Abstract
BACKGROUND AND PURPOSE
Previously, 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine (KMUP-3) has been shown to induce aortic smooth muscle relaxation through KATP channel opening and endothelial nitric oxide synthase (eNOS) enhancement. We further investigated whether KMUP-3 protects against myocardial remodelling after myocardial infarction (MI), and whether KMUP-3 increases the expression of eNOS in MI rats.
EXPERIMENTAL APPROACH
Wistar rats were randomly allocated into three groups: MI (n= 10), MI + KMUP-3 group (n= 10) and sham group (n= 10). MI was induced by ligation of the left anterior descending coronary artery. After recovery, the MI + KMUP-3 group received KMUP-3 (0.3 mg·kg−1·day−1) infusion for 4 weeks, while the MI and sham group received vehicle only. To further confirm that the effect of KMUP-3 is dependent on eNOS, KMUP-3 was applied in the culture of transforming growth factor-β-stimulated human cardiac fibroblasts.
KEY RESULTS
KMUP-3 treatment attenuated cardiac hypertrophy post-MI and improved cardiac function. The fibrotic area was reduced by KMUP-3 both in central-, peri- and non-infarction areas. KMUP-3 enhanced the expression of eNOS and tissue inhibitor of metalloproteinase-1 (TIMP-1), but reduced matrix metalloproteinase-9 (MMP-9) expression. In vitro, the activities of KMUP-3 were blocked by pretreatment with the eNOS inhibitor Nω-nitro-L-arginine methyl ester.
CONCLUSIONS AND IMPLICATIONS
The KATP channel opener KMUP-3 preserved cardiac function after MI by enhancing the expression of eNOS. In addition, KMUP-3 restored the myocardial MMP-9/TIMP-1 balance and attenuated ventricular remodelling by an eNOS-dependent mechanism.
Keywords: KMUP-3, endothelial nitric oxide synthase, KATP channel, matrix metalloproteinase-9, myocardial infarction, heart failure
Introduction
Left ventricular (LV) remodelling and congestive heart failure are still a major cause of death in survivors of acute myocardial infarction (MI) (Hellermann et al., 2002). Growing evidence indicates that the inhibition of cardiomyocyte apoptosis and reduction of pro-inflammatory cytokines contribute to improved cardiac function in post-infarction heart failure (Sia et al., 2002; Li et al., 2009). ATP-dependent potassium (KATP) channels are expressed in cardiomyocytes at high levels and exert cardioprotective effects (Noma, 1983). In acute situations, for example, the opening of KATP channels results in cardioprotection against ischaemia/reperfusion injury with decreased cell death and reduced infarction size (Lee et al., 2003). Recently, long-term administration of KATP channel activators has also been shown to attenuate the ventricular remodelling after MI (Lee et al., 2008). A xanthine-based vasorelaxant synthesized in our laboratory, 7-[2-[4-(4-Nitrobenzene)-piperazinyl]ethyl]-1,3-dimethylxanthine (KMUP-3), has been demonstrated to elevate cyclic nucleotide levels, inhibit phosphodiesterase and affect KATP channel opening (Wu et al., 2005; 2006;). In previous studies, KMUP-3 induced aortic smooth muscle relaxation through a KATP channel-dependent pathway. The vasorelaxant effect of KMUP-3 was attenuated by the KATP channel blocker glibenclamide and elevated extracellular K+ levels (80 mM) (Wu et al., 2005; Lin et al., 2006).
KMUP-1, an analogue of KMUP-3, has been shown to have a cardioprotective effect in hypertensive rats, reducing cardiac hypertrophy and fibrosis (Wu et al., 2001; Yeh et al., 2010). KMUP-3, a more potent phosphodiesterase inhibitor than KMUP-1, also has the ability to enhance endothelial nitric oxide synthase (eNOS), as demonstrated in human umbilical vein endothelial cells (HUVECs) (Wu et al., 2005; Lin et al., 2006). By releasing the chemical mediator nitric oxide (NO), eNOS is involved in a variety of inflammatory responses. Recently, transgenic mice with eNOS overexpression have been reported to have less lung injury, induced via the inhibition of matrix metalloproteinase-9 (MMP-9), in a bleomycin-induced lung fibrosis model (Yoshimura et al., 2006). This implies that eNOS may not only be involved in acute inflammation but also in chronic fibrosis. However, the relationship between eNOS and MMP-9 in chronic heart failure has not been established.
MMP-9 is a zinc-dependent endopeptidase involved in the breakdown of extracellular matrix in both normal physiological processes and tissue remodelling in pathological conditions (Spinale et al., 2000a). In the Framingham Heart Study, elevated plasma MMP-9 levels were associated with increased left ventricular end-diastolic dimension (LVEDD) and wall thickness, indicating the potential role of MMP-9 in LV remodelling (Sundström et al., 2004; Lin et al., 2007). Enhanced MMP-9 activity has been shown both in the acute phase of MI and chronic heart failure (Spinale et al., 2000b; Takai et al., 2007). In previous studies, targeted deletion of MMP-9 or use of MMP-9 inhibitors prevented the LV dysfunction after MI (Romanic et al., 2002). For these reasons, MMP-9 is thought to play a major role in the ventricular remodelling process. The MMP-9 inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1), has also been shown to be a regulator of myocardial healing in MI (Halapas et al., 2008), and a reduction of TIMP-1 exacerbates LV remodelling in MI mice (Creemers et al., 2003).
In this study we aimed to investigate whether KMUP-3 protects against myocardial remodelling after MI, and if so, whether KMUP-3 increases the expression of eNOS and affects the balance of MMP-9/TIMP-1.
Methods
Animal preparation
Male Wistar rats (250–300 g) were provided by the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan) and housed under constant temperature and controlled illumination. Food and water were available ad libitum. MI was induced by ligation of the left anterior descending (LAD) coronary artery. Briefly, under general anaesthesia with pentobarbital sodium (30 mg·kg−1, i.p.), the heart was exposed via a small left thoracotomy (Liang et al., 2006). The LAD was ligated with 6.0 silk at 2 mm from the origin, and the wound was closed with primary suture. After the operation, the animals were observed in a supervised setting until fully conscious. Sham animals underwent similar thoracotomy and pericardiotomy, except that the ligature around the coronary artery was not tied. All the animals were administered s.c. doses of analgesia (ketoprofen; 3 mg·kg−1) and antibiotics (gentamicin; 0.7 mg·kg−1) for 2 days. This study was approved by the Animal Care and Use Committee at Kaohsiung Medical University.
Experimental design, grouping and implantation
Surviving rats were fitted with ALZET osmotic minipumps (Model 2ML4, DURECT Corporation, Cupertino, CA, USA) immediately after recovery. At this time, most of the rats were still anaesthetized from the previously administered pentobarbital sodium; a further bolus dose of pentobarbital sodium (15 mg·kg−1) was given if necessary. These Alzet osmotic minipumps had a 2 mL capacity and a mean pumping rate of 2.5 µL·h−1 for 4 weeks. In the treatment group, the minimumps were filled with hydrochloride of KMUP-3 (KMUP-3·HCl; 0.3 mg·kg−1·day−1) synthesized in this laboratory (Lin et al., 2006). In the sham and MI groups, the minipumps were filled with saline.
Haemodynamic measurements and echocardiography
Echocardiography was performed in all animals at the time points before and 4 weeks post-surgery. Each rat was anaesthetized with pentobarbital sodium (30 mg·kg−1, i.p.). The anterior chest wall was shaved and acoustic coupling gel was applied. We used an echocardiography system (Hewlett-Packard Sonos 1500, 5-MHz probe, Andover, MA, USA) through M-mode longitudinal and transverse parasternal views to measure LV end-systolic dimension (LVESD) and LVEDD. LV fractional shortening (FS) was analysed from the LV dimensions using the following formula (Louhelainen et al., 2007):
After echocardiography, a PE-50 catheter was inserted through the right carotid artery and passed into the LV to measure the LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP) and maximum rates of pressure development (LV +dP/dt) and relaxation (LV –dP/dt). At the end of the recording, the hearts were perfused through the aorta with NaCl 0.9% buffer to wash out plasma components. The inferior vena cava and pulmonary veins were opened to avoid fluid overload. The hearts were then excised and the atria and right ventricles were dissected out. Sections of the LV were embedded in mounting medium and the remaining tissues were transferred to a liquid nitrogen tank for further evaluation. Throughout the whole procedure, a further bolus dose of pentobarbital sodium (15 mg·kg−1) was given if required.
Determination of infarct size and histology
All hearts were sectioned across the ventricles for histological analyses. Some rats were randomly chosen to be analysed for area of risk after coronary artery ligature by Evan blue staining as described by Harada et al. (2005). Infarct size was determined by 2,3-,5-triphenyltetrazolium chloride (TTC) staining, and was expressed as ratio of the surface area of the infarct wall to the entire surface area of the LV (Vandegriff et al., 2008). The heart section was also stained with Masson's trichrome to assess fibrosis as previously described (Lin et al., 2009; Yeh et al., 2010). The infarct area was visible as white and non-infarct as red after being stained with TTC. Peri-infarct area was defined as the region of myocardium extending 1 mm from the infarct scar. Ten sections in each heart were analysed for the percentage of fibrosis. The mean fibrotic change after MI was then compared between study groups and the Masson's trichrome staining of the sham rats' hearts as control. The total LV and infarct area and fibrosis region were measured by an investigator without knowledge of the treatment group using NIH ImageJ, 1.42q (US National Institutes of Health, Bethesda, MD, USA).
Culture of human cardiac fibroblasts
Human cardiac fibroblasts (HCFs; catalogue number: 306–05f) were purchased at passage 2 from Cell Applications Inc. (San Diego, CA, USA). The cells were cultured as monolayers in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum at 37°C in 95% humidity and 5% CO2. Cells were harvested at passage 3–6 for experiment. Before treatment, HCFs were washed twice with serum-free medium and switched to serum-free medium for 24 h. To stimulate MMP-9 expression, the cultures were treated with 10 ng·mL−1 transforming growth factor-β (TGF-β) for 24 h.
Western blot analysis
The hearts were sonicated in 50 mmol·L−1 Tris for 10 s three times and centrifuged at 13 000 rpm at 4°C for 30 min. The protein concentration of the supernatants was determined by using bovine serum albumin as the standard. The cell extracts were then boiled in ratio of 4:1 with sample buffer (100 mmol·L−1 Tris, pH 6.8, 20% glycerol, 4% SDS, and 0.2% bromophenol blue). Electrophoresis was performed using 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Millipore Corporation, Billerica, MA, USA). The membranes were blocked with Tris-buffered saline (20 mmol·L−1 Tris and 137 mmol·L−1 NaCl, pH 7.6) containing 0.1% Tween-20 (TTBS) and 5% non-fat milk at room temperature for 1 h, washed with TTBS, and then incubated overnight at 4°C with one of the three primary antibodies of MMP-9, TIMP-1 (Millipore, Temecula, CA, USA), or eNOS (BD Transduction Laboratories, Franklin Lakes, NJ, USA). The membranes were washed in TTBS before being incubated with horseradish peroxidase-conjugated antibody against mouse or rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h. The membranes were then washed in TTBS and developed with the enhanced chemiluminescence for the detection of the specific antigen. The intensity of the bands was quantified by densitometry.
Statistical analysis
Data are expressed as mean ± SE for continuous variables. The values were compared by use of Student's t-test or one-way analysis of variance followed by Bonferroni post hoc test where significant differences were found. Analyses were carried out using SPSS for Windows 11.0 (SPSS Inc., Chicago, IL, USA) and P < 0.05 was defined as statistically significant.
Results
Cardiac structure and haemodynamics
Early surgery-related death assigned to severe thoracic bleeding within 6 h after surgery was similar in all groups (9%) and these rats were therefore excluded from final analysis. None of the animals died during the post-MI treatment period. As indicated in Table 1, MI induced significant cardiac hypertrophy with an increased heart weight to body weight ratio. Treatment with KMUP-3 prevented the cardiac remodelling process. The LV +dP/dt decreased in the MI group, while administration of KMUP-3 (0.3 mg·kg−1·day−1) for 4 weeks improved cardiac systolic function. The LVSP and LV –dP/dt also tended to be decreased in the MI group but none of these differences reached statistical significance. A small increase in heart rate occurred after KMUP-3 treatment, but this was not statistically significant.
Table 1.
Effects of KMUP-3 on cardiac structure and haemodynamics
| Sham | MI | MI + KMUP-3 | |
|---|---|---|---|
| n | 10 | 10 | 10 |
| HW (mg) | 1228 ± 72 | 1300 ± 32 | 1219 ± 39 |
| BW (g) | 446 ± 30 | 441 ± 10 | 448 ± 10 |
| HW/BW (mg/g) | 2.76 ± 0.05 | 2.96 ± 0.06* | 2.72 ± 0.04# |
| LVSP (mmHg) | 132.1 ± 11.6 | 117.6 ± 7.4 | 140.6 ± 4.2 |
| LVEDP (mmHg) | 9.3 ± 0.7 | 12.1 ± 1.7 | 12.6 ± 2.3 |
| LV +dP/dt (mm Hg·s−1) | 7348 ± 986 | 4565 ± 394* | 7637 ± 333## |
| LV −dP/dt (mm Hg·s−1) | −5633 ± 911 | −3603 ± 353 | −5379 ± 441 |
| HR (bpm) | 370 ± 21 | 380 ± 22 | 411 ± 5 |
BW, body weight; + dP/dt and − dP/dt, maximum rate of rise and full of pressure; HR, heart rate; HW, heart weight; KMUP-3, 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; MI, myocardial infarction.
P < 0.05 versus sham.
P < 0.05.
P < 0.01 versus MI rats.
LV remodelling
Figure 1 shows the increased LVEDD and LVESD of MI rats, compared with the sham-operated group. Decreased systolic function was also demonstrated by the decrease in FS in the MI group. All this cardiac remodelling and dysfunction were prevented by the administration of KMUP-3 for 4 weeks (Figure 1).
Figure 1.
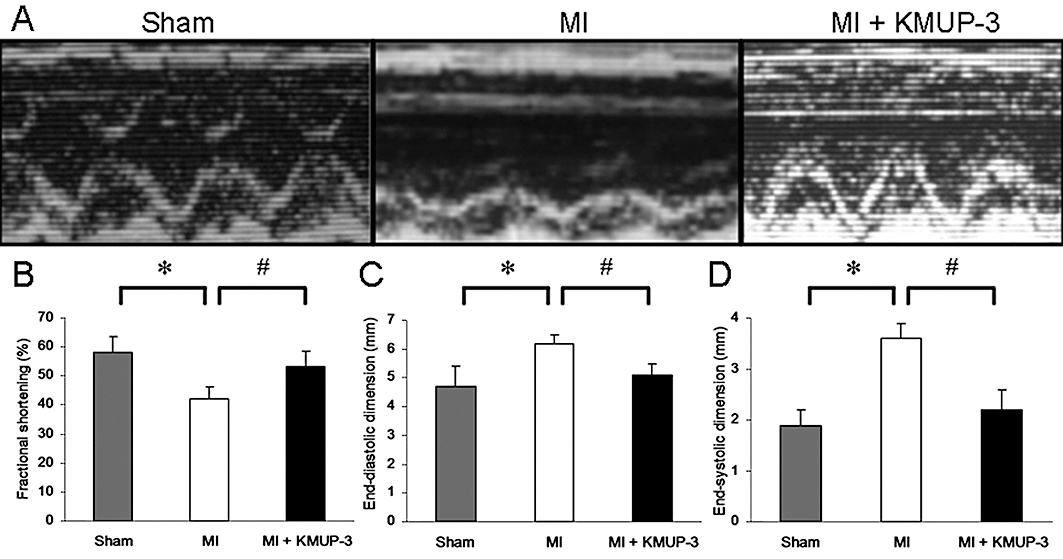
Effects of 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine (KMUP-3) on left ventricular remodelling (n= 10 per group). (A) Representative echocardiography obtained from each group. Data are shown as mean ± SE. Fractional shortening (B) and left ventricular dimension at end of diastole (C) and systole (D). *P < 0.05 versus sham group; #P < 0.05 versus myocardial infarction (MI) group.
Cardiac fibrosis and infarction size
The area of risk after coronary artery ligature was determined by Evans blue staining, and there was no difference between treatment groups (Figure 2A). Infarction size, determined by TTC staining, decreased significantly after KMUP-3 treatment (47.4 ± 3.7% vs. 33.6 ± 1.7%, respectively, P < 0.05) (Figures 2B,C). KMUP-3 attenuated cardiac fibrosis in both the central-infarct and peri-infarct areas as determined by Masson's trichrome staining (Figure 3). The anti-fibrotic effect of KMUP-3 was also found in the non-infarcted area, as the percentage of fibrosis was comparable with that of the sham-operated hearts.
Figure 2.
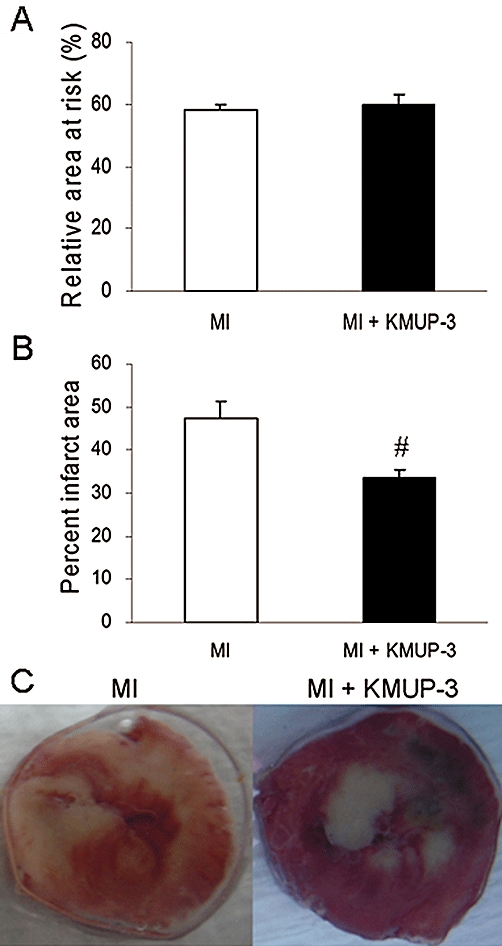
Effects of 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine (KMUP-3) on myocardial infarction (MI) size. (A) The area of risk after coronary artery ligature was determined by Evans blue staining, and there was no difference between treatment groups (n= 5). (B) Quantification of infarct area as a % (n= 10). (C) Representative images of 2,3,5-triphenyltetrazolium chloride staining. #P < 0.05 versus MI group.
Figure 3.
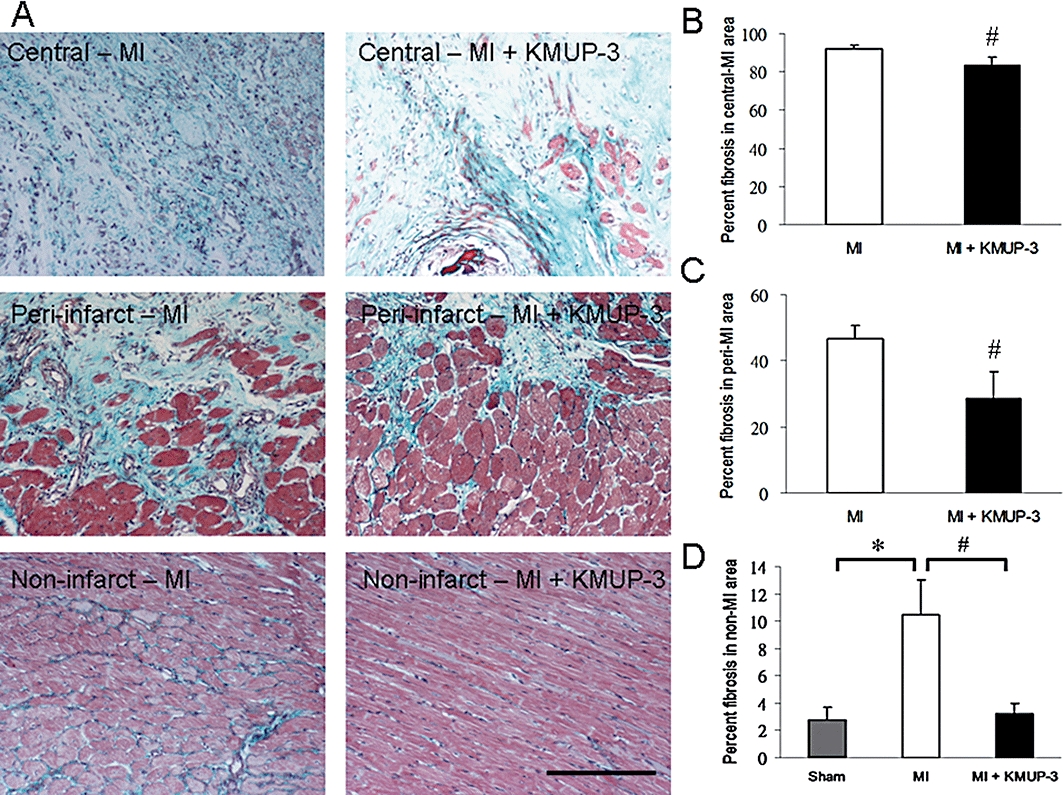
Effects of 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine (KMUP-3) on cardiac fibrosis after myocardial infarction (MI). (A) Fibrosis stained blue by Masson's trichrome in rat hearts. Original magnification, x200. (B) Fibrosis as a % of the central-infarct area (n= 10). (C) Fibrosis as a % of the peri-infarct area (n= 10). (D) Fibrosis as a % of the non-infarct area (n= 10). The size of the bar is 100 µm. *P < 0.05 versus sham group; #P < 0.05 versus MI group.
Myocardial eNOS, MMP-9 and TIMP-1
MMP-9 expression increased significantly in MI rats, compared with sham-operated rats. The administration of KMUP-3 decreased MMP-9 expression in MI rats (Figure 4A). It was also noted that the expression of the MMP-9 inhibitor TIMP-1 increased significantly after treatment with KMUP-3 (Figure 4B). A small decrease in eNOS expression was noted in MI rats. After treatment with KMUP-3, the eNOS expression increased significantly compared with rats in the MI group (Figure 4C).
Figure 4.
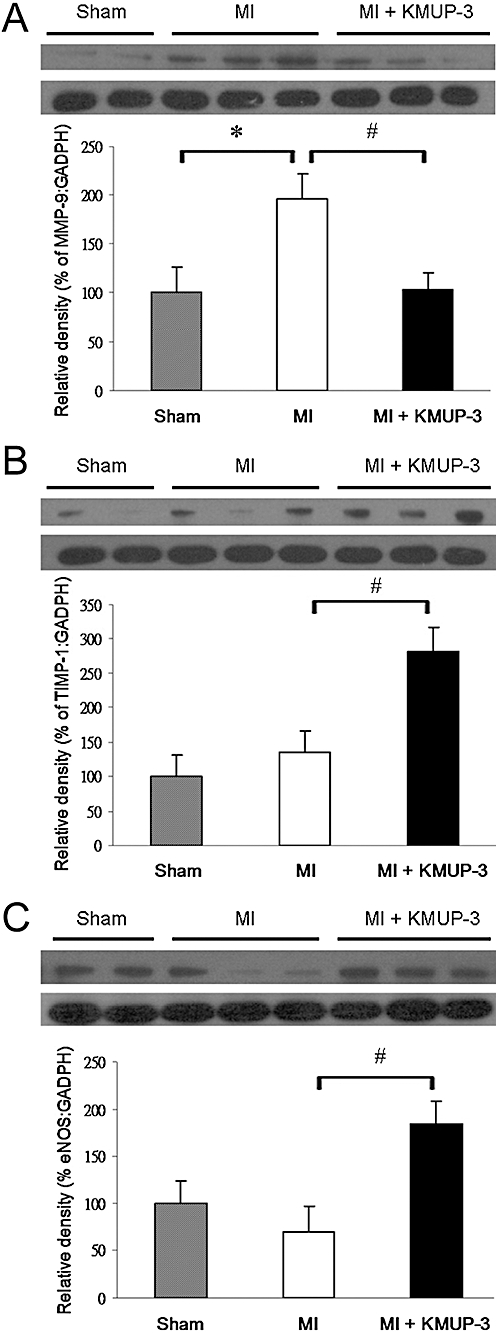
Effects of KMUP-3 on levels of MMP-9 (A), TIMP-1 (B) and eNOS (C) protein expression in rat hearts, determined by Western blot analysis and densitometry (n= 9–10). *P < 0.05 versus sham group; #P < 0.05 versus MI group. eNOS, endothelial nitric oxide synthase; KMUP-3, 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine; MI, myocardial infarction; MMP-9, matrix metalloproteinase-9; TIMP-1, tissue inhibitor of metalloproteinases-1.
eNOS, MMP-9 and TIMP-1 in cardiac fibroblasts
To further investigate the mechanism of eNOS-dependent cardiac protection in vitro, HCFs were stimulated with TGF-β (10 ng·mL−1) and the expression of MMP-9 and TIMP-1 were measured. MMP-9 expression increased significantly following TGF-β stimulation (Figure 5A) and the administration of KMUP-3 (10 µmol·L−1) attenuated the expression of MMP-9. Pretreatment with the eNOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; 100 µmol·L−1) reversed the inhibition of MMP-9 expression (Figure 5A). At the same time, TIMP-1 expression was significantly enhanced by the treatment with KMUP-3, and the enhancement was blocked by the pretreatment with L-NAME (Figure 5B).
Figure 5.
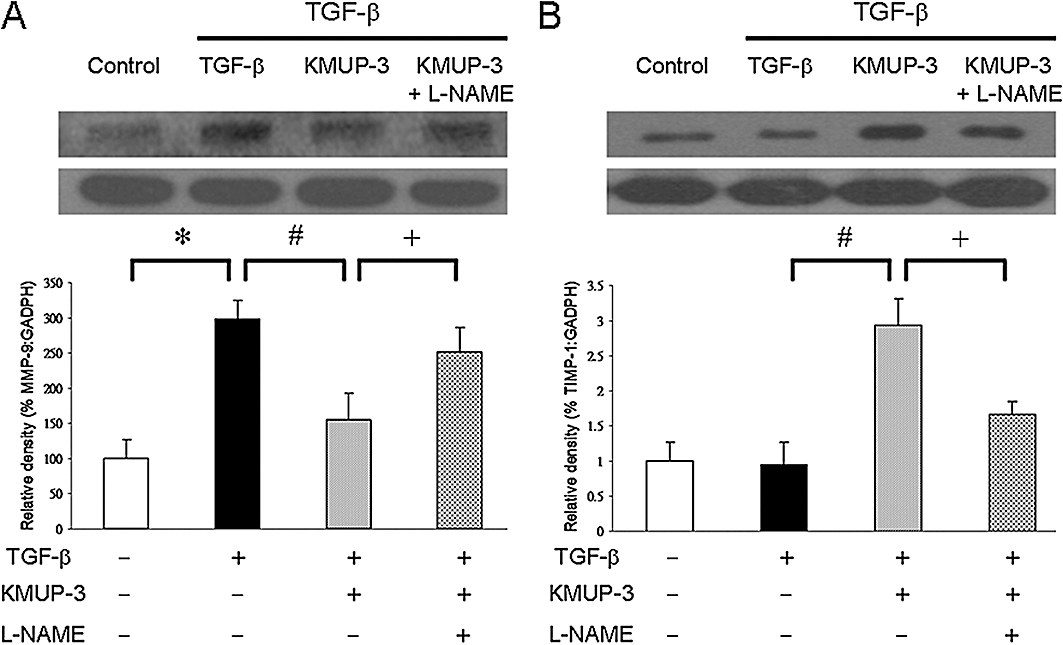
Effects of KMUP-3 on levels of MMP-9 (A) and TIMP-1 (B) protein expression in human cardiac fibroblasts (HCFs), determined by Western blot analysis and densitometry. HCFs were pretreated with KMUP-3 (10 µmol·L−1) with or without L-NAME (100 µmol·L−1) for 1 h before addition of 10 ng·mL−1 TGF-β for 24 h. Each experiment was repeated three times. *P < 0.05 versus control; #P < 0.05 versus TGF-β; +P < 0.05 versus KMUP-3. KMUP-3, 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine; L-NAME, Nω-nitro-L-arginine methyl ester; MMP-9, matrix metalloproteinase-9; TGF-β, transforming growth factor-β; TIMP-1, tissue inhibitor of metalloproteinases-1.
Discussion
This study showed that KMUP-3 treatment for 4 weeks attenuated unfavourable myocardial remodelling after MI. The protective effects of KMUP-3, which probably involve inhibition of the fibrosis pathway, were demonstrated by the prevention of myocardial hypertrophy, reduction in infarction size and fibrotic area, and preservation of cardiac function.
Despite numerous studies in animals showing that KATP channel activators have a cardioprotective effect through their preconditioning mimetic properties, there is still a paucity of evidence on the clinical effect of pure KATP activators in humans suffering from MI (Kloner and Rezkalla, 2004). A possible explanation is that in most KATP activation studies, the acute ischaemia-reperfusion injury model is used; however, the role of KATP channel opening in MI-induced chronic heart failure has been seldom evaluated. In contrast, the eNOS/NO pathway, by substantially inhibiting myocardial fibrosis in a chronic manner, may be analogous to the cardioprotection induced with KATP activators. In this study, KMUP-3 combined the KATP channel opening and eNOS activating activity; and protected against heart failure after MI in a long-term animal model, which may elicit new research directions and have potential therapeutic implications.
NO produced by eNOS is known to be a potent antioxidant and modulates many processes in LV remodelling. Following MI, decreased NO production by eNOS may result in persistent endothelial dysfunction. In contrast, enhanced NO bioavailability attenuates the capillary loss and potentiates new vessel formation during MI-induced heart failure (Jones et al., 2003). In addition, eNOS may counteract the effects of adrenergic stimulation through cGMP-dependent inhibition of L-type voltage-gated calcium channels (Campbell et al., 1996) and protein kinase G-dependent phosphorylation of troponin I (Lincoln and Corbin, 1978). Our research group previously demonstrated that KMUP-3 enhances eNOS expression in HUVECs. KMUP-3 also has the ability to enhance cGMP, which in turn, may augment the antagonism of the calcium channel by eNOS (Wu et al., 2005; 2006;). In the present study, an elevated eNOS expression in the myocardium of KMUP-3 treatment group contributed, at least in part, to the attenuation of cardiac remodelling after MI.
The phosphoinositide 3-kinase (PI3k)/Akt signalling pathway, one of the most characterized survival-related systems, can directly phosphorylate and activate eNOS, leading to NO production (Iwakiri et al., 2002). Cross-talk between PI3k/Akt and mitogen-activated protein kinase (MAPK) pathways has triggered intense study interests and a close relationship between eNOS expression and MAPK phosphorylation has been suggested (Peng et al., 2010). In a recent report, the cardioprotective effect of eNOS has been shown to be related to the inhibition of inflammation and suppression of MAPK signalling (Chen et al., 2010). In our previous study, increased eNOS expression attenuated isoprenaline-induced cardiac hypertrophy and fibrosis. This cardioproctective effect was blocked by the administration of the NOS inhibitor L-NAME (Yeh et al., 2010). Consistent with these previously published results, this present study demonstrated a reduction in cardiac fibrosis after MI through eNOS enhancement by KMUP-3.
Multiple studies have reported that NO reduces the expression and activation of MMPs (Eberhardt et al., 2000). However, studies on the impact of eNOS on MMP expression have generated conflicting results. In aortic smooth muscle cells, eNOS gene transfer inhibited transwell migration and decreased MMP-2 and MMP-9 activities (Gurjar et al., 1999), whereas a decreased expression of MMP-9 was found under basal and oestradiol-stimulated conditions after MI in eNOS-null mice (Iwakura et al., 2006). In the present study, KMUP-3 administration resulted in enhanced eNOS expression in concert with a reduced expression of MMP-9. In TGF-β-stimulated HCFs, the deactivating effect of KMUP-3 on MMP-9 was reversed by L-NAME, suggesting the involvement of the eNOS/NO pathway in MMP-9 regulation.
TIMP-1 serves as one of the major regulators of MMP-9. In a human mesangial cell line, treatment with the NOS inhibitor L-NAME potentiated the increase in MMP-9 production, but prevented the suppression of TIMP-1 production following treatment with tumour necrosis factor-α (Nee et al., 2008). In a bleomycin-induced lung fibrosis model, MMP-9 levels were significantly decreased in eNOS transgenic mice compared with bleomycin-injured wild-type mice. In contrast, the TIMP-1 levels increased significantly in bleomycin-treated eNOS transgenic mice compared with wild-type mice (Yoshimura et al., 2006). These results indicate that eNOS contributes to the altered MMP-9/TIMP-1 balance. In the present study, the stimulation of HCFs with TGF-β resulted in a significantly increased MMP-9 expression without a concomitant suppression of TIMP-1 expression. After KMUP-3 administration, MMP-9 expression decreased significantly with a markedly increased TIMP-1 expression (Figure 5). Blockade of eNOS activity by L-NAME reversed this effect. It seems that KMUP-3, by activating eNOS, not only inhibited the MMP-9 expression but also increased the TIMP-1 expression. The major limitation of this study is that we did not use KATP or eNOS inhibitors in the experimental animals. Further studies are needed to clarify the interaction between the KATP channel opening and eNOS/NO pathway.
In conclusion, KMUP-3 preserved heart function in MI rats by KATP channel opening and eNOS enhancement. The attenuation of cardiac fibrosis and unfavourable LV remodelling was partly achieved through the restoration of the MMP-9/TIMP-1 balance.
Acknowledgments
This work was supported by the National Science Council of Taiwan, grants NSC 97-2323-B-037-005-CC2 and NSC 96-2320-B-037-026-MY3. We would also like to thank Ms Chiu Yu-Yuan for technical assistance.
Glossary
Abbreviations
- AMI
acute myocardial infarction
- + dP/dt and – dP/dt
maximum rate of rise and full of pressure
- eNOS
endothelial nitric oxide synthase
- FS
fractional shortening
- HCF
Human cardiac fibroblast
- HUVEC
human umbilical vein endothelial cell
- KATP channel
ATP-dependent potassium channel
- LAD
left anterior descending coronary artery
- L-NAME
Nω-nitro-L-arginine methyl ester
- LVEDD
left ventricular end-diastolic dimension
- LVESD
left ventricular end-systolic dimension
- LVSP
left ventricular systolic pressure
- MI
myocardial infarction
- MMP-9
matrix metalloproteinase-9
- NO
nitric oxide
- PKG
protein kinase G
- TGF-β
transforming growth factor-β
- TIMP-1
tissue inhibitor of metalloproteinase-1
Conflict of interest
The authors state no conflict of interest.
References
- Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Zhu TB, Yin H, Huang J, Wang LS, Cao KJ, et al. Inhibition of MAPK signaling by eNOS gene transfer improves ventricular remodeling after myocardial infarction through reduction of inflammation. Mol Biol Rep. 2010;37:3067–3072. doi: 10.1007/s11033-009-9879-6. [DOI] [PubMed] [Google Scholar]
- Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, et al. Deficiency of TIMP-1 exacerbates LV remodeling after MI in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–H371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- Eberhardt W, Beeg T, Beck KF, Walpen S, Gauer S, Böhles H, et al. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000;57:59–69. doi: 10.1046/j.1523-1755.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- Gurjar MV, Sharma RV, Bhalla RC. eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol. 1999;19:2871–2877. doi: 10.1161/01.atv.19.12.2871. [DOI] [PubMed] [Google Scholar]
- Halapas A, Zacharoulis A, Theocharis S, Karavidas A, Korres D, Papadopoulos K, et al. Serum levels of the osteoprotegerin, receptor activator of nuclear factor kappa-B ligand, metalloproteinase-1 (MMP-1) and tissue inhibitors of MMP-1 levels are increased in men 6 months after acute MI. Clin Chem Lab Med. 2008;46:510–516. doi: 10.1515/CCLM.2008.091. [DOI] [PubMed] [Google Scholar]
- Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- Hellermann JP, Jacobsen SJ, Gersh BJ, Rodeheffer RJ, Reeder GS, Roger VL. Heart failure after myocardial infarction: a review. Am J Med. 2002;113:324–330. doi: 10.1016/s0002-9343(02)01185-3. [DOI] [PubMed] [Google Scholar]
- Iwakiri Y, Tsai MH, McCabe TJ, Gratton JP, Fulton D, Groszmann RJ, et al. Phosphorylation of eNOS initiates excessive NO production in early phases of portal hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H2084–H2090. doi: 10.1152/ajpheart.00675.2001. [DOI] [PubMed] [Google Scholar]
- Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- Jones SP, Greer JJ, van Haperen R, Duncker DJ, de Crom R, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci USA. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol. 2004;44:276–286. doi: 10.1016/j.jacc.2004.03.068. [DOI] [PubMed] [Google Scholar]
- Lee TM, Chou TF, Tsai CH. Differential role of KATP channels activated by conjugated estrogens in the regulation of myocardial and coronary protective effects. Circulation. 2003;107:49–54. doi: 10.1161/01.cir.0000043243.49875.2e. [DOI] [PubMed] [Google Scholar]
- Lee TM, Lin MS, Chang NC. Effect of ATP-sensitive potassium channel agonists on ventricular remodeling in healed rat infarcts. J Am Coll Cardiol. 2008;51:1309–1318. doi: 10.1016/j.jacc.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Li GH, Shi Y, Chen Y, Sun M, Sader S, Maekawa Y, et al. Gelsolin regulates cardiac remodeling after myocardial infarction through DNase I-mediated apoptosis. Circ Res. 2009;104:896–904. doi: 10.1161/CIRCRESAHA.108.172882. [DOI] [PubMed] [Google Scholar]
- Liang JC, Chen HR, Chiu CC, Liou SF, Chen IJ, Yeh JL. Protective effect of labedipinedilol-A, a novel dihydropyridine-type calcium channel blocker, on myocardial apoptosis in ischemia-reperfusion injury. Life Sci. 2006;79:1248–1256. doi: 10.1016/j.lfs.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Lin RJ, Wu BN, Lo YC, An LM, Dai ZK, Lin YT, et al. A xanthine-based epithelium-dependent airway relaxant KMUP-3 (7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine) increases respiratory performance and protects against tumor necrosis factor-alpha-induced tracheal contraction, involving nitric oxide release and expression of cGMP and protein kinase G. J Pharmacol Exp Ther. 2006;316:709–717. doi: 10.1124/jpet.105.092171. [DOI] [PubMed] [Google Scholar]
- Lin TH, Chiu HC, Lee YT, Su HM, Juo SH, Voon WC, et al. The C-allele of tissue inhibitor of metalloproteinases 2 is associated with increased magnitude of QT dispersion prolongation in elderly Chinese – 4-year follow-up study. Clin Chim Acta. 2007;386:87–93. doi: 10.1016/j.cca.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Lin X, Jo H, Ishii TM, Fujita M, Fu M, Tambara K, et al. Controlled release of matrix metalloproteinase-1 plasmid DNA prevents left ventricular remodeling in chronic myocardial infarction of rats. Circ J. 2009;73:2315–2321. doi: 10.1253/circj.cj-09-0379. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Corbin JD. Purified cyclic GMP-dependent protein kinase catalyzes the phosphorylation of cardiac troponin inhibitory subunit (TN-1) J Biol Chem. 1978;253:337–339. [PubMed] [Google Scholar]
- Louhelainen M, Vahtola E, Kaheinen P, Leskinen H, Merasto S, Kytö V, et al. Effects of levosimendan on cardiac remodeling and cardiomyocyte apoptosis in hypertensive Dahl/Rapp rats. Br J Pharmacol. 2007;150:851–861. doi: 10.1038/sj.bjp.0707157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee L, O'Connell S, Nolan S, Ryan MP, McMorrow T. Nitric oxide involvement in TNF-α and IL-1β-mediated changes in human mesangial cell MMP-9 and TIMP-1. Nephron Exp Nephrol. 2008;110:e59–e66. doi: 10.1159/000158524. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Peng XQ, Damarla M, Skirball J, Nonas S, Wang XY, Han EJ, et al. Protective role of PI3-kinase/Akt/eNOS signaling in mechanical stress through inhibition of p38 mitogen-activated protein kinase in mouse lung. Acta Pharmacol Sin. 2010;31:175–183. doi: 10.1038/aps.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, et al. Myocardial protection from ischemia/ reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res. 2002;54:549–558. doi: 10.1016/s0008-6363(02)00254-7. [DOI] [PubMed] [Google Scholar]
- Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL. Improved post-myocardial infarction survival with probucol in rats: effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Coll Cardiol. 2002;39:148–156. doi: 10.1016/s0735-1097(01)01709-0. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Coker ML, Bond BR, Zellner JL. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc Res. 2000a;46:225–238. doi: 10.1016/s0008-6363(99)00431-9. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, et al. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000b;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, et al. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- Takai S, Jin D, Inagaki S, Yamamoto D, Tanaka K, Miyazaki M. Significance of matrix metalloproteinase-9 in cardiac dysfunction during the very acute phase after myocardial infarction in hamsters. Eur J Pharmacol. 2007;572:57–60. doi: 10.1016/j.ejphar.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Vandegriff KD, Young MA, Lohman J, Bellelli A, Samaja M, Malavalli A, et al. CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol. 2008;154:1649–1661. doi: 10.1038/bjp.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BN, Lin RJ, Lin CY, Shen KP, Chiang LC, Chen IJ. A xanthine-based KMUP-1 with cyclic GMP enhancing and K(+) channels opening activities in rat aortic smooth muscle. Br J Pharmacol. 2001;134:265–274. doi: 10.1038/sj.bjp.0704231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BN, Chen IC, Lin RJ, Chiu CC, An LM, Chen IJ. Aortic smooth muscle relaxantsKMUP-3 and KMUP-4, two nitrophenylpiperazine derivatives of xanthine, display cGMP-enhancing activity: roles of endothelium, phosphodiesterase, and K+ channel. J Cardiovasc Pharmacol. 2005;465:600–608. doi: 10.1097/01.fjc.0000180900.32489.f9. [DOI] [PubMed] [Google Scholar]
- Wu BN, Chen CW, Liou SF, Yeh JL, Chung HH, Chen IJ. Inhibition of proinflammatory tumor necrosis factor-{alpha}-induced inducible nitric-oxide synthase by xanthine-based 7-[2-[4-(2-chlorobenzene) piperazinyl]ethyl]- 1,3- dimethylxanthine (KMUP-1) and 7-[2-[4-(4-nitrobenzene)piperazinyl]ethyl]-1, 3-dimethylxanthine (KMUP-3) in rat trachea: the involvement of soluble guanylate cyclase and protein kinase G. Mol Pharmacol. 2006;70:977–985. doi: 10.1124/mol.106.024919. [DOI] [PubMed] [Google Scholar]
- Yeh JL, Hsu JH, Wu PJ, Liou SF, Liu CP, Chen IJ, et al. KMUP-1 attenuates isoprenaline-induced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br J Pharmacol. 2010;159:1151–1160. doi: 10.1111/j.1476-5381.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Nishimura Y, Nishiuma T, Yamashita T, Kobayashi K, Yokoyama M. Overexpression of nitric oxide synthase by the endothelium attenuates bleomycin-induced lung fibrosis and impairs MMP-9/TIMP-1 balance. Respirology. 2006;5:546–556. doi: 10.1111/j.1440-1843.2006.00894.x. [DOI] [PubMed] [Google Scholar]


