Abstract
Siglecs have emerged as an important family of immunomodulatory glycan-binding proteins that can bind sialoside ligands both on the same cell surface, in cis, and on other cells, in trans. Expression of siglecs varies among a variety of immune cells, and tools to probe siglecs on these cells are crucial to understanding their function. In designing synthetic ligands, competition by cis ligands requires the use of multivalency to achieve sufficient avidity to stably bind siglecs on native cells. This chapter describes the use of multivalent ligands to probe cell surfaces, as well as to investigate ligand binding to recombinant siglecs.
Keywords: Siglec, multivalency, polyacrylamide, CD22, sialic acid
1. Introduction
1.1 Sialic acid-binding immunoglobulin-like lectins (Siglecs)
Many glycan-binding proteins are involved in the regulation of the immune system, through both activating and inhibitory mechanisms, as well as cell-cell adhesion, homing of immune cells and pathogen recognition. The sialic acid-binding immunoglobulin-like lectin, or Siglec, family comprises glycan-binding proteins believed to be involved in all of these functions.(1) Sharing a common sialic acid-binding function via the terminal V-set Ig domain and variable numbers of C2-set Ig-like domains, these receptors nevertheless have overlapping but distinct cell type distribution and specificity for the underlying glycan (Table 1). Many contain intracellular signaling motifs, such as the immunoreceptor tyrosine inhibitory motif (ITIM), immunoreceptor tyrosine activatory motif (ITAM) or Grb-binding domain.(1) Four of the siglecs are highly conserved amongst species, including Sialoadhesin (Siglec-1), CD22 (Siglec-2), CD33 (Siglec-3), and MAG (Siglec-4), while the remaining, known as CD33-like siglecs, are rapidly evolving, presumably due to adaptive pressures from viruses and microorganisms that have gained the ability to incorporate sialic acid.(2, 3) The expression of siglecs predominantly on immune cells and the presence of intracellular signaling motifs suggest a role in immunomodulation for siglecs, which has been validated for many, though the role of glycan binding is still poorly understood.(4)
Table 1.
Glycan-binding specificity and cellular distribution of siglecs
| Siglec (other names) | Murine ortholog or paralog | Sialoside Preferencea | Cell type Expressionb |
|---|---|---|---|
| Sialoadhesin (SAD, Sn, Siglec-1) | Sialoadhesin (mSiglec-1, mSn) | 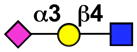 |
tissue macrophages (Activated monocytes) |
| CD22 (Siglec-2) | mCD22 (mSiglec-2) | 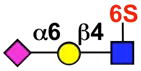 |
B cells |
| CD33 (Siglec-3) | mCD33 (mSiglec-3) | 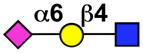 |
Monocytes, basophils, CD34+ cells, dendritic cells, macrophages, mast cells, neutrophils (granulocytes, myeloid progenitors) |
| MAG (Siglec-4) | mMAG (mSiglec-4) | 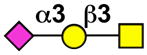 |
oligodendrocytes, Schwann cells |
| Siglec-5 | - | 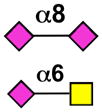 |
neutrophils, monocytes, basophils, CD34+ cells, macrophages, mast cells (B cells) |
| Siglec-6 | - | 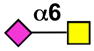 |
Basophils, mast cells, placental trophoblasts (B cells) |
| Siglec-7 | - | NK cells, dendritic cells, monocytes, CD8+ T cells (monocytes) | |
| Siglec-8 | Siglec-F | 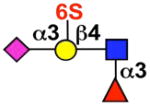 |
eosinophils, mast cells (basophils) |
| Siglec-9 | - | 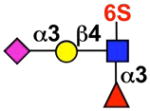 |
monocytes, neutrophils, dendritic cells, CD34+ cells, CD8+ T cells (NK cells) |
| Siglec-10 | Siglec-G | 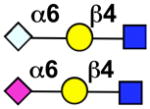 |
B cells, CD34+ cells, dendritic cells, monocytes, NK cells (eosinophils) |
| Siglec-11 | - | 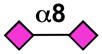 |
Monocytes, macrophages, brain microglia |
| Siglec-14 | - | 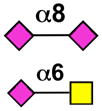 |
Not determined, but expected to be similar to Siglec-5 based on sequence homology |
| Siglec-15 | mSiglec-15 | 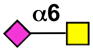 |
macrophages, monocytes, dendritic cells |
| Siglec-16 | - | 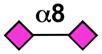 |
macrophages (brain microglia) |
| - | Siglec-E | 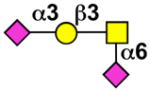 |
neutrophils, monocytes, dendritic cells |
| - | Siglec-H | plasmatoid dendritic cells (macrophages) |
Key:  NeuAc
NeuAc  NeuGc
NeuGc  Gal
Gal  GalNAc
GalNAc  GlcNAc
GlcNAc  Fuc
Fuc  Sulfate
Sulfate
Sialoside preferences are taken from recent reviews (1, 5, 6), data from the Consortium for Functional Glycomics http://www.functionalglycomics.org, or inferred from the binding preferences of highly homologous siglecs. Carbohydrate sequences shown refer to preferences of the human counterparts, with the exception of Siglec-E.
1.2 Glycan-binding specificity and cell-type expression
Synthetic sialoside ligands of siglecs have been developed to probe their function and glycan-binding specificity, and to detect siglecs on different cell types. This chapter will address the detection of siglecs on cells using ligand-based probes, which requires consideration of both cell-type expression and glycan-binding specificity. As shown in Table 1, siglecs are expressed on a variety of cells, most of which are immune cells.(1, 5, 6) Certain siglecs, such as CD22 and Siglec-8, are expressed predominantly on one cell type, B cells and eosinophils, respectively. Others can be expressed on many several cell types, such as Siglec-9 on monocytes, dendritic cells, and neutrophils. Table 1 also shows the preferred glycan(s) for each siglec that has been shown to bind sialic acid. Similar to cellular distribution, some siglecs have strict specificity, while others can bind several different glycan structures. Specificity can be considered from the perspective of the siglec and of the carbohydrate ligand, which may also have one or more cognate binding partners. CD22 is highly specific for sialosides with the alpha-2,6 linkage, but other more promiscuous siglecs can bind this sialoside as well, precluding specific targeting of this sequence to CD22. The discovery that the preferred ligand of human CD22 includes a sulfate group on the 6-position of GlcNAc may improve the ability to achieve more selective binding.(7, 8) Siglec-7 exhibits a clear preference for glycans with the NeuAcα2,8-NeuAcα2,3-Galβ1,4-GlcNAc sequence, but also bind NeuAcα2,3-Galβ1,4-GlcNAc and NeuAcα2,6-Galβ1,4-GlcNAc (O’Reilly and Paulson, unpublished results). Siglec-8, expressed on eosinophils, binds preferentially to 6′-sulfo-sialyl LewisX. As an example of specificity from the perspective of the ligand, a polyacrylamide polymer of 6′-sulfo-sialyl LewisX binds selectively to only eosinophils among leukocytes in a sample of whole blood.(9) Several labs have explored the use of sialic acid analogs to achieve enhanced binding and selectivity for one siglec over others.(10, 11) A biphenyl substitution at the 9-position of sialic acid was able to enhance the affinity of CD22 for the ligand, NeuAcα2,6-Galβ1,4-GlcNAc, by 100-fold, for example.(12) The use of glycan arrays is greatly accelerating the structure-activity relationship for siglec ligands, although more work is needed before the goal of a specific ligand for each siglec can be achieved.
1.3 Cis- and trans-ligand binding
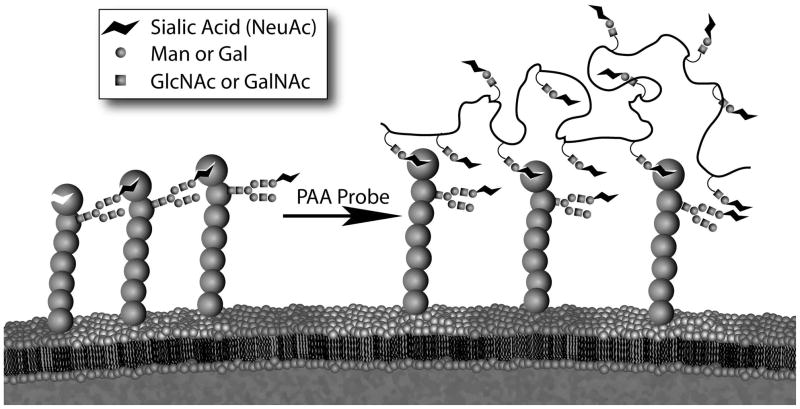
In nature, siglecs can bind glycans terminating in sialic acid both on the same cell (in cis) or on other cells, glycoproteins, viruses, etc. (in trans). The masking effect of cis ligands on siglecs (Figure 1) has been known since the demonstration that binding of a synthetic multivalent CD22 ligand to CD22 on B cells could be enabled or enhanced by removal of sialic acids from the cell surface or destruction of the sialic acid glycerol side chain, a key binding determinant.(13) While the highest affinities exhibited by siglecs for their preferred ligands is micromolar (Kd),(14) the concentration of sialic acids on the cell are estimated to be in the millimolar range (eg. ~25 mM in the glycocalyx of B-cells(15)). The endogenous ligands have not been identified for all siglecs, but CD22 has been shown to predominantly bind to the glycans of other molecules of CD22 in cis,(16) and to the B cell receptor, IgM, in trans with other B cells.(17) The ability of CD22 to bind glycans on other cells in trans was demonstrated by using fluorescence microscopy to visualize the co-localization of CD22 at the site of cell-cell contact between two B cells.(15) Importantly, this localization was dependent on the expression of α2,6 sialosides on the trans cell. Binding to glycans in trans on pathogenic organisms has been documented for several siglecs, including HIV-1 to sialoadhesin, Campylobacter jejuni to Siglec-7, Group B streptococcus to Siglec-9, and Neisseria meningitidis to Siglec-F.(3, 18–23) Presumably these interactions are influenced by the degree of cis ligand masking, making the composition of cis ligands on the cell surface a possible determinant for recognition of pathogens and the immune response. This scenario highlights the need for ligand-based methods of siglec detection. While specific antibodies can be used to probe cell types for siglec expression, only multivalent ligand-based probes can define the functional availability of siglecs. Many factors are involved in modulation of masking, including expression levels of sialyltransferases and sialidases, as well as enzymes regulating the biosynthesis of underlying glycan structures. An addition level of regulation is achieved by post-glycosylational modifications, including sulfation, acetylation, and sialic acid cyclization, which are regulated by other enzymes.(24, 25)
Figure 1. Schematic of competition between trans ligands and cis ligands of siglecs.
Using CD22 as an example, cis ligand binding leads to masking of the ligand-binding site. Only with sufficient avidity (or removal of sialic acids) can trans ligands compete with cis ligands to achieve stable binding at the cell surface.
1.4 Multivalent scaffolds for siglec ligands
Due to the low affinity of siglec-ligand interactions and competition from cis ligands, multivalency is needed to achieve the avidity required of synthetic ligands (Figure 1). Polymers have provided a convenient scaffold for siglec ligands with defined lengths and substitution densities. Ruthenium-catalyzed olefin metathesis polymerization (ROMP) has been used to prepare polymers of the CD22 ligand to study CD22 function.(26, 27) The study of siglecs and other glycan-binding proteins has drawn heavily on the use of polyacrylamides (PAA) constructed with pendant carbohydrate ligands and biotin groups.(28, 29) This chapter will focus primarily on polyacrylamide polymer-based siglec ligands with a brief section on univalent biotinylated ligands because the reagents needed for these methods are readily available, and no further synthesis is required. Other multivalent scaffolds for siglec ligands have been developed more recently, which have the benefit of being more rigid and structurally defined. Viral capsids (eg. cowpea mosaic virus and bacteriophage Qβ) have been chemoenzymatically decorated with a high-affinity CD22 ligand with remarkable control over spacing and valency.(30) These were able to bind to CD22 on native B cells. Another system proved that with the proper spacing and geometry, valency becomes less important. A heterobifunctional CD22 ligand bearing a hapten is able to drive the self-assembly of CD22-IgM complexes at the surface of native B cells.(30) The maximum valency of this complex is ten, and in fact the same ligand was able to mediate stable complex formation between CD22 and the lower valency antibodies, IgA and IgG. For purposes of in vivo drug delivery to specific cell types, liposomes decorated with CD22 ligand and loaded with doxorubicin have been shown to bind and kill native B cells, to prolong life in a murine model of disseminated B cell lymphoma, and to kill malignant B cells in samples taken from lymphoma patients.(31) Liposomes, viral capsids, and heterobifunctional ligands may also be used to probe immune cells as described herein, but given the labor-intensive preparation of these alternate platforms, it is more practical to use the available polyacrylamide or biotinylated probes. Finally, glycan arrays are commonly used to probe the binding specificity of glycan-binding proteins such as siglecs.(7, 10, 32) The analogous experiment described in this chapter would be soluble siglec-Fc binding to PAA probes immobilized on magnetic beads. The glycan array may be preferred for a broad screening due to the high-throughput nature and the relatively miniscule amounts of glycan required. However, the array has not yet been optimized for screening of siglec-expressing cells, and it is often desirable to investigate siglecs in a more native-like environment, considering such effects as lateral mobility and masking by cis ligands.
2. Materials and Methods
2.1. Reagents and cells
Siglec-expressing cells can be primary cells from human or murine origin, cell lines that natively express siglecs, or cells transfected with siglecs, most commonly CHO cells. Some commonly used B cell lines used to probe CD22 include BJAB, Daudi, and Raji, all of which are maintained in RPMI media containing 10% fetal bovine serum (FBS). The BJAB (K20) cell line is of particular interest due to a mutation in an epimerase that is required to synthesize sialic acid.(33) Growing BJAB (K20) cells in serum-free media results in asialo cells that thus lack cis ligands of CD22. To wean BJAB (K20) cells off of serum, cells are initially grown in RPMI containing 10% FBS and 50 μM 2-mercaptoethanol, and then switched to half of the previous media and half serum-free HYQ-SFM medium for two days, replacing with fresh media every day, and then replacing with 100% serum-free HYQ-SFM. Cells become semi-adherent, and 2 mM EDTA can be used to dislodge cells. At this point media is changed daily. Many of the siglecs have also been cloned and stably transfected into CHO cells as a convenient and comparable model for studying in situ siglec function.(34–36) These are grown in 1:1 DMEM:F12, 10% FBS, and, if cloned as previously described, 250 μg/mL Hygromycin B (Roche Diagnostics). As this is an adherent cell line, Trypsin/EDTA should be used to dislodge the cells for passaging, but only 2 mM EDTA should be used to harvest cells for experiments, as trypsin will degrade cell surface proteins. It should be noted that CHO cells do not express appreciable amounts of alpha2-6 sialosides. CD22 on CHO cells is therefore unmasked and removal of cis ligands is unnecessary. Siglec-Fc chimerae are also a common source of siglecs, and represent truncated fusions of the siglecs that comprise part of the extracellular portion of the siglec, including the entire N-terminal V-set carbohydrate-binding domain, and an IgG constant fragment (Fc) at the C-terminus. The Fc domain dimerizes, which provides bivalency to the siglec, while it also enables detection, complexation, and/or immobilization of the siglec by using anti-IgG antibodies. These constructs can be expressed in COS cells as previously described.(37–39) Alternatively, many siglec-Fc fusions are commercially available from R&D Systems.
Both biotinylated PAA probes and biotinylated univalent ligands are commercially available from Glycotech (Gaithersburg, MD) or can be requested by participating investigators from the Consortium for Functional Glycomics (http://www.functionalglycomics.edu). PAA probes are water-soluble and can be stored in solution for months to years at 4°C or below. Extended storage can lead to precipitation, in which case the probe can be coaxed back into solution with gentle mixing and/or warming. PAA probes obtained from the Consortium for Functional Glycomics are substituted with 20 mol% carbohydrate ligands and 5 mol% biotin. Many are available as either a low molecular weight version (30 kDa) or a high molecular weight version (1500 kDa). Glycotech probes are provided as 30-kDa polymers in which typically every 5th amide is substituted with biotin in a 4:1 ratio. In principle, PAA probes could be pre-complexed with streptavidin prior to the binding. On one hand, this may greatly increase valency if multiple polymer chains are cross-linked by the tetravalent streptavidin. On the other hand, pre-complexation may greatly restrict the degrees of freedom available to the polymer backbone. The diminished flexibility of the chain may dampen the ability to bind its cognate siglec. This effect may be particularly important if the siglec does not have lateral mobility, such as recombinant siglec immobilized on beads, or even on cells at 4°C. Another consideration of pre-complexation of the probe is that it is likely that if PAA probes can be sufficiently cross-linked with streptavidin, the complex may be too large to be internalized by clathrin-dependent endocytosis, enabling binding assays at 37 °C without the complication of internalization.
2.2 Preparation of Siglec-expressing cells
Due to the high concentration of sialosides at the cell surface, siglecs are constitutively bound by neighboring ligands residing on the same cell surface (cis ligands). One notable exception to this is sialoadhesin, which because of 17 extracellular Ig-domains, extends beyond the cellular glycocalyx and is thought to be the only unmasked siglec. Synthetic multivalent ligands have been designed that can compete with these cis ligands to achieve stable binding to siglecs on the cell surface, but for purposes of siglec detection, removal of these ligands may in some cases be desirable or necessary. Two simple methods are available to achieve this purpose, and include removal of sialic acid with neuraminidases, and destruction of the glycerol side chain of sialic acid by periodate oxidation.
A commonly used neuraminidase for the purpose of removing cis ligands is the Arthrobacter ureafaciens sialidase (AUS), which is available from Roche. Cells are suspended in Hank’s Balanced Salt Solution (Gibco) containing 5 mg/mL bovine serum albumin (Sigma) (HBSS/BSA) at a density of 0.2 – 1 × 107 cells/ml. AUS is then added to a final concentration of 200 mU/mL and the cells are incubated at 37 °C for 30 minutes. After washing twice with cold HBSS/BSA, cells are ready for probing with the exogenous ligand.
Periodate oxidation is another convenient method to destroy cis ligands. Cells are resuspended to 1 × 106/mL, and 1 mM sodium periodate (Sigma-Aldrich, cat. no. 311448) is added from a 200 mM stock solution in water. Cells are incubated at 4 °C for 10 minutes before quenching the reaction by adding equimolar glycerol. After washing the cells twice in cold HBSS/BSA, they are ready for analysis.
An important consideration for subsequent use of periodate-oxidized cells is that this treatment only addresses glycans that are located on the cell surface at the time of treatment, since periodate is cell-impermeable at 4 °C. Due to the rapid turnover of the cell surface, there is a constant replenishment of glycoproteins and glycolipids from de novo biosynthesis and recycling from intracellular compartments. Even a brief warming of the cells to 37 °C could lead to a significant re-population of cis ligands at the cell surface. While this effect may be less of a concern after sialidase treatment, which is done at 37 °C and could address rapidly recycling factors as they reach the cell surface, it is still a consideration for newly emerging glycans.
2.3 PAA probe to siglec-expressing cells
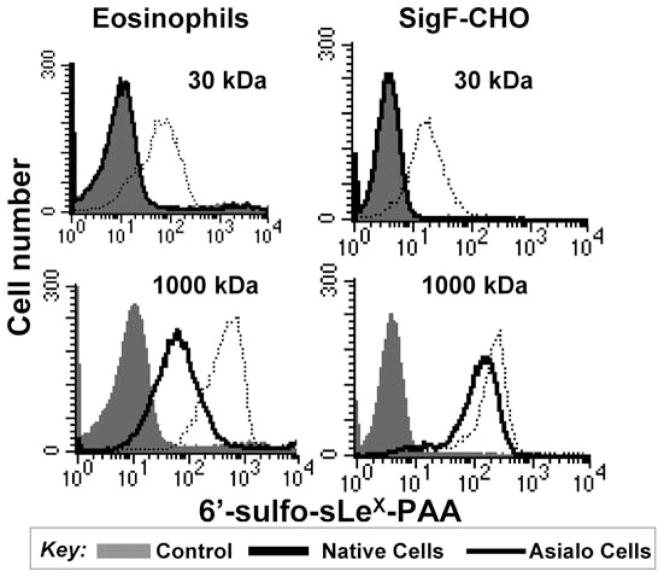
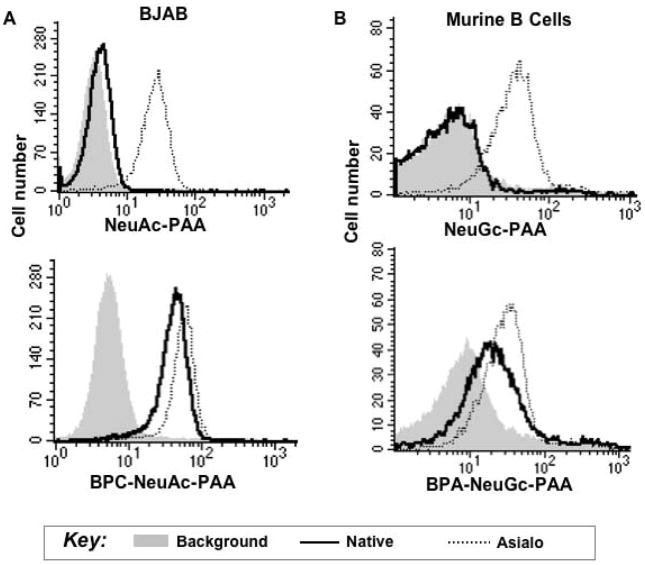
When staining siglec-expressing cells with ligand-conjugated PAA probes, there are several considerations. If the lower molecular weight polymer is being used, then cells will most likely need to be treated with sialidase or periodate, unless the cells are devoid of cis ligands, as is the case of CD22 expressed on CHO cells. For example, eosinophils and Siglec-F expressing CHO cells had to be pre-treated with sialidase to achieve binding of a 30-kDa polymer of 6′sulfo-sialyl LewisX, while the high-molecular weight polymer could bind both treated and untreated cells (Figure 2). Another consideration is the intrinsic affinity of the ligand. For example, NeuGc-LacNAc-PAA that is not substituted with the affinity-enhancing BPA group at the 9 position does not bind to CD22 on native B cells even if it is incorporated into a high molecular weight polymer (Figure 3). These data also show that the binding of the native ligands is much more sensitive to the masking effect of cis ligands compared to the high-affinity versions. While modest binding of the NeuGc probe to native murine B cells has since been observed, the BPA-substituted version is a much more robust binding partner.(40) In the latter example, a lower concentration of probe was used (1.25 μg/mL). Thus, some optimization may be advantageous for each siglec-probe combination.
Figure 2. PAA probe binding to siglec-expressing cells reveals importance of valency.
6′-sulfo-sialyl-Lewisx-PAA of low molecular weight (30 kDa, approximately 15-mer) or high molecular weight (1000 kDa, approximately 500-mer) was incubated with eosinophils, which express Siglec-F, or CHO cells transfected with Siglec-F. Asialo cells were prepared by pre-treatment of native cells with sialidase. Reproduced with permission.
Figure 3. High-affinity PAA probe binding to siglec-expressing cells shows the importance of intrinsic affinity in overcoming cis ligands.
A human BJAB B cell line (A) or murine B cells (B) were probed with native (NeuAc(Gc)α2,6-LacNAc-) or high-affinity BPC(BPA)NeuAc(Gc)α2,6-LacNAc- ligands appended to PAA. Reproduced with permission.
Check that no precipitation of the polymer has occurred, and if it has, pipet the solution up and down to re-dissolve the polymer.
Wash and resuspend cells at a density of 2 × 106/mL in 100 μL of HBSS/BSA
Add 0.1–1 μg of polymer for a final probe concentration of 1–10 μg/mL
Incubate at 4 °C for 1 hour with end-over-end rotation or occasional mixing
Pellet cells by centrifugation in an Eppendorf centrifuge (5415D or similar model) at 2,000 rpm for 5 min at 4°C.
Wash cells twice with 1 mL/ea ice-cold HBSS/BSA, pelleting after each wash as in step 5.
Resuspend in 100 μL of HBSS/BSA.
Add 1 μL of DTAF-Streptavidin (Jackson Laboratories, catalog # 016-010-084) and incubate at 4 °C for 30 minutes.
Wash away unbound streptavidin with HBSS/BSA as above 9. Resuspend cells in 200 μL HBSS/BSA
Analyze by flow cytometry (10,000 cells/sample).
It is important that these experiments be carried out at 4 °C to prevent endocytosis, which would cause internalization of the probe and thus an underestimation of binding. Alternatively, if performing the experiment at 37 °C is desired, maintaining the cells in hypertonic media will prevent endocytosis by preventing the formation of clathrin-coated pits.(41) We should note, however, that this method only affects siglec that undergo clathrin-dependent internalization. Siglec-F for example, are endocytosed by a clathrin-indepent mechanism (36), and the mechanism for many others is not yet known. Cells are first incubated for 45 minutes at 37 °C in media or buffer containing 0.45 M sucrose, then washed twice with the same sucrose-containing media at 4 °C. Since this inhibitory effect is reversible, cells must be kept in this high concentration of sucrose throughout the experiment. We have shown that this environment does not affect CD22 binding to synthetic multivalent ligands (unpublished observations, O’Reilly and Paulson).
2.4 PAA probes to Siglec-Fc beads
While there are certain advantages to testing the binding of glycans to siglecs in their native environment, it can also be useful to examine specificity by using recombinant siglec-Fc fusion proteins, which enables a quantitative measure without the complexity of the cellular environment. This section will discuss immobilized siglec-Fc, while section 2.5 will describe their use in the soluble form. Commercially available magnetic beads conjugated to Protein A (Dynabeads® Protein A for Immunoprecipitation, Cat. No. 100-01, Invitrogen) are used for immobilization through the Fc portion. These beads have a capacity of approximately 8 μg human IgG per milligram of beads, and are supplied as 30 mg/mL. Either purified siglec-Fc or culture supernatant from siglec-Fc expressing COS cells is applied to Protein A beads for immobilization as previously described.(30) One advantage of this method is that a siglec-Fc purification step is built in. In fact, it may be preferable to use culture media from siglec-Fc expressing cells to load the beads if these are available because the conditions used to purify the fusion protein can lead to loss of binding activity. This direct method negates the harsh elution step. These beads can then be probed with the carbohydrate-conjugated PAA probes as described for siglec-expressing cells in section 2.3, except that instead of using centrifugation for the washing steps, beads are isolated using a magnetic tube rack (MagneSphere® Technology Magnetic Separation Stand (twelve-position), catalog # Z5342, Promega).
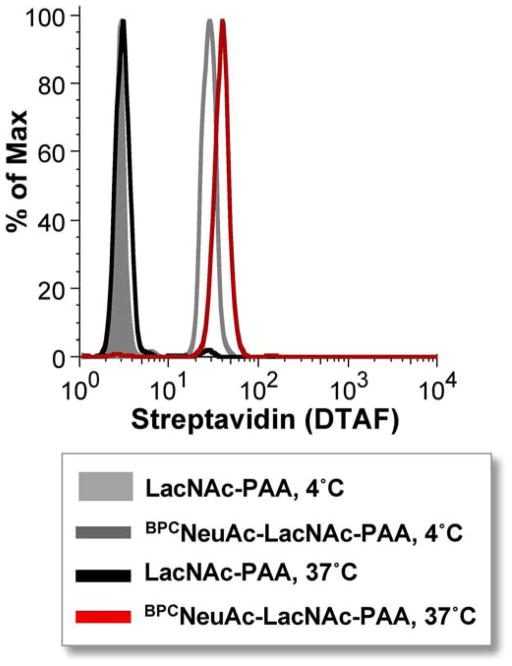
Using beads as a model for siglec-expressing cells has the benefit of removing complications due to both cis ligands and siglec endocytosis at elevated temperatures. Therefore, binding can be examined at a variety of temperatures. This was done using hCD22-coated beads and BPCNeuAc-LacNAc-PAA, revealing that at least for these interacting partners, binding may be improved at elevated temperatures (Figure 4). Using beads therefore may be a way to achieve binding where binding to cells at 4 °C is not observed. Because PAA probes are not very structurally well defined, increased temperatures may help the polymer to overcome any secondary structure and increase the opportunity for favorable binding. Another advantage of using beads is that very long incubations are possible to ensure that binding has reached equilibrium. Long incubations may compromise the integrity of cells or cause other unknown changes. Disadvantages include lack of lateral mobility of siglecs.
Figure 4. Siglec-Fc beads enable binding at elevated temperature without endocytosis to reveal improved binding.
Human CD22-Fc beads were probed with high molecular weight LacNAc-PAA or BPCNeuAcα2,6-LacNAc-PAA at 4 °C or 37 °C for 16 hours.
When analyzing the binding of probes to beads by flow cytometry, we have noticed that the predominant population by dot plot (forward scatter vs. side scatter) is accompanied by a series of less abundant populations with increasing forward and side scatter. The abundance of each population decreases with increasing scattering properties, and the degree to which this effect occurs seems to correlate with the amount of ligand bound. It is possible that the multivalent ligands can cross-link beads to change their light scattering properties, though this effect has not been further investigated.
2.5 Siglec-Fc to PAA probe beads
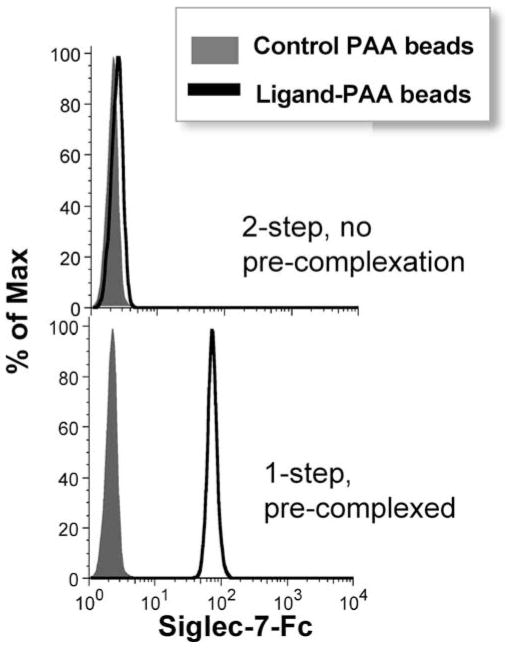
As opposed to immobilizing the siglec, the reverse experiment can be carried out by immobilizing the PAA probe. With this method, it is important to realize that despite the bivalency of the siglec-Fc, we do not observe any binding unless the siglec-Fc is pre-complexed with the secondary antibody, FITC-anti-human IgG. Figure 5 gives the example of Siglec-7 binding to beads coated with NeuAcα2,8-NeuAcα2,3-LacNAc-PAA, but this principle applies to each siglec-Fc that we have tested. Pre-complexation leads to greater valency due to crosslinking of at least two, but possibly many more, siglec chimera dimers, depending on the isotype of the secondary antibody. In practice, pre-complexation is not performed as a separate step, but rather by adding the secondary antibody and siglec-Fc simultaneously to PAA beads.
Figure 5. Pre-complexation of Siglec-Fc is key to engaging with ligand-PAA-coated beads.
Siglec-7-Fc from culture supernatant was incubated with streptavidin beads coated with unsubstituted PAA or NeuAcα2,8-NeuAcα2,3-Galβ1,4-GlcNAc-PAA, either in the absense (top, 2-step) or presence (botton, 1-step) of the detection antibody, FITC-anti-human IgG. For the 2-step method (top), unbound probe was washed away prior to adding the detection antibody.
Remove a 5-μL aliquot (~3–3.5 × 106 beads) of streptavidin-coated Dynal microbeads (Dynabeads® M-280 Streptavidin Cat. No. 112-05D, Invitrogen) and wash with HBSS/BSA
Add 6 μg of PAA probe in 0.5 mL HBSS/BSA and incubate at 25 °C for 1 hour
Wash beads twice using magnetic stand with 1 mL/ea HBSS/BSA and resuspend to 50 μL
Combine 2 μL of beads with 50 μL of culture supernatant from siglec-Fc expressing cells and 1 μL of FITC-anti-human IgG (Jackson Immunoresearch, cat. no. 109-095-098)
Incubate at 4 °C with end-over-end rotation for 30 minutes.
Isolate beads using a magnetic stand to isolate beads and wash twice with 1 mL/ea HBSS/BSA
Resuspend beads with 200 μL HBSS/BSA and analyze bead fluorescence by flow cytometry (10,000 beads/sample).
2.6 Siglec-Fc to biotinyated free saccharide-coated beads
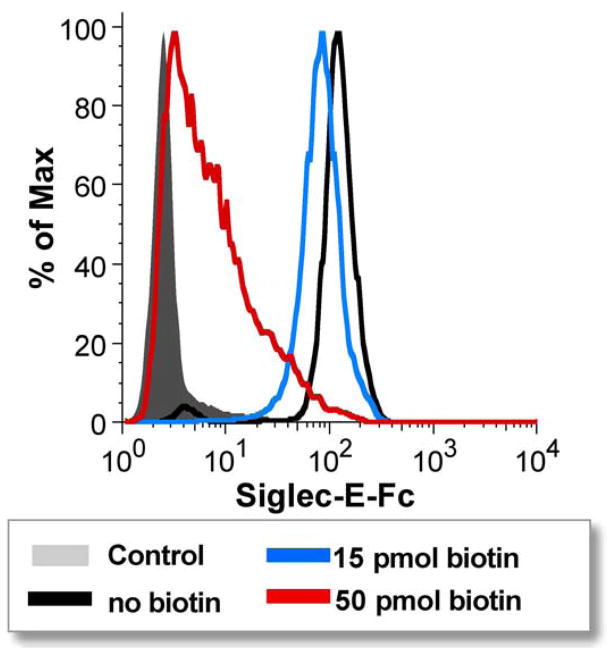
Similar to immobilizing the PAA probe to streptavidin beads, free ligands conjugated to biotin can also be used to load streptavidin beads, and these reagents are available both commercially and from the Consortium for Functional Glycomics. Like the PAA-loaded beads, this platform provides high valency. It differs from PAA-loaded beads in certain ways that may be desirable for some applications. For instance, titrating in free biotin can govern the loading of the bead, and thus the avidity (Figure 6). PAA probes, on the other hand, are not as easy to control in terms of valency. Because of the effectively irreversible binding of biotin to streptavidin, the polymers may become trapped in conformations with unknown numbers of ligands being accessible for siglec binding. For competition assays with free inhibitor, lower avidity may be desired, whereas higher avidity may be needed in other circumstances to achieve measurable binding. Also, biotinylated free ligands are easier to access synthetically, so candidate ligands could be tested without having to synthesize the entire polymer. The procedure used to analyze siglec binding in the experiment shown in Figure 6 is as follows, and can be modified to use different ligands, siglecs, and loading densities.
Figure 6. Tuning the density of biotinylated ligand-coated beads to modulate avidity of siglec-Fc binding.
Streptavidin beads were coated with biotinylated NeuAcα2,8-NeuAcα2,3-Galβ1,4-GlcNAc after pre-treatment of beads with varying concentrations of free biotin to adjust the density of carbohydrate ligands. Beads were then probed with siglec-E-Fc from culture supernatant in the presence of the detection antibody, FITC-anti-human IgG. Control beads had neither biotin nor ligand.
Retrieve a 20-μL aliquot of Dynamax beads (Invitrogen)
Wash twice with 1 mL of PBS, using the magnetic eppendorf rack to collect the beads
Resuspend in 0.4 mL of PBS and remove four 100-μL aliquots
Add 0, 5, 15, or 50 pmol, respectively, of 5-(biotinamido)pentylamine from a 50 μM stock in PBS
Incubate for 1 hour at 25 °C with end-over-end rotation
Wash each sample twice with 1 mL HBSS/BSA
Resuspend each in 50 μL HBSS/BSA
Add 1 μL of biotinylated ligand (from 55 μM stock) and incubated for 1 hour at 25 °C with end-over-end rotation.
Wash each sample twice with 1 mL HBSS/BSA
Resuspend each in 50 μL HBSS/BSA
Add 5-μL aliquots of beads to 200-μL aliquots of culture supernatant from siglec-Fc-expressing cells
Immediately add 4 μL of FITC-anti-human-IgG and incubate at 4 °C for 30 minutes with end-over-end rotation.
Wash each sample twice with HBSS/BSA
Resuspend beads in 200-μL aliquots of HBSS/BSA and analyze by flow cytometry (10,000 beads/sample).
2.7 CHO-Siglec cells to PAA probe beads
Higher ligand valency may be achieved by coating synthetic beads with the PAA probe of interest. Combining these beads with cells may prove a better model for cell-cell adhesion than soluble probes. This method has the advantage of providing very high valency for both ligand and siglec. Microscopy can then be used to visualize the cell-bead interactions. This method may also be of interest for experiments done at elevated temperatures. At 37 °C, most siglecs will undergo clathrin-dependent or independent endocytosis, transporting the ligand inside the cell. We have observed that with a sufficiently large ligand scaffold, such as a highly cross-linked anti-NP IgM, internalization is precluded (O’Reilly and Paulson, unpublished observations. The procedure for PAA bead-cell adhesion has been published previously, and was used to demonstrate bead-bound BPANeuGc-PAA binding to murine B cells (Figure 7).(42) These results are consistent with the free probe-binding results shown in Figure 3. Therefore, as an alternative, the PAA probe can be bound to the cells first, followed by washing and then exposure to the streptavidin-coated magnetic beads. This procedure has also been described.(42)
Figure 7. High-affinity ligand is required for adhesion between-PAA-coated beads and CD22-expressing cells.
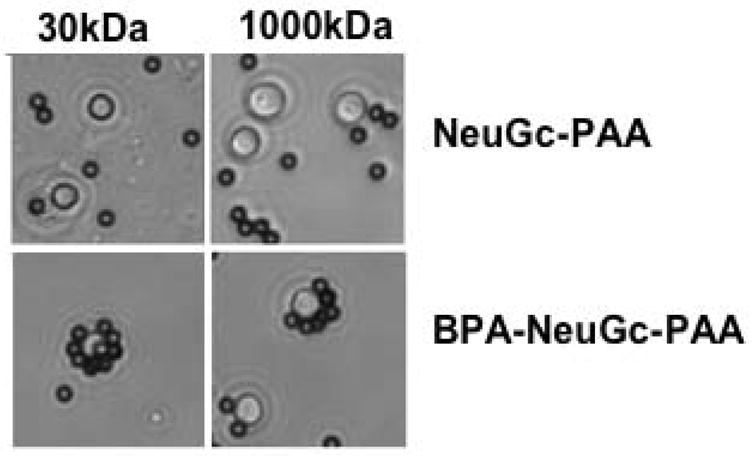
Streptavidin beads coated with 30 kDa or 1000 kDa versions of NeuGca2,6-LacNAc-PAA or BPANeuGca2,6-LacNAc-PAA were exposed to primary murine B cells. Reproduced with permission.
3. Conclusions and future directions
Discovery of ligand analogs with enhanced affinity and selectivity will improve the precision with which siglecs can be studied. Substituents at various positions, most commonly the 5 and 9 position of sialic acid, have been shown to enhance or diminish siglec affinity, thus setting the stage to develop ligands with a high degree of selectivity for specific siglecs.(10, 11) These tools will enable improved detection and targeting of siglecs in complex biological systems. Depending on the application, different permutations of carbohydrate probe and siglec immobilization will serve different needs for the detection and analysis of siglecs in their native environment. Siglecs are already considered targets for immunotherapy of cancers, autoimmunity and other inflammatory disorders.(5) With the continued development of selective probes will come improved methods for assessing changes in the availability of siglec binding sites on different cells and under different conditions (ie. transformed cells, microenvironments of inflammation, etc.). This can in turn lead to enhanced targeting strategies using glycan-based drug delivery vehicles. These probes will also be useful for investigating the innate functions of siglecs and the contribution of glycan binding.
Acknowledgments
The authors wish to thank Anna Tran-Crie for her assistance in preparation of the manuscript, and Cory Rillahan, Dr. Hua Tian, and Dr. Christoph Rademacher for careful reading of the manuscript and helpful suggestions. This work was supported by NIH R01AI050143 & R01GM60938 to J.C.P., and an American Cancer Society postdoctoral fellowship to M.K.O.
Abbreviations
- NeuAc
N-acetyl neuraminic acid
- BPC
biphenylcarbonyl
- BPA
biphenylacetyl
- Gal
galactose
- GlcNAc
N-acetylglucosamine
- HBSS
Hank’s Balanced Salt Solution
- BSA
bovine serum albumin
- FBS
fetal bovine serum
- CHO
Chinese hamster ovary
- PAA
Polyacrylamide
- FITC
fluorescein isothiocyanate
- Ig
immunoglobulin
- PBS
phosphate-buffered saline
- DTAF
Dichlorotriazinylaminofluorescein
References
- 1.Crocker PR, Paulson JC, Varki A. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 2.Angata T. Mol Divers. 2006;10:555–66. doi: 10.1007/s11030-006-9029-1. [DOI] [PubMed] [Google Scholar]
- 3.Severi E, Hood DW, Thomas GH. Microbiology. 2007;153:2817–22. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 4.Crocker PR, Redelinghuys P. Biochem Soc Trans. 2008;36:1467–71. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- 5.O’Reilly MK, Paulson JC. Trends Pharmacol Sci. 2009;30:240–8. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Gunten S, Bochner BS. Ann N Y Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Proc Natl Acad Sci U S A. 2004;101:17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura N, Ohmori K, Miyazaki K, Izawa M, Matsuzaki Y, Yasuda Y, Takematsu H, Kozutsumi Y, Moriyama A, Kannagi R. J Biol Chem. 2007;282:32200–7. doi: 10.1074/jbc.M702341200. [DOI] [PubMed] [Google Scholar]
- 9.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. J Pharmacol Exp Ther. 2009;330:608–12. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blixt O, Han S, Liao L, Zeng Y, Hoffmann J, Futakawa S, Paulson JC. J Am Chem Soc. 2008;130:6680–1. doi: 10.1021/ja801052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chokhawala HA, Huang S, Lau K, Yu H, Cheng J, Thon V, Hurtado-Ziola N, Guerrero JA, Varki A, Chen X. ACS Chem Biol. 2008 doi: 10.1021/cb800127n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelm S, Gerlach J, Brossmer R, Danzer CP, Nitschke L. J Exp Med. 2002;195:1207–13. doi: 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razi N, Varki A. Proc Natl Acad Sci U S A. 1998;95:7469–74. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakker TR, Piperi C, Davies EA, Merwe PA. Eur J Immunol. 2002;32:1924–32. doi: 10.1002/1521-4141(200207)32:7<1924::AID-IMMU1924>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Proc Natl Acad Sci U S A. 2004;101:6104–9. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S, Collins BE, Bengtson P, Paulson JC. Nat Chem Biol. 2005;1:93–7. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 17.Ramya TN, Weerapana E, Liao L, Zeng Y, Tateno H, Yates JR, 3rd, Cravatt BF, Paulson JC. Mol Cell Proteomics. 2010 doi: 10.1074/mcp.M900461-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avril T, Wagner ER, Willison HJ, Crocker PR. Infect Immun. 2006;74:4133–41. doi: 10.1128/IAI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlin AF, Chang YC, Areschoug T, Lindahl G, Hurtado-Ziola N, King CC, Varki A, Nizet V. J Exp Med. 2009;206:1691–9. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Blood. 2009;113:3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones C, Virji M, Crocker PR. Mol Microbiol. 2003;49:1213–25. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 22.Rempel H, Calosing C, Sun B, Pulliam L. PLoS ONE. 2008;3:e1967. doi: 10.1371/journal.pone.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Kuyl AC, van den Burg R, Zorgdrager F, Groot F, Berkhout B, Cornelissen M. PLoS ONE. 2007;2:e257. doi: 10.1371/journal.pone.0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H, Chen X. Org Biomol Chem. 2007;5:865–72. doi: 10.1039/b700034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cariappa A, Takematsu H, Liu H, Diaz S, Haider K, Boboila C, Kalloo G, Connole M, Shi HN, Varki N, Varki A, Pillai S. J Exp Med. 2009;206:125–38. doi: 10.1084/jem.20081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Proc Natl Acad Sci U S A. 2009;106:2500–5. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang ZQ, Puffer EB, Pontrello JK, Kiessling LL. Carbohydr Res. 2002;337:1605–13. doi: 10.1016/s0008-6215(02)00270-7. [DOI] [PubMed] [Google Scholar]
- 28.Chinarev AA, Galanina OE, Bovin NV. Methods Mol Biol. 2010;600:67–78. doi: 10.1007/978-1-60761-454-8_5. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport EM, Pazynina GV, Sablina MA, Crocker PR, Bovin NV. Biochemistry (Mosc) 2006;71:496–504. doi: 10.1134/s0006297906050051. [DOI] [PubMed] [Google Scholar]
- 30.Kaltgrad E, O’Reilly MK, Liao L, Han S, Paulson JC, Finn MG. J Am Chem Soc. 2008;130:4578–9. doi: 10.1021/ja077801n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. Blood. 2010 doi: 10.1182/blood-2009-12-257386. Epub ahead of print, PMID: 20181615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. J Biol Chem. 2005;280:4307–12. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 33.Hinderlich S, Berger M, Keppler OT, Pawlita M, Reutter W. Biol Chem. 2001;382:291–7. doi: 10.1515/BC.2001.036. [DOI] [PubMed] [Google Scholar]
- 34.Khatua B, Ghoshal A, Bhattacharya K, Mandal C, Saha B, Crocker PR. FEBS Lett. 2010;584:555–61. doi: 10.1016/j.febslet.2009.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munday J, Kerr S, Ni J, Cornish AL, Zhang JQ, Nicoll G, Floyd H, Mattei MG, Moore P, Liu D, Crocker PR. Biochem J. 2001;355:489–97. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC. Mol Cell Biol. 2007;27:5699–710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nath D, van der Merwe PA, Kelm S, Bradfield P, Crocker PR. J Biol Chem. 1995;270:26184–91. doi: 10.1074/jbc.270.44.26184. [DOI] [PubMed] [Google Scholar]
- 38.van der Merwe PA, Crocker PR, Vinson M, Barclay AN, Schauer R, Kelm S. J Biol Chem. 1996;271:9273–80. [PubMed] [Google Scholar]
- 39.Vinson M, van der Merwe PA, Kelm S, May A, Jones EY, Crocker PR. J Biol Chem. 1996;271:9267–72. doi: 10.1074/jbc.271.16.9267. [DOI] [PubMed] [Google Scholar]
- 40.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson J, Nemazee D. J Exp Med. 2010;207:173–87. S1–4. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heuser JE, Anderson RG. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins BE, Blixt O, Han S, Duong B, Li H, Nathan JK, Bovin N, Paulson JC. J Immunol. 2006;177:2994–3003. doi: 10.4049/jimmunol.177.5.2994. [DOI] [PubMed] [Google Scholar]