Abstract
BACKGROUND AND PURPOSE
Protocatechuic acid (PCA) is plentiful in edible fruits and vegetables and is thus one anti-oxidative component of normal human diets. However, the molecular mechanisms involved in the chemopreventive activity of PCA are poorly understood. Here, we investigated the mechanism(s) underlying the anti-metastatic potential of PCA.
EXPERIMENTAL APPROACH
We used AGS cells in a wound healing model and Boyden chamber assays in vitro and injection of B16/F10 melanoma cells in mice (metastasis model in vivo) to analyse the effect of PCA on cancer cell invasion and metastasis. The activities and expression of molecular proteins were measured by zymographic assay, real-time RT-PCR and Western blotting.
KEY RESULTS
PCA inhibited cell migration and invasion at non-cytotoxic concentrations. Decreased expression of matrix metalloproteinase (MMP)-2 and a coincident increase in tissue inhibitor of MMP followed treatment with PCA. The PCA-inhibited MMP-2 activity and expression was accompanied by inactivation of NF-κB. All these effects of PCA could be mediated via the RhoB/ protein kinase Cε (PKCε) and Ras/Akt cascade pathways, as demonstrated by inhibition of PKCε and transfection of PKCε siRNA and ras overexpression vector. Finally, PCA inhibited metastasis of B16/F10 melanoma cells to the liver in mice.
CONCLUSION AND IMPLICATIONS
Our data imply that PCA down-regulated the Ras/Akt/NF-κB pathway by targeting RhoB activation, which in turn led to a reduction of MMP-mediated cellular events in cancer cells and provides a new mechanism for the anti-cancer activity of PCA.
Keywords: PCA, invasion, metastasis, MMP-2, RhoB/PKCε, Ras/Akt/NF-κB
Introduction
Protocatechuic acid (3, 4-dihydroxybenzoic acid; PCA) (Lin et al., 2007), a simple phenolic acid, is a precursor for the synthesis of other more complex molecules, such as cyanidin 3-O-β-D-glucoside and vanillin (Kampa et al., 2004). This compound is found in edible vegetables, fruits and nuts (Masella et al., 1999), brown rice (Hudson et al., 2000) and pecans (Stich, 1991), in plant-derived beverages like tea (Yen and Hsieh, 2000), Hibiscus sabdariffa (Tseng et al., 1996) and white grape wine (Li et al., 1993), and in herbal medicines (Liu et al., 1992; Lee and Yang, 1994) and exerts contradictory biologic effects. Studies in animals have recently shown PCA to be effective in inhibiting the carcinogenic action of diethylnitrosamine in the liver (Tanaka et al., 1993), azoxymethane in the colon (Kawamori et al., 1994), N-nitrosobis(2-oxopropyl)amine in pancreas (Nakamura et al., 2000) and N-methyl-N-nitrosourea in glandular stomach tissue (Tanaka et al., 1995). In vitro, PCA induced cell cycle arrest and apoptotic cell death through multiple signalling pathways from the mitogen activated protein kinase (MAPK) to the subsequent mitochondria- and/or Fas-mediated caspase activation in human gastric adenocarcinoma (AGS) cells and other tumour cells of digestive organs (Tseng et al., 2000; Lin et al., 2007). However, the anti-invasive and anti-metastatic activities of PCA have not been demonstrated up to now; thus, the objective of the present study was to determine whether PCA would be useful in suppressing cancer growth.
Tumour metastasis occurs by a series of steps, including vessel formation, cell attachment, invasion and cell proliferation, and is regulated by highly complicated mechanisms (Fidler, 2005). The degradation of basement membranes and stromal extracellular matrix (ECM) are crucial steps for tumour invasion and metastasis. Matrix metalloproteinases (MMPs), a family of human zinc-dependent endopeptidases, is responsible for the degradation of the ECM (Parks and Shapiro, 2001). Among them, the gelatinases (MMP-2 and MMP-9) efficiently degrade native collagen types IV and V, fibronectin and elastin. The expression of MMP genes is primarily regulated at the transcriptional, through activator protein-1 (AP-1) or nuclear factor-κB (NF-κB) via MAPK or phosphatidylinositol 3-kinase (PI3K)/Akt pathways, at post-transcriptional levels and at the protein level via their activators or inhibitors and their cell surface localization (Westermarck and Kähäri, 1999; Chen et al., 2008). MMPs and their regulatory pathways have been considered as promising targets for anti-cancer drugs and chemotherapeutic agents (Rao, 2003).
A major mechanism considered pivotal in human cancer progression is the PI3K/Akt signalling pathway (Vivanco and Sawyers, 2002). This pathway is activated by low-molecular-weight GTP/GDP binding GTPases, such as Ras, which is found oncogenically mutated in 30% of all human cancers (Barbacid, 1987). The ability of the Ras/PI3K/Akt pathway to induce deregulated proliferation and survival of human cancer cells may depend not only on the activation of genes that stimulate cellular proliferation, migration and metastasis, but also on the inhibition of those genes that suppress proliferation and/or induce apoptosis (Jiang et al., 2004). Closely related family members of Ras, such as Ras-homologous (Rho) small GTPases, are also involved in the regulation of a variety of cellular processes, such as organization of actin cytoskeleton, genotoxic stress-induced signalling and malignant transformation. Recent studies further confirmed the role of the Rho proteins in cancer by showing their involvement in cell transformation, invasion, metastasis and angiogenesis. The major members of the Rho subfamily comprise the RhoA, RhoB and RhoC proteins. RhoB is quite different from RhoA and RhoC in many aspects, although it shares 87% homology (Jiang et al., 2004). For example, RhoB has a tumour-suppressive role, including inhibiting cell proliferation and inducing apoptosis in several human cancer cells, and inhibiting tumour growth in a nude mouse xenograft model, while activation of RhoA promotes cell malignant transformation, proliferation, invasion and metastasis, like other small GTPases such as Ras, Rac1 and Cdc42 (Du et al., 2004). Furthermore, RhoB, unlike the constitutively expressed RhoA, is inducible by genotoxic stress, such as u.v. light, some growth factors (TGFβ) and chemotherapeutic drugs (cisplatin and 5-FU) (Fritz et al., 1995; Chen et al., 2006).
Previous studies on functions of PCA have been mainly focused on its anti-oxidative activity or the suppression of tumour cell proliferation, whereas the effect of PCA on migration and invasion of tumour cells has been less extensively investigated. As cancer metastasis and invasion are strongly correlated with degradation of the ECM, intercellular adhesion and cellular motility, this study examined the effects of PCA on MMP expression, as well as Akt and NF-κB activities in AGS cells to explore the underlying mechanism for the action of PCA in cancer cell invasion. Additionally, we used B16/F10 mouse melanoma cells, widely used as model systems in studying metastasis, to demonstrate the effect of PCA on tumour cell invasion in vivo.
Methods
Cell culture and treatment
Human gastric carcinoma AGS cells were maintained in F-12 Nutrient Mixture medium (Gibco/BRL, Gaithersburg, MD, USA). B16/F10 mouse melanoma cells were cultured in RPMI 1640. Cells were cultured at 37°C in 5% CO2 in medium supplemented with 10% FBS and antibiotics (100 U·mL−1 of penicillin and 100 µg·mL−1 of streptomycin). Cells were seeded at a density of 7 × 105 cells onto 10 mm Petri dishes 24 h before treatment. For the inhibition test, GF 109203X (GFX) (Calbiochem, Bad Soden, Germany) was added 30 min before PCA (purity 99%) (Sigma Chemical Co., St. Louis, MO, USA) treatment.
Wound healing and invasion assay
The wound healing assay was performed as described previously (Chen et al., 2008). AGS cells were grown to confluent monolayer in six well Petri dishes for 24 h in serum-free medium. The medium was replaced with serum-containing medium following treatment with PCA at various concentrations (0, 0.1, 0.5, 1.0 and 2.0 mM), and the cells in the monolayer were disrupted –‘wounded’– by scraping them off with a P200 micropipette tip. At the indicated times (0, 12, 24 and 48 h) after scraping, the cells were washed twice with phosphate-buffered saline (PBS). The number of cells in the denuded (scraped) zone of each dish was counted at ×100 magnification (without knowledge of the treatments). Each dish was counted three times to ensure accuracy.
Cells, treated as described above, were placed in the upper part of a Boyden chamber (Neuro Probe, Cabin John, MD, USA) at a density of 3 × 105 cells·mL−1 in 50 µL of serum free medium. The in vitro invasiveness was tested by the Boyden chamber invasion assay, as described previously (Chu et al., 2004).
Gelatin zymography
The activities of MMP-2 and MMP-9 in the conditioned medium were measured by gelatin-zymogram protease assays, as described previously (Chu et al., 2004).
Real-time quantitative RT-PCR
Total RNA was isolated from cells with a guanidinium chloride procedure and the mRNA levels were analysed by real-time quantitative RT-PCR using a Bio-Rad iCycler system (Bio-Rad, Hercules, CA, USA), as described previously (Chen et al., 2008).
Western blot analysis
Western blotting was performed according to a method described earlier (Lin et al., 2007). The preparation of cytosolic and nuclear fractions of the cells was performed according to the procedures described by Chen et al. (2008).
Electrophoretic mobility shift assay (EMSA)
The DNA-binding activity of NF-κB in nuclear extracts was assessed by EMSA (Chen et al., 2008) using the Lightshift kit from Pierce (Rockford, IL, USA) with biotin-labelled double-stranded NF-κB oligonucleotide (Promega, Madison, WI, USA). For competition assays, the reaction mixtures were pre-incubated with the indicated non-labelled NF-κB oligonucleotides probe for 15 min before addition of labelled κB site oligo.
Active GTPase pull-down assay
The small GTPase activity was assessed by GST–protein binding domain (PBD) Affinity Precipitation (Ren et al., 1999) and Thermo Scientific Active GTPase Pull-Down and Detection Kits from Pierce. Cell lysates (500 µg) were clarified by centrifugation at 13 000×g at 4°C for 10 min, and equal volumes of lysates were incubated with GST–PBD (20 mg) beads at 4°C for 45 min. The beads were washed four times with buffer B (Tris buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 10 mg·mL−1 each of leupeptin and aprotinin, and 0.1 mM PMSF). Bound small GTPase proteins were detected by Western blotting using a monoclonal antibody against Ras, RhoA, RhoB, Rac1 and Cdc42 (Santa Cruz Biotech, Santa Cruz, CA, USA).
The amount of PBD-bound small GTPase was normalized by the total amount of small GTPase in cell lysates for the comparison of small GTPase activity (level of GTP-bound small GTPase) in different samples. Depending on cell conditions and types, and different batches of GST–PBD, the PBD-bound small GTPase accounts for ∼0.5–5% of total small GTPase.
Immunoprecipitation
Cell lysates were prepared using the lysis buffer. Protein (500 µg) from cell lysates was pre-cleared with protein A-Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ, USA), followed by immunoprecipitation using polyclonal anti-IκBα or anti-PKCε antibodies. Immune complexes were harvested with protein A, and immunoprecipitated proteins were analysed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), as previously mentioned. Immunodetection was carried out using polyclonal anti-Ubiquitin (Ub) or anti-RhoB antibodies (Santa Cruz Biotech).
Transient transfection
Transient transfection assay was carried out as described previously (Lin et al., 2007). Liposome-mediated transfection was performed using Lipofectamine™ (Invitrogen, Carlsbad, CA, USA) on AGS cells with a control pcDNA3.1 empty vector or an expression construct for K-ras cDNA in pcDNA3.1 (a gift from Ko and co-workers) (Weng et al., 2005), or PKCε siRNA (Santa Cruz Biotech).
Cell growth experiment
Cells (1 × 104 cells·mL−1) were seeded in 12 well plates and treated with PCA (1.0 mM) or dimethyl sulphoxide (DMSO) as control for 24, 48 and 72 h. The number of cells was measured by the Trypan blue dye exclusion assay.
Experimental metastasis and histologic evaluation
All animal care and experimental procedures were according to the guidelines of the Institutional Animal Care and Use Committee of Chung Shan Medical University (IACUC, CSMC) for the care and use of laboratory animals. Cells of the B16/F10 melanoma line of C57/BL6 origin were washed and resuspended in PBS. A cell suspension containing 106 cells in 0.1 mL of PBS was injected into the lateral tail vein of 6-week-old C57/BL6 mice (supplied by the Laboratory Animal Center, Hualien, Taiwan). At the same time, two of the groups were orally treated with PCA at doses of 5 mg and 10 mg per mouse (equivalent to 20 mg and 40 mg per 100 g), and this treatment was continued daily for 6 weeks. Mice were killed after 6 weeks after injection of the BF16F10 cells, as our preliminary study of this animal model showed that B16/F10 cells developed numerous liver metastatic nodules in this time. Immediately after death, the size and number of tumour nodules for each liver tissue section were measured and counted automatically by the software of the image analysis program Image-Pro Plus 4.5 (Media Cybernetics, Silver Spring, MD, USA), according to the different greyscale intensity of normal and pathologic areas under a microscope. All organs were examined for metastasis formation (Jiang et al., 2004). The histologic evaluation was performed as described previously (Chen et al., 2008).
Data analysis
Data are reported as means ± standard deviation of three independent experiments and evaluated by one-way analysis of variance (anova). Significant differences were established at P < 0.05.
Results
Effects of PCA on the motility and invasive ability of AGS cells
The concentrations of PAC (0.1 to 2.0 mM) used in the present experiments were shown to be non-cytotoxic to AGS cells, in our previous study (Lin et al., 2007). The effect of PCA on AGS cell migration was determined by wound-healing assays in which cells were stimulated to migrate by physical ‘wounding’ of cell monolayers on fibronectin pre-coated plates. As shown in Figure 1A, an apparent and gradual increase of cells in the denuded zone was observed in the cells treated with control or DMSO for 24 and 48 h under light microscopy. AGS cells exposed to 0.5, 1.0 and 2.0 mM of PCA showed a reduced ability to migrate and fill the wounded area, compared to the control cells. The quantitative data in Figure 1B revealed that PCA inhibited the migration of AGS cells in a dose- and time-dependent manner. To further examine the effect of PCA on the invasive ability of AGS cells, a Boyden chamber coated with Matrigel was used. The results showed that the number of cells invading the lower chamber was significantly reduced by 24 h treatment with PCA, and such reduction was concentration-dependent with a 70% decrease (P < 0.005) when PCA was used at 2.0 mM (Figure 1C).
Figure 1.
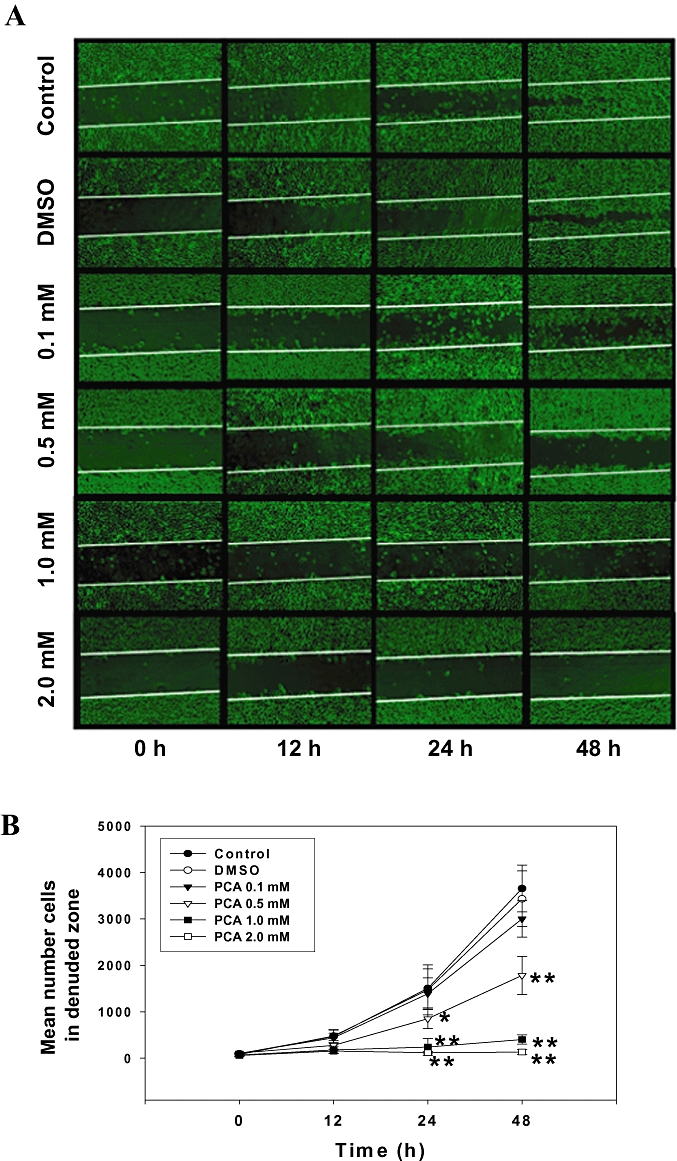
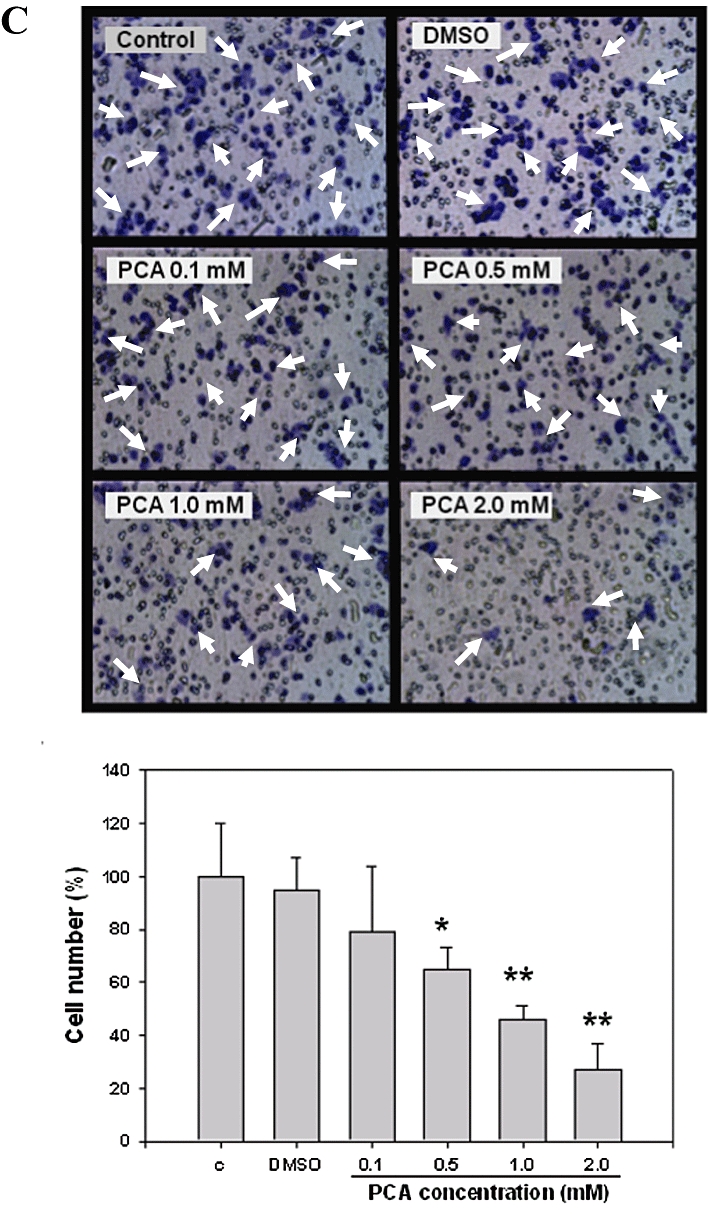
Effects of PCA on AGS cell motility and invasion in vitro. (A) Monolayers of AGS cells treated with various concentrations (0–2.0 mM) of PCA or dimethyl sulphoxide (DMSO) were scraped and the number of cells in the denuded zone was photographed and quantified at the times indicated (0, 12, 24 and 48 h). (B) Quantitative assessment of the mean number of cells in denuded zone represents the average of three independent experiments ± SD *P < 0.05, **P < 0.005 compared with the corresponding time point of control group. (C) AGS cells were treated with various concentrations of PCA for 24 h. Invasion assay was performed using Boyden chamber. Representative photomicrographs of the membrane-associated cells (arrow) were assayed by Giemsa stain. ‘% of control’ denotes the mean number of the cells expressed as a proportion of the untreated group. Data shown are the average of three independent experiments ± SD *P < 0.05, **P < 0.005 compared with the control. Control (C) was the untreated PCA sample. DMSO (0.2%) served as the solvent control.
Effects of PCA on the activities and expression of MMPs
Because ECM degradation is crucial to cellular invasion, implying the crucial involvement of matrix-degrading proteinases (Parks and Shapiro, 2001; Chen et al., 2008), the effect of PCA on MMP activities was investigated by gelatin-zymography under conditions of serum starvation to clarify the contribution of MMPs to the inhibition by PCA of the invasive ability of cells. As shown in Figure 2A (left panel), MMP-2 activity was reduced by PCA in a concentration-dependent manner, whereas there was little effect on MMP-9 activity. Time-course experiments with PCA at a lower concentration of 1.0 mM revealed a prolonged inhibition of MMP-2 activity in AGS cells, in a time-dependent manner (Figure 2A, right panel). In order to further understand the down-regulatory effects of PCA on MMP-2 and its endogenous inhibitors, tissue inhibitors of metalloproteinases-2 (TIMP-2), quantitative RT-PCR analysis was performed. Although the mRNA level of MMP-2 was significantly reduced, that of TIMP-2 was elevated after incubation with PCA (Figure 2B, left panel). These PCA-induced changes in the mRNA levels of MMP-2 and TIMP-2 coincided well with their protein levels, as shown by Western blots (Figure 2B, right panel), indicating that PCA might regulate MMP-2 and TIMP-2 expressions at the transcriptional level.
Figure 2.
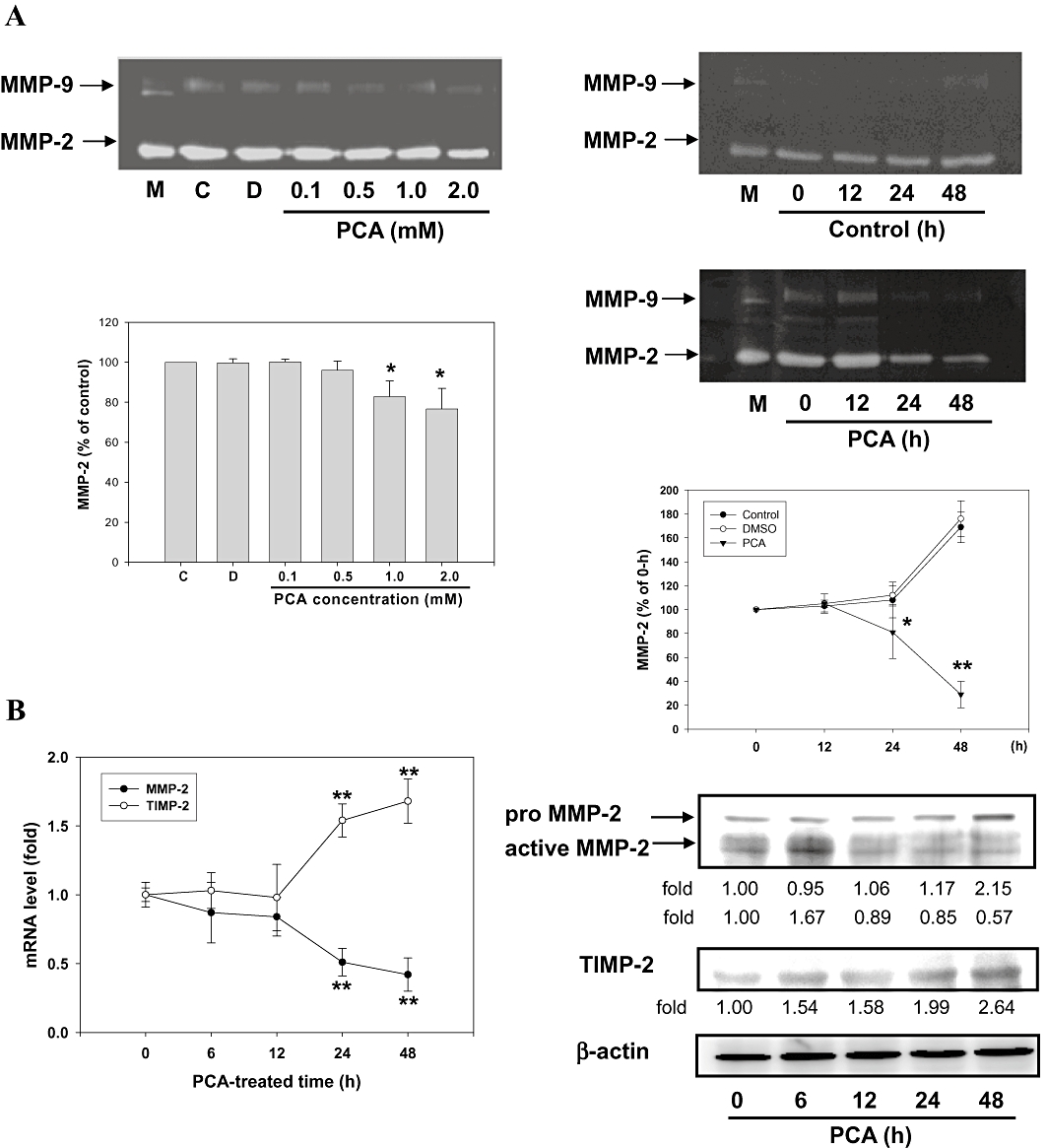
Effects of PCA on the MMPs activities and expressions. (A) AGS cells in serum-free medium were treated with various concentrations (0–2.0 mM) of PCA for 24 h (left panel) or treated with 1.0 mM of PCA for the times indicated (0, 12, 24 and 48 h) (right panel). The culture medium of cells after treatment was subjected to gelatin-zymography to analyse the activity of MMPs. (B) Real-time quantitative RT-PCR (left panel) and Western blot (right panel) analysis of mRNA and protein levels of MMP-2 and TIMP-2 in cells treated with 1.0 mM of PCA and harvested at the times indicated. β-actin served as an internal control of protein level. The quantitative data are means ± SD of three repeats from one independent study. *P < 0.05, **P < 0.005 compared with the control or the corresponding time point of control group. Control (C) was the untreated PCA sample. Dimethyl sulphoxide (DMSO) (0.2%, D) served as the solvent control.
Effects of PCA on the nuclear translocation of NF-κB
Previous reports have demonstrated that the MMP-2 promoter contains several transcription factor-binding elements, including binding sites for AP-1 and NF-κB (Westermarck and Kähäri, 1999; Chu et al., 2004). Therefore, the signal transduction pathway of AP-1 and NF-κB may play important roles in the regulation of MMP-2 expression. Next, we tested whether PCA disrupted the translocation of AP-1 and NF-κB into the nucleus in AGS cells by immunoblotting analysis of the nuclear extracts prepared from treated cells. The data in Figure 3A demonstrated that the nuclear level of NF-κB (p65) was decreased to 52%-63% of control values after a 24 h treatment with PCA at 0.1–2.0 mM, while no noticeable change was observed in the nuclear translocation of c-Jun and c-Fos (components of the transcription factor AP-1). EMSA analysis also confirmed a decrease in the DNA-binding activity of the nuclear translocated NF-κB, but not AP-1 (data not shown), in the cells treated with PCA for 24 h (Figure 3B). The specificity of NF-κB binding activity was established by using a competition assay with an unlabeled NF-κB oligonucleotide. Therefore, it is possible that the inhibitory effect of PCA on the motility and invasion of AGS cells was due to inactivation of NF-κB that led to a reduction in MMP-2 expression. The time-course (0–48 h) of expression of nuclear NF-κB revealed that 1.0 mM of PCA treatment of AGS cells induced a decrease in nuclear NF-κB, beginning 6 h after treatment and persisting through the next 12–48 h, a time span that corresponds to the increase in the amount of cytosolic IκBα protein (Figure 3C). As activation of NF-κB correlates with rapid proteolytic degradation of IκBα, prevention of IκBα degradation was also studied as a marker of inhibition of NF-κB activation by PCA. As shown in Figure 3D, treatment of AGS cells with 1.0 mM PCA for 6 h prevented the ubiquitin-dependent degradation of IκBα. In Supplementary Figure 1, the transfection of NF-κB p65 cDNA into AGS cells resulted in increased expression of NF-κB, concomitantly with a marked induction of cell invasion. These findings suggested that the inhibition of cell migration/invasion by PCA may involve suppression of NF-κB signalling.
Figure 3.
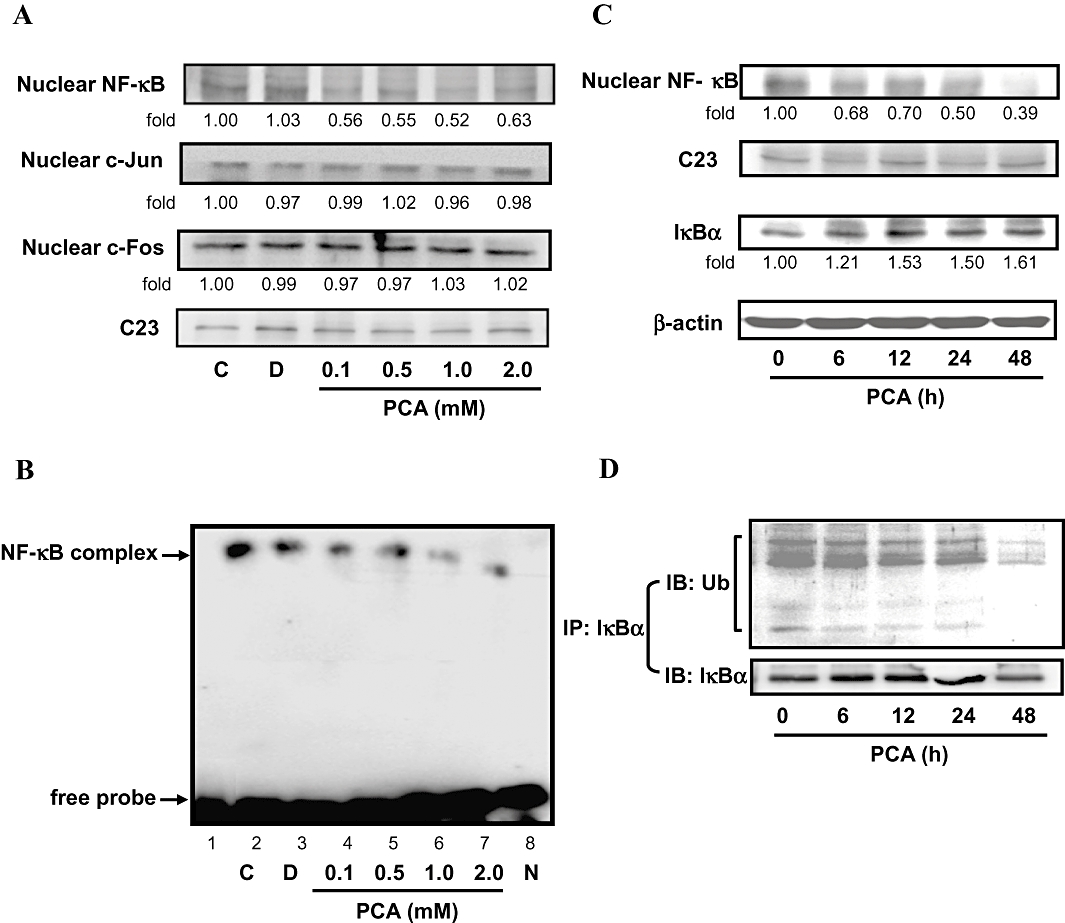
Effects of PCA on the nuclear translocation of NF-κB. (A) AGS cells were treated with various concentrations (0–2.0 mM) of PCA for 24 h, after which cells were harvested and the nuclear fraction analysed for the expression of NF-κB, c-Jun and c-Fos. The nuclear protein levels were determined by Western blotting. (B) The nuclear extracts were analysed for NF-κB DNA-binding activity using biotin-labelled NF-κB specific oligonucleotide by EMSA. Lane 1 represents nuclear extracts incubated with unlabeled oligonucleotide (free probe) to confirm the specificity of binding. Lane 8 represented nuclear extracts with the indicated double-stranded the non-labelled NF-kB sequence (N) as a competitor. (C) Western blot analysis of nuclear NF-κB and IκBα protein expression in cells at the times indicated following treatment with PCA (1.0 mM). (D) Cell extracts prepared from the same treatment condition were immunoprecipitated with IκBα. The precipitated complexes were examined for immunoblotting using Ub antibody. C23 and β-actin were served as a nuclear and cytosolic internal control, receptively. The quantitative data are means ± SD of three repeats from one independent study. Control (C) was the untreated PCA sample. Dimethyl sulphoxide (0.2%, D) served as the solvent control.
Effects of PCA on the expressions of PI3K/Akt and small GTPase family
MAPK, Akt and focal adhesion kinase (FAK) have been shown to be involved in MMP-2 induction in various tumour types (Westermarck and Kähäri, 1999; Hwang and Lee, 2008). To examine whether the activities of these protein kinases were down-regulated by PCA, we analysed their phosphorylation in AGS cells after treatment with PCA (0.1–2.0 mM) for 24 h. Immunoblot analysis showed that PCA inhibited the phosphorylation of Akt and, to a greater effect, PI3K (Figure 4A). Densitometric determination indicated that the treatment of AGS cells with 2.0 mM PCA (24 h) resulted in a 50% reduction in the amount of phosphorylated Akt, without affecting MAPK or FAK (Supplementary Figure 2).
Figure 4.
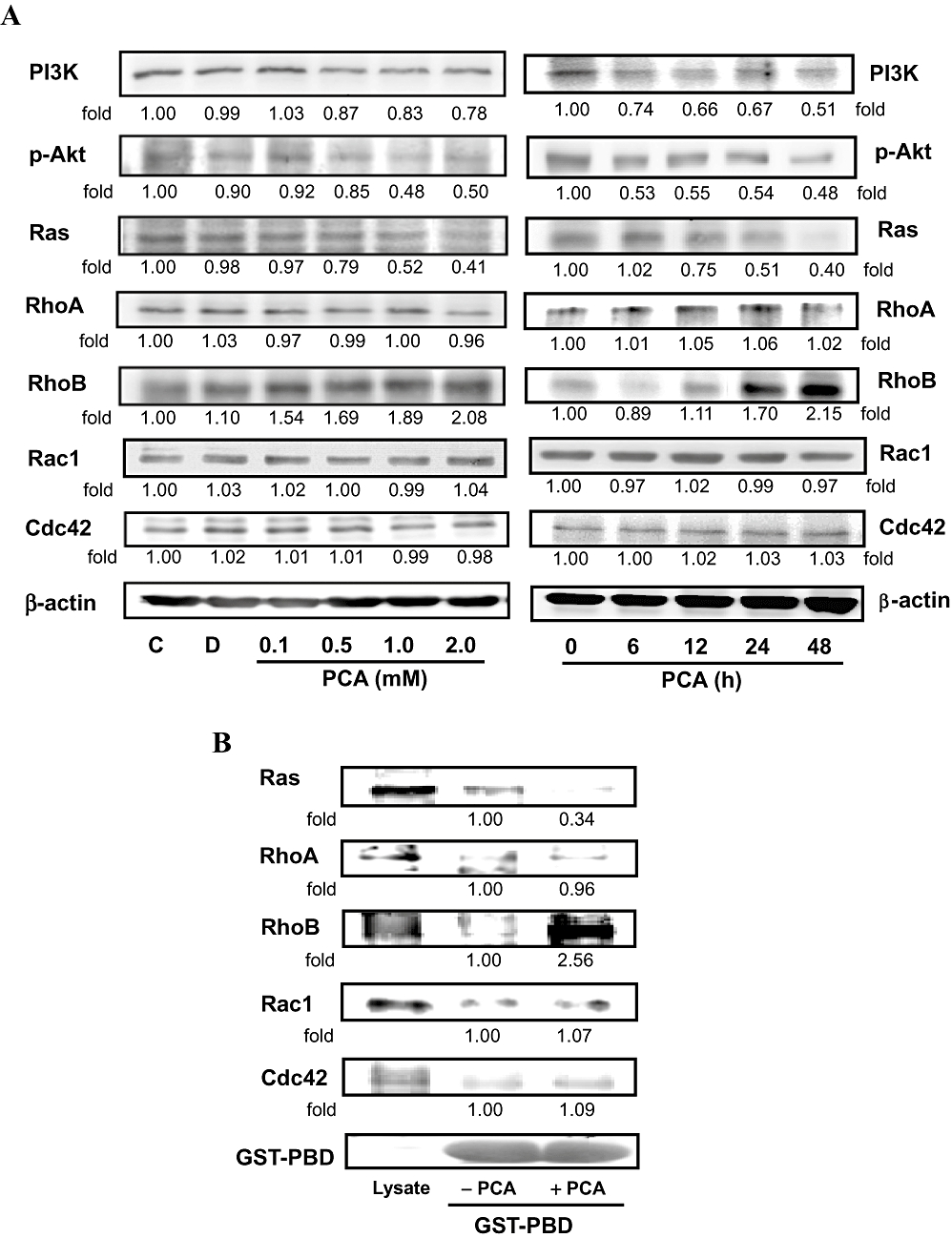
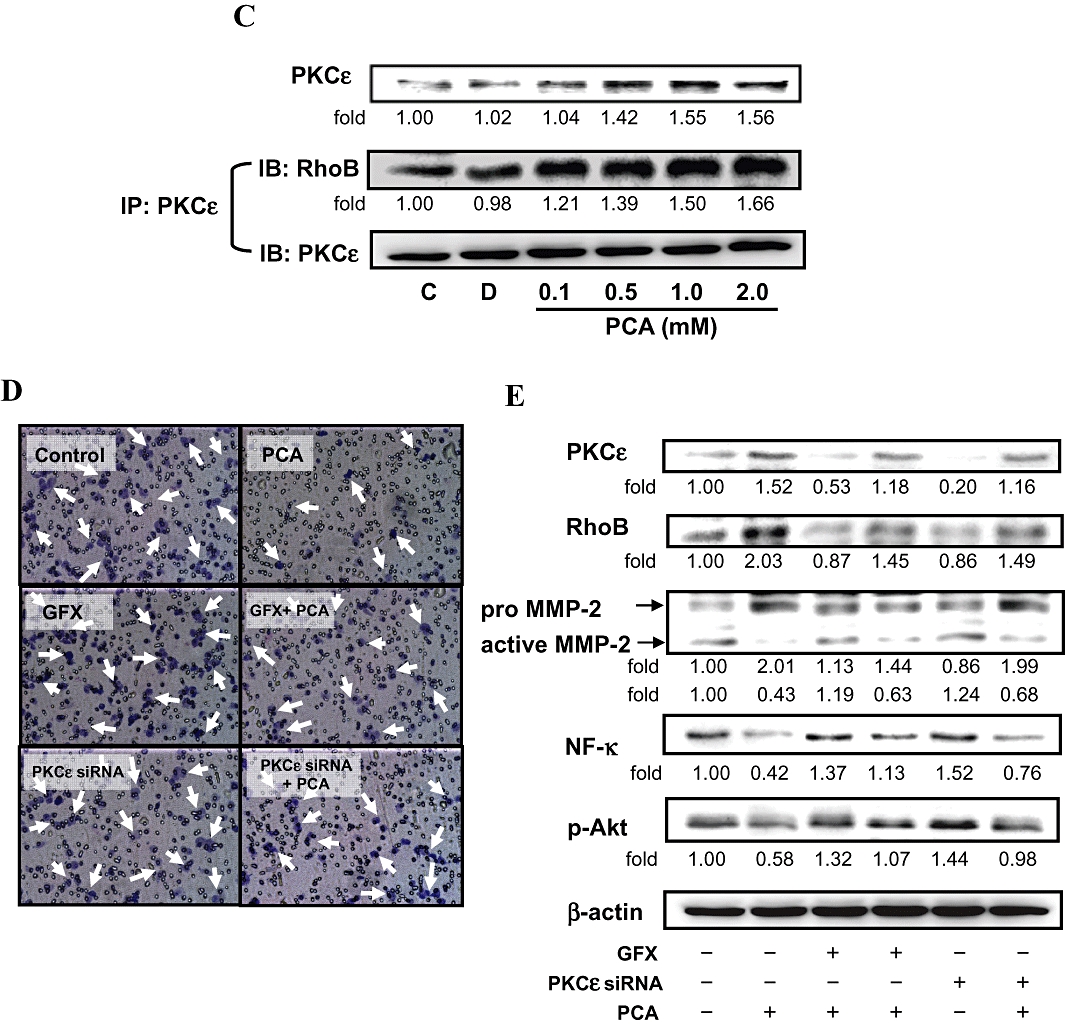
Effects of PCA on the expressions of PI3K/Akt and the small GTPase family. (A) AGS cells were treated with various concentrations (0–2.0 mM) of PCA for 24 h or 1.0 mM of PCA for the times indicated (0, 6, 12, 24 and 48 h), after which cells were harvested and analysed for the levels of PI3K, p-Akt, and small GTPase family proteins. Equal amounts of cell lysates (50 µg) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose and probed with specific PI3K, p-Akt, PKCε, small GTPase family proteins antibodies, including Ras, RhoA, RhoB, Rac1 and Cdc42, and PKCε (C) antibodies. (B) Extracts of the cells treated with 1.0 mM of PCA (24 h) were incubated with GST–PBD glutathione beads. After washing, the bound proteins were analysed by Western blotting with monoclonal antibodies against small GTPase family proteins. A portion of lysate (10 µg) was loaded as a blotting control. Duplicate gels (bottom line) were run and probed with specific GST–PBD antibodies to show fusion protein loading. (C) Cell extracts prepared from the same treatment condition were immunoprecipitated with PKCε. The precipitated complexes were examined for immunoblotting using RhoB antibody. Control (C) was the untreated PCA sample. Dimethyl sulphoxide (0.2%, D) served as the solvent control. (D) AGS cells were pre-treated with GFX for 30 min or transfected with PKCε siRNA, and then treated with 1.0 mM PCA for 24 h. Cell invasion was analysed by Boyden chamber assay. (E) The same samples were also analysed for PKCε, RhoB, MMP-2, NF-κB and p-Akt by Western blotting. β-actin was served as an internal control of protein level. The quantitative data are means ± SD of three repeats from one independent study.
It is well known that the small GTPase family, which includes Ras, RhoA, RhoB, Rac1 and Cdc42, is involved in the regulation of actin cytoskeleton remodelling, cell migration and invasion (Jiang et al., 2004). To assess the contribution of these proteins to the anti-migration effects of PCA on AGS cells, the expression of small GTPase family proteins was measured by Western blot analysis. In the presence of PCA, the protein level of Ras was decreased in proportion to the increased concentrations and incubation times with PCA, whereas the protein levels of RhoA, Rac1 and Cdc42 remained unchanged. An inhibitory effect on Ras activity (GTP-bound Ras) was also observed when the cells were incubated with 1.0 mM PCA for 24 h, as measured by the active GTPase pull-down assay (Figure 4B). The amount of RhoB, but not RhoA, Rac1 and Cdc42, bound to the PBD beads was increased by treating cells with PCA. PCA also induced the protein level and activity of RhoB (Figure 4A and B), which subsequently activated the expression of a downstream effector of RhoB, PKCε (line 1, Figure 4C). Using immunoprecipitation, we confirmed that the addition of PCA (0.5–2.0 mM) for 24 h, up-regulated the formation of RhoB/PKCε complexes in AGS cells (Figure 4C).
Effects of PCA on the RhoB/PKCε pathway
Since the activity of MMP-2 and cellular levels of p-Akt and nuclear NF-κB were decreased at the same time as the PKCε and RhoB expression increased in the PCA-treated AGS cells for 24 h, we next attempted to determine whether the PCA-mediated cellular events were dependent on the RhoB/PKCε pathway. Pre-treatment with GFX, a known blocker of RhoB/PKCε signalling (Liu et al., 2006), before the addition of 1.0 mM of PCA, prevented the effect of PCA on invasion by AGS cells (Figure 4D). GFX also completely reversed the PCA-induced changes in the related proteins (Figure 4E), while GFX alone had no affect. To examine the involvement of PKCε in the negative regulation of PCA on the Akt/NF-κB-mediated cell invasion, endogenous PKCε was depleted by specific siRNA. We found that the effect of this siRNA were similar to those of GFX (Figure 4D and E). These results suggested that the RhoB/PKCε pathway mediated the action of PCA to inactivate MMP-2, NF-κB and p-Akt that were involved in the migration/invasion of AGS cells.
Effects of mutant Ras expression vector on PCA-mediated cellular events
The role of Ras in the PCA-mediated cellular events was further defined by a genetic approach to over-express Ras in AGS cells. The results of Western blots showed that the cells expressing a control vector, indeed, had a diminished level of Ras when cells were treated with PCA (Figure 5A). These suppressive effects of PCA on the level of Ras and cell growth were reversed by an atypical over-expression of Ras (line 2 and 4, Figure 5A and B). The expression of constitutively active Ras also enhanced invasion ability and MMP-2 activity of AGS cells that were originally inhibited by PCA as analysed by transwell (bottom, Figure 5C) and zymography assay (line 3 and 4, Figure 5D).
Figure 5.
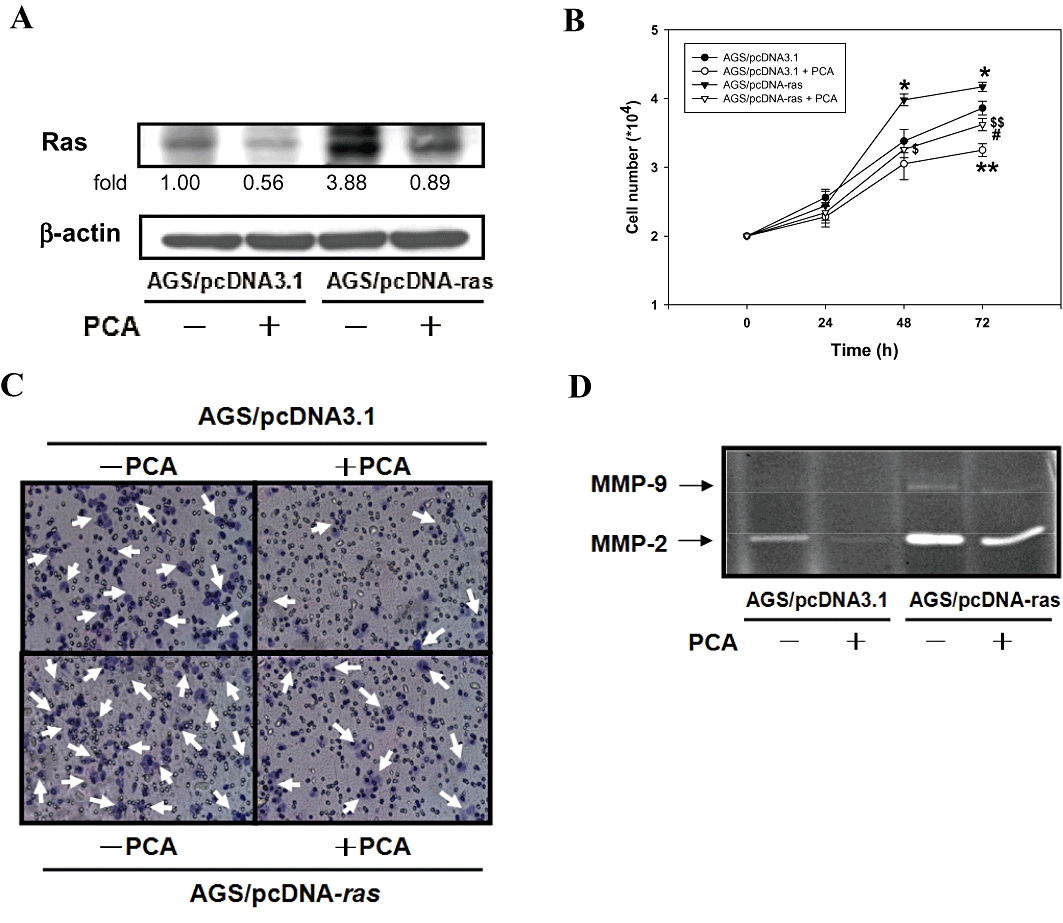
Effects of mutant Ras expression vector on PCA-mediated cellular events. AGS cells were transfected with an empty vector or a ras cDNA (activated) and treated with or without 1.0 mM of PCA for 24 h. (A) The cellular level of Ras was analysed by Western blotting. β-actin was served as an internal control. (B) The number of cells was counted by Trypan blue dye exclusion assay. The quantitative data were presented as means ± SD of three repeats from one independent study. *P < 0.05, **P < 0.005 significantly different from control (AGS/pcDNA3.1) in the respective time point. #P < 0.005 PCA-treated AGS/pcDNA-ras significantly different from PCA-treated AGS/pcDNA3.1 at the corresponding time point. $P < 0.05, $$P < 0.005 PCA-treated AGS/pcDNA-ras significantly different from AGS/pcDNA-ras at the corresponding time point. Cell invasion (C) and MMP-2 activity (D) were analysed by Boyden chamber and zymography assay. Results are representative of at least three independent experiments.
Effects of PCA on B16/F10 metastasis to the liver in a mouse model
The work described earlier clearly showed that PCA was able to repress the migration and invasion of cancer cells in models in vitro. As AGS cells do not easily form tumour xenografts in mice (Gong et al., 2008), the highly metastatic B16F10 melanoma cell line was used to assay the metastasis of cancer cells in vivo (Jiang et al., 2004; Reischer et al., 2007). C57/Bl mice injected with B16/F10 cells were treated orally without or with PCA at doses of 5 mg or 10 mg per mouse. Six weeks later, animals were examined for metastasis and the disseminated tumours, formed principally in the liver.
The results of liver metastatic colonization are summarized in Table 1. In group 2 mice receiving only the B16/F10 melanoma cells, a significant number of metastatic nodules were present after 6 weeks. In contrast, the mice without injection of B16/F10 cells (group 1) or treated with PCA alone (data not shown) did not form any metastatic nodules. Treatment with PCA inhibited the number of B16/F10 metastatic nodules, the hepatocellular tumour volume and the weight of liver (groups 3 and 4), compared to the corresponding values in group 2 (B16/F10 cells alone).
Table 1.
Effects of PCA on metastasis of B16/F10 melanoma cells in C57/BL6 mice
| Liver metastasis per mouse ± SD | ||||
|---|---|---|---|---|
| Groups | No. of mice | No. of nodules | Tumour volume (mm3) | Weight (mg) |
| 1.Vehicle (PBS) | 12 | ND | ND | 1220 ± 132 |
| 2.B16/F10 cells | 12 | 4.66 ± 3.78a | 168 ± 85a | 1730 ± 240a |
| 3.B16/F10 cells + PCA 5 mg per mouse | 12 | 3.44 ± 1.09a,b | 131 ± 54a,b | 1154 ± 110a,b |
| 4.B16/F10 cells + PCA 10 mg per mouse | 12 | 1.02 ± 0.57a,b | 57 ± 24a,b | 1242 ± 183a,b |
Effect of PCA on liver colonization by B16/F10 cells was determined as described in the text. The mice were killed 6 weeks after cell injection, and the number and tumour volume of liver metastases and the whole liver weight were measured. Liver nodules >0.5 mm in diameter were counted.
ND = non-detected; PBS = phosphate-buffered saline.
P < 0.05 compared with vehicle (group 1).
P < 0.05 compared with B16/F10 group (group 2).
Also, levels of MMP were increased in liver tissues obtained from group 2 animals were reduced by treatment with PCA (Figure 6A). Furthermore, the metastasis-related proteins were readily expressed in the liver tissues colonized by B16/F10 cells, as shown by Western blotting (Figure 6B). Treatment with PCA down-regulated the expression of Ras and p-Akt to 43% and 14% of group 2 (P < 0.005). The levels of RhoB and PKCε, which appeared to be involved in PCA-inhibited invasion in vitro (see earlier discussion) were also enhanced in the samples obtained from the PCA-treated animals.
Figure 6.
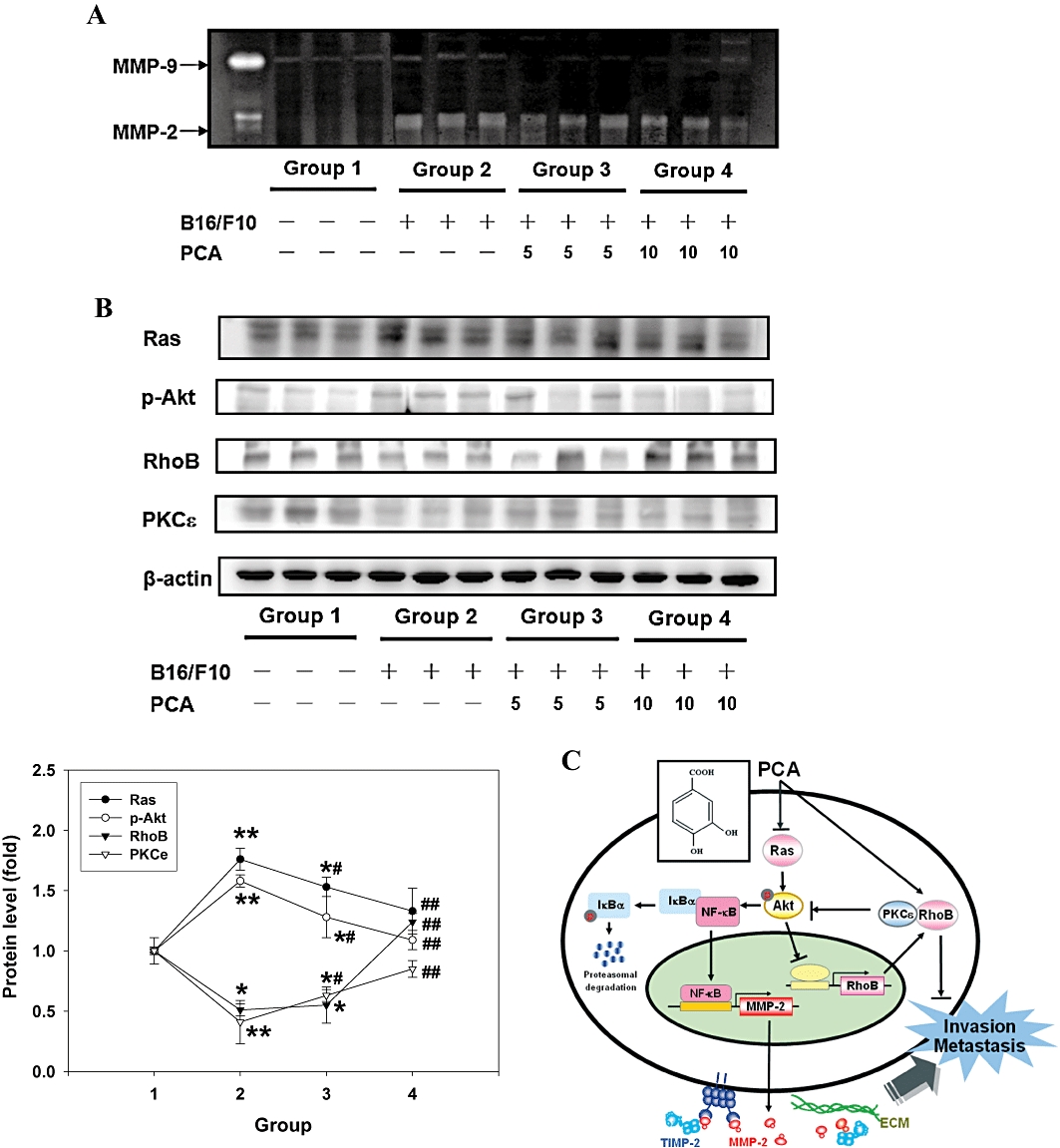
Effects of PCA on the activity of MMPs and the levels of metastasis-related proteins in vivo. 106 B16/F10 cells were injected into the lateral tail vein of 6-week-old C57/BL6 mice. At the same time, two of the groups were orally treated with PCA at doses of 5 mg or 10 mg per mouse. The mice were killed after 6 weeks, and zymography assay (A) and Western blotting (B) were carried out with liver tissue extracts. Results were representative of 12 different mice. The quantitative data were presented as means ± SD of three repeats from one independent study. *P < 0.05, **P < 0.005 significantly different from group 1. #P < 0.05, ##P < 0.005 significantly different from group 2. Group no. 1. vehicle (phosphate-buffered saline); 2. B16/F10; 3. B16/F10 + PCA 5 mg per mouse; and 4. B16/F10 + PCA 10 mg per mouse. (C) A proposed model for the PCA-mediated inhibition of human cancer cell migration, invasion and metastasis. See the text for discussion.
Discussion
In recent years, attention has been focused on the anti-cancer properties of several components of food for application in cancer chemoprevention. The simple phenolic compound PCA is one of the major benzoic acid derivatives from vegetables and fruits with a strong anti-oxidative effect, 10-fold higher than that of α-tocopherol (Ueda et al., 1996). PCA, even at 100 ppm in a diet, shows potent chemopreventive effects on liver, colon, pancreas and gastric carcinogenesis in rats (Tanaka et al., 1993; 1995; Kawamori et al., 1994; Nakamura et al., 2000). Our recent study (Lin et al., 2007) demonstrated the effectiveness of PCA against tumour proliferation in human gastric adenocarcinoma AGS cells. The result of the present study is in agreement with this previous study concerning the inhibitory activity of PCA against tumorigenesis in the stomach. In some cancer cells, PCA treatment suppressed cell adhesion and the production of interleukin (IL)-6, IL-8, vascular endothelial growth factor and the intercellular adhesion molecule-1, which would further attenuate angiogenic and metastatic actions (Yin et al., 2009). However, the molecular mechanisms underlying the PCA-mediated inhibition of cancer cell migration, invasion and metastasis have not been elucidated until our present study, showing that the down-regulation of the Ras/PI3K/Akt signalling pathway is likely to be responsible for the inhibitory effects of PCA.
An increased expression of MMP-2 and MMP-9 has been shown to be related to an invasive phenotype of cancer cells. It is also relevant to note that green tea polyphenol, epigallocatechin gallate (EGCG), suppressed matrix degradation in rat hepatic stellate cells by altering the balance between MMP-2 and TIMP-2 (Zhen et al., 2006). Recent findings have demonstrated that high levels of expression of MMP-2 in epithelial cells in gastric cancer is associated with poor survival, and that aggressive forms of gastric cancer are associated with low TIMP-2 expression (Alakus et al., 2008). The present study demonstrated that PCA inhibited the secretion of MMP-2, but not MMP-9, by AGS cells (Figure 2A). PCA not only had an effect on the activity and expression of MMP-2, but also altered the balance between MMP-2 and TIMP-2 (Figure 2B). The transcription of MMP-2 gene is regulated by upstream regulatory elements, including NF-κB and AP-1 binding sites (Westermarck and Kähäri, 1999; Chen et al., 2008). Indeed, one or more of these binding sites have been implicated in mediating the effects of a diverse set of agents. Consequently, suppression of the activity of NF-κB, c-Fos and c-Jun, or blockade of their binding to respective regulatory elements, potentially inhibited tumour invasion (Bergman et al., 2003; Philip and Kundu, 2003). Consistent with these findings, the treatment of AGS cells with PCA suppressed NF-κB DNA binding activity (Figure 3B), which was accompanied by a decrease in nuclear translocation of this transcription factor (Figure 3A) and decreased degradation of IκBα (Figure 3C and D). Therefore, it is possible that the inhibitory effect of PCA on the motility and invasion of AGS cells was via the inactivation of NF-κB that subsequently down-regulated the expression of MMP-2.
Multiple genetic changes take place during the process of carcinogenesis. Identifying key proteins, such as PI3K, Akt and MAPK involved in these processes is vital for understanding carcinogenesis and devising new therapies. Further, NF-κB is a MAPK and Akt-responsive promoter element (Woo et al., 2005). Therefore, MAPK and Akt signal transduction pathway may play an important role in the regulation of MMP-2 expression. Increasing evidence suggests that the PI3K/Akt signalling pathway is involved in cell migration and invasion (Tanno et al., 2001; Vivanco and Sawyers, 2002), and inhibition of this signalling pathway is a promising approach in cancer treatment (Siddiqui et al., 2004). In agreement with these reports, we observed that PCA caused a dose- and time-dependent decrease in cellular levels of PI3K and phosphorylated Akt (Figure 4A), without a noticeable change in total and phosphorylated levels of MAPK (Figure S2).
Many traditional herbal medicines have been reported to exert different functions in different cell types at different concentrations (Chiu and Wan, 1999; Chan et al., 2000). As our study has shown, PCA at lower concentrations (0.1–2.0 mM) inhibited cell migration and invasion via a sustained inactivation of the PI3K/Akt signal in AGS cells (Figures 1 and 4A), and, on the other hand, a higher dosage (2.0 mM) induced apoptosis via p53 signalling and the p38 MAPK/FasL cascade pathway (Lin et al., 2007). Our findings have, therefore, provided evidence of a dose-dependent range of effects of PCA on AGS cells.
When AGS cells were treated with PCA (0.1–2.0 mM) for 24 h, there was an increase in the level and activity of RhoB, but not of the other small GTPase family proteins (Figure 4A and B). This is the first report documenting the polyphenol-induced anti-invasive and anti-metastatic activities of RhoB. It is an important finding, since RhoB has been mostly associated with carcinogenesis to date, such as deregulation of RhoB repression in tumorigenesis, rather than being involved in the action of chemopreventive agents (Karlsson et al., 2009). In this study, PCA was shown to inhibit the expression of MMP-2, NF-κB, PI3K and Akt, accompanied by an increase in active RhoB. Thus, PCA may target the activation of RhoB as a mechanism to inhibit the Akt/NF-κB/MMP-2 pathway in AGS cells. Consistent with this is the demonstration by Jiang et al., (2004) that one possible mechanism by which RhoB inhibits tumour migration/invasion is by blocking the ability of the Ras/PI3K/Akt pathway to activate NF-κB binding to the MMP promoter. However, the mechanism by which Ras/Akt inhibits RhoB expression remains unknown. It is well established that the promoter region of human RhoB gene contains binding sites for NF-Y, AP2, SP1 and c-myb, as determined by mat-inspector analysis (Delarue et al., 2007; Kim et al., 2010). The earlier study had showed that Ras/Akt signalling can affect the activity of many transcription factors, including AP2 and c-myb. This raises the possibility that Ras/Akt prevents the binding of transcription factors such as AP2, by recruiting histone deacetylase to the promoter. Such an inhibition of transcription factor binding will facilitate histone deacetylation and decrease transcription of the RhoB promoter selectively (Delarue et al., 2007). Therefore, the Akt signal transduction pathway may play an important role in the regulation of RhoB expression. Additional studies are needed to elucidate the molecular basis for the Akt-blocked expression of RhoB and their association with the regulation of cancer cell invasion.
The involvement of the RhoB/PKCε signal pathway in the inhibitory effect of PCA on cell invasion was further confirmed in the experiments using GFX and PKCε siRNA (Figure 4D and E), implying that an increase in the RhoB/PKCε complex could either indirectly inhibit the phosphorylation of Akt that subsequently prevents downstream factors required for cell migration/invasion, or directly retard cell migration/invasion. Additionally, recent studies have proposed PKCε as a negative regulator of Akt activation (Matsumoto et al., 2001; Liu et al., 2006). Our results would support these reports and demonstrate that the RhoB/PKCε complex was involved in the suppression of Akt-mediated cell migration and invasion by PCA (Figure 4C–E).
Through our experiments, a new link has been established between MMP-2 expression and Ras activation, which alters both cell migration and invasion. AGS cells transfected with pcDNA-ras (active form) showed increases in cell growth, migration/invasion, MMP-2 expression (Figure 5) and also in the levels of PI3K/Akt signal proteins. Our genetic evidence demonstrated that Ras signalling played a crucial role in the suppressive effect of PCA on cell migration/invasion. Because of its inhibitory effects on Ras/Akt/NF-κB, PCA might, in turn, have a significant impact on the mechanism to inhibit the MMP-mediated cellular events in AGS cells.
Based on these results, we have produced a schematic presentation of possible mechanisms for the effects of PCA on migration and invasion of AGS cells (Figure 6C). The results clearly demonstrated that PCA regulated an antagonistic interaction between the oncogenic Ras/Akt/NF-κB tumour progression pathway and RhoB. Most significantly, PCA given with the B16/F10 melanoma cells reduced dramatically their metastasis to the liver in the animal model (Table 1). Our data also showed that PCA inhibited both the invasion and metastatic potential of malignant carcinoma cells in vitro and in vivo through the regulation of MMP activity, which was mediated through the cross-talk of Ras/Akt and RhoB/PKCε (Figure 6A and B). These results demonstrated that PCA inhibited the progression of cancer cells by several mechanisms: repression of migration, decreased matrix degradation and inhibition of metastasis. Taken together, these results suggested that PCA could decrease the invasiveness of cancer cells and, therefore, may be of value in developing as an anti-cancer agent.
Acknowledgments
This work was supported by the grant from the National Science Council (NSC94-2320-B-040-046), Taiwan.
Glossary
Abbreviations
- AGS cells
human gastric carcinoma cells
- AP-1
activator protein-1
- ECL
enhanced chemiluminescence
- ECM
extracellular matrix
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor-κB
- PCA
protocatechuic acid
- PI3K
phosphatidylinositol 3-kinase
- Rho
Ras-homologous
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- TBS
Tris-buffered saline
Conflicts of interest
No conflicts of interest were stated.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effects of mutant NF-kB expression vector on PCA-inhibited cell invasion. AGS cells were transfected with a control empty pUC vector or a NF-kB p65 cDNA and treated with or without 1.0 mM of PCA for 24 h. (A) The cellular level of NF-kB was analysed by Western blot. β-actin was served as an internal control. The quantitative data were presented as means ± SD of three repeats from one independent study. (B) Cell invasion was analysed by Boyden chamber assay. Results are representative of at least three independent experiments.
Figure S2 Effects of PCA on MAPK and FAK activation. AGS cells were treated with various concentrations (0–2.0 mM) of PCA for 24 h, after which cells were harvested and analysed for the levels and phorylations of p38, JNK, ERK, and FAK proteins. Equal amounts of cell lysates (50 μg) were resolved by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose and probed with specific p-MAPK, MAPK, p-FAK and FAK antibodies. β-actin was served as an internal control of protein level. Results are representative of at least three independent experiments. Control (C) was the untreated PCA sample. Dimethyl sulphoxide (DMSO) (0.2%, D) served as the solvent control.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alakus H, Grass G, Hennecken JK, Bollschweiler E, Schulte C, Drebber U, et al. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–923. doi: 10.14670/HH-23.917. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bergman MR, Cheng S, Honbo N, Piacentini L, Karliner JS, Lovett DH. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem J. 2003;369:485–496. doi: 10.1042/BJ20020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000;160:219–228. doi: 10.1016/s0304-3835(00)00591-7. [DOI] [PubMed] [Google Scholar]
- Chen YX, Li BZ, Diao F, Cao DM, Fu CC, Lu J. Up-regulation of RhoB by glucocorticoids and its effects on the cell proliferation and NF-κB transcriptional activity. J Steroid Biochem Mol Biol. 2006;101:179–187. doi: 10.1016/j.jsbmb.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Chen JH, Lin HH, Chiang TA, Ho HH, Lee YC, Wang CJ. Gaseous nitrogen oxide promotes human lung cancer cell line A549 migration, invasion, and metastasis via iNOS-mediated MMP-2 Production. Toxicol Sci. 2008;106:364–375. doi: 10.1093/toxsci/kfn195. [DOI] [PubMed] [Google Scholar]
- Chiu LC, Wan JM. Induction of apoptosis in HL-60 cells by eicosapentaenoic acid (EPA) is associated with downregulation of bcl-2 expression. Cancer Lett. 1999;145:17–27. doi: 10.1016/s0304-3835(99)00224-4. [DOI] [PubMed] [Google Scholar]
- Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40:143–149. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- Delarue FL, Adnane J, Joshi B, Blaskovich MA, Wang DA, Hawker J, et al. Farnesyltransferase and geranylgeranyltransferase I inhibitors upregulate RhoB expression by HDAC1 dissociation, HAT association and histone acetylation of the RhoB promoter. Oncogene. 2007;26:633–640. doi: 10.1038/sj.onc.1209819. [DOI] [PubMed] [Google Scholar]
- Du J, Jiang B, Coffey RJ, Barnard J. Raf and RhoA cooperate to transform intestinal epithelial cells and induce growth resistance to transforming growth factor beta. Mol Cancer Res. 2004;2:233–241. [PubMed] [Google Scholar]
- Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2005;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- Fritz G, Kaina B, Aktories K. The ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J Biol Chem. 1995;270:25172–25177. doi: 10.1074/jbc.270.42.25172. [DOI] [PubMed] [Google Scholar]
- Gong M, Meng L, Jiang B, Zhang J, Yang H, Wu J, et al. p37 from Mycoplasma hyorhinis promotes cancer cell invasiveness and metastasis through activation of MMP-2 and followed by phosphorylation of EGFR. Mol Cancer Ther. 2008;7:530–537. doi: 10.1158/1535-7163.MCT-07-2191. [DOI] [PubMed] [Google Scholar]
- Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2000;9:1163–1170. [PubMed] [Google Scholar]
- Hwang ES, Lee HJ. Benzyl isothiocyanate inhibits metalloproteinase-2/-9 expression by suppressing the mitogen-activated protein kinase in SK-Hep1 human hepatoma cells. Food Chem Toxicol. 2008;46:2358–2364. doi: 10.1016/j.fct.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Jiang K, Sun J, Cheng J, Djeu JY, Wei S, Sebti S. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol Cell Biol. 2004;24:5565–5576. doi: 10.1128/MCB.24.12.5565-5576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, Hatzoglou A, et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res. 2004;6:R63–R74. doi: 10.1186/bcr752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Tanaka T, Kojima T, Suzui M, Ohnishi M, Mori H. Suppression of azoxymethane-induced rat colon aberrant crypt foci by dietary protocatechuic acid. Jpn J Cancer Res. 1994;85:686–691. doi: 10.1111/j.1349-7006.1994.tb02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Won M, Choi CH, Ahn J, Kim BK, Song KB, et al. Increase of RhoB in gamma-radiation-induced apoptosis is regulated by c-Jun N-terminal kinase in Jurkat T cells. Biochem Biophys Res Commun. 2010;391:1182–1186. doi: 10.1016/j.bbrc.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Lee IR, Yang MY. Phenolic compounds from Duchesnea chrysantha and their cytotoxic activities in human cancer cell. Arch Pharm Res. 1994;17:476–479. doi: 10.1007/BF02979129. [DOI] [PubMed] [Google Scholar]
- Li P, Wang XQ, Wang HZ, Wu YN. High performance liquid chromatographic determination of phenolic acids in fruits and vegetables. Biomed Environ Sci. 1993;6:389–398. [PubMed] [Google Scholar]
- Lin HH, Chen JH, Huang CC, Wang CJ. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int J Cancer. 2007;120:2306–2316. doi: 10.1002/ijc.22571. [DOI] [PubMed] [Google Scholar]
- Liu H, Qiu Y, Xiao L, Dong F. Involvement of protein kinase Cepsilon in the negative regulation of Akt activation stimulated by granulocyte colony-stimulating factor. J Immunol. 2006;176:2407–2413. doi: 10.4049/jimmunol.176.4.2407. [DOI] [PubMed] [Google Scholar]
- Liu GT, Zhang TM, Wang BE, Wang YW. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol. 1992;43:147–152. doi: 10.1016/0006-2952(92)90271-j. [DOI] [PubMed] [Google Scholar]
- Masella R, Cantafora A, Modesti D, Cardilli A, Gennaro L, Bocca A, et al. Antioxidant activity of 3,4-DHPEA-EA and protocatechuic acid: a comparative assessment with other olive oil biophenols. Redox Rep. 1999;4:113–121. doi: 10.1179/135100099101534792. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Ogawa W, Hino Y, Furukawa K, Ono Y, Takahashi M, et al. Inhibition of insulin-induced activation of Akt by a kinase-deficient mutant of the isozyme of protein kinase C. J Biol Chem. 2001;276:14400–14406. doi: 10.1074/jbc.M011093200. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nishikawa A, Furukawa F, Kasahara K, Miyauchi M, Son HY, et al. Inhibitory effects of protocatechuic acid on the post-initiation phase of hamster pancreatic carcinogenesis induced by N-nitrosobis(2-oxopropyl)amine. Anticancer Res. 2000;20:3423–3427. [PubMed] [Google Scholar]
- Parks WC, Shapiro SD. Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip S, Kundu GC. Osteopontin induces nuclear factor kappa B mediated promatrix metalloproteinase-2 activation through I kappa B alpha/ IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J Biol Chem. 2003;278:14487–14497. doi: 10.1074/jbc.M207309200. [DOI] [PubMed] [Google Scholar]
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- Reischer D, Heyfets A, Shimony S, Nordenberg J, Kashman Y, Flescher E. Effects of natural and novel synthetic jasmonates in experimental metastatic melanoma. Br J Pharmacol. 2007;150:738–749. doi: 10.1038/sj.bjp.0707146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui IA, Adhami VM, Afaq F, Ahmad N, Mukhtar H. Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J Cell Biochem. 2004;91:232–242. doi: 10.1002/jcb.10737. [DOI] [PubMed] [Google Scholar]
- Stich HF. The beneficial and hazardous effects of simple phenolic compounds. Mutat Res. 1991;259:307–324. doi: 10.1016/0165-1218(91)90125-6. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kojima T, Kawamori T, Yoshimi N, Mori H. Chemoprevention of diethylnitrosamine-induced hepatocarcinogenesis by a simple phenolic acid protocatechuic acid in rats. Cancer Res. 1993;53:2775–2779. [PubMed] [Google Scholar]
- Tanaka T, Kojima T, Kawamori T, Mori H. Chemoprevention of digestive organs carcinogenesis by natural product protocatechuic acid. Cancer. 1995;75:1433–1439. doi: 10.1002/1097-0142(19950315)75:6+<1433::aid-cncr2820751507>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589–593. [PubMed] [Google Scholar]
- Tseng TH, Wang CJ, Kao ES, Chu HY. Hibiscus protocatechuic acid protects against oxidative damage induced by tert-butylhydroperoxide in rat primary hepatocytes. Chem Biol Interact. 1996;101:137–148. doi: 10.1016/0009-2797(96)03721-0. [DOI] [PubMed] [Google Scholar]
- Tseng TH, Kao TW, Chu CY, Chou FP, Lin WL, Wang CJ. Induction of apoptosis by hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem Pharmacol. 2000;60:07–15. doi: 10.1016/s0006-2952(00)00322-1. [DOI] [PubMed] [Google Scholar]
- Ueda J, Saito N, Shimazu Y, Ozawa T. A comparison of scavenging abilities of antioxidants against hydroxyl radicals. Arch Biochem Biophys. 1996;333:77–84. doi: 10.1006/abbi.1996.0404. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Weng MW, Lai JC, Hsu CP, Yu KY, Chen CY, Lin TS, et al. Alternative splicing of MDM2 mRNA in lung carcinomas and lung cell lines. Environ Mol Mutagen. 2005;46:1–11. doi: 10.1002/em.20118. [DOI] [PubMed] [Google Scholar]
- Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH, Kim WK, et al. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem Biophys Res Commun. 2005;335:1017–1025. doi: 10.1016/j.bbrc.2005.07.174. [DOI] [PubMed] [Google Scholar]
- Yen GC, Hsieh CL. Reactive oxygen species scavenging activity of Du-zhong (Eucommia ulmoides Oliv.) and its active compounds. J Agric Food Chem. 2000;48:3431–3436. doi: 10.1021/jf000150t. [DOI] [PubMed] [Google Scholar]
- Yin MC, Lin CC, Wu HC, Tsao SM, Hsu CK. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: potential mechanisms of action. J Agric Food Chem. 2009;57:6468–6473. doi: 10.1021/jf9004466. [DOI] [PubMed] [Google Scholar]
- Zhen MC, Huang XH, Wang Q, Sun K, Liu YJ, Li W, et al. Green tea polyphenol epigallocatechin-3-gallate suppresses rat hepatic stellate cell invasion by inhibition of MMP-2 expression and its activation. Acta Pharmacol Sin. 2006;27:1600–1607. doi: 10.1111/j.1745-7254.2006.00439.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


