Abstract
Crystal structures of the bacterial amino acid transporter LeuT have provided the basis for understanding the conformational changes associated with substrate translocation by a multitude of transport proteins with the same fold. Biochemical and modeling studies led to a “rocking bundle” mechanism for LeuT that was validated by subsequent transporter structures. These advances suggest how coupled solute transport might be defined by the internal symmetry of proteins containing inverted structural repeats.
Alternating Access as a Mechanism for Transmembrane Solute Transport
For the last 40 years, our understanding of the mechanistic basis for solute transport across membranes has been shaped by the alternating access mechanism (22, 37, 56). In this model, transported solutes bind to a site in the transporter that can be exposed by conformational changes to one side of the membrane or the other. Early mechanistic interpretations of this model suggested that the transporter (carrier) bound its substrate on one side of the membrane and then migrated to the other side, where substrate was released (8). Although this moving carrier model is probably accurate for ionophores such as valinomycin, nigericin, and 2,4-dinitrophenol, our current understanding of membrane protein structure suggests that the energetic barrier for such movement is prohibitive.
Peter Mitchell suggested that, rather than a moving carrier, we should think about the transport process in terms of a moving barrier (34). The binding site for substrate could be at a fixed point within the transporter structure, but the permeability barrier between that site and the aqueous phases on either side of the membrane could move so that the site was accessible alternately from only one side of the membrane at a time. Although the moving carrier and moving barrier models for transport are quite different in their mechanistic details, they both predict similar “carrier kinetics,” which explain such phenomena as counterflow and accelerative exchange diffusion (52). Until recently, when the first crystal structures of transporters were solved, evaluation of specific mechanisms has not been possible. Now that such structures are appearing with regularity, the issue of specific transporter mechanism is at the forefront of the field. Conformational mechanisms of transport have been proposed for P-type ATPases, based on the numerous crystal structures of the SERCA Ca2+ pump (36, 53). Another mechanism has been proposed for lac permease, a H+-coupled transporter, based on abundant biochemical and biophysical evidence (24, 32, 50).
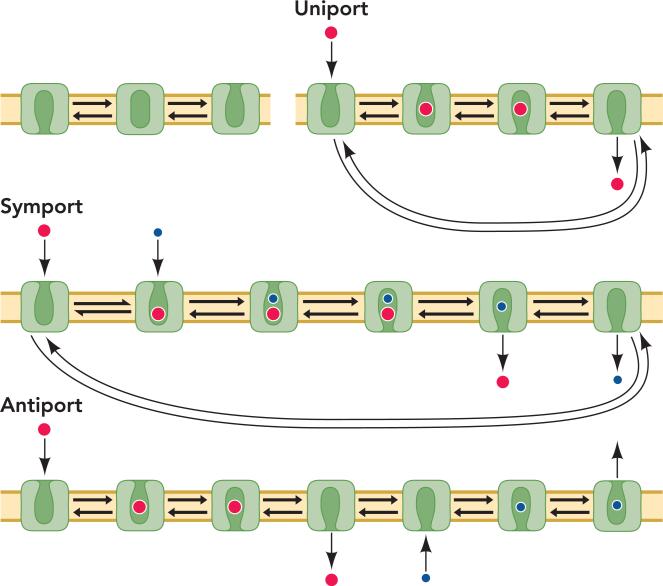
A compelling feature of the alternating access model is that it can account for many types of transport. In the simplest of these, uniport (also facilitated or mediated diffusion), the transporter allows passage of a single solute across the membrane. An example of this process is the transport of glucose into mammalian cells catalyzed by the GLUT family of transporters (5). For alternating access to account for uniport, the substrate would bind to the transporter from one side of the membrane, and a conformational change would close access to the binding site from that side and open access to the opposite side. The solute would then dissociate, and another conformational change would bring the transporter back to its original conformation (FIGURE 1).
FIGURE 1. Alternating-access mechanisms.
A simple mechanism for alternately exposing a substrate binding site to either side of the membrane involves interconversion through an occluded intermediate (top left). This mechanism is sufficient to explain transport of a single solute (uniport), two or more solutes in the same direction (symport), and movement of solutes in opposite directions (antiport). For efficient symport, the interconversion of outward- and inward-facing transporter conformations cannot occur when only one substrate is bound, and for efficient antiport the interconversion can occur only when one of the substrates is bound.
The alternating access mechanism also accounts for coupling the flux of two or more solutes. This process is sometimes referred to as secondary active transport to distinguish it from primary active transport, which is driven by metabolic energy such as ATP hydrolysis. In symport (co-transport) the solutes cross the membrane in the same direction, and in antiport (counter-transport, exchange) they cross in opposite directions. For symport, two or more solutes bind and are released to the opposite side of the membrane in the same way as in uniport, followed by reorientation of the transporter as with uniport (FIGURE 1). In antiport, one or more solutes is transported across the membrane (like uniport or symport), but then before reorientation of the transporter, a different solute binds and is subsequently transported in the opposite direction (FIGURE 1). In both symport and antiport, the energy of a solute transmembrane concentration gradient can thus be used to concentrate another solute on one side of the membrane.
If different transporters use this same general mechanism to catalyze uniport, symport, or antiport, there must be additional parameters that determine which kind of transport occurs in each case. Jencks (23) postulated that there must be “rules” followed by different coupled vectorial processes to account for different modes of coupling. In alternating-access transport mechanisms, these rules must determine when the transporter may undergo the conformational change that switches binding site access from one side of the membrane to the other. For example, a transporter will catalyze symport if it undergoes the conformational change when either both solutes or none are bound, but not if only one is bound. Antiport, in contrast, requires that the conformational change occurs only when a substrate is bound but not when the binding site is empty (see FIGURE 1).
The conformational change that transforms a transporter from outward- to inward-facing is likely to go through some intermediate state. One could imagine states in which the binding site is accessible simultaneously from both sides of the membrane and others in which it is accessible from neither side, as in FIGURE 1. A transporter that moves its substrate against a concentration gradient is less efficient if there is an intermediate from which the substrate could bind and dissociate to either side of the membrane. Such an intermediate would allow uncoupled flux of the bound substrate and would be equivalent to a channel through the membrane, albeit one selective for the substrate. A stark example is the Na+-K+-ATPase, which can be converted from an ATP-coupled transporter to a channel for Na+ and K+ by binding of the marine natural product palytoxin (4). In its channel mode, the protein dissipates cation gradients much faster than they can be generated by ATP hydrolysis. Consequently, we expect that transport by alternating access requires an occluded intermediate in which the substrate binding site is not accessible from either side of the membrane (FIGURE 1). Nevertheless, there are transporters with associated uncoupled ion fluxes, and these may occur through intermediate states in which the pathway is incompletely occluded (13, 31, 51).
A reasonable expectation of many proposed implementations of the alternating access mechanism is that the substrate binding site would be located near the center of the membrane so that the path from either side of the membrane would be of similar length. Indeed, structures of transporters with bound ligands have borne out the expectation of a binding site at the midpoint of the permeability barrier (1, 18, 38, 59, 60). These findings highlight the intrinsic symmetry of the transport process. Binding of substrate from one side of the membrane is the converse of dissociation of that substrate to the opposite side of the membrane. The process of substrate transport can thus be reduced to a set of binding and dissociation steps on either side of the membrane, in addition to the opening and closing of permeability barriers between a central binding site and the aqueous solution on either side.
Mechanistic Implications of Transporter Structure: LeuT as an Example
The neurotransmitter sodium symporter (NSS) family illustrates key elements in transporter structure and function. In 2005, Eric Gouaux and his colleagues presented the first atomistic structure of a member of this family. It was a high-resolution X-ray diffraction structure of LeuT, a transporter from the thermophilic bacterium Aquifex aeolicus (59). This structure was remarkable in many ways, but perhaps the most significant is that it made sense of a wealth of biochemical data collected from neurotransmitter transporters. Although there is only 20–25% amino acid identity between LeuT and the mammalian NSS transporters, the regions of highest homology cluster around the positions where a leucine molecule and two Na+ ions were found. LeuT is now understood to be an amino acid transporter, and leucine is a very poor substrate but one that binds tightly to the protein (49). Crystal structures are now available for LeuT with other substrate and non-substrate amino acids bound, and in complex with tricyclic antidepressants—compounds that inhibit the homologous transporters SERT and NET (which transport serotonin and norepinephrine, respectively) (48, 62).
In the original LeuT structure with leucine bound (59), an aqueous pathway is clearly visible from the extracellular medium, almost reaching the binding site for amino acid and Na+ ions. However, between the bound amino acid and the cytoplasmic side of the membrane, there is no pathway for substrate diffusion. Rather, 20 Å of packed protein structure prevent dissociation of the amino acid to the cytoplasm. Indeed, diffusion of bound leucine to the extracellular side is also blocked (59), although only a few amino acid side chains constitute this steric barrier. Thus, although the overall structure resembles an extracellular-facing conformation, leucine is occluded within its binding site by barriers to both sides of the membrane, similar to the prediction from alternating access models of transport.
LeuT structures have since been reported with bound alanine, glycine, methionine, tyrosine, or tryptophan (49). In all but one of these, the protein structure is almost identical to that of the LeuT-leucine structure. However, the LeuT-tryptophan structure is different. Although tryptophan was found in the same binding pocket, its greater bulk results in a protein conformation that is more open, providing a continuous path from the extracellular medium to the binding site. Tryptophan is not a substrate for LeuT and acts as a competitive inhibitor of leucine and alanine transport. Its inhibitory action seems to be that of a classical competitive inhibitor: it competes with substrate for binding to LeuT but is not itself transported.
In contrast to competitive inhibition by tryptophan, tricyclic antidepressants inhibit LeuT non-competitively (48). In other words, although inhibition by tryptophan can always be overcome by adding higher concentrations of substrate (49), no amount of substrate can overcome LeuT inhibition by a tricyclic compound like clomipramine or desipramine (48). The structural basis for this non-competitive inhibition was revealed in a series of crystal structures with both leucine and antidepressant bound to LeuT (48, 62, 63). The substrate site was occupied with leucine, whereas the antidepressant molecule was bound in the permeation pathway leading from the extracellular medium to the leucine site. Antidepressant binding, by preventing closure of the extracellular pathway, could thus block transport if opening of the cytoplasmic pathway is coupled to closing the extracellular one. Because the antidepressant was bound to a site distinct from that for leucine, increasing the leucine concentration could not overcome inhibition, and the kinetics were classically non-competitive.
The cytoplasmic pathway
Through what pathway do substrates bound to LeuT and other NSS transporters diffuse to the cytoplasm? By measuring the reactivity of cysteine residues placed in SERT, we found that many positions were accessible to reagents from the cytoplasmic side of the membrane despite the fact that these positions were predicted, by comparison with the structure of LeuT, to be buried in the protein interior (16). These reactive positions delineated a pathway from the substrate binding site to the cytoplasm composed of one face each of TMs 1, 5, 6, and 8 (FIGURE 2A). Similar reactivity was subsequently found in the corresponding residues of TM8 in GAT-1 (6).
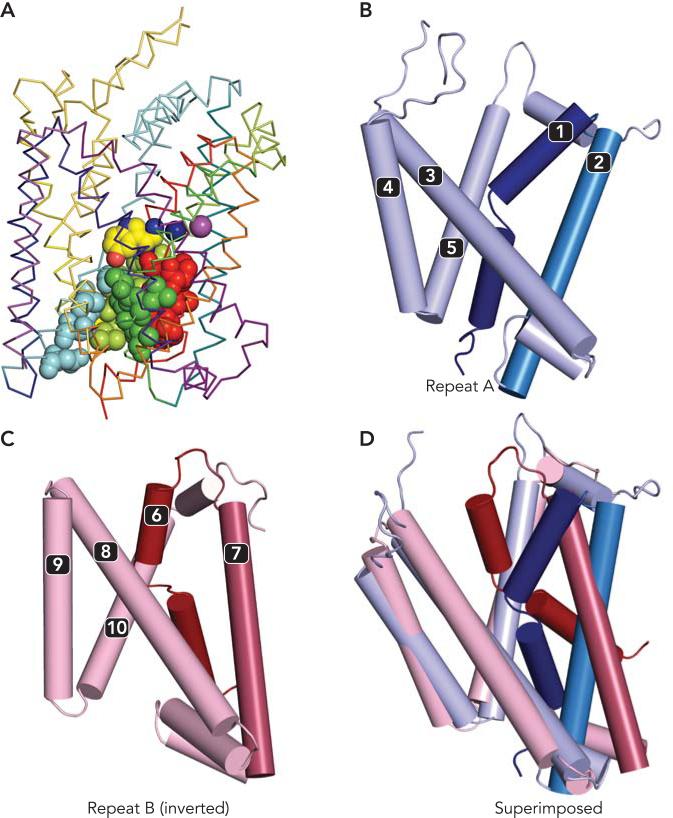
FIGURE 2. Cytoplasmic pathway of SERT and repeat structure of LeuT.
A: Cytoplasmic pathway of serotonin transporter SERT is lined by amino acid residues (spheres) from transmembrane helices 1 (red), 5 (pale green), 6 (green), and 8 (cyan). The model of SERT was built by homology to LeuT and is viewed from the plane of the membrane with the backbone trace in ribbons, with bound serotonin (yellow), chloride (magenta), and sodium ions (dark blue) shown as spheres. B–D: the LeuT fold contains inverted topology repeats of five trans-membrane helices. Repeat A contains transmembrane helices 1–5 (B), and repeat B consists of transmembrane helices 6–10 (C), according to the LeuT numbering. Superposition (D) of the last three transmembrane helices from each of those repeats (pale colors) indicates a relative difference in the orientation of the first two transmembrane helices (dark colors). These two conformations of the repeats may contain the essence of the two alternating-access states of the transporter.
The location of the cytoplasmic permeation pathway was confirmed by changes in its accessibility in response to ligands and substrates. For several positions in this pathway, addition of serotonin increased the reactivity of inserted cysteines, and this increase required Na+ and Cl–, both of which are also required for serotonin-dependent conformational changes in transport (61). Almost all positions in this pathway were less accessible in the presence of cocaine, which binds from the cell exterior, holding the extracellular pathway open and the cytoplasmic pathway closed (61). The opposite was observed with ibogaine, an inhibitor that favors the SERT conformation in which the cytoplasmic pathway is open and the extracellular pathway is closed (16, 21).
“. . . the involvement of repeated structural elements is clearly an elegant and appealing solution to the problem of bringing the substrate into the binding site from one side of the membrane and allowing it to exit on the opposite side . . .”
Inverted repeats
An unexpected feature of the LeuT structure is that it contains an inverted structural repeat. This structural feature provided a key to understanding how the cytoplasmic pathway (which was not visible in any LeuT structure) opens and closes. Within the 12 transmembrane (TM) helices that define the LeuT structure, helices 1–5 (repeat A) are in a strikingly similar arrangement to that of TMs 6–10 (repeat B), despite the lack of significant sequence homology between these two regions. Because the repeated structure contains an odd number of transmembrane spans, the topological orientation of repeat A is inverted with respect to that of repeat B. Specifically, repeat A starts with TM1 on the cytoplasmic side of the membrane and ends with TM5 on the extracellular side, and repeat B starts with TM6 on the extracellular side and ends with TM10 on the cytoplasmic side. Each part of the repeat has a structural characteristic that is echoed in the other repeat (FIGURE 2, B AND C). The unwound region in TM1, which contributes to the substrate and Na+ binding site, is complemented by an unwound region of TM6, which also contributes to these sites. TMs 1 and 6 are found within a four-helix bundle also containing TMs 2 and 7. The long, tilted helix of TM3 lies next to TM8, which is also a long and tilted helix. This pair of helices is nestled within two V-shaped helix pairs, formed by TM4–5 and TM9–10, where the short loop between the helix pairs resides on the cytoplasmic side for TM4–5 and on the extracellular side for TM9–10. Furthermore, the residues from TMs 1, 5, 6, and 8 that were accessible in the cytoplasmic pathway of SERT (16) correspond to residues from TMs 6, 10, 1, and 3, respectively, which line the extracellular vestibule in LeuT and which belong to the other repeat (49, 59). Thus the 10-TM core of LeuT containing these repeats is likely to represent the functional transport machinery. Indeed, although most members of the NSS family have 12 TMs, some have only 11 (39), and in other families a similar 10-TM core is flanked by a variable number of additional helices (see below).
Further inspection reveals that each repeat consists of two distinct elements, which are extremely structurally conserved between the two repeats (16). Specifically, comparison of the first two TM segments of each repeat (i.e., TMs 1–2 with TMs 6–7) reveals that they have very similar arrangements, including the location of the unwound segment of TMs 1 and 6. The remaining fragment of each repeat, the last three transmembrane helices, also have a greater similarity than is expected from the comparison of the whole repeats. This analysis suggests that the main difference between the two 5-TM repeats is the relative orientation of the first two TM helices with respect to the rest of the repeat (FIGURE 2D).
This difference in orientation of the first two TMs in each repeat provided an important clue for understanding the mechanism of transport. As mentioned above, these TMs together form a four-helix bundle in LeuT. Within the context of the rest of the structure, this bundle is mostly protected from the lipid by the rest of the protein, which forms a sort of scaffold around the bundle. Furthermore, the different orientation of the bundle helices in the two repeats relative to the scaffold helices reflects the fact that the four-helix bundle is itself tilted by 25° relative to the scaffold, in such a way that the extracellular pathway is formed. Thus the 5-TM structural motifs may be able to form the two required states of the transporter simply by changing the tilt of the bundle, and thereby switching between the two conformations of the repeats observed in the extracellular-facing structure.
The Cytoplasm-Facing Conformation and the Rocking Bundle Mechanism of Transport
One intriguing implication of the above observations is that it should be possible to generate a model of the unknown conformation opposite to that of the crystal structure of LeuT, a cytoplasm-facing conformation, simply by swapping the conformations of the repeats. A model of LeuT was therefore generated in which the sequence of TMs 1–5 was threaded onto the structure of TMs 6–10 and vice versa. Remarkably, this “swapped” model was extremely consistent with an alternating-access mechanism (16): a cytoplasmic access pathway to the central binding site appeared, composed of residues buried in the extracellular-facing structure that became exposed to the cytoplasmic solution. Simultaneously, the extracellular pathway to that site became occluded (FIGURE 3A).
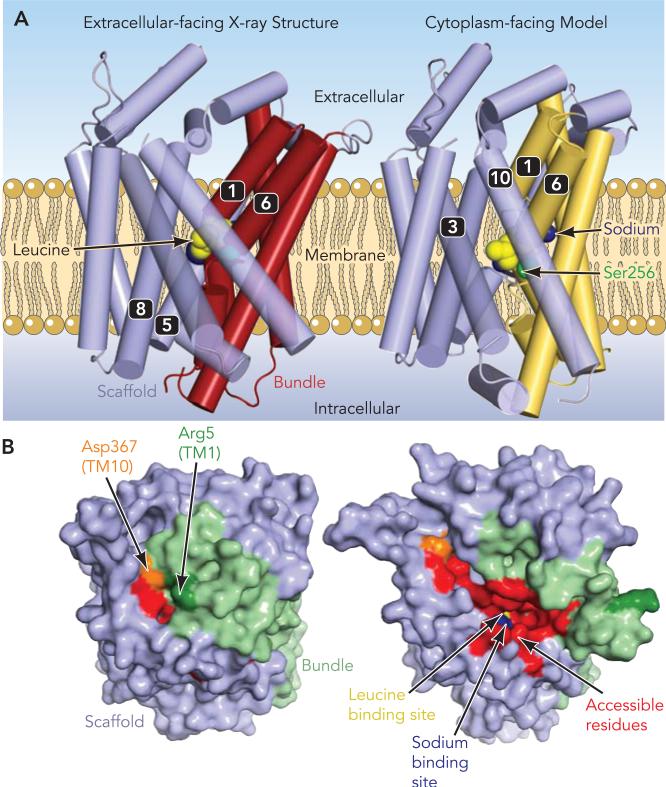
FIGURE 3. Comparison of LeuT X-ray structure and cytoplasm-facing model.
A: a model of the cytoplasm-facing conformation of leucine transporter, LeuT (right), constructed by swapping the conformations of the two five-transmembrane-helix repeats, compared with the X-ray crystal structure bound to leucine (left), viewed from the plane of the membrane, showing how the bundle (red or yellow) pivots around the central binding site at approximately the position of Ser256. B: residues found to be accessible to cysteine-modifying reagents in serotonin transporter (red or orange) are not exposed in the extracellular-facing X-ray structure of LeuT but become exposed in the cytoplasm-facing model. The primary motion is an outward rotation of the cytoplasmic half of the bundle (light green), with respect to the scaffold (light blue); for reference, two residues (Arg-5 and Asp-367) are colored dark green and orange, respectively. Structures are viewed from the cytoplasmic side of the membrane. Leucine (yellow) and sodium (blue) bound at the central binding site are visible at the base of the cytoplasmic pathway in the model but not in the X-ray structure.
The cytoplasmic pathway revealed in this model was lined by the four transmembrane domains identified in the cytoplasmic pathway by accessibility measurements in SERT, namely, TMs 1, 5, 6, and 8. Moreover, the calculated accessibility of residues in these helices agreed extremely well with the experimental measurements (FIGURE 3B). Thus the model generated by swapping the conformations of the two repeats of LeuT is highly consistent with a cytoplasm-facing state conformation of the whole protein.
Comparison of the LeuT X-ray structure with the cytoplasm-facing model highlights those regions that may change during transport. The greatest observed change between the LeuT crystal and the model is in the orientation of the bundle of TMs 1–2 and 6–7 with respect to the scaffold. The bundle orientation differs by a tilt of ~25° around an axis running through the binding site region (FIGURE 3A). In effect, the cytoplasmic ends of TMs 1 and 6, which are packed against the scaffold helices 5 and 8 in the structure of Yamashita et al. (59), are separated from these regions in the cytoplasm-facing conformation. In addition, in the model, the extracellular ends of TMs 1 and 6 pack closely against TMs 3 and 10 on the other side of the binding site. A simple molecular interpretation therefore would be that “rocking” of this bundle between two alternate orientations would suffice to open and close the pathway in a coordinated manner.
Other, more limited, differences were identified between the X-ray structure and the cytoplasm-facing model, for example, for the long, tilted helices of TMs 3 and 8, which curve slightly toward or away from the binding site, respectively, in the X-ray structure. In the model, the curvature of theses helices is exchanged, as if each helix was flexing during the conformational change. Such a flexing motion may help to exaggerate the pathway opening on one side or the other but also requires subtle movements of the two V-shaped pairs of helices (TMs 4–5 straddling TM8 and TMs 9–10 straddling TM3). Although these movements may contribute to the accessibility changes in the binding site, they are probably not sufficient to expose the binding site to the cytoplasm, a transition that appears to require movement of the TM1, 2, 6, 7 bundle.
The LeuT structure and proposed mechanism for transport by LeuT, SERT, and other members of this family fulfill many requirements of an alternating access transport process. For example, a mechanism by which accessibility of the central binding site is switched from side to side by tilting or rocking of the four-helix bundle serves to couple the opening of the cytoplasmic permeation pathway with the closing of the extracellular pathway and vice versa. Moreover, by pivoting around the central binding site during this motion, residues in contact with the substrate and symported ions move relatively little compared with the large motions at the extreme ends of the bundle.
According to coupling rules for symport and antiport, the conformational changes required for transport should be controlled by ligand binding events. According to the rocking-bundle mechanism, the binding sites would be located at the interface between the moving bundle and a stationary scaffold, providing a way for the ligand to control the orientation of the bundle through direct interactions. A recent proposal suggests that, in LeuT, binding of a second molecule of leucine in the extracellular pathway facilitates the conformational change (47). Studies testing this hypothesis and others are critical to understanding the mechanisms that couple solute binding to conformational change in transport proteins.
Inverted Repeats in New LeuT-Like Structures
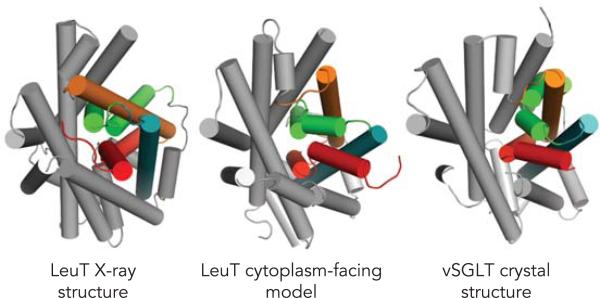
Four years after the publication of the LeuT structure, crystallographic studies of several other transporter proteins originally categorized in very different families from NSS have revealed that the 5-TM inverted-repeat fold is more common than expected. In 2008, Faham et al. reported a 2.7 Å-resolution X-ray diffraction structure of a bacterial homolog of the sodium:glucose symporter SGLT1 from the solute sodium symporter (SSS) family (12). A relationship to the NSS family had been suggested by comparisons of hydrophobicity involving alignments of so-called hydropathy plots (28, 29) and from careful interpretation of biochemical data on the related sodium-iodide symporter (10). Nevertheless, the presence of a trans-membrane helix that precedes the 5-TM repeats, of a total of 14 TM domains, as well as the extremely low sequence identity, were sufficient to confound any general expectation of a structural relationship. Thus it was a surprise that the Vibrio parahaemolyticus vSGLT structure in fact has a close relationship to that of LeuT. Given that it probably represents a cytoplasm-facing conformation, it is useful to compare the structure of vSGLT with the cytoplasm-facing model of LeuT described above. Most remarkably, the cytoplasmic pathway in vSGLT consists of the very TM domains (2, 6, 7, and 9) that correspond to those forming the pathway in the cytoplasm-facing model of LeuT (1, 5, 6, and 8) (FIGURE 4). This observation suggests that LeuT and vSGLT use the same rocking bundle mechanism and that the two crystal structures represent the two main conformational states. The vSGLT structure also confirms predictions that the two pathways in these proteins are symmetrical and are formed by the corresponding regions of the structural repeats, on opposite sides of a pseudo-symmetric binding site (16, 59). According to the suggestion of Abramson and Wright (2), we will subsequently refer to TM helices in vSGLT and other proteins that share the LeuT fold according to the corresponding helix in LeuT so that repeat A contains TMs 1–5 and repeat B contains TMs 6–10 in each case.
FIGURE 4. Comparison of LeuT with vSGLT.
The cytoplasmic pathway in LeuT-fold structures, viewed from the cytoplasm. A model of LeuT in a cytoplasm-facing conformation (center), generated by swapping the conformations of the two five-transmembrane repeats in LeuT (left), differs from the X-ray structure of LeuT (left) but resembles that of a structure of vSGLT (right), also in a cytoplasm-facing conformation. Structures are shown with helices as cylinders, showing how the cytoplasmic halves of TMs 1 (red), 2 (orange), 6 (green), and 7 (blue) in the bundle peel away from the scaffold (gray) in the cytoplasm-facing conformations but are packed against the scaffold in the extracellular-facing conformation of LeuT (left).
Subsequently, the structure of a sodium:benzylhydantoin symporter Mhp1 from Microbacterium liquefaciens and belonging to the nucleobase:cation symporter (NCS) family revealed it to be even more similar to LeuT, and also in an extracellular-facing orientation (58). Two X-ray crystal structures were reported for this protein, one at 2.9 Å resolution without bound substrate and a second at lower resolution (4.0 Å), showing a clear density for substrate, coupled with a conformational change of an extracellular loop that completely closes the pathway on the extracellular side. Mhp1 contains twelve transmembrane domains, with structural repeats in TMs 1–5 and 6–10 as for LeuT. TMs 11 and 12 are also peripheral to the core domain in Mhp1 and pack against different parts of the structure than in LeuT.
An X-ray crystal structure of a sodium:betaineglycine transporter, BetP from Corynebacterium glutamicum at 3.35-Å resolution has also been reported (42). This structure also contains a LeuT-like fold but has two TM helices preceding the first repeat, which contains TMs 3–7, whereas the second repeat contains TMs 8–12. The BetP structure is apparently in an occluded, substrate-bound state: no pathway is apparent on the periplasmic side, although a narrow pathway on the cytoplasmic side is formed by helices corresponding to those lining the pathway in vSGLT and in the cytoplasm-facing model of LeuT. Interestingly, the two repeats in the BetP structure are more similar to one another than in the other structures, consistent with a more intermediate conformation. Under resting conditions with low osmolarity, as found in the crystallization conditions, BetP is inactive. Thus it is likely that this occluded state reflects a down-regulated, inactive state of the protein. However, it is also possible that this intermediate conformation occurs as part of the normal transport cycle of BetP.
Recently, consistent with bioinformatic analysis (30), three structures representing another major transporter family, the amino acid/polyamine/organocation or APC superfamily, have been published. The two proteins, AdiC and ApcT, both adopt the LeuT fold (14, 17, 46). The APC (or SLC7) family is itself related by sequence homology to the SLC12, SLC32, SLC36, and SLC38 families that catalyze cationchloride symport, amino acid/H+ antiport, amino acid/H+ symport, and amino acid-Na+ symport/H+ antiport, respectively. The variety of substrates and coupling modes suggests that this basic protein fold has evolved to allow wide mechanistic flexibility within the same structural framework. The finding that this transporter fold accommodates both symport and antiport mechanisms portends that uniport also may be catalyzed by some members of this structural family and that movement of the four-helix bundle may be the key step in transport for all of these proteins.
Scaffold and Bundle Movements in Proteins with the LeuT Fold
The scaffold of the 5TM-inverted-repeat structures appears to be relatively well conserved, both between repeats and across proteins. Between any two proteins of this fold, the entire scaffold region (TMs 3–5 + 8–10) shows strong similarities, even between structures in different states. These observations argue against a mechanism of transporter conformational change in which large movements within the scaffold region are responsible for changing the accessibility to the binding site as proposed for Mhp1 (58). Nevertheless, we note that in the structures of Mhp1 and vSGLT, and in molecular dynamics simulations of BetP (Khafizov KF, Forrest LR, unpublished results), there is evidence for movement of the loop regions connecting TM4–5 and TM9–10, which are both in the scaffold (58). These movements may serve to occlude the substrate site from one side of the membrane and may reflect an intrinsic flexibility of these regions but appear not to be involved in opening the pathway from the opposite side of the membrane.
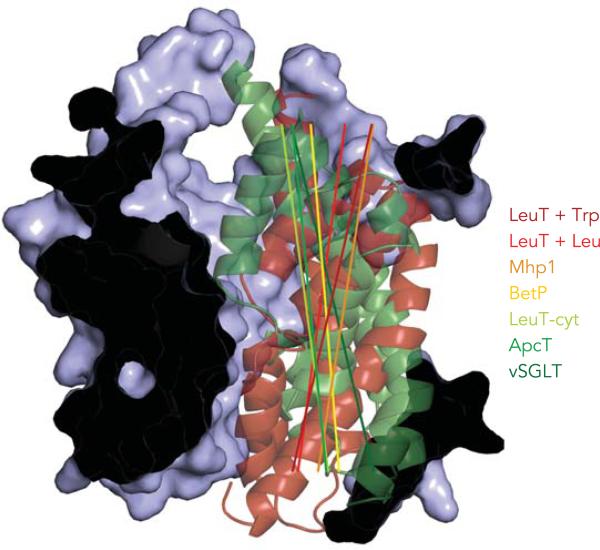
The most significant difference between the various LeuT fold structures is the orientation of the bundle relative to the scaffold. The angle between the bundle axis and the vertical scaffold helices varies from +15° in LeuT (with Trp bound) to –14° in vSGLT, with Mhp1, BetP, and ApcT at intermediate angles (FIGURE 5) (Forrest LR, unpublished analysis). In all these structures, the four bundle helices are packed together. This integrity of the bundle structure, and the short loops connecting its helices, argue in favor of it moving essentially as a unit in the transition from extracellular- to cytoplasm-facing orientations, as suggested by the rocking-bundle mechanism. Such a mechanism in which the bundle remains intact during the transition from extracellular- to cytoplasm-facing conformations assures the integrity of the binding site for Cl– found in SERT and GAT-1 (15, 54, 64), which is formed by residues from TMs 2, 6, and 7. Similarly, the substrate binding sites in vSGLT and BetP, which are formed by residues in TMs 1, 2, 6, and 7 (12, 42), would also be maintained. Furthermore, moving the bundle as a unit provides a means for coupling the opening and closing of the two pathways, through the positioning of the long, unbroken TM helices 2 and 7. That is, helices 2 and 7 may serve as semi-rigid splints behind the broken TMs 1 and 6. Thus, if one pathway is opened by the swinging out of 1 and 6, then the splints would help ensure that the other pathway is simultaneously closed. However, this mechanism does not require the four-helix bundle unit to move as a perfectly rigid body, and some internal rearrangements between the helices are no doubt possible, as illustrated by the LeuT-Trp structure, in which the extracellular ends of the helices are swung further outward. Furthermore, accessibility and cross-linking measurements on the related GABA transporter GAT-1 and the amino acid transporter KAAT1 indicate some rearrangement of the bundle helices during transport (7, 43). In any case, this mechanism does not rule out the possibility of additional intermediate and/or occluded states.
FIGURE 5. Rocking of the bundle within the LeuT transporter fold structure results in opening and closing of pathways on either side of the membrane.
The surface of the scaffold region from LeuT is shown as a blue cut-away surface, viewed from the plane of the membrane. The axis of the four-helix bundle consisting of TMs 1, 2, 6, and 7 is shown as a line, colored from red for extracellular-facing through green for cytoplasm-facing conformations. Axes were defined as the line joining the average coordinates of all helical residues in the upper and lower leaflets of the membrane, after superposition of the residues in the scaffold onto those of LeuT. Four-helix bundles are shown as cartoons for the X-ray structures of tryptophan-bound LeuT (dark red) and for vSGLT (dark green), which are in the fully open extracellular-facing and cytoplasm-facing conformations, respectively.
In addition to the cytoplasmic and extracellular halves of the four-helix bundle, which function as large gating elements that open and close the permeation pathways, there are a few side chains that can separate bound substrate from the open pathways in LeuT and vSGLT (26). We speculate that these thin central “gates” (D404, R30, Y108, and F253 in LeuT; M73, Y87, and F424 in vSGLT) serve an important role in transport by preventing substrate dissociation as the four-helix bundle reorients. The gates therefore would fulfill the prediction of the alternating access model by preventing the formation of a continuous open pathway that would function as an uncoupled substrate channel. In transporters with uncoupled ion conductances (13, 31, 51), it may be irregularities in the closing of these thin gates or the motion of the bundle that are responsible for the leak.
Inverted Repeats, Evolution, and Mechanism in Other Membrane Proteins
Repeated structural elements are a very common motif in membrane proteins, observed in proteins with diverse functions, including primary active transporters (27, 55), secondary transporters (11) and channels (35). For example, the SecY translocon, responsible for primary transport of newly translated proteins, contains a pseudo-symmetric repeat of five TM helices with a different fold from that of LeuT (55). In many cases, these relationships were unexpected, due to a lack of obvious sequence homology, and revealed only by resolution of their atomic structures. Nevertheless, bioinformatic analysis suggests that such repeats originate from internal gene duplications of smaller membrane proteins and thus reflect a common evolutionary pathway for membrane proteins (44). For example, the last 3-TM segment of some major-facilitator superfamily (MFS) transporters is related in sequence to a voltage-gated sodium channel of a similar size (20). In the specific case of repeats with inverted topologies, their evolution appears to have occurred via half-sized intermediate proteins that were able to adopt both of the two transmembrane topologies, followed by internal gene duplication (40, 41, 45, 57). In any case, the commonality of such topologies suggests that additional novel structures containing inverted repeats are likely to be discovered.
For some proteins, such as LeuT, the use of inverted-topology repeats may have had the added advantage, beyond the mechanism of evolution, of creating a symmetric pathway leading from either side of the membrane toward a central substrate binding site. That is, each of the structural elements that make up the extracellular permeation pathway has a counterpart in the other repeat that contributes to the cytoplasmic pathway. Aside from LeuT, this is potentially also the case for the sodium-proton antiporters (e.g., NhaA), for which a repeat of three TM helices has been reported (and can, moreover, be extended to include two additional TM domains, providing a case of two 5-TM inverted segments separated by an insertion of two TM helices), although the ion pathway in these antiporters remains to be characterized (19). Structures of proteins from the ClC family, on the other hand, clearly reveal a similar situation to that in LeuT, namely that a twofold symmetric repeat (with seven TM segments in this case) creates a pseudo-symmetric central pathway (11). The function of the ancestral ClC proteins, which is probably chloride-proton antiport, as in the E. coli protein (3), may well have benefited from such a symmetrical arrangement. Interestingly, however, some eukaryotic homologs of these antiporters have subsequently lost their ability to couple to proton gradients (33), resulting in passive channels for chloride that nevertheless retain the pseudo-symmetric structure.
Like the chloride channels of the ClC family, proteins in the aquaporin (Aqp) and Amt/MEP/Rh families also provide examples of channels in which inverted-topology repeats contribute to a pseudo-symmetric pathway: in the case of the Aqp family, the repeat consists of three TM helices plus a short helix in a so-called re-entrant loop (35), whereas the Amt/MEP/Rh proteins contain twofold symmetric repeats of five TM helices (25). It is tempting to speculate that the channels in these families, like the ClC channels, are also “broken” transporters.
Although the involvement of repeated structural elements is clearly an elegant and appealing solution to the problem of bringing the substrate into the binding site from one side of the membrane and allowing it to exit on the opposite side, it is obviously not the only solution, since major classes of transport proteins have structures without this twofold symmetry. An example is the mitochondrial carriers typified by the adenine nucleotide exchanger (38).
Beyond the formation of symmetric pathways, the case of LeuT also suggests that inverted-topology repeats allow for a dynamic opening and closing of those pathways through generation of two symmetry-related conformations of each repeat (FIGURE 2). Recently, inverted-topology repeats with different conformations have also been identified in the extra-cellular-facing conformation of the aspartate transporter GltPh (9, 42a). This protein belongs to the DAACS/EAAT families, whose structures are very different from that of LeuT (60). The repeats in GltPh were also used to construct a model for the cytoplasm-facing form of the aspartate transporter that is pseudosymmetrical relative to the extracellular-facing structure (9) and that is close agreement with a recent crystal structure of the cytoplasm-facing state (42a). In contrast to the rocking bundle mechanism of LeuT, GltPh is predicted to function by a shuttling movement of a substrate-carrying domain through the protein—locking the gate to one side of the membrane while allowing the other gate to open.
A similar exchange between two alternate conformations of inverted repeats may also occur in MFS transporters, typified by the lactose permease LacY. These proteins contain two 6-TM domains, each containing a 3-TM inverted-topology repeat (1). If TM segments 1–3 and 10–12 are considered to constitute one non-contiguous repeat, whereas TMs 4–6 and 7–9 constitute a second repeat, then exchanging their conformations should lead to a similar exposure of pseudo-symmetrical pathways. It will be interesting to see whether such analysis of structural repeats can indeed prove to be a generally useful approach to deduce transport mechanisms from yet-tobe-discovered transporter structures. Fundamentally, however, these observations suggest that inverted-topology repeats not only contribute to formation of symmetric pathways in channels and transporters but provide an elegant mechanism by which nature can create a transporter protein capable of adopting two symmetry-related conformational states.
References
- 1.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 2.Abramson J, Wright EM. Structure and function of Na+-symporters with inverted repeats. Curr Opin Struct Biol. 2009;19:425–432. doi: 10.1016/j.sbi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl– channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- 4.Artigas P, Gadsby DC. Ion channel-like properties of the Na+/K+ Pump. Ann NY Acad Sci. 2002;976:31–40. doi: 10.1111/j.1749-6632.2002.tb04711.x. [DOI] [PubMed] [Google Scholar]
- 5.Barrett MP, Walmsley AR, Gould GW. Structure and function of facilitative sugar transporters. Curr Opin Cell Biol. 1999;11:496–502. doi: 10.1016/s0955-0674(99)80072-6. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Yona A, Kanner BI. Transmembrane domain 8 of the gamma-aminobutyric acid transporter GAT-1 lines a cytoplasmic accessibility pathway into its binding pocket. J Biol Chem. 2009;284:9727–9732. doi: 10.1074/jbc.M809423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castagna M, Soragna A, Mari SA, Santacroce M, Bette S, Mandela PG, Rudnick G, Peres A, Sacchi VF. Interaction between lysine 102 and aspartate 338 in the insect amino acid cotransporter KAAT1. Am J Physiol Cell Physiol. 2007;293:C1286–C1295. doi: 10.1152/ajpcell.00190.2007. [DOI] [PubMed] [Google Scholar]
- 8.Crane RK, Forstner G, Eichholz A. Studies on the mechanism of the intestinal absorption of sugars. Biochim Biophys Acta. 1965;109:467–477. doi: 10.1016/0926-6585(65)90172-x. [DOI] [PubMed] [Google Scholar]
- 9.Crisman TJ, Qu S, Kanner BI, Forrest LR. The inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats. PNAS. doi: 10.1073/pnas.0908570106. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De la Vieja A, Reed MD, Ginter CS, Carrasco N. Amino acid residues in transmembrane segment IX of the Na+/I– symporter play a role in its Na+ dependence and are critical for transport activity. J Biol Chem. 2007;282:25290–25298. doi: 10.1074/jbc.M700147200. [DOI] [PubMed] [Google Scholar]
- 11.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 12.Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Jayaram H, Shane T, Kolmakova-Partensky L, Wu F, Williams C, Xiong Y, Miller C. Structure of a prokaryotic virtual proton pump at 3.2 A resolution. Nature. 2009;460:1040–1043. doi: 10.1038/nature08201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrest LR, Tavoulari S, Zhang YW, Rudnick G, Honig B. Identification of a chloride ion binding site in Na+/Cl–-dependent transporters. PNAS. 2007;104:12761–12766. doi: 10.1073/pnas.0705600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci USA. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao X, Lu F, Zhou L, Dang S, Sun L, Li X, Wang J, Shi Y. Structure and mechanism of an amino acid antiporter. Science. 2009;324:1565–1568. doi: 10.1126/science.1173654. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 19.Hunte C, Screpanti E, Venturi M, Rimon A, Padan E, Michel H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature. 2005;435:1197–1202. doi: 10.1038/nature03692. [DOI] [PubMed] [Google Scholar]
- 20.Hvorup RN, Saier MH., Jr Sequence similarity between the channel-forming domains of voltage-gated ion channel proteins and the C-terminal domains of secondary carriers of the major facilitator superfamily. Microbiology. 2002;148:3760–3762. doi: 10.1099/00221287-148-12-3760. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs MT, Zhang YW, Campbell SD, Rudnick G. Ibogaine, a noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. J Biol Chem. 2007;282:29441–29447. doi: 10.1074/jbc.M704456200. [DOI] [PubMed] [Google Scholar]
- 22.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 23.Jencks WP. Utilization of binding energy and coupling rules for active transport and other coupled vectorial processes. Methods Enzymol. 1989;171:145. doi: 10.1016/s0076-6879(89)71010-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khademi S, O'Connell J, 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2009;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locher KP, Lee AT, Rees DC. The E-coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 28.Lolkema JS, Slotboom DJ. Estimation of structural similarity of membrane proteins by hydropathy profile alignment. Mol Membr Biol. 1998;15:33–42. doi: 10.3109/09687689809027516. [DOI] [PubMed] [Google Scholar]
- 29.Lolkema JS, Slotboom DJ. Hydropathy profile alignment: a tool to search for structural homo-logues of membrane proteins. FEMS Microbiol Rev. 1998;22:305–322. doi: 10.1111/j.1574-6976.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 30.Lolkema JS, Slotboom DJ. The major amino acid transporter superfamily has a similar core structure as Na+-galactose and Na+-leucine transporters. Mol Membr Biol. 2008;25:567–570. doi: 10.1080/09687680802541177. [DOI] [PubMed] [Google Scholar]
- 31.Mager S, Min C, Henry DJ, Chavkin C, Hoffman BJ, Davidson N, Lester HA. Conducting states of a mammalian serotonin transporter. Neuron. 1994;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 32.Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR. Single-molecule chemistry and biology special feature: single-molecule FRET reveals sugar-induced conformational dynamics in LacY. PNAS. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mindell JA. The chloride channel's appendix. Nat Struct Mol Biol. 2008;15:781–783. doi: 10.1038/nsmb0808-781. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell P. Osmochemistry of solute translocation. Res Microbiol. 1990;141:286–289. doi: 10.1016/0923-2508(90)90002-8. [DOI] [PubMed] [Google Scholar]
- 35.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature. 2006;443:173–179. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 36.Olesen C, Picard M, Winther AML, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450:1036–1042. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 37.Patlak CS. Contributions to the theory of active transport: II. The gate type non-carrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull Math Biophys. 1957;19:209–235. [Google Scholar]
- 38.Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 39.Quick M, Yano H, Goldberg NR, Duan LH, Beuming T, Shi L, Weinstein H, Javitch JA. State-dependent conformations of the translocation pathway in the tyrosine transporter Tyt1, a novel neurotransmitter : sodium symporter from Fusobacterium nucleatum. J Biol Chem. 2006;281:26444–26454. doi: 10.1074/jbc.M602438200. [DOI] [PubMed] [Google Scholar]
- 40.Rapp M, Granseth E, Seppala S, von Heijne G. Identification and evolution of dual-topology membrane proteins. Nat Struct Mol Biol. 2006;13:112–116. doi: 10.1038/nsmb1057. [DOI] [PubMed] [Google Scholar]
- 41.Rapp M, Seppala S, Granseth E, von Heijne G. Emulating membrane protein evolution by rational design. Science. 2007;315:1282–1284. doi: 10.1126/science.1135406. [DOI] [PubMed] [Google Scholar]
- 42.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na+/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 42a.Reyes N, Ginter C, Boudker O. Transport mechanism of bacterial homologue of glutamate transporters. Nature. doi: 10.1038/nature08616. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg A, Kanner BI. The substrates of the gamma-aminobutyric acid transporter GAT-1 induce structural rearrangements around the interface of transmembrane domains 1 and 6. J Biol Chem. 2008;283:14376–14383. doi: 10.1074/jbc.M801093200. [DOI] [PubMed] [Google Scholar]
- 44.Saier MH. Tracing pathways of transport protein evolution. Mol Microbiol. 2003;48:1145–1156. doi: 10.1046/j.1365-2958.2003.03499.x. [DOI] [PubMed] [Google Scholar]
- 45.Schuldiner S. When biochemistry meets structural biology: the cautionary tale of EmrE. Trends Biochem Sci. 2007;32:252–258. doi: 10.1016/j.tibs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. Structure and mechanism of a Na+-independent amino acid transporter. Science. 2009;325:1010–1014. doi: 10.1126/science.1176088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter: inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh S, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- 49.Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. PNAS. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonders M, Zhu S, Zahniser N, Kavanaugh M, Amara S. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein WD. The Movement of Molecules Across Cell Membranes. Academic; New York: 1967. [Google Scholar]
- 53.Takahashi M, Kondou Y, Toyoshima C. Interdomain communication in calcium pump as revealed in the crystal structures with transmembrane inhibitors. Proc Natl Acad Sci USA. 2007;104:5800–5805. doi: 10.1073/pnas.0700979104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tavoulari S, Forrest LR, Rudnick G. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J Neurosci. 2009;29:9635–9643. doi: 10.1523/JNEUROSCI.0440-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van den Berg B, Clemons WM, Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 56.Vidaver GA. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J Theor Biol. 1966;10:301–306. doi: 10.1016/0022-5193(66)90128-7. [DOI] [PubMed] [Google Scholar]
- 57.von Heijne G. Membrane-protein topology. Nature Rev Mol Cell Biol. 2006;7:909–918. doi: 10.1038/nrm2063. [DOI] [PubMed] [Google Scholar]
- 58.Weyand S, Shimamura T, Yajima S, Suzuki Si Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O'Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PCJ, Iwata S, Henderson PJF, Cameron AD. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322:709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl–-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 60.Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 61.Zhang YW, Rudnick G. The cytoplasmic substrate permeation pathway of serotonin transporter. J Biol Chem. 2006;281:36213–36220. doi: 10.1074/jbc.M605468200. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Z, Zhen J, Karpowich N, Goetz R, Law C, Reith M, Wang D. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science. 2007;317:1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zomot E, Bendahan A, Quick M, Zhao Y, Javitch JA, Kanner BI. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449:726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]