Abstract
There is widespread recognition among both patients and caregivers that breast cancer patients often experience debilitating deficiencies in their ability to achieve thermal comfort, feeling excessively hot or cold under circumstances when others are comfortable. However, this symptom receives little clinical or scientific attention beyond identification and testing of drugs that minimise menopausal-like symptoms. Could some of these symptoms represent an important prognostic signal? Could thermal discomfort be among other cytokine-driven sickness behaviour symptoms seen in many breast cancer patients? While the literature reveals a strong link between treatment for breast cancer and some menopausal vasomotor symptoms (e.g. hot flashes also known as “hot flushes”), there is little data on quantitative assessment of severity of different types of symptoms and their possible prognostic potential. However, recent, intriguing studies indicating a correlation between the presence of hot flashes and reduced development of breast cancer recurrence strongly suggests that more study on this topic is needed. In comparison to reports on the phenomenon of breast cancer-associated hot flashes, there is essentially no scientific study on the large number of women who report feeling excessively cold after breast cancer treatment. Since similar acquired thermal discomfort symptoms can occur in patients with cancers other than breast cancer, there may be as yet unidentified cancer – or treatment-driven factor related to temperature dysregulation. In general, there is surprisingly little information on the physiological relationship between body temperature regulation, vasomotor symptoms, and cancer growth and progression. The goal of this article is twofold: (1) to review the scientific literature egarding acquired deficits inthermoregulation among breast cancer survivors and (2) to propose some speculative ideas regarding the possible basis for thermal discomfort among some of these women. Specifically, we suggest a potential association with excessive proinflammatory cytokine activity, similar to other cytokine-driven symptoms experienced after breast cancer, including fatigue and depression. We highlight the similarity of some breast cancer-associated thermal discomfort symptoms to those which occur during fever, suggesting the possibility that there may be common underlying changes in pro-inflammatory cytokine activity in both conditions. We anticipate that this contribution will stimulate additional scientific interest among researchers in identifying potential mechanisms and prognostic significance of this under-studied aspect of breast cancer biology and survivorship.
Keywords: fever, menopausal vasomotor symptoms, pro-inflammatory cytokines, sickness behaviour symptoms
Introduction
This article was prepared to familiarise cancer researchers and thermal medicine specialists with the fact that a large percentage of patients report the onset of a significant degree of acquired thermal discomfort symptoms after cancer. While patients with various types of cancer report this symptom, breast cancer far outweighs the other cancers in terms of reports of thermal discomfort symptoms. Breast cancer patients frequently feel excessively hot and/or cold under ambient temperature conditions in which others are able to adjust easily to achieve thermal comfort. Some patients report feeling quite cold for long periods of time. As judged from the large anecdotal information available regarding this problem on various breast cancer websites (see below) this problem can create significant quality of life problems which may be largely underappreciated by caregivers, who in general, may simply attribute these symptoms to annoying menopausal symptoms induced by treatment. However, whether all symptoms of thermal discomfort in breast cancer patients are actually a result of treatment-induced menopause is not clear. For example, as discussed in more detail below, it is already known that there are important differences in some menopausal symptoms in women with breast cancer compared to those experienced in normal women [1]. Further, recent studies indicate that the presence of hot flashes is an independent predictor of reduced recurrence of breast cancer [2, 3]. What is the relationship between body temperature regulation, thermal discomfort symptoms, and breast cancer recurrence? While there is as yet little information on this topic, it may be important to note that recent data from the field of cardiovascular disease research [4-6] suggests that menopausal vasomotor symptoms may be an independent risk factor associated with adverse cardiovascular risk profile. These data raise new concerns about whether vasomotor symptoms may be associated with systemic vascular dysfunction beyond the peripheral vascular changes associated with hot flashes and strongly suggests that these symptoms should be regarded as more than simply a nuisance [4].
The ability to achieve thermal comfort, feeling neither too hot nor too cold under different ambient temperatures, is normally controlled by conscious and unconscious mechanisms regulated by a homeostatic process known as thermoregulation. There appear to be two major types of breast cancer-related defects in thermoregulation: (1) excessive, rapid overheating, similar to that which occurs with the vasomotor symptoms associated with menopause (i.e. ‘hot flashes’) and (2) excessive, persistent chills. However, many women report the occurrence of both symptoms and studies to identify whether there are different, identifiable patterns of thermal discomfort after breast cancer have not been conducted.
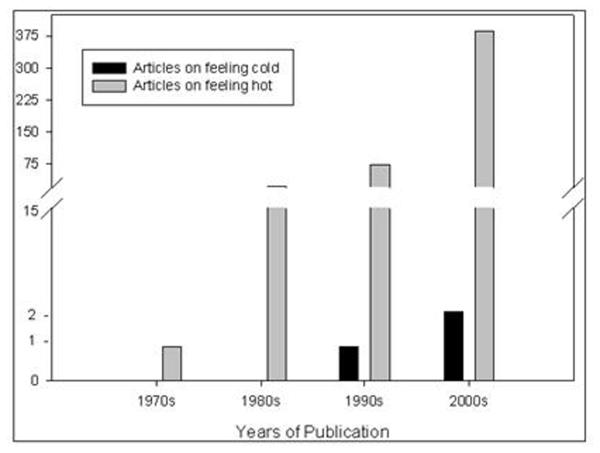
Most attention in this field has been devoted to development and testing of drugs which may alleviate menopausal vasomotor symptoms including hot flashes and sweating [7], generally concluded to be side effects of treatment with hormone therapies [8]. In comparison to studies on hot flashes, (and identification of treatments to alleviate them) almost no attention has been given to understanding symptoms of feeling inappropriately and persistently cold, which to date have only been reported anecdotally and have not been scientifically researched (see comparison of publications in Figure 1). Whether there are actual changes in body temperature with either symptom is not known. Further, neither symptom has been studied carefully in regards to possible associations with specific types of treatment and even in the case of treatment-associated hot flashes, the actual similarity to true menopause is not clear. Indeed, little attention has been devoted to quantifying the severity of these symptoms or determining whether they may arise from similar or different underlying mechanisms. Although there seems to be a general tendency by caregivers to lump together symptoms of thermal discomfort into simply being annoying menopause-like side effects of various breast cancer treatments, it is clear that the actual basis of thermal discomfort after breast cancer may be much more complex. Much of the discussion related to thermal discomfort among breast cancer patients is currently limited to anecdotal sources, including internet support groups and personal communications. Indeed, a web search of the terms ‘feeling cold’ and ‘breast cancer’ yields a remarkably large number of sites in which this symptom is vigorously discussed among patients and caregivers. From these anecdotal sources it appears that following breast cancer diagnosis and treatment many individuals have had problems regulating their body temperature. These complaints are manifested not only by the expected complaints of hot flashes but also, and perhaps more interestingly, in complaints of feeling persistently colder than others in the same ambient temperature. Websites such as Cancer Survivors Network, American Cancer Society®, and Cancer Compass® (as accessed during January - March 2010) show that people who have various cancers including breast, lung, and prostate experience long term, uncomfortable chills on a regular basis. Because these symptoms have not been researched yet, it will be important to first scientifically document and characterize this subset of patients who feel persistently cold and then determine whether these symptoms have any prognostic significance. Whether body temperature is actually lowered is not known. Given the increase in interest on the effects of mild hyperthermia on the immune system [9, 10], and its involvement in the positive outcome of many clinical trials utilising hyperthermia as an adjunct to radiation and/or chemotherapy for cancer [11-13], it is especially important to understand whether body temperature defects negatively influence the generation of anti-tumour immunity. Before questions like this can be addressed however, the next step must be to study the underlying etiological mechanisms for cancer- and/or treatment-induced alterations in thermoregulation, which lead to these symptoms.
Figure 1.
Quantification of scientific literature pertaining to “feeling cold and cancer” reveals far fewer references than those studying “hot flashes and cances”. Moreover, literature involving hot flashes and cancer has been steadily increasing in numbers over the past decade while articles pertaining to feeling cold remain infrequent.
In this article we summarise some of the published and anecdotal data related to thermal discomfort after breast cancer for those interested in learning more about this phenomenon (see Table I) and also, we propose possible connections between thermal discomfort symptoms and certain sickness behavior symptoms commonly experienced by breast cancer patients, such as fatigue and depression. Since several of the same pro-inflammatory cytokines thought to be involved in sickness behaviour symptoms are also known to drive fever, a condition in which patients usually have heightened immune system activity and feel intermittent episodes of excessive heat and may also ‘feel’ cold despite rising body temperature, we hypothesise that there may be as yet unknown links between symptoms of thermal discomfort and abnormal pro-inflammatory cytokine activity. If this association is demonstrated in breast cancer patients, this research could suggest new avenues for the use of thermal therapies designed to modulate inflammatory cytokine production, as well as improve at least some of the debilitating symptoms of thermal discomfort in breast cancer patients.
Table I.
Summaries of symptoms of thermal dysregulation in cancer patients are provided separated by cancer site. Studies are also organized by year of publication.
| Thermal Dysfunction Studies in Cancer Patients | |||
|---|---|---|---|
| Cancer Site | Thermal Dysfunction | Reference | Year |
| Breast | |||
| DB, PC, CO, RT showed a reduction in hot flashes with 20 mg twice a day of medroxyprogesterone acetate compared to placebo. |
[58] | 1994 | |
| DB, PC, CO, RT showed that clonidine significantly reduced hot flash frequency and severity however results were only moderate clinically. |
[132] | 1994 | |
| Phase II trial found that venlafaxine at 12.5 mg twice a day alleviated sweating. |
[133] | 1998 | |
| PC, CO, RT showed that, although statistically significant, 800 IU/day of vitamin E only showed a marginal improvement in the clinical magnitude of hot flashes. |
[134] | 1998 | |
| A single patient with recurrent breast cancer reported feeling cold and was found to have elevated levels of IGF-2. |
[135] | 1999 | |
| Pilot study found 83% of participants chose to continue the experimental paroxetine hydrochloride to reduce hot flashes and warrant further study through a DB, PC, RT. |
[136] | 2000 | |
| DB, PC, RT determined that venlafaxine reduced hot flashes and found a 61% decrease in median hot flash score in patient's taking 150 mg of venlafaxine for 4 weeks compared to a 27% median decrease in those taking a placebo. |
[96] | 2000 | |
| BD, PC, RT found that 0.1mg/day of oral clonidine was effective in reducing hot flashes in patients taking tamoxifen. |
[99] | 2000 | |
| DB, CO, RT of soy products found no difference in the reduction if hot flashes in women taking 50 mg of soy isoflavones compared to those taking a placebo. |
[104] | 2000 | |
| RT of black cohosh did not find a significant difference the reduction of number of intensity of hot flashes. |
[95] | 2001 | |
| Demonstrated that a short cycle of intramuscular depot medroxyprogesterone acetate injections provided significant and long-lasting relief from postmenopausal hot flashes compared to oral megestrol. |
[137] | 2002 | |
| DB, PC, CO, RT found that 20 mg/day of fluoxetine modestly improved hot flash symptoms based on unadjusted analysis. |
[92] | 2002 | |
| Cross sectional analysis failed to find a significant difference in hot flash quality or triggers in breast cancer survivors compared to healthy women. |
[53] | 2002 | |
| DB, PC, RT found that beverages containing soy phytoestrogens had no more of an effect of hot flashes in postmenopausal women than a placebo beverage. |
[103] | 2002 | |
| Group of 13 patients showed a significant decrease in hot flash severity with 20 mg/day of paroxetine. |
[138] | 2002 | |
| BD, PC, RT in which participants were given paroxetine controlled release had significantly reduced hot flash frequency compared to those given placebo. |
[91] | 2003 | |
| Cross-sectional study shows that sleep disturbance is not related to hot flashes in menopausal women who were not treated for their hot flashes. |
[139] | 2004 | |
| CYP2D6 genetic variants may alter the effects of tamoxifen resulting in increased hot flashes than women without this genotype. |
[140] | 2004 | |
| Tamoxifen treated patients with CYP2D6 *4/*4 genotype have a lower incidence of hot flashes suggesting that this gene is, at least in part, responsible for the metabolism of tamoxifen. |
[141] | 2005 | |
| Literature review concludes that SSRI, SNRI, clonidine, and gabapentin trials provide evidence for efficacy but are not as effective as estrogen in treatment of hot flashes. |
[61] | 2006 | |
| DB, PC CO, RT showed that sertraline significantly reduced hot flash severity compared to placebo in patients taking tamoxifen. |
[90] | 2006 | |
| DB, PC, RT found a significant decrease in hot flash severity in those taking gabapentin compared to those taking a placebo. |
[142] | 2005 | |
| Intramuscular medroxyprogesterone acetate daily is more effective than oral venlafaxine for treatment of hot flashes. |
[98] | 2006 | |
| Two DB, PC, RT showed that venlafaxine reduced hot flashes but was not efficient for alleviating other treatment related symptoms such as fatigue and sleep disturbance. |
[97] | 2007 | |
| DB, RT (Phase III) showed that 37.5 mg twice a day of venlafaxine was significantly more effective in reducing hot flashes compared to 0.075 mg twice a day of clonidine. |
[143] | 2007 | |
| Found that hypnosis resulted in a 59% decrease in daily hot flashes, a 70% decrease in weekly hot flashes, and a decrease in the degree to which hot flashes interfered with normal daily activities in a small 16 patient sample. |
[144] | 2007 | |
| Hot flash frequency in was reduced following acupuncture but no significance was found when compared to sham acupuncture. |
[145] | 2007 | |
| Hot flashes were a better predictor of recurrence than age, hormone receptor status, or even the difference in the stage of the cancer at diagnosis (Stage I versus Stage II). |
[72] | 2008 | |
| PC, RT showed that A diet high in vegetables, fruit, and fiber and low in fat decreased additional risk of secondary breast cancer events in women without hot flashes compared with that in women with hot flashes, possibly through lowered concentrations of circulating estrogens. |
[146] | 2009 | |
| Prostate | |||
| DB, PC, CO, RT showed a reduction in hot flashes with 20 mg twice a day of Medroxyprogesterone acetate compared to placebo. |
[58] | 1994 | |
| Found that radiation therapy combined with 3.6 mg of goserelin (subcutaneously), improves local control and survival in patients with advanced cancer but 62% of participants receiving goserelin experienced hot flashes. |
[106] | 1997 | |
| Details five individuals who were prescribed sertraline for anxiety and depression, all whom experienced reductions in their hot flash symptoms. |
[93] | 1998 | |
| Megestrol acetate use was found to be safe and effective in reducing hot flash symptoms for up to three years. |
[107] | 1999 | |
| Venlafaxine hydrochloride in doses of 12.5 mg twice a day alleviated hot flashes in men undergoing androgen ablation therapy |
[147] | 1999 | |
| Lung | |||
| 7% of patients experienced hot flashes that were severe and limiting but these symptoms were limited to women. |
[148] | 2004 | |
| Ovary | |||
| Study found a positive association between occurrence of hot flashes and the relative risk of ovarian cancer |
[149] | 1992 | |
| Testes | |||
| Men reported feeling cold more often that the general male population. |
[115] | 2002 | |
DB=double bind, PC=placebo controlled, RT=randomized trial
Thermoregulation
Homeostatic mechanisms underlying maintenance of thermal comfort
Thermoregulation is the homeostatic process through which individuals respond to temperature cues from either their external or internal environment. When individuals experience significant and persistent inability to achieve thermal comfort through simple conscious changes in clothing or ambient temperature, or through unconscious events, such as changes in blood flow patterns, this may signal the onset of a defect in the individual's ability to optimally control its body temperature. A core/brain temperature of 37C is classically viewed as the temperature at which the body will operate most efficiently [14], with some studies showing that the optimal body temperature for humans may be slightly lower, at 36.8C [15]. Moreover, it is important to note that internal temperatures of different parts of the body are not homogenous; temperatures in the extremities are normally much lower than those in the core [16]. This is particularly true for larger mammals, such as humans, which are homeothermic and are able to sustain their core temperature through a wide range of ambient temperatures, except those at the extreme. In endotherms, heat is generated in the body through various exothermic reactions including the combustion of sugars and contraction of muscles. Core body temperature and the cooler temperature of the skin and extremities are then maintained primarily through the circulatory and autonomic nervous systems as well as conscious stimuli that prompt changes in clothing or room temperature [17].
‘Thermoneutral’ temperature is the ambient temperature at which an individual feels comfortable with minimal clothing. Any environmental change that occurs to change the external temperature below this point of thermal equilibrium causes an immediate response from the body [18]. In this situation, both heat production and conservation of heat become the body's priorities. To conserve heat, the body will employ mechanistic behaviours involving vasoconstriction and counter-current heat exchange. Blood vessels are constricted, blood flow to the skin and organs is limited, and skin temperature declines. This leads to a decreased temperature gradient between the skin and the ambient environment, and results in a reduction in heat loss from the body as well as raised blood pressure and increased heart rate [19].
Thermoregulation depends upon many neurological signals that influence bodily changes in order to control body temperature. Body heat is primarily produced by deep organs and by contraction of skeletal muscles, with most heat being dispersed on the surface of the skin [20]. Because many factors can induce a change in body temperature, such as ambient temperature [21], food consumption [22], and exercise [23, 24], it is essential to have a stable and reliable homeostatic mechanism to respond to these events. Thermoregulation is highly dependent upon conscious and unconscious neural signals controlled by the preoptic/anterior hypothalamus (PO/AH) [25, 26]. Information regarding core and skin temperatures is conveyed to the PO/AH, which directs an appropriate effecter response [26, 27]. In hot conditions, the hypothalamus signals blood vessels to expand (vasodilation), which brings more heat and blood to the skin and allows loss of excess heat through convection and conduction [28]. In contrast, under cold conditions when body temperature drops, the hypothalamus signals blood vessels to constrict (vasoconstriction), which reroutes blood away from the skin and towards the warmer core [28]. The hypothalamus also signals muscles to contract, causing shivering, which increases heat production and raises body temperature [28].
Energy costs of thermoregulation
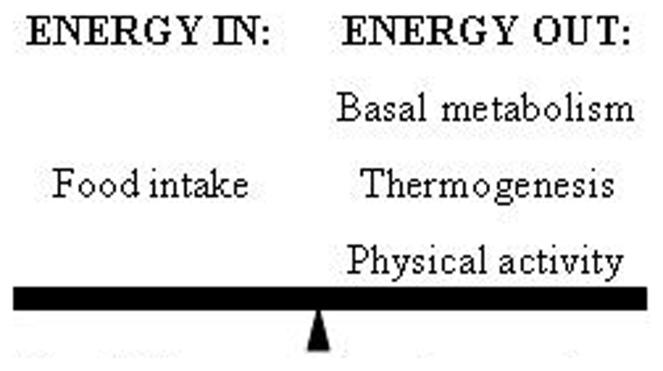
Given the extent of the behavioural and neurovascular homeostatic processes involved in endothermic thermoregulation, maintaining thermal comfort is energetically costly [29]. A major feature of the metabolism of endotherms is the maintenance of higher internal body temperatures compared to their surroundings, a process that requires a large amount of energy to support [30]. Energy balance (see Figure 2) encompasses the biological homeostatic processes by which the body takes in and expends energy, and is explained by the following equation:
Figure 2.
Humans must balance the amount of energy they consume, in the form of food, with the amount of energy they expend, through basal metabolism (homeothermy and biological maintenance), thermogenesis, and physical activity. When excess energy is required for one process utilizing energy, less energy is available to sustain other processes.
In the above equation, energy intake accounts for food energy consumed [31, 32], and the sum of the energy used to maintain basal metabolic levels, produce internal heat, and perform physical activity/exercise [31]. Energy output differs between individuals [31, 33] and is dependent on several variables including body weight, exercise, and genetic makeup [34]. The majority of one's energy output is accounted for by basal metabolism or resting metabolic rate (RMR) which is made up of a series of biological processes required by humans [35]. RMR includes homeothermy, the ability to sustain a constant core temperature despite changing external temperatures, and maintenance of cellular integrity [31]. The remainder of one's energy output is comprised of physical exercise, including all voluntary and involuntary movement, and thermogenesis, the dissipation of energy in the form of heat in response to processing recently consumed foods or facing an extremely cold environment [35-39]. Therefore, when the body is exposed to cold, additional energy has to be generated to maintain core temperatures.
As one's energy must remain balanced, there are biological consequences to changes in the energy usage of an individual (see Figure 2). If energy intake, in the form of food, exceeds the amount of energy expended, an individual will likely gain weight as the excess energy will be stored, primarily as fat [31]. Conversely, if one requires more energy than is taken in nutritionally, the body will utilise stored energy sources, and in order to sustain biological functionality, the individual must replenish its energy supply quickly by eating [40]. In those with chronic conditions such as breast cancer, there may be additional strain on the system, especially after treatments such as chemotherapy and radiation. Could this be the basis for alteration of the normal energy available for thermoregulation creating defects in normal temperature regulation in some women after breast cancer treatment? More study is needed to answer this question.
Effects of aging on thermoregulation
Several studies have looked at differences in thermoregulation between different aged populations and have found an inverse correlation between age and efficiency in temperature regulation. Elderly people tend to have lower body temperatures compared to those in younger age groups [41-43] and core body temperature has been shown to decrease with age [44, 45]. An early study, reported in 1971, found a sample of 40 elderly women to have a mean body temperature of 36.2°C with 14 (35%) women having body temperatures below 36°C compared to a mean temperature of 36.7°C measured in a group of college-aged students [44]. The mechanisms leading to declining body temperatures in aging populations are unclear, but include clinical and environmental influences such as nutrition and medication [45]. Interestingly, body temperature in elderly individuals with various morbidities are significantly lower compared to body temperature in young adults, while temperatures among healthy elderly individuals remain similar to their younger counterparts [46]. This observation could present interesting ramifications in cancer research as cancer patients (putatively unhealthy) may be more prone to additional thermal regulatory issues not seen in other age-matched populations.
In addition, the elderly respond to cold stress differently from younger individuals. Several studies report that the elderly are less efficient at maintaining core body temperature under cold stress [45, 47, 48], which appears to be mediated by an impaired ability to undergo vasoconstriction [49, 50]. Heat stress, however, does not seem to pose as much of a problem among older adults, and has not been shown to correlate to warmer body temperatures [51, 52]. In addition, older individuals have a lower RMR than younger individuals, which may indicate metabolic deterioration and alter overall energy balance [53]. Several factors including sodiumpotassium pump activity, fat mass, maximal aerobic power, and menopausal status are important factors influencing the decline of RMR in the elderly [54].
Effects of thyroid hormones on thermoregulation
The thyroid gland may be another target of investigation for a better understanding of temperature dysfunction in breast cancer patients. Thyroid hormones are responsible for the increased heat production normally required for humans to maintain body temperature above that of the environment [55]. Also, a direct association between breast cancer and enlarged thyroid glands has been shown. Both an increased mean thyroid volume and larger percentages of individuals with enlarged thyroid glands were shown to be significantly greater in women with breast cancer than age-matched controls [56]. Clearly, further investigation of the effects of breast cancer treatments on the thyroid gland and production of thyroid hormones, and on whether these effects are present in the same patients with defective thermoregulation, are needed. If an association is revealed, it may stimulate new research on identification of new thyroid-related targets through which thermal discomfort may be alleviated.
Feeling too hot: Menopausal vasomotor symptoms of overheating/sweating among cancer patients
Hot flashes and the role of sex hormones
A very common thermoregulatory alteration is the experience of hot flashes, which are characterised by sudden episodes of flushing and/or sweating and a sensation of heat, often preceded or followed by chills [57-59]. These sensations are a normal occurrence in about 75% of healthy menopausal women [60]. In healthy women, hot flashes follow a circadian rhythm similar to that of their core body temperature, with hot flash frequency and intensity increasing when core body temperature is at its apex [61, 62]. However, as will be detailed later, the correlation between core body temperature and the circadian patterns of hot flashes is disrupted in cancer patients [63]. Studies have found that women susceptible to hot flashes show a reduction in their ‘thermoregulatory null zone’, which is the temperature range between sweating and the onset of shivering [64-66]. Among symptomatic women with reduced thermoregulatory null zones, changes in core body temperatures are more detectable, which induces changes in hormones and/or neurotransmitters that lead to a hot flash [63, 66].
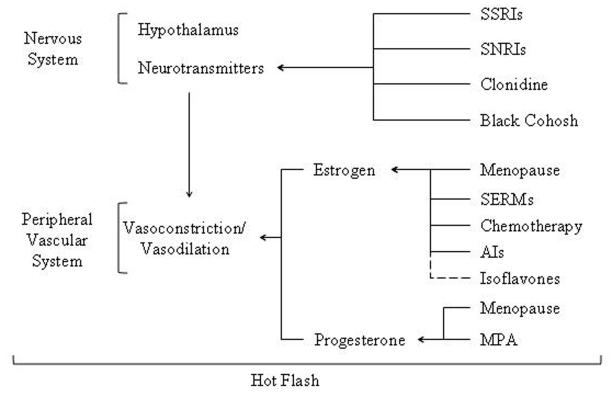
Menopause, either natural or therapeutically induced, is considered to be a key instigator for temperature dysregulation. As shown in Figure 3, female reproductive hormones, estrogen and/or progesterone, affect the mechanisms regulating blood flow to the skin [67-70], and hot flashes have been found to be influenced by the diameter of blood vessels that deliver blood to the skin and the volume of blood in these vessels [26]. Estrogen promotes vasodilation and therefore reductions in estrogen Thermoregulation defects after cancer occurring during menopause restrict the body's ability to efficiently dissipate heat [26]. Estrogen therapy has been shown to alleviate some of these symptoms by decreasing body temperature and lowering the temperature at which vasodilation is initiated [68, 69]. Conversely, progesterone has been suggested as an inhibitor of vasodilation [71]. Studies have found that estrogen replacement therapies reduce the incidence of hot flashes when combined with a progestin, which mimics progesterone, although the effect is not additive [72]. Although decreased estrogen levels are implicated as a major factor in thermoregulatory control, few studies have been conducted to determine the precise physiological mechanism(s) by which estrogen controls thermoregulation. Moreover, estrogen deprivation alone is not a sufficient cause of hot flashes as estrogen levels do not differ between symptomatic and asymptomatic postmenopausal women [60, 73-75], and frequency and severity of hot flashes have not been correlated to plasma [60,76], urinary [60, 77], or vaginal [60, 77] estrogen measurements. Thus, other mechanisms are likely to be involved in the etiology of hot flashes [78].
Figure 3.
Summary of therapeutic and physiological influences on the thermoregulatory pathway and hot flashes. Generally, menopause reduces estrogen and progesterone levels. See text for additional details. SSRIs: selective serotonin reuptake inhibitors; SNRIs: serotonin-norepinephrine reuptake inhibits; SERMs: selective estrogen replacement moudulators; AIs Aromatase Inhibitors; MPA: medroxyprogesterone acetate.
Menopausal symptoms among breast cancer patients
Although most women experience hot flashes as they age and become menopausal, women with a history of breast cancer appear to have more severe symptoms [63, 79, 80]. Sudden onset of treatment-induced menopausal symptoms among breast cancer patients are common, with these individuals being over five times more likely to report hot flashes than those with no history of breast cancer [81]. Hot flashes have been postulated to be an independent predictor of tamoxifen efficacy among breast cancer patients, and data from the Women's Healthy Eating and Living (WHEL) randomised trial of 1,551 women found that women who reported hot flashes among those taking tamoxifen were less likely to develop recurrent breast cancer than those who did not report hot flashes [2]. Similarly in the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial, the appearance of new vasomotor symptoms or joint symptoms in response to estrogen depletion was associated with lower subsequent recurrence compared to women who not report these symptoms [3]. Despite being a possible predictor of better disease prognosis menopausal symptoms lead to declines in quality of life among breast cancer patients by interfering with daily activities, sleep patterns, and self esteem [58, 82]. Because of the prominence of these symptoms following hormone suppression treatments, it is important to understand the causal mechanism for these symptoms in order to develop alleviation treatments without affecting the prognosis or efficacy of breast cancer treatments prescribed. Subsequent improvements in quality of life would be expected to promote treatment adherence, particularly with respect to long-term use of anti-estrogens, and would be expected to optimise disease prognosis.
Hormone suppression medications commonly used in breast cancer treatment regimens include use of selective estrogen replacement modulators (SERMs) and aromatase inhibitors (AIs) [83-86]. Chemotherapeutic drugs also contribute to the high degree of thermal dysfunction in breast cancer patients because of their negative impact on ovarian function often causing premature and unnatural menopause due to rapid declines in estrogen levels [63, 80, 87] (see Figure 3).
In addition to hormone-mediated effects, hot flashes among breast cancer patients appear to be potentially influenced by a number of other factors. For instance, recent studies show that serum interleukin-8 (IL-8) oncentrations in women who report hot flashes are significantly higher compared to women who do not experience hot flashes [88]. Given that IL-8, a pro-inflammatory cytokine involved in immune function, has been associated with breast cancer invasiveness and angiogenesis [89], understanding the biological relationship between thermoregulation and immune function, if any, may be important for disease prognosis (see below).
The pathophysiology of breast cancer itself may also make breast cancer patients more susceptible to hot flashes. Breast cancer can disrupt circadian rhythms, thereby altering the release of reproductive hormones [90], and altering circadian control of body temperature [62, 63]. The thermoregulatory null zone sets the bounds within which core body temperature is regulated in humans [64-66]. When the thermoregulatory null zone is reduced in women experiencing a hot flash, increases in core body temperatures are more detectable by the individual and therefore induce additional discomfort [63, 66]. It is important to note that hot flashes do not result from an increased heating of blood, but instead from signals sent to the hypothalamus resulting in the release of large amounts of blood into regions that are normally set to remain cooler, such as the skin [26]. Whether these physiological factors are involved in reducing risk of breast cancer recurrence in patients who experience hot flashes (as in the case reported by the WHEL and ATAC trials described above) is not known.
Treatments used to alleviate hot flashes among breast cancer patients
Although a great deal of current scientific literature has already been dedicated to studying patients who feel too hot after cancer treatment (see Table I), many questions remain. Understanding the mechanisms targeted by drugs used to alleviate hot flash symptoms may help researchers gain insight into why hot flashes and other thermal regulatory issues arise in patients following cancer. Most hot flash treatments work by mimicking or supplementing estrogen allowing for increased vasodilation and heat to be efficiently dissipated from the body. The use of estrogen supplements to reduce hot flash symptoms has been shown to be effective, but comes with increased risk of heart disease and breast cancer. Therefore, these supplements are generally used only as a last resort in healthy women [91] and not recommended for breast cancer patients, particularly those with estrogen receptor positive disease [92]. Non-hormonal treatment for hot flashes is currently an active research area in both healthy women and women with breast cancer. This topic has been recently reviewed by a number of authors and is summarised in Figure 3 [70, 78, 93, 94].
Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are generally prescribed as antidepressants, but have been shown to reduce hot flash symptoms in users. SSRIs have been shown to reduce hot flashes in the general population [95,96] as well as in breast [97-99] and prostate [100] cancer patients. Encouragingly, sertraline is safe in combination with tamoxifen and the combination of these drugs results in fewer and less severe hot flashes [97]. However, the effects of these drugs are not consistent between individuals. Unfortunately, no obvious factor such as age or health has been identified to determine the strength of an individual's response to SSRI treatment on hot flash occurrence [95]. Black cohosh, a plant extract that acts on serotonin by an uncertain mechanism [101], however, has not been found to decrease the frequency or intensity of hot flash [102].
Venlafaxine is another antidepressant used to alleviate hot flashes in breast cancer patients [103,104]. Venlafaxine differs from sertraline because it is a serotonin-norepinephrine reuptake inhibitor (SNRI), which in addition to acting on serotonin also acts on norepinephrine, although its efficacy in relieving hot flashes is lower than that associated with medroxyprogesterone acetate (MPA), a progestin [105]. Additional alternative therapies are being investigated to alleviate hot flash symptoms. Clonidine, a drug used to treat high blood pressure, has been found safe to use in conjunction with tamoxifen and is capable of reducing hot flashes resulting from breast cancer treatment [106]. The effects of isoflavones, such as soya and clover, are inconsistent in the current literature. Soya works as a phytoestrogen in humans as it binds to estrogen receptors and has been shown in some studies to reduce hot flash symptoms [107-109] while others report no significant differences between soya and placebo [110, 111] and red clover and placebo [112]. Magnetic therapy has been found to be unsuccessful in hot flash treatment [58].
Occurrence of hot flashes in cancer populations other than breast cancer
While the phenomenon of hot flashes is most widely reported among breast cancer patients, hot flashes are also reported for other cancer sites, especially those in which hormone suppression treatments are common (Table I). Men with prostate cancer who undergo chemical or surgical castration to lower sex hormone levels have a high frequency of hot flashes following treatment [113, 114]. Alleviation of hot flashes in these patients has been achieved with low doses of megestrol acetate [86, 114]. Another common treatment for prostate cancer is the use of gonadotropin-releasing hormone agonist goserelin, which reduces the secretion of testosterone by reducing gonadotropin secretion and inducing hypogonadism. Goserelin is used as an adjuvant in combination with irradiation. The combination of these treatments will improve control and survival in prostate cancer patients but up to 62% of patients receiving this treatment report hot flashes [113]. Anti-depressants have been shown to help relieve hot flashes in male patients recovering from prostate cancer [59] similarly as in women with breast cancer. It is possible that anti-depressants relieve hot flashes in prostate cancer patients due to a stabilising effect on the autonomic nervous system [100]. In addition, hot flashes have been reported among ovarian cancer patients treated with leuprolide acetate, a gonadotropin releasing hormone agonist [115-117], which lowers estrogen levels.
Feeling too cold: Symptoms of persistent chill among breast cancer patients
Evidence for symptoms of cold stress among breast cancer patients
Much less recognised in the scientific community is the possibility that a subset of cancer patients report experiencing symptoms of being persistently and inappropriately cold after cancer diagnosis and treatment. To date, this symptom has primarily been reported anecdotally, particularly among women participating in breast cancer support groups. Interestingly, one report on the economics of hidden costs associated with breast cancer mentions the increased need for extra ‘heating, bedding, clothing, electric blanket, heater, thermal underwear, baths, towels and high calorie foods’ identified by women as needed to deal with excessive coldness [118]. This symptom is frequently clustered together clinically and scientifically with reports of hot flashes and attributed to menopause or hormone suppression therapy. However, we propose that the symptom of feeling too cold should not be ascribed to menopausal symptoms because this may overlook the importance of different thermoregulatory symptoms that may occur among cancer patients.
Much of the clinical and scientific evidence indicating that some cancer patients might experience cold stress after cancer diagnosis either comes from case reports or indirectly from studies that were focused on some other primary hypotheses. As a result, this symptom has not been explored rigorously. On careful examination however, findings from some studies do indicate that symptoms related to cold stress might be part of a distinct pathological mechanism that is separate from menopausal and hormone-related causes. For example, a study that used factor analysis to validate a survey measuring pain among 100 early stage organ non-specific cancer outpatients receiving chemotherapy (38 men, 62 women) identified feeling numb and being cold as important clusters loading onto a distinct factor [119]. Another study reviewing cancer-related fatigue indicated that changes in body processes, including feeling cold, occurred only in fatigued or exhausted patients [120]. Chemotherapy has also been linked to feeling cold; a study of 40 women receiving chemotherapy reported that 14% of women receiving six cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) experienced ‘feeling cold in the chest and arm’ following their therapy [121]. In addition to breast cancer, feeling cold has been associated with testicular cancer, lasting several years following treatment [122]. A study of 277 testicular cancer survivors and 392 non-cancer controls showed that the cases felt significantly colder when compared to controls [122].
Some recent studies may shine further light on molecular pathways that may be involved in the manifestation of symptoms of cold stress. Endothelin-1 (ET-1) can alter temperature detection thresholds among cancer patients. ET-1 acts as a growth factor in various malignancies [123], is overexpressed in breast carcinomas, and has been linked to poorer disease prognosis [124]. In a randomized study, Hans et al. [125] examined the effect of ET-1 injection, a known vasoconstrictor, on spontaneous pain and temperature perception in healthy male volunteers. They found that high doses of ET-1 altered both cold and heat detection thresholds. The cold thresholds were significantly increased by a 10−10M dose of ET-1 after 60 min (p<0.05) whereas all doses above 10−6M elicited a significant dose-dependent increase in heat detection threshold (p<0.05) [125]. They concluded that the observed changes in heat detection developed sooner, lasted longer and were more pronounced than the changes observed in cold detection [125]. These finding raise the possibility that ET-1 may alter temperature preferences. Since ET-1 expression has also been observed in breast cancer tissue, it warrants further investigation into the epidemiological and clinical factors that contribute to altered ET-1 concentrations and their influence on temperature regulation and disease prognosis in this group.
In future studies aimed at characterising this thermal symptom and elucidating its clinical significance, several questions should be posed. What proportion of breast cancer patients have symptoms of being persistently chilled? What is the frequency and severity of these symptoms? What degree of distress and interference in activities of daily living and timing do these symptoms have? When do breast cancer patients experience these symptoms with respect to disease diagnosis and treatment? How long do these symptoms persist after treatment? What are the perceived reasons for their experience and what are the treatments patients have tried to cope with these symptoms? Also important is determining whether there is an accompanying change in body temperature and whether changes in body temperature or symptoms of feeling persistently cold are related to disease course and/or treatment efficacy. If it is found, through observational epidemiological studies, that an actual increase in body temperature occurs, is it possible that breast cancer patients develop deficiencies in their ability to perceive thermal comfort. Conversely, if a decline in body temperature is observed, is it possible that this is a physiological response to toxic breast cancer treatments, similar to that seen in animal models in response to harmful exposures. Several of these possibilities are discussed below.
Evidence for hypothermia among animal models
While scientific study examining cold stress in clinical populations and its significance is in its infancy (see Figure 1), there is a growing body of evidence in animal models indicating that hypothermia, induced by immune mediators, occurs in response to harmful environmental exposures. Murine models have shown evidence that body temperatures may drop in response to various exposures, such as bacterial lipopolysaccharide (LPS) [18, 126]. Additionally, studies in various animal models report decreased core body temperature in response to adverse events such as food restriction [127], hypoglycaemia [128, 129], hypoxia [130, 131], dehydration [132], and infection [133-136]. These studies suggest that declines in body temperature could be a possible mechanism for defending the body against harmful exposures. This idea is further supported by studies showing that exposure to nickel or cadmium metal decreases metabolic rates in mice making them hypothermic [137]. The decrease in temperature helps the body fight toxins in two ways: first by attenuating the toxicity of the chemical by reducing its conversion into an active intermediate, and second, by decreasing the rate of respiration and further uptake of toxin [18]. It is possible that chemotherapy may be perceived as a toxin and therefore result in declining body temperature among breast cancer patients as they undergo and complete breast cancer treatment. A second possibility regarding feelings of excessive chill may relate to symptoms which occur during fever, in which an individual can feel quite cold, despite normal or even elevated body temperatures. This provocative possibility is discussed below.
Potential link between thermoregulation, pro-inflammatory cytokines, and immune function
Pro-inflammatory cytokines and sickness behavior symptoms in breast cancer patients
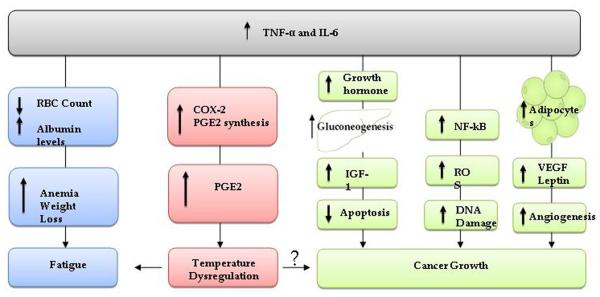
Chemotherapy treatment in breast cancer patients promotes increases in plasma levels of proinflammatory cytokines [156, 157]. Additionally patients unresponsive to chemotherapy have significantly higher IL-6 levels than responsive patients [158]. Elevated cytokine levels, including IL-1, IL-6, IL-8, and IL-18 have been correlated with disease stage and progression of cancer [159, 160]. These cytokines have been etiologically implicated in a number of sickness behaviours experienced by breast cancer patients, including fatigue [161-163], sleep disturbance [162], depressed mood [164], and loss of appetite [165]. While symptom severity often declines with time, some symptoms remain for years after the initial cancer diagnosis [161], possibly indicating a long-term effect of pro-inflammatory cytokines on disease-related symptoms as well as outcomes. These symptoms have been well studied, and found to be related to increased cytokine levels of IL-1, tumour necrosis factor-alpha (TNF-α), and/or IL-6 [156, 166]. As shown in Figure 4, increased levels of TNF- α and IL-6 are correlated with decreased red blood cell production, higher levels of albumin, weight loss, anaemia, and fatigue [166]. In addition, TNF-α and IL-6 have various metabolic actions including increased adipocyte production and gluconeogenesis [167].
Figure 4.
The same cytokines are responsible for metabolic factors leading to cancer growth, fatigue, and temperature dysregulation (fever). High levels of TNF-α and IL-6 affect blood concentration and makeup leading to fatigue (blue). Increases in TNF-α and IL-6 promote metabolic syndrome (green) which induces gluconeogenesis and adipocyte production. These bodily changes lead to an upregulation of various immune regulators that result in cancer development and growth. These cytokines also lead to fever via a PGE-2 dependent pathway (red). Temperature dysregulation (feeling cold) has been related to fatigue and may also be associated with cancer growth. RBC: red blood cell; COX-2: inducible cyclo-oxygenase, PGE2: prostaglandin E2; IGF-1: insulin-like growth factor 1; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; ROS: reactive oxygen species; DNA: deoxyribonucleic acid; VEGF: vascular endothelial growth factor
The pathway through which these cytokine mediators act may also promote cancer growth [167] (see Figure 4) and this is an important research area which has received much recent attention. For instance, TNF- α and IL-6 promote up-regulation of growth hormone receptors in the liver which stimulate gluconeogenesis and insulin-like growth factor 1 (IGF-1) production [168]. Overproduction of IGF-1 promotes cancer growth by induction of anti-apoptotic events [169]. TNF- α activates nuclear factor kappa-light-chain-enhancer of activated B cells (NFκ-B) that increases levels of reactive oxygen species (ROS) [170]. ROS might induce DNA damages such as deletions, frame shifts, and rearrangements leading to tumour progression [171]. Visceral fat accumulation is associated with increased production of vascular endothelial growth factor (VEGF), that aids in cell proliferation and migration [172]. Finally, increased adipocyte production initiates various processes that ultimately promote tumour growth. Leptin, a hormone that plays roles in various biological pathways, is produced redominantly by adipose tissue. In humans, plasma levels of leptin correlate with total body fat, with high concentrations present in obese women [173]. Leptin promotes cancer growth through angiogenesis via increased levels of metalloproteinase in various cancer sites including the prostate, colon, endometrium, and breast [174].
Importantly, we are intrigued by the fact that the same pro-inflammatory cytokines implicated in supporting cancer growth are known to play a critical role in the generation of fever, a condition in which patients also report feelings of thermal discomfort (e.g. feeling intermittently excessively chilled or overheated). Thus, as outlined further below, we speculate that elevated levels of pro-inflammatory cytokines in breast cancer patients may also be linked to at least some symptoms of thermal dysregulation.
Fever – a natural mechanism that can create feelings of excessive chills and overheating
Fever is defined as 'a state of elevated core temperature, which is often, but not necessarily, part of the defensive response of multicellular organisms (host) to the invasion of live microorganisms or inanimate matter recognised as pathogenic or alien by the host [175]. Pyrogenic cytokines are produced by phagocytic cells as part of the innate immune system and these signalling molecules cause an increase in the thermoregulatory set-point in the hypothalamus thereby creating a febrile response [176]. Several pro-inflammatory cytokines are critical in generating the hyperthermic condition of fever (see Figure 4), and also in the regulation of the immune responses. Normally secreted in small amounts, pyrogenic cytokines including IL-1, IL-6, and TNF-α, are able to mediate fever caused by infection, with cancer patients often secreting abnormally large amounts [166]. These circulating cytokines are thought to affect centres of thermoregulation in the hypothalamus by inducing expression of cyclooxygenase 2 (COX-2), which leads to increased production of prostaglandins [177]. Specifically, increased concentration of prostaglandin E2 (PGE2) is thought to affect thermoregulatory neurons and lead to a rise in core body temperature [15]. PGE2 plays a predominant role in the inflammatory response and modulates a variety of immune responses, including cytokine production.
Studies dealing with fever are often difficult to compare, because of differing opinions as to what constitutes normal body temperature [15]. Furthermore, various demographic characteristics, such as age, sex, and weight, as well as experimental variables including time of day, may affect temperature readings. Importantly, when an individual has a fever, and even before febrile temperatures are recorded, he/she will often report feeling cold [178, 179] rather than feeling hot, and will exhibit heat-seeking behaviour. After the new set point in body temperature is reached, the same individual may experience the sensation of overheating and may even notice extensive sweating. We speculate that some of the same heat-seeking behaviour that accompanies a fever-generated feeling of cold could be involved in patients who report excessive and persistent chills after breast cancer. Generation of a fever-like state could also help to explain intermittent feelings of being too cold and too hot in some breast cancer patients. If this relationship between cytokine production and thermal discomfort is found in breast cancer patients, it could indicate previously unrecognized relationships between the mechanisms underlying thermoregulation and thermal comfort, and cytokine driven signals from the immune system. Furthermore, it would strongly support the need for further study devoted to particular thermal discomfort symptoms since these symptoms may provide important prognostic information.
The relationship between thermoregulation and immune response
Current evidence suggests that inflammation and immune function play a significant role in thermoregulation. Further, there is growing evidence that use of mild hyperthermia as part of cancer therapy may positively influence the anti-tumour immune system [11]. Systemic inflammation is associated with both fever and hypothermia [180]. Fever occurs as a response to mild systemic inflammation, which is mediated by COX-2, whereas, severe inflammation results in hypothermia and appears to be mediated by COX-1, but not COX-2 [181]. In rat models of systemic inflammation induced by bacterial LPS, core body temperature changes appear to depend upon the ambient temperature and the LPS dose [182]. At neutral or slightly warm temperatures, fever is the common response and is monophasic when the dose of LPS is low, but is polyphasic when the dose is high [180]. In contrast, at cooler ambient temperatures, hypothermia followed by fever is the predominant response, with the magnitude of the hypothermia increasing with the LPS dose [183, 184]. A number of studies have shown that animals respond to LPS with warmthseeking behaviour and fever, although at high LPS doses, emulating systemic inflammation, animals will first demonstrate cold-seeking behaviour and hypothermia followed by warmth-seeking behavior and fever [185].
Romanovsky et al. and others have suggested that while fever may be beneficial because of its immunostimulant and antimicrobial effects, these benefits may be offset by the high energetic cost associated with maintaining a high body temperature [133]. As a result, it is possible that when an inflammatory stimulus is severe enough to threaten energy reserves, processes that conserve energy may come into effect. In this context, leptin, a hormone which plays a central role in both energy homeostasis and the inflammatory response, may serve as the signal that ties together energy balance and inflammation [186]. Immune activation of leptin production is thought to involve neuroendocrine pathways, although these mechanisms are still poorly understood [186].
Because pro-inflammatory cytokines have been identified as performing key roles in thermal dysregulation (fever), cancer-related sickness behaviour, and various metabolic functions it is reasonable to hypothesize that there may be a relationship between the production of pro-inflammatory cytokines and symptoms of thermal discomfort among breast cancer patients. In addition, these symptoms may be clustered with other cytokine-related symptoms reported among breast cancer patients. Epidemiological and clinical studies to address this important possibility are currently needed.
Thermal discomfort, thermal therapy and possible connections to the immune system?
Straub et al. suggest that elevated pro-inflammatory cytokine levels act as a signal for the need of energyrich fuels by the immune system [187]. Available energy levels in the body are significantly influenced by the requirements for heat production and/or heat dissipation. Fever, which is most often indicative of heightened immune activity due to infectious agents, is accompanied by an increase in body temperature due in large part to signals in the brain encouraging heat-seeking behaviour, as demonstrated in many animal models [188]. When breast cancer patients feel inappropriately cold, is this a signal employing physiological and behavioural mechanisms to conserve and generate heat energy for other activities, such as the immune system? Since metabolic energy could be drained by a cold individual attempting to attain thermal comfort, less energy will be available for other homeostatic functions, which might include the immune response unless that individual obtains body temperature support by finding warmer ambient conditions (e.g. adding more clothes or turning up the thermostat). Thus, we wonder whether feeling persistently cold has a negative impact on the immune system and could even signal poorer disease prognosis among cancer patients and that appropriate energy conserving interventional strategies (e.g. hyperthermia or warming thermal therapy) should be employed. Indeed, because cold stress among breast cancer patients may be indicative of changes in underlying immune function (e.g. production of pro-inflammatory cytokines) or increased metabolic needs, there could be therapeutic benefit in the use of thermal therapies to help support overall energy balance. For example, if a patient exhibits persistent chills, and this is found to be associated with other symptoms of metabolic sickness, perhaps benefit would be obtained by interventions involving frequent mild thermal therapies designed to alleviate thermal discomfort and reduce excessive pro-inflammatory cytokine production. Moreover, supporting the energy requirements of maintaining body temperature may be expected to allow redirection of energy use in the body to support other energy requiring functions, such as enhanced immune function.
Indications that temperature manipulation may be a potentially effective treatment strategy to enhance anti-tumour immune function are supported indirectly by findings from several preclinical and clinical studies showing that mild systemic whole body hyperthermia can potentiate the anti-tumour effects of various cytotoxic agents and can stimulate the immune system [11, 12, 189, 190].
Mild systemic fever-range whole body hyperthermia both in vitro and in vivo can regulate the production of pro-inflammatory cytokines such as IL-6 and TNF-α from activated macrophages [191]. Several immune activities have been shown to be enhanced by mild heating [192-195]. IL-6 has been shown to play a critical role in mediating at least some of the immunological effects of fever range hyperthermia on T-lymphocytes [196, 197]. Immunological changes have also been observed in both cancer patients and healthy volunteers whose core temperatures were increased modestly in a warm water bath [198]. In summary, while considerable data supports the notion that providing mild hyperthermia could enhance the immune system, much more data is needed in regard to the antitumour immune response. However, an attractive rationale for a new clinical indication for mild hyperthermia may be the goal of alleviation of the persistent excessive chills experienced by many women with breast cancer.
Opportunities for future research directions
The information provided here supports the need for the cancer research community to take a more rigorous approach to the study of thermal discomfort symptoms among breast cancer survivors. In order to obtain a precise and accurate assessment of the effects of thermal dysfunction, a wide range of interdisciplinary studies will be necessary. A combination of epidemiological, clinical, biological, and immunological studies will need to be employed to determine the risk factors and underlying etiologic mechanisms for thermal dysregulation among breast cancer patients, and the significance of these symptoms with disease prognosis. Although there are an overwhelming number of studies published on hot flashes, greater emphasis is needed to improve understanding of causal mechanisms for these vasomotor menopausal symptoms in breast cancer patients. Since these symptoms can be quite debilitating, affecting patient compliance with the use of anti-estrogen therapies for example, it is important to learn how to alleviate them without affecting treatment efficacy or risk of disease recurrence. Recent findings from the WHEL and ATAC studies [2, 3] support the provocative idea that better treatment outcomes are related to development of menopausal vasomotor symptoms. If confirmed, clinical studies can be designed to test the use of these symptoms as a means of providing therapy tailored to breast cancer patients.
In comparison to studies on hot flashes, the possibility that some cancer patients may feel persistently cold has never been scientifically recognised, and studies to characterise this symptom and understand the underlying etiological mechanism have never been conducted. A first step in conducting this research might be to design an observational epidemiological study that will provide a thoughtful prospective examination of potential relationships between body temperature, thermal discomfort experienced by women with breast cancer, immune phenotype, disease prognosis, and cytokine-driven symptoms of cancer-associated sickness. Identification of key immune patterns related to breast cancer prognosis, body temperature, symptoms of thermal discomfort, and cytokinerelated symptoms experienced by breast cancer patients will be critical for the design of future intervention studies aimed at altering or supporting body temperature as a potential strategy for supporting immune function among cancer patients. Such studies may be able to target these cytokines as intermediate biomarkers of long-term prognosis. If body temperature and/or feelings of being persistently cold are found in initial observation studies to be robust prognostic factors for breast cancer, it will be important to identify modifiable risk factors for these conditions. Greater understanding of these relationships will provide insight into potentially modifiable factors and interventions that may be designed to impact body temperature and/or symptoms of thermal discomfort, which may in turn improve anti-tumour immune activity and disease prognosis.
Conclusions
This article has highlighted the phenomenon of thermal discomfort which is highly prevalent in breast cancer patients. Overall, the information presented here supports the idea that patients' reports of being too hot or too cold should not be simply discounted as an annoying side effect of treatment-induced menopausal symptoms. The emerging evidence indicating that some vasomotor symptoms may be associated with treatment outcomes and disease recurrence is intriguing. Further study is needed to determine whether this easily recognisable symptom can provide a simple means for assessing treatment efficacy among individual breast cancer patients in order to support individualized therapies optimising disease outcomes. A second intention of this review is to draw attention to the possibility that some breast cancer survivors may feel persistently and inappropriately cold and have diminished ability to easily maintain thermal comfort. We hypothesize that similar to other sickness behaviours, thermal discomfort may reflect changes in the levels or activity of pro-inflammatory cytokines.
The established links between febrile symptoms, the immune response and pro-inflammatory cytokines, combined with a growing literature indicating a positive relationship between mild hyperthermia and the immune system present several compelling hypotheses regarding thermal discomfort symptoms in breast cancer patients. Addressing these hypotheses will optimally require interdisciplinary study by scientists interested in breast cancer epidemiology and thermal physiology/immunology, as well as in metabolism and inflammation.
Acknowledgements
The authors would like to thanks Drs. Bonnie Hylander and Christine Ambrosone and Maegan Capitano and Chen-Ting Lee for their comments and help with this manuscript. We would also like to acknowledge many very helpful suggestions from an expert reviewer.
This work was supported in part by grants from the NCI R01 CA071599 and P01 CA094045, and by a US Department of Defense – Congressionally Directed Medical Research Program on Breast Cancer Multidisciplinary Postdoctoral Fellowship Award (W81XWH-06-1-0101), and a New York State Department of Health, New York State Breast Cancer Research and Education Fund, Postdoctoral Fellowship (C020918). The authors alone are responsible for the content and writing of the paper.
Footnotes
Declaration of interest: The authors report no conflicts of interest.
References
- 1.Carpenter J, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nursing Forum. 2002;29(3):E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 2.Mortimer J, Flatt S, Parker B, Gold E, Wasserman L, Natarajan L, Pierce J. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108(3):421–426. doi: 10.1007/s10549-007-9612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Cella D, Fallowfield L. Treatmentemergent endocrine symptoms and the risk of breast cancer recurrence: A retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9(12):1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 4.Szmuilowicz ED. Menopausal vasomotor symptoms:Symptom or signal? Endocrine Today. 2009 February 29; [Google Scholar]
- 5.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 6.Gast GCM, Grobbee DE, Pop VJM, Keyzer JJ, Wijnands-van Gent CJM, Samsioe GN, Nilsson PM, van der Schouw YT. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51(6):1492–1498. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- 7.Grond S, Zech D, Diefenbach C, Bischoff A. Prevalence and pattern of symptoms in patients with cancer pain: A prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage. 1994;9(6):372–382. doi: 10.1016/0885-3924(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 8.Stearns V, Hayes D. Approach to menopausal symptoms in women with breast cancer. Curr Treat Options Oncol. 2002;3(2):179–190. doi: 10.1007/s11864-002-0064-6. [DOI] [PubMed] [Google Scholar]
- 9.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner C, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010;11(6):561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vujaskovic Z, Kim DW, Jones E, Lan L, McCall L, Dewhirst MW, Craciunescu O, Stauffer P, Liotcheva V, Betof A, et al. A phase I/II study of neoadjuvant liposomaldoxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia. 2010;26(5):514–521. doi: 10.3109/02656731003639364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peer A, Grimm M, Zynda E, Repasky E. Diverse immune mechanisms may contribute to the survival benefit seen in cancer patients receiving hyperthermia. Immunol Res. 2010;46(1):137–154. doi: 10.1007/s12026-009-8115-8. [DOI] [PubMed] [Google Scholar]
- 12.Skitzki JJ, Repasky EA, Evans SS. Hyperthermia as an immunotherapy strategy for cancer. Current opinion in investigational drugs. 2009;10(6):550–558. [PMC free article] [PubMed] [Google Scholar]
- 13.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 14.Campbell NA. Benjamin/Cummings series in the life sciences. Benjamin/Cummings; Menlo Park, CA: 1993. Biology. [Google Scholar]
- 15.Mackowiak PA. Concepts of fever. Arch Int Med. 1998;158(17):1870–1881. doi: 10.1001/archinte.158.17.1870. [DOI] [PubMed] [Google Scholar]
- 16.Cabanac M. Temperature regulation. Ann Rev Phys. 1975;37(1):415–439. doi: 10.1146/annurev.ph.37.030175.002215. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RH. The autonomic nervous system and body temperature. Proc Royal Soc Med. 1966;59(5):463–466. [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon CJ. Temperature regulation in laboratory rodents. Cambridge University Press; Cambridge: 1993. [Google Scholar]
- 19.Frisancho AR. Human adaptation and accommodation. University of Michigan Press; Michigan: 1995. [Google Scholar]
- 20.Guyton AC, Hall JE. Textbook of Medical Physiology. 11th edn. Saunders; Philadelphia: 2006. [Google Scholar]
- 21.Lu SH, Dai YT. Normal body temperature and the effects of age, sex, ambient temperature and body mass index on normal oral temperature: A prospective, comparative study. Int J Nurs Stud. 2009;46(5):661–668. doi: 10.1016/j.ijnurstu.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Brobeck JR. Food intake as a mechanism of temperature regulation. Yale J Bio Med. 1948;20(6):545–552. [PMC free article] [PubMed] [Google Scholar]
- 23.Rhind SG, Gannon GA, Shephard RJ, Buguet A, Shek PN, Radomski MW. Cytokine induction during exertional hyperthermia is abolished by core temperature clamping: Neuroendocrine regulatory mechanisms. Int J Hyperthermia. 2004;20(5):503–516. doi: 10.1080/02656730410001670651. [DOI] [PubMed] [Google Scholar]
- 24.Mostardi R, Kubica R, Veicsteinas A, Margaria R. The effect of increased body temperature due to exercise on the heart rate and on the maximal aerobic power. Eur J Applied Physiol. 1974;33(3):237–245. doi: 10.1007/BF00421151. [DOI] [PubMed] [Google Scholar]
- 25.Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201(4350):16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- 26.Charkoudian N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin Proc. 2003;78(5):603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- 27.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31:S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- 28.Cameron JR, Skofronick JG, Grant RM. Physics of the body (Medical Physics Series) 2nd edn. Medical Physics Publishing; Madison: 1999. [Google Scholar]
- 29.Frank SM, Raja SN, Bulcao CF, Goldstein DS. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J Appl Physiol. 1999;86(5):1588–1593. doi: 10.1152/jappl.1999.86.5.1588. [DOI] [PubMed] [Google Scholar]
- 30.Bennett AF. Evolution of the control of body temperature: Is warmer better? In: Dejours P, Taylor CR, Weibel ER, editors. Comparative Physiology: Life in Water and on Land. Litvia Press; Padova: 1987. pp. 421–431. [Google Scholar]
- 31.Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW. Is obesity associated with lower body temperatures? Core temperature: A forgotten variable in energy balance. Metabolism. 2009;58(6):871–876. doi: 10.1016/j.metabol.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchard C, Tremblay A, Despres JP, Nadeau A, Lupien PJ, Theriault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322(21):1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 35.Spiegelman B. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 36.Landsberg L, Saville ME, Young JB. Sympathoadrenal system and regulation of thermogenesis. Am J Physiol Endocrin Metab. 1984;247(2):E181–189. doi: 10.1152/ajpendo.1984.247.2.E181. [DOI] [PubMed] [Google Scholar]
- 37.Doucet E, St Pierre S, Almras N, Desprs JP, Bouchard C, Tremblay A. Evidence for the existence of adaptive thermogenesis during weight loss. Br J Nutr. 2001;85:715–723. doi: 10.1079/bjn2001348. [DOI] [PubMed] [Google Scholar]
- 38.Doucet E, Imbeault P, St-Pierre S, Alméras N, Mauriège P, Després JPP, Bouchard C, Tremblay A. Greater than predicted decrease in energy expenditure during exercise after body weight loss in obese men. Clin Sci. 2003;105(1):89–95. doi: 10.1042/CS20020252. [DOI] [PubMed] [Google Scholar]
- 39.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 40.Woods SC, Seeley RJ, Porte D, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280(5368):1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor A. Really? - Is it true that body temperature declines with age? New York Times. 2009 December 28; [Google Scholar]
- 42.Castle SC, Norman DC, Yeh M, Miller D, Yoshikawa TT. Fever response in elderly nursing home residents: Are the older truly colder? J Am Geriatr Soc. 1991;39(9):853–857. doi: 10.1111/j.1532-5415.1991.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 43.Günes UYY, Zaybak A. Does the body temperature change in older people? J Clin Nurs. 2008;17(17):2284–2287. doi: 10.1111/j.1365-2702.2007.02272.x. [DOI] [PubMed] [Google Scholar]
- 44.Salvosa CB, Payne PR, Wheeler EF. Environmental conditions and body temperatures of elderly women living alone or in local authority home. Br Med J. 1971;4(5788):656–659. doi: 10.1136/bmj.4.5788.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox RH, Woodward PM, Exton-Smith AN, Green MF, Donnison DV, Wicks MH. Body temperatures in the elderly:A national study of physiological, social, and environmental conditions. Br Med J. 1973;1(5847):200–206. doi: 10.1136/bmj.1.5847.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenney WL, Munce TA. Invited review: Aging and human temperature regulation. J Appl Physiol. 2003;95(6):2598–2603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- 47.Collins KJ, Dore C, Exton-Smith AN, Fox RH, MacDonald IC, Woodward PM. Accidental hypothermia and impaired temperature homoeostasis in the elderly. Br Med J. 1977;1(6057):353–356. doi: 10.1136/bmj.1.6057.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horvath SM, Rochelle RD. Hypothermia in the aged. Environ Health Perspect. 1977;20:127–130. doi: 10.1289/ehp.7720127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenney WL, Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men. J Appl Physiol. 1996;80(2):512–515. doi: 10.1152/jappl.1996.80.2.512. [DOI] [PubMed] [Google Scholar]
- 50.Falk B, Bar-Or O, Smolander J, Frost G. Response to rest and exercise in the cold: Effects of age and aerobic fitness. J Appl Physiol. 1994;76(1):72–78. doi: 10.1152/jappl.1994.76.1.72. [DOI] [PubMed] [Google Scholar]
- 51.Drinkwater BL, Bedi JF, Loucks AB, Roche S, Horvath SM. Sweating sensitivity and capacity of women in relation to age. J Appl Physiol. 1982;53(3):671–676. doi: 10.1152/jappl.1982.53.3.671. [DOI] [PubMed] [Google Scholar]
- 52.Shoenfeld Y, Udassin R, Shapiro Y, Ohri A, Sohar E. Age and sex difference in response to short exposure to extreme dry heat. J Appl Physiol. 1978;44(1):1–4. doi: 10.1152/jappl.1978.44.1.1. [DOI] [PubMed] [Google Scholar]
- 53.Krems C, Lührmann PM, Strassburg A, Hartmann B, Neuhäuser-Berthold M. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr. 2005;59(2):255–262. doi: 10.1038/sj.ejcn.1602066. [DOI] [PubMed] [Google Scholar]
- 54.Poehlman ET, Arciero PJ, Goran MI. Endurance exercise in aging humans: Effects on energy metabolism. Exerc Sport Science Rev. 1994;22:251–284. [PubMed] [Google Scholar]
- 55.Danforth E, Burger A. The role of thyroid hormones in the control of energy expenditure. Clin Endocrin Metab. 1984;13(3):581–595. doi: 10.1016/s0300-595x(84)80039-0. [DOI] [PubMed] [Google Scholar]
- 56.Smyth PP, Smith DF, McDermott EW, Murray MJ, Geraghty JG, O'Higgins NJ. A direct relationship between thyroid enlargement and breast cancer. J Clin Endocrinol Metab. 1996;81(3):937–941. doi: 10.1210/jcem.81.3.8772554. [DOI] [PubMed] [Google Scholar]
- 57.Kronenberg F. Hot flashes: Phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994;29(3–4):319–336. doi: 10.1016/0531-5565(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 58.Carpenter JS, Wells N, Lambert B, Watson P, Slayton T, Chak B, Hepworth JT, Worthington WB. A pilot study of magnetic therapy for hot flashes after breast cancer. Cancer Nurs. 2002;25(2):104–109. doi: 10.1097/00002820-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Kouriefs C, Georgiou M, Ravi R. Hot flashes and prostate cancer: Pathogenesis and treatment. Br J Urology Intl. 2002;89(4):379–383. doi: 10.1046/j.1464-4096.2001.01761.x. [DOI] [PubMed] [Google Scholar]
- 60.Freedman R. Hot flashes: Behavioral treatments, mechanisms, and relation to sleep. Am J Med. 2005;118(12):124–130. doi: 10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 61.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80(8):2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 62.Carpenter JS, Gautam S, Freedman RR, Andrykowski M. Circadian rhythm of objectively recorded hot flashes in postmenopausal breast cancer survivors. Menopause. 2001;8(3):181–188. doi: 10.1097/00042192-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause. 2004;11(4):375–381. doi: 10.1097/01.gme.0000113848.74835.1a. [DOI] [PubMed] [Google Scholar]
- 64.Brück K. Adaptive changes in thermoregulation and their neuropharmacological basis. Pharmacol Ther. 1987;35(1–2):163–215. doi: 10.1016/0163-7258(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 65.Mekjavic IB, Sundberg CJ, Linnarsson D. Core temperature ‘null zone’. J Appl Physiol. 991;71(4):1289–1295. doi: 10.1152/jappl.1991.71.4.1289. [DOI] [PubMed] [Google Scholar]
- 66.Freedman R, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181(1):66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 67.Loprinzi CL, Michalak JC, Quella SK, O'Fallon JR, Hatfield AK, Nelimark RA, Dose AM, Fischer T, Johnson C, Klatt NE, et al. Megestrol Acetate for the Prevention of Hot Flashes. N Engl J Med. 1994;331(6):347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 68.Brooks EM, Morgan AL, Pierzga JM, Wladkowski SL, O'Gorman JT, Derr JA, Kenney WL. Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol. 1997;83(2):477–484. doi: 10.1152/jappl.1997.83.2.477. [DOI] [PubMed] [Google Scholar]
- 69.Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: Thermoregulatory responses to exercise in the heat. J Appl Physiol. 1992;73(4):1238–1245. doi: 10.1152/jappl.1992.73.4.1238. [DOI] [PubMed] [Google Scholar]
- 70.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA. 2006;295(17):2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 71.Charkoudian N, Johnson JM. Female reproductive hormones and thermoregulatory control of skin blood flow. Exerc Sport Science Rev. 2000;28(3):108–112. [PubMed] [Google Scholar]
- 72.Greendale G. Symptom relief and side effects of postmenopausal hormones: Results from the postmenopausal estrogen/ progestin interventions trial. Obstet Gynecol. 1998;92(6):982–988. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- 73.Hutton JD, Jacobs HS, Murray MA, James VH. Relation between plasma oestrone and oestradiol and climacteric symptoms. Lancet. 1978;1(8066):678–681. doi: 10.1016/s0140-6736(78)90796-1. [DOI] [PubMed] [Google Scholar]
- 74.Freedman RR, Dinsay R. Clonidine raises the sweating threshold in symptomatic but not in asymptomatic postmenopausal women. Fertil Steril. 2000;74(1):20–23. doi: 10.1016/s0015-0282(00)00563-x. [DOI] [PubMed] [Google Scholar]
- 75.Freedman R. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82(1):138–144. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 76.Aksel S, Schomberg DW, Tyrey L, Hammond CB. Vasomotor symptoms, serum estrogens, and gonadotropin levels in surgical menopause. Am J Obstet Gynecol. 1976;126(2):165–169. doi: 10.1016/0002-9378(76)90270-2. [DOI] [PubMed] [Google Scholar]
- 77.Stone SC, Mickal A, Rye PH. Postmenopausal symptomatology, maturation index, and plasma estrogen levels. Obstet Gynecol. 1975;45(6):625–627. doi: 10.1097/00006250-197506000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Younus J, Kligman L. Management of aromatase inhibitorinduced arthralgia. Curr Oncol. 2010;17:87–90. doi: 10.3747/co.v17i1.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carpenter JS. Hot flashes and their management in breast cancer. Semin Oncol Nurs. 2000;16(3):214–225. doi: 10.1053/sonc.2000.8116. [DOI] [PubMed] [Google Scholar]
- 80.Carpenter JS, Andrykowski MA, Cordova M, Cunningham L, Studts J, McGrath P, Kenady D, Sloan D, Munn R. Hot flashes in postmenopausal women treated for breast carcinoma: Prevalence, severity, correlates, management, and relation to quality of life. Cancer. 1998;82(9):1682–1691. [PubMed] [Google Scholar]
- 81.Harris P. Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symp Man. 2002;23(6):501–509. doi: 10.1016/s0885-3924(02)00395-0. [DOI] [PubMed] [Google Scholar]
- 82.LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS. Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause. 2007;14(2):191–202. doi: 10.1097/01.gme.0000230347.28616.1c. [DOI] [PubMed] [Google Scholar]
- 83.Love RR. Tamoxifen therapy in primary breast cancer: Biology, efficacy, and side effects. J Clin Oncol. 1989;7(6):803–815. doi: 10.1200/JCO.1989.7.6.803. [DOI] [PubMed] [Google Scholar]
- 84.Love RR, Cameron L, Connell BL, Leventhal H. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Int Med. 1991;151(9):1842–1847. [PubMed] [Google Scholar]
- 85.Pasacreta JV, McCorkle R. Providing accurate information to women about tamoxifen therapy for breast cancer: Current indications, effects, and controversies. Oncol Nurs Forum. 1998;25(9):1577–1583. [PubMed] [Google Scholar]
- 86.Loprinzi CL, Barton DL, Sloan JA, Novotny PJ, Dakhil SR, Verdirame JD, Knutson W, Kelaghan J, Christensen BB. Mayo Clinic and North Central Cancer Treatment Group hot flash studies. Menopause. 2008;15(4):655–660. doi: 10.1097/gme.0b013e3181679150. [DOI] [PubMed] [Google Scholar]
- 87.Reichman BS, Green KB. Breast cancer in young women: Effect of chemotherapy on ovarian function, fertility, and birth defects. J Nat Cancer Inst. 1994;16:125–129. [PubMed] [Google Scholar]
- 88.Yasui T, Uemura H, Tomita J, Miyatani Y, Yamada M, Kuwahara A, Matsuzaki T, Maegawa M, Tsuchiya N, Yuzurihara M, et al. Association of interleukin-8 with hot flashes in premenopausal, perimenopausal, and postmenopausal women and bilateral oophorectomized women. J Clin Endocrinol Metab. 2006;91(12):4805–4808. doi: 10.1210/jc.2006-1100. [DOI] [PubMed] [Google Scholar]
- 89.Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, Huang R. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109(4):507–515. doi: 10.1002/ijc.11724. [DOI] [PubMed] [Google Scholar]
- 90.Mormont MC, Lévi F. Circadian-system alterations during cancer processes: A review. Int J Cancer. 1997;70(2):241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 91.Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: Scientific review. JAMA. 2004;291(13):1610–1620. doi: 10.1001/jama.291.13.1610. [DOI] [PubMed] [Google Scholar]
- 92.Kontos M, Agbaje OF, Rymer J, Fentiman IS. What can be done about hot flashes after treatment for breast cancer? Climacteric. 2010;13(1):4–21. doi: 10.3109/13697130903291058. [DOI] [PubMed] [Google Scholar]
- 93.Hickey M, Saunders C, Partridge A, Santoro N, Joffe H, Stearns V. Practical clinical guidelines for assessing and managing menopausal symptoms after breast cancer. Ann Oncol. 2008;19(10):1669–1680. doi: 10.1093/annonc/mdn353. [DOI] [PubMed] [Google Scholar]
- 94.Bordeleau L, Pritchard K, Goodwin P, Loprinzi C. Therapeutic options for the management of hot flashes in breast cancer survivors: An evidence-based review. Clin Therapeutics. 2007;29(2):230–241. doi: 10.1016/j.clinthera.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Gordon PR, Kerwin JP, Boesen KGG, Senf J. Sertraline to treat hot flashes: A randomized controlled, double-blind, crossover trial in a general population. Menopause. 2006;13(4):568–575. doi: 10.1097/01.gme.0000196595.82452.ca. [DOI] [PubMed] [Google Scholar]
- 96.Stearns V, Slack R, Greep N, Henry-Tilman R, Osborne M, Bunnell C, Ullmer L, Gallagher A, Cullen J, Gehan E, et al. Paroxetine is an effective treatment for hot flashes: Results from a prospective randomized clinical trial. J Clin Oncol. 2005;23(28):6919–6930. doi: 10.1200/JCO.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 97.Kimmick GG, Lovato J, McQuellon R, Robinson E, Muss HB. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006;12(2):114–122. doi: 10.1111/j.1075-122X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 98.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: A randomized controlled trial. JAMA. 2003;289(21):2827–2834. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 99.Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, Halyard MY, Pruthi S, Novotny PJ, Rummans TA. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 100.Roth AJ, Scher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology. 1998;7(2):129–132. doi: 10.1002/(SICI)1099-1611(199803/04)7:2<129::AID-PON294>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 101.Powell SL, Gödecke T, Nikolic D, Chen SN, Ahn S, Dietz B, Farnsworth NR, van Breemen RB, Lankin DC, Pauli GF, et al. In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent. J Agric Food Chem. 2008;56(24):11718–11726. doi: 10.1021/jf803298z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, Kinne D, Lo KM, Moore A, Rosenman PJ, Kaufman EL, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19(10):2739–2745. doi: 10.1200/JCO.2001.19.10.2739. [DOI] [PubMed] [Google Scholar]
- 103.Loprinzi C, Kugler J, Sloan J, Mailliard J, Lavasseur B, Barton D, Novotny PJ, Dakhil SR, Rodger K, Rummans TA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: A randomised controlled trial. Lancet. 2000;356(9247):2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 104.Carpenter JS, Storniolo AM, Johns S, Monahan PO, Azzouz F, Elam JL, Johnsona CS, Sheltonb RC. Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist. 2007;12(1):124–135. doi: 10.1634/theoncologist.12-1-124. [DOI] [PubMed] [Google Scholar]
- 105.Loprinzi CL, Levitt R, Barton D, Sloan JA, Dakhil SR, Nikcevich DA, Bearden JD, Mailliard JA, Tschetter LK, Fitch TR, et al. Phase III comparison of depomedroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24(9):1409–1414. doi: 10.1200/JCO.2005.04.7324. [DOI] [PubMed] [Google Scholar]
- 106.Pandya KJ, Raubertas RF, Flynn PJ, Hynes HE, Rosenbluth RJ, Kirshner JJ, Pierce HI, Dragalin V, Morrow GB. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: A University of Rochester Cancer Center Community Clinical Oncology Program Study. Ann Intern Med. 2000;132(10):788–793. doi: 10.7326/0003-4819-132-10-200005160-00004. [DOI] [PubMed] [Google Scholar]
- 107.Nagata C, Takatsuka N, Kawakami N, Shimizu H. Soy product intake and hot flashes in Japanese women: Results from a community-based prospective study. Am J Epidemiol. 2001;153(8):790–793. doi: 10.1093/aje/153.8.790. [DOI] [PubMed] [Google Scholar]
- 108.Scambia G, Mango D, Signorile PG, Angeli RA, Palena C, Gallo D, Bombardelli E, Morazzoni P, Riva A, Mancuso S. Clinical effects of a standardized soy extract in postmenopausal women. Menopause. 2000;7(2):105–111. doi: 10.1097/00042192-200007020-00006. [DOI] [PubMed] [Google Scholar]
- 109.Faure ED, Chantre P, Mares P. Effects of a standardized soy extract on hot flashes: A multicenter, double-blind, randomized, placebo-controlled study. Menopause. 2002;9(5):329–334. doi: 10.1097/00042192-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, Hislop TG, Templeton E, Wattie A, Prior JC. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: A randomized, controlled clinical trial. J Clin Oncol. 2002;20(6):1449–1455. doi: 10.1200/JCO.2002.20.6.1449. [DOI] [PubMed] [Google Scholar]
- 111.Quella SK, Loprinzi CL, Barton DL, Knost JA, Sloan JA, LaVasseur BI, Swan D, Krupp KR, Miller KD, Novotny PJ. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: A North Central Cancer Treatment Group Trial. J Clin Oncol. 2000;18(5):1068þ. doi: 10.1200/JCO.2000.18.5.1068. [DOI] [PubMed] [Google Scholar]
- 112.Tice JA, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings SR. Phytoestrogen supplements for the treatment of hot flashes: The isoflavone clover extract (ICE) study: A randomized controlled trial. JAMA. 2003;290(2):207–214. doi: 10.1001/jama.290.2.207. [DOI] [PubMed] [Google Scholar]
- 113.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 114.Quella S, Loprinzi C, Sloan J, Vaught N, Dekrey W, Fischer T, Finck G, Pierson N, Pisansky T. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. J Urology. 1999;161(1):360. [PubMed] [Google Scholar]
- 115.Kavanagh JJ, Roberts W, Townsend P, Hewitt S. Leuprolide acetate in the treatment of refractory or persistent epithelial ovarian cancer. J Clin Oncol. 1989;7(1):115–118. doi: 10.1200/JCO.1989.7.1.115. [DOI] [PubMed] [Google Scholar]
- 116.Miller DS, Brady MF, Barrett RJ. A phase II trial of leuprolide acetate in patients with advanced epithelial ovarian carcinoma. A Gynecologic Oncology Group study. Am J Clin Oncol. 1992;15(2):125–128. doi: 10.1097/00000421-199204000-00006. [DOI] [PubMed] [Google Scholar]
- 117.Tresukosol D, Kudelka AP, Edwards CL, Silva EG, Kanojia M, Kavanagh JJ. Leuprolide acetate in advanced ovarian serous tumor of low malignant potential. A case report. J Reproductive Med. 1996;41(5):363–366. [PubMed] [Google Scholar]
- 118.Hidden costs of breast cancer 2005. Available from: http://www.countrydoctor.co.uk/precis/precis%20-%20Breast%20 cancer%20costs.htm (accessed 1 July 2010)
- 119.Clark W. Factor analysis validates the cluster structure of the dendrogram underlying the Multidimensional Affect and Pain Survey (MAPS) and challenges the a priori classification of the descriptors in the McGill Pain Questionnaire (MPQ) Pain. 2003;106(3):357–363. doi: 10.1016/j.pain.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 120.Olson K. A new way of thinking about fatigue: A reconceptualization. Oncol Nurs Forum. 2007;34(1):93–99. doi: 10.1188/07.ONF.93-99. [DOI] [PubMed] [Google Scholar]
- 121.McDaniel RW, Rhodes VA. Development of a preparatory sensory information videotape for women receiving chemotherapy for breast cancer. Cancer Nurs. 1998;21(2):143–148. doi: 10.1097/00002820-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 122.Rudberg L, Carlsson M, Nilsson S, Wikblad K. Selfperceived physical, psychologic, and general symptoms in survivors of testicular cancer 3 to 13 years after treatment. Cancer Nurs. 2002;25(3):187–195. doi: 10.1097/00002820-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 123.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: Emerging role in cancer. Nat Rev Cancer. 2003;3(2):110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 124.Wülfing P, Diallo R, Kersting C, Wülfing C, Poremba C, Rody A, Greb RR, Böcker W, Kiesel L. Expression of endothelin-1, endothelin-a, and endothelin-b receptor in human breast cancer and correlation with long-term followup. Clin Cancer Res. 2003;9(11):4125–4131. [PubMed] [Google Scholar]
- 125.Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: A behavioral study. Neurosci Lett. 2007;418(2):117–121. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 126.Leon LR. Hypothermia in systemic inflammation: Role of cytokines. Frontiers in Bioscience. 2004;9:1877–1888. doi: 10.2741/1381. [DOI] [PubMed] [Google Scholar]
- 127.Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, Vinson C, Reitman ML. Torpor in mice is induced by both leptin-dependent and –independent mechanisms. Proc Natl Acad Sci. 1999;96(25):14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Buchanan TA, Cane P, Eng CC, Sipos GF, Lee C. Hypothermia is critical for survival during prolonged insulin-induced hypoglycemia in rats. Metabolism. 1991;40(3):330–334. doi: 10.1016/0026-0495(91)90118-g. [DOI] [PubMed] [Google Scholar]
- 129.Graf R, Krishna S, Heller HC. Regulated nocturnal hypothermia induced in pigeons by food deprivation. Am J Physiol. 1989;256(3):R733–738. doi: 10.1152/ajpregu.1989.256.3.R733. [DOI] [PubMed] [Google Scholar]
- 130.Malvin GM, Wood SC. Behavioral hypothermia and survival of hypoxic protozoans Paramecium caudatum. Science. 1992;255(5050):1423–1425. doi: 10.1126/science.1542790. [DOI] [PubMed] [Google Scholar]
- 131.Rausch RN, Crawshaw LI, Wallace HL. Effects of hypoxia, anoxia, and endogenous ethanol on thermoregulation in goldfish, Carassius auratus. Am J Phys. 2000;278(3):R545–R555. doi: 10.1152/ajpregu.2000.278.3.R545. [DOI] [PubMed] [Google Scholar]
- 132.Ibuka N, Fukumura K. Unpredictable deprivation of water increases the probability of torpor in the Syrian hamster. Physiol Behav. 1997;62(3):551–556. doi: 10.1016/s0031-9384(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 133.Romanovsky A, Szekely M. Fever and hypothermia: Two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses. 1998;50(3):219–226. doi: 10.1016/s0306-9877(98)90022-6. [DOI] [PubMed] [Google Scholar]
- 134.Klein MS, Conn CA, Kluger MJ. Behavioral thermoregulation in mice inoculated with influenza virus. Physiol Behav. 1992;52(6):1133–1139. doi: 10.1016/0031-9384(92)90472-e. [DOI] [PubMed] [Google Scholar]
- 135.Lagerspetz KY, Väätäinen T. Bacterial endotoxin and infection cause behavioural hypothermia in infant mice. Comp Biochem Phys. 1987;88(3):519–521. doi: 10.1016/0300-9629(87)90074-0. [DOI] [PubMed] [Google Scholar]
- 136.Muller CB, Schmid-Hempel P. Exploitation of cold temperature as defence against parasitoids in bumblebees. Nature. 1993;363(6424):65–67. [Google Scholar]
- 137.Gordon CJ, Stead AG. Effect of nickel and cadmium chloride on autonomic and behavioral thermoregulation in mice. Neurotoxicology. 1986;7(3):97–106. [PubMed] [Google Scholar]
- 138.Goldberg RM, Loprinzi CL, O'Fallon JR, Veeder MH, Miser AW, Mailliard JA, Michalak JC, Dose AM, Rowland KM, Jr, Burnham NL. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12(1):155–158. doi: 10.1200/JCO.1994.12.1.155. [DOI] [PubMed] [Google Scholar]
- 139.Loprinzi CL, Pisansky TM, Fonseca R, Sloan JA, Zahasky KM, Quella SK, Novotny PJ, Rummans TA, Dumesic DA, Perez EA. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol. 1998;16(7):2377–2381. doi: 10.1200/JCO.1998.16.7.2377. [DOI] [PubMed] [Google Scholar]
- 140.Barton DL, Loprinzi CL, Quella SK, Sloan JA, Veeder MH, Egner JR, Fidler P, Stella PJ, Swan DK, Vaught NL. Prospective evaluation of vitamin E for hot flashes in breast cancer survivors. J Clin Oncol. 1998;16(2):495–500. doi: 10.1200/JCO.1998.16.2.495. [DOI] [PubMed] [Google Scholar]
- 141.Bessell EM, Selby C, Ellis IO. Severe hypoglycaemia caused by raised insulin-like growth factor II in disseminated breast cancer. J Clin Path. 1999;52(10):780–781. doi: 10.1136/jcp.52.10.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stearns V, Isaacs C, Rowland J, Crawford J, Ellis MJ, Kramer R, Lawrence W, Hanfelt JJ, Hayes DF. A pilot trial assessing the efficacy of paroxetine hydrochloride (Paxil) in controlling hot flashes in breast cancer survivors. Ann Oncol. 2000;11(1):17–22. doi: 10.1023/a:1008382706068. [DOI] [PubMed] [Google Scholar]
- 143.Bertelli G, Venturini M, Del Mastro L, Bergaglio M, Sismondi P, Biglia N, Venturini S, Porcile G, Pronzato P, Costantini M. Intramuscular depot medroxyprogesterone versus oral megestrol for the control of postmenopausal hot flashes in breast cancer patients: A randomized study. Ann Oncol. 2002;13(6):883–888. doi: 10.1093/annonc/mdf151. [DOI] [PubMed] [Google Scholar]
- 144.Weitzner MA, Moncello J, Jacobsen PB, Minton S. A pilot trial of paroxetine for the treatment of hot flashes and associated symptoms in women with breast cancer. J Pain Symptom Manage. 2002;23(4):337–345. doi: 10.1016/s0885-3924(02)00379-2. [DOI] [PubMed] [Google Scholar]
- 145.Carpenter J, Elam J, Ridner S, Carney P, Cherry G, Cucullu H. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncol Nursing Forum. 2004;31(3):591–598. doi: 10.1188/04.onf.591-598. [DOI] [PubMed] [Google Scholar]
- 146.Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 147.Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 148.Pandya KJ, Morrow GR, Roscoe JA, Zhao H, Hickok JT, Pajon E, Sweeney TJ, Banerjee TK, Flynn PJ. Gabapentin for hot flashes in 420 women with breast cancer: A randomised double-blind placebo-controlled trial. Lancet. 2005;366(9488):818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Loibl S, Schwedler K, von Minckwitz G, Strohmeier R, Mehta KM, Kaufmann M. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients–A double-blind, randomized study. Ann Oncol. 2007;18(4):689–693. doi: 10.1093/annonc/mdl478. [DOI] [PubMed] [Google Scholar]
- 150.Elkins G, Marcus J, Stearns V, Hasan Rajab M. Pilot evaluation of hypnosis for the treatment of hot flashes in breast cancer survivors. Psychooncology. 2007;16(5):487–492. doi: 10.1002/pon.1096. [DOI] [PubMed] [Google Scholar]
- 151.Deng G, Vickers AJ, Yeung KS, D'Andrea GM, Xiao H, Heerdt AS, Sugarman S, Troso-Sandoval T, Seidman AD, Hudis CA, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. 2007;25(35):5584–5590. doi: 10.1200/JCO.2007.12.0774. [DOI] [PubMed] [Google Scholar]
- 152.Pierce JP, Natarajan L, Caan BJ, Flatt SW, Kealey S, Gold EB, Hajek RA, Newman VA, Rock CL, Pu M, et al. Dietary change and reduced breast cancer events among women without hot flashes after treatment of early-stage breast cancer: Subgroup analysis of the Women's Healthy Eating and Living Study. Am J Clin Nutr. 2009;89(5):1565 S–1571. doi: 10.3945/ajcn.2009.26736F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Quella S, Loprinzi C, Sloan J, Novotny P, Perez E, Burch P, Antolak SJ, Pisansky TM. Pilot evaluation of venlafaxine for the treatment of hot flashes in men undergoing androgen ablation therapy for prostate cancer. J Urology. 1999;162(1):98–102. doi: 10.1097/00005392-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 154.Gift AG, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004;31(2):202–212. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- 155.Badawy YA, Bayoumi DM. An epidemiologic study of ovarian cancer. Part 11: Oral contraceptive use and menstrual events. J Egypt Public Health Assoc. 1992;67(5–6):579–591. [PubMed] [Google Scholar]
- 156.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 157.Konsman J. Cytokine-induced sickness behaviour:Mechanisms and implications. Trends Neurosci. 2002;25(3):154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 158.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19(2B):1427–1432. [PubMed] [Google Scholar]
- 159.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88(11):1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.DeMichele A, Martin AMM, Mick R, Gor P, Wray L, Klein-Cabral M, Athanasiadis G, Colligan T, Stadtmauer E, Weber B. Interleukin-6 -174G–4C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Res. 2003;63(22):8051–8056. [PubMed] [Google Scholar]
- 161.Meeske K, Smith A, Alfano C, McGregor B, McTiernan A, Baumgartner K, Malone KE, Reeve BB, Ballard-Barbash R, Bernstein L. Fatigue in breast cancer survivors two to five years post diagnosis: A HEAL study report. Qual Life Res. 2007;16(6):947–960. doi: 10.1007/s11136-007-9215-3. [DOI] [PubMed] [Google Scholar]
- 162.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 163.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 164.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. Br Med J. 2005;330(7493):702–706. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 166.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92(S6):1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 167.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006;169(5):1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: A review of the evidence. J Nutr. 2001;131(11):3109 S–3120. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 169.Ibrahim Y, Yee D. Insulin-like growth factor-I and cancer risk. Growth Hormone IGF Res. 2004;14(4):261–269. doi: 10.1016/j.ghir.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 170.Sonnenberg GE, Krakower GR, Kissebah AH. A novel pathway to the manifestations of metabolic syndrome. Obesity Res. 2004;12(2):180–186. doi: 10.1038/oby.2004.24. [DOI] [PubMed] [Google Scholar]
- 171.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266(1):37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 172.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46(11):1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 173.Rose DP, Gilhooly EM, Nixon DW. Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review) Int J Oncol. 2002;21(6):1285–1292. [PubMed] [Google Scholar]
- 174.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg Res. 2004;116(2):337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 175.Glossary of terms for thermal physiology. Eur J Physiol. 1987;410(4):567–587. doi: 10.1007/BF00586542. [DOI] [PubMed] [Google Scholar]
- 176.Gordon CJ. Temperature and toxicology: An integrative, comparative, and environmental approach. Taylor & Francis; Boca Raton: 2005. pp. 169–171. [Google Scholar]
- 177.Netea MG, Kullberg BJ, Van der Meer JWM. Circulating cytokines as mediators of fever. Clin Infect Dis. 2000;31:S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- 178.Schmitt BD. Fever in childhood. Pediatrics. 1984;74(5):929–936. [PubMed] [Google Scholar]
- 179.Stern RC. Pathophysiologic basis for symptomatic treatment of fever. Pediatrics. 1977;59(1):92–98. [PubMed] [Google Scholar]
- 180.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: Recent discoveries and revisions. Frontiers Biosci. 2005;10:2193–2216. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- 181.Steiner AA, Hunter JC, Phipps SM, Nucci TB, Oliveira DL, Roberts JL, Scheck AC, Simmons DL, Romanovsky AA. Cyclooxygenase-1 or -2 – Which one mediates lipopolysaccharide-induced hypothermia? Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R485–494. doi: 10.1152/ajpregu.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kageyama R, Ohkubo H, Nakanishi S. Induction of rat liver angiotensinogen mRNA following acute inflammation. Biochem Biophys Res Com. 1985;129(3):826–832. doi: 10.1016/0006-291x(85)91966-7. [DOI] [PubMed] [Google Scholar]
- 183.Blanque R, Meakin C, Millet S, Gardner C. Hypothermia as an indicator of the acute effects of lipopolysaccharides: Comparison with serum levels of IL-1, IL-6 and TNF-α. Gen Pharmacol: Vasc Sys. 1996;27(6):973–977. doi: 10.1016/0306-3623(95)02141-8. [DOI] [PubMed] [Google Scholar]
- 184.Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock: Thermoregulatory mechanisms. Am J Physiol Regul Integr Comp Physiol. 1996;270(4):R693–703. doi: 10.1152/ajpregu.1996.270.4.R693. [DOI] [PubMed] [Google Scholar]
- 185.Almeida MC, Steiner AA, Branco LGS, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neursci. 2006;23(12):3359–3367. doi: 10.1111/j.1460-9568.2006.04854.x. [DOI] [PubMed] [Google Scholar]
- 186.Steiner AA, Romanovsky AA. Leptin: At the crossroads of energy balance and systemic inflammation. Prog Lipid Res. 2007;46(2):89–107. doi: 10.1016/j.plipres.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine immune control in chronic inflammatory diseases. J Int Med. 2010;267(6):543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 188.Kluger MJ. Fever: Role of pyrogens and cryogens. Physiol Rev. 1991;71(1):93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dayanc BE, Beachy SH, Ostberg JR, Repasky EA. Dissecting the role of hyperthermia in natural killer cell mediated antitumor responses. Int J Hyperthermia. 2008;24(1):41–56. doi: 10.1080/02656730701858297. [DOI] [PubMed] [Google Scholar]
- 190.Bull JM, Scott GL, Strebel FR, Nagle VL, Oliver D, Redwine M, Rowe RW, Ahn CW, Koch SM. Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: A description of a phase I-II protocol. Int J Hyperthermia. 2008;24(8):649–662. doi: 10.1080/02656730802104740. [DOI] [PubMed] [Google Scholar]
- 191.Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J Leukoc Biol. 2000;68(6):815–820. [PubMed] [Google Scholar]
- 192.Ostberg JR, Patel R, Repasky EA. Regulation of immune activity by mild (fever-range) whole body hyperthermia: Effects on epidermal Langerhans cells. Cell Stress Chaperones. 2000;5(5):458–461. doi: 10.1379/1466-1268(2000)005<0458:roiabm>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82(5):1322–1331. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 194.Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol. 1998;177(1):137–147. doi: 10.1002/(SICI)1097-4652(199810)177:1<137::AID-JCP15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 195.Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sciences. 2005;62(1):10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PubMed] [Google Scholar]
- 196.Chen Q, Wang WC, Bruce R, Li H, Schleider DM, Mulbury MJ, Wallace PK, Baumann H, Evans SS. Central role of il-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity. 2004;20(1):59–70. doi: 10.1016/s1074-7613(03)00358-3. [DOI] [PubMed] [Google Scholar]
- 197.Evans SS, Fisher DT, Skitzki JJ, Chen Q. Targeted regulation of a lymphocyte-endothelial-interleukin-6 axis by thermal stress. Int J Hyperthermia. 2008;24(1):67–78. doi: 10.1080/02656730701772498. [DOI] [PubMed] [Google Scholar]
- 198.Blazícková S, Rovenský J, Koska J, Vigas M. Effect of hyperthermic water bath on parameters of cellular immunity. Int J Clin Pharm Res. 2000;20(1–2):41–46. [PubMed] [Google Scholar]