAL SEPTUM ON ETHANOL-INDUCED LOSS OF RIGHTING REFLEX*
| Treatment | Sleep Time (Min ± SEM) |
|---|---|
| Control | 102 ± 11 |
| Electrical Stimulation | 37 ± 5†‡ |
| Procaine (70 μg) | 112 ± 5 |
The animals (N≥5 per group) received 3.0 g/kg ethanol 30 min prior to treatment. The electrical stimulation consisted of 160 μA square waves, 120 Hz, 1.5 msec duration. The stimulation was applied until the animals regained their righting reflex or ten min transpired. The procaine (70 μg) was microinjected into the medial septum 30 min after the ethanol administration.
Only one of the five animals in this group did not regain his righting reflex within the ten minute stimulation period.
p<0.01, two-tailed t-test.
DISCUSSION
The intial finding in these studies was that microinjection of TRH into the medial septum potently antagonized ethanol’s impairment of the righting reflex in rats, while microinjection of TRH into the nucleus accumbens or the area of the raphe obscurus did not alter the course of ethanol’s depressant effects. This localization of TRH’s analeptic action with respect to ethanol mirrors the basic findings of both Kalivas and Horita [7] and Sharp et al. [14] where phentobarbital was used as the depressant agent. Also, a comparison of the results from the later two studies reinforces the conclusion that within the septum, the medial portion is more sensitive to TRH’s analeptic action. Both groups of investigators used a 40 mg/kg dose of pentobarbital, but the median effective dose of TRH in the medial septum was 85 ng [7] while bilateral microinjections into the lateral septi required 1000 ng of TRH to significantly antagonize the pentobarbital depression [14]. This dose discrepancy becomes further magnified when other brain areas, known to mediate TRH effects and contain TRH receptors [10], were tested in the present studies. The area of the raphe obscurus is very sensitive with respect to TRH’s actions on respiratory function (100 ng significantly alters respiratory timing) [11], yet microinjection of 1000 ng of TRH had no effect on ethanol’s impairment of the righting reflex. In the nucleus accumbens, high doses of TRH are necessary to reverse pentobarbital-induced sedation (5 μg) [14] and even higher doses of TRH are necessary to increase locomotor activity (20 μg) [12]. In the present studies, 2 μg of TRH microinjected into the nucleus accumbens had no effect on the depressant action of ethanol. Clearly, the medial septum appears most sensitive to the analeptic action of TRH against ethanol-induced sedation. Presumably, this interaction of TRH with ethanol involves a neuronal mechanism, because previous studies have shown that intracerebral administration of 30 μg of TRH does not alter ethanol concentrations in the brain [2].
One possible explanation for this TRH effect would be a non-specific irritation of neurons in the medial septum. Such an irritation, like mechanical trauma, might increase neuronal firing and hence, shorten the time necessary for the animals to regain their righting reflex. The studies using the TRH Fab fragments do not support this possibility. The microinjection of the TRH Fab fragments, prior to the microinjection of TRH into the medial septum, completely abolished the effects of the TRH, while microinjection of the TRH Fab fragments alone had no effects on the loss of righting by ethanol. Apparently, the binding of the exogenously applied TRH by the TRH Fab fragments prevented the action of the exogenous TRH on neurons in the medial septum.
The actual effect of TRH on the neurons in the medial septum could be a net increase or decrease in neuronal activity. For example, Farber et al. [5] found that microinjection of lidocaine into the hypothalamus mimicked TRH-induced increases in respiratory rate, and Winokur and Beckman [16] found that iontophoretic application of TRH in the septum generally decreased cell firing. In contrast, Lamour et al. [8] reported that TRH increased the activity of identified septohippocampal neurons. Since electrical stimulation of the medial septum mimicked the effects of TRH in antagonizing ethanol’s impairment of the righting reflex, while procaine only tended to prolonged ethanol’s effects, it appears that the net effect of TRH microinjection into the medial septum is to increase neuronal activity. It could also be argued that the electrical stimulation used in these experiments actually hyperpolarized the neurons, leading to a decrease in the net output from the medial septum. However, when one considers that ethanol administration causes an increase in the afterdischarge threshold most potently in the medial septum [9], the net effect of ethanol on the medial septum appears to be inhibitory in nature. Therefore, antagonism by TRH would involve conceptually an increase in the net output. Certainly, this increased output could arise from inhibitory action of TRH on neurons that serve an inhibitory function in the medial septum.
As mentioned in the introduction, the dissection of ethanol’s mechanism(s) of action on the CNS depends upon the identification of a site that is linked to some ethanol-induced behavioral change. Given such an interaction, then one could attempt to define what neurochemical events, occurring within that specific anatomical area, parallel the behavioral effects induced by ethanol. Furthermore, one could also determine if pharmacological manipulation of the neurochemical systems in this area alter the effects of ethanol on the particular behavior. From the present findings, we would hypothesize that the medial septum is intimately involved in alterting functions which contribute to the ability of an animal to right itself, and the course of effects ethanol has on the righting reflex. However, it is equally plausible that the medial septum serves an alerting role in the CNS that is distinctly different from the neurons ethanol acts upon, and the action of TRH merely alters the balance between opponent neuronal systems. Therefore, it will be necessary to assess if any neuronal measures in the medial septum follow temporally ethanol’s impairment of the righting reflex. If so, then one could postulate a correlation between ethanol’s action on this behavior and neuronal activity in the medial septum, as well as utilize the medial septum as an in vivo model for the study of ethanol’s mechanism of action on the CNS.
Acknowledgments
We thank Carolyn Reams for her assistance in the preparation of this manuscript. This work was supported by North Carolina Alcoholism Research Authority Grant #8211 and NICHD-HD-14005.
References
- 1.Breese GR, Frye GD, McCown TJ, Mueller RA. Comparison of the CNS effects induced by TRH and bicuculline after microinjection into the medial septum, substantia nigra and inferior colliculus: Absence of support for a GABA antagonist action for TRH. Pharmacol Biochem Behav. 1984;21:145–149. doi: 10.1016/0091-3057(84)90144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breese GR, Coyle S, Towle AC, Mueller RA, McCown TJ, Frye GD. Ethanol-induced locomotor stimulation in rats after thryrotropin-releasing hormone. J Pharmacol Exp Ther. 1984;229:731–737. [PubMed] [Google Scholar]
- 3.Chin JH, Goldstein DB. Effects of low concentrations of ethanol on the fluidity of spin-labled erthrocyte and brain membranes. Mol Pharmacol. 1977;13:435–441. [PubMed] [Google Scholar]
- 4.Cott JM, Breese GR, Cooper BR, Barlow TS, Prange AJ. Investigations into the mechanism of reduction of ethanol sleep by thyrotropin-releasing hormone (TRH) J Pharmacol Exp Ther. 1976;196:594–604. [PMC free article] [PubMed] [Google Scholar]
- 5.Farber JP, Connors AF, Gisolfi CV, McCaffree DR, Smith RM. Effects on breathing of putative transmitters in the rostral hypothalamus of the rat. Brain Res Bull. 1981;6:13–17. doi: 10.1016/s0361-9230(81)80064-0. [DOI] [PubMed] [Google Scholar]
- 6.Harris RA, Schroeder F. Ethanol and the physical properties of brain membranes: flourescence studies. Mol Pharmacol. 1981;20:128–137. [PubMed] [Google Scholar]
- 7.Kalivas PW, Horita A. Thyrotropin-releasing hormone: neurogenesis of actions in the pentobarbital narcotized rat. J Pharmacol Exp Ther. 1980;212:203–210. [PubMed] [Google Scholar]
- 8.Lamour Y, Dutar P, Jobert A. Effects of TRH, cyclo-(His-Pro) and (3-Me-His2) TRH on identified septohippocampal neurons in the rat. Brain Res. 1985;331:342–347. doi: 10.1016/0006-8993(85)91560-4. [DOI] [PubMed] [Google Scholar]
- 9.Leslie H, Harper RK. Regional effects of ethanol on limbic afterdischarge activity. Brain Res Bull. 1984;13:519–525. doi: 10.1016/0361-9230(84)90034-0. [DOI] [PubMed] [Google Scholar]
- 10.Mantyh PW, Hunt SP. Thyrotropin-releasing hormone (TRH) receptors. J Neurosci. 1985;5:557–561. doi: 10.1523/JNEUROSCI.05-02-00551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCown TJ, Hedner JA, Towle AC, Mueller RA, Breese GR. Brain stem localization of a thyrotropin-releasing hormone (TRH)-induced change in respiratory function. Brain Res. doi: 10.1016/0006-8993(86)90330-6. in press. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto M, Nagawa Y. Mesolimbic involvement in the locomotor stimulant action of thryrotropin-releasing hormone (TRH) in rats. Eur J Pharmacol. 1977;44:143–152. doi: 10.1016/0014-2999(77)90100-5. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- 14.Sharp T, Tulloch IF, Bennett GW, Marsden CA, Metcalf G, Dettmar PW. Analeptic effects of centrally injected TRH and analogues of TRH in the pentobarbitone-anesthetized rat. Neuropharmacology. 1984;23:339–348. doi: 10.1016/0028-3908(84)90197-7. [DOI] [PubMed] [Google Scholar]
- 15.Walgren H, Barry H., III . Actions of Alcohol. Vol. 1. New York: Elsevier Publishing Co.; 1970. pp. 209–273. [Google Scholar]
- 16.Winokour A, Beckman AL. Effects of thyrotropin-releasing hormone, norepinephrine and acetylcholine on the activity of neurons in the hypothalamus, septum and cerebral cortex of the rat. Brain Res. 1978;150:205–209. doi: 10.1016/0006-8993(78)90668-6. [DOI] [PubMed] [Google Scholar]
- 17.Youngblood WW, Moray LJ, Busby WH, Jr, Kizer JS. Bovine serum albumin-GABA-His-Pro-NH2: an immunogen for production of higher affinity antisera for TRH. J Neurosci Methods. 1983;9:367–373. doi: 10.1016/0165-0270(83)90067-5. [DOI] [PubMed] [Google Scholar]


