The role of cGMP as a second messenger in physiological and pathophysiological processes is widely appreciated 1–3. The successes of drugs that target the cGMP-signaling pathway [nitrovasodilators and PDE5 inhibitors (Viagra, Cialis, and Levitra)] have inspired interest in other cardiovascular benefits that might derive from a better understanding of this pathway 2, 4–7. Elevation of cGMP in cardiomyocytes or intact heart is associated with a negative inotropic effect 8, blunting and/or reversal of cardiac hypertrophy 5, protection against ischaemia/reperfusion injury 4, 7 and changes in apoptosis 9–10. Activation of the cGMP-dependent protein kinase I (PKGI) and phosphorylation of target proteins is involved in each of these processes although the precise mechanisms that bring about these effects are not fully understood 1–2. Cyclic GMP activation of PKG in cardiomyocytes lowers cellular calcium, which can reduce contractility and counter calcineurin-mediated dephosphorylation/activation/nuclear translocation of NFAT (nuclear factor of activated T-cells), which promotes expression of a cadre of pro-hypertrophic genes 2, 5–6. PKG-mediated phosphorylation of an unknown protein increases opening of mitochondrial K+/ATP channels, thereby diminishing damages resulting from ischaemia/reperfusion or myocardial infarction 11–12. Moreover, cGMP elevation suppresses β-adrenergic signaling in the heart and is associated with activation of PKG phosphorylation of troponin I (TnI) 8, 13–14. PKG activation increases the GTPase activity of RGS2 (regulator of G-protein coupled signaling 2) and thereby interferes with Gαq/11-coupled signaling 15.
Several groups have provided evidence for spatially and functionally distinct cellular pools of cGMP 16–17. In this issue of Circulation Research, Castro et al. document surprising new complexities involved in modulating cGMP level in cardiomyocytes in response to atrial natriuretic peptide (ANP) or nitric oxide (NO) 18. Distinct cGMP pools are generated by action of the particulate guanylyl cyclase (pGC), which is located on the plasma membrane and activated by ANP, and the cytosolic NO-stimulated GC (NO-GC) 16–17, 19. Fischmeister’s group has previously shown that the cGMP pool near the plasma membrane is controlled primarily by the action of the cGMP-hydrolyzing phosphodiesterase-2 (PDE2), while PDE5 action limits the cytosolic cGMP pool (Fig. 1) 16, 20. The biological effects of both pools are apparently mediated by activation of PKGI.
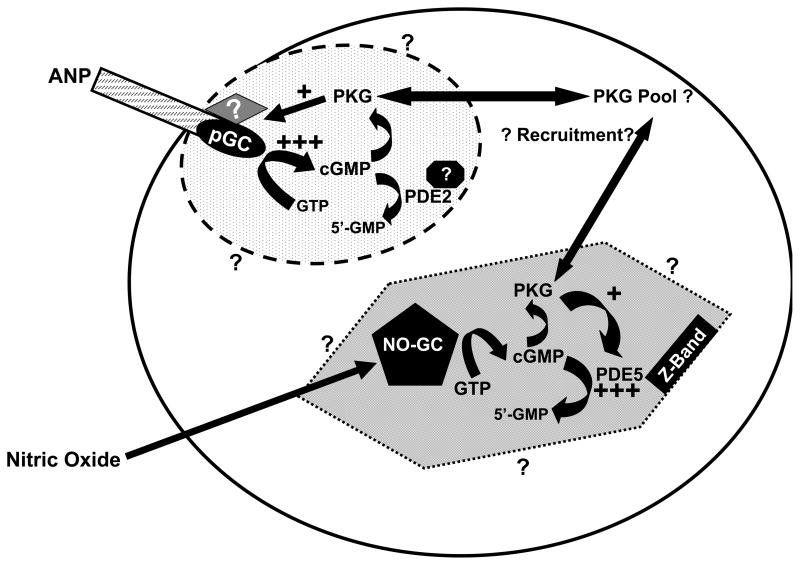
Figure.
Diagram depicting effect of activation of PKG on cGMP synthesis and breakdown in cardiomyocytes. The cGMP pools are indicated by the oval and hexagon containing cGMP-signaling proteins. Dotted boundaries indicate that these pools are restricted, but not impermeable; surrounding question marks indicate that spatial dimensions of the pools are poorly understood. ANP activation of pGC increases cGMP production in the subsarcolemmal region resulting in PKG activation. The activated PKG acts on ANP-activated pGC to increase cGMP synthesis (+++) and generate feed-forward regulation. The diamond with a question mark indicates that the target of PKG could be a protein that influences pGC activity and/or ANP sensitivity. Interactions that localize PDE2 to this region are unknown and indicated by the approximated black octagon. NO-activation of the NO-GC increases cGMP synthesis in the cytosolic pool; PKG activated by the increase in cGMP phosphorylates/activates PDE5 (+++), which rapidly hydrolyzes cGMP resulting in negative feedback regulation of cGMP in this pool.
The report by Castro et al. demonstrates that PKG activation in response to ANP-activation of pGC elicits a strong feed-forward mechanism that further enhances cGMP production in the subsarcolemmal pool (Fig. 1). The protein target of PKG that elicits this effect is unknown. Notably, this is the first feed-forward effect to be defined for cGMP signaling in any tissue. Surprisingly, it appears that there is little activation of PDE2 activity through cGMP binding to its allosteric sites, which should counter the effect, and the mechanism for terminating the feed-forward signal is not determined. Moreover, the mechanism whereby PDE2 is selectively localized to this cGMP pool is unknown.
In contrast, increased cGMP production by NO-GC elicits the opposite effect on cGMP levels by activating a negative feedback regulation of cytosolic cGMP; this is mediated by activation of PKG, which phosphorylates and activates PDE5. The resulting increased cGMP breakdown blunts further elevation of cGMP and lowers cytosolic cGMP. In the absence of PDE inhibitors, there is modest increase in cGMP in response to NO. Allosteric cGMP binding in PDE5 and phosphorylation by PKG increase catalytic activity and are important in negative feedback regulation of cGMP in several tissues 1, 21–22. Surprisingly, in the current report, only a role for PDE5 phosphorylation is indicated 18. Allosteric cGMP binding in PDE5 increases the rate of phosphorylation by PKG but is not required for phosphorylation or PDE5 activation 18. However, the allosteric site has higher affinity for cGMP than does the catalytic site; presumably both sites on a PDE5 molecule would bind nearby cGMP based on their respective affinities for cGMP. However, there may be unknown influences that impact PDE5 regulatory mechanisms in intact cells. By all accounts, PDE5 in cardiomyocytes is very low and localized to z-bands 2, 5; this localization is sensitive to PKGI action and sustained NO-GC activity, which would be predicted to foster allosteric cGMP binding by PDE5 as well as phosphorylation by PKGI. Curiously, it appears that the cytosolic cGMP pool in cardiomyocytes is confined by PDE5 which is low in abundance and not free to diffuse in that pool. The results reported by Castro et al. 18 provide exciting insights into the contrasting mechanisms controlling cGMP signaling in the heart, but there is still controversy in the field regarding the effects of PKGI and PDE5 in cardiomyocytes 23–25. The conflicting results could have several explanations. However, compelling results from numerous studies (including this one) support a role for cGMP and PKG action in cardiac function.
These new findings 18 raise many questions regarding cGMP signaling in the heart and use of therapies that target this pathway. Although the GCs and PDEs that define cellular cGMP pools are confined to particular regions, the localization of PKG is unclear. Are PKGs that mediate the effects in these regions persistently localized therein or recruited following cGMP elevation? What mechanism provides for PKG localization/recruitment? Is the same PKGI isoenzyme involved in each pool? PKGIα and PKGIβ differ in affinity for cGMP as well as in substrates in some instances, which could influence signaling 1, 26. What is the role of the relatively abundant PDE1, which participates in controlling cGMP levels in some region of the cardiomyocyte and effects biologically meaningful changes 27? How is pGC activated by PKG, and what mechanism provides for termination of this feed-forward process? What is the role of PKGI, PDE5, and cGMP-signaling in normal cardiomyocytes since there are minimal effects on cardiac function when PKGI is absent or when PDE5 is blocked in individuals taking PDE5-selective inhibitors 28–29? Acute effects of PDE5 inhibition in cardiomyocytes 8 and in studies utilizing mice and humans are modest 13. Will chronic use of PDE5 inhibitors alter regulation of the cGMP pools and/or roles of PDEs 1, 2, and 5 in cardiac functions? If cGMP signaling is primarily cardioprotective against stressors, e.g., ischaemia/reperfusion or pressure-overload, how is this regulated? Lastly, species differences in proteins involved in signaling pathways and changes that occur when cells are cultured present a challenge to extrapolating findings to functions in human tissues. However, this elegant piece of work by Castro and colleagues provides a significant advance in understanding cGMP signaling and opens new avenues for investigation of this complex pathway.
Acknowledgments
Financial Support for Author: National Institutes of Health DK40029 and Vanderbilt University School of Medicine
Non-Standard Abbreviations
- ANP
Atrial Natriuretic Peptide
- NFAT
Nuclear factor of Activated T Cells
- NO
Nitric Oxide
- NO-GC
Nitric Oxide Stimulated Guanylyl Cyclase
- pGC
Particulate Guanylyl Cyclase
- PKGI
cGMP-Dependent Protein Kinase I
- RGS2
Regulator of G-Protein Coupled Signaling 2
- TnI
Troponin I
Footnotes
Disclosures: None
References
- 1.Francis SH, Busch JA, Corbin JD. Cyclic GMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–63. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122:216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res. 2007;101:1084–1095. doi: 10.1161/CIRCRESAHA.107.162511. [DOI] [PubMed] [Google Scholar]
- 4.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 5.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 6.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol. 2010;48:713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Roldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DI, Vahebi S, Tocchetti CG, Barouch LA, Solaro RJ, Takimoto E, Kass DA. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol. 2010;105:337–347. doi: 10.1007/s00395-010-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 10.Wu CF, Bishopric NH, Pratt RE. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem. 1997;272:14860–14866. doi: 10.1074/jbc.272.23.14860. [DOI] [PubMed] [Google Scholar]
- 11.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 12.Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, Kukreja RC. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–458. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation. 2005;112:2642–2649. doi: 10.1161/CIRCULATIONAHA.105.540500. [DOI] [PubMed] [Google Scholar]
- 14.Takimoto E, Champion HC, Belardi D, Noslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 15.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, Bedja D, Gabrielson KL, Blanton R, Siderovski DP, Mendelsohn ME, Kass DA. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation. 2007;115:2159–2167. doi: 10.1161/CIRCULATIONAHA.106.643536. [DOI] [PubMed] [Google Scholar]
- 18.Castro LR, Schittl J, Fischmeister R. Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res. 2010;107:XXX–XXX. doi: 10.1161/CIRCRESAHA.110.226712. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn M. Function and dysfunction of mammalian membrane guanylyl cyclase receptors: lessons from genetic mouse models and implications for human diseases. Handb Exp Pharmacol. 2009:47–69. doi: 10.1007/978-3-540-68964-5_4. [DOI] [PubMed] [Google Scholar]
- 20.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 21.Koesling D, Mullershausen F, Lange A, Friebe A, Mergia E, Wagner C, Russwurm M. Negative feedback in NO/cGMP signalling. Biochem Soc Trans. 2005;33:1119–1122. doi: 10.1042/BST20051119. [DOI] [PubMed] [Google Scholar]
- 22.Wyatt TA, Naftilan AJ, Francis SH, Corbin JD. ANF elicits phosphorylation of the cGMP phosphodiesterase in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 1998;274:H448–H455. doi: 10.1152/ajpheart.1998.274.2.H448. [DOI] [PubMed] [Google Scholar]
- 23.Lukowski R, Rybalkin SD, Loga F, Leiss V, Beavo JA, Hofmann F. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci U S A. 2010;107:5646–5651. doi: 10.1073/pnas.1001360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandeput F, Krall J, Ockaili R, Salloum FN, florio V, Corbin JD, Francis SH, Kukreja RC, Movsesian MA. cGMP-hydrolytic activity and its inhibition by sildenafil in normal and failing human and mouse myocardium. J Pharmacol Exp Ther. 2009;330:884–891. doi: 10.1124/jpet.109.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kass DA, Takimoto E. Regulation and role of myocyte cyclic GMP-dependent protein kinase-1. Proc Natl Acad Sci U S A. 2010;107:E98. doi: 10.1073/pnas.1003889107. author reply E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann F. The biology of cyclic GMP-dependent protein kinases. J Biol Chem. 2005;280:1–4. doi: 10.1074/jbc.R400035200. [DOI] [PubMed] [Google Scholar]
- 27.Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen YF, Li JD, Blaxall BC, Abe J, Yan C. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis SH, Corbin JD. Phosphodiesterase-5 inhibition: the molecular biology of erectile function and dysfunction. Urol Clin North Am. 2005;32:419–429. vi. doi: 10.1016/j.ucl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Carson CC., 3rd Efficacy and safety of sildenafil citrate in men with erectile dysfunction and stable coronary artery disease. Curr Urol Rep. 2004;5:449–450. doi: 10.1016/j.amjcard.2003.09.030. [DOI] [PubMed] [Google Scholar]