Abstract
BACKGROUND AND PURPOSE
We investigated whether high-fat diet (HFD)-induced obesity was associated with changed function of components of the mesenteric innervation (adrenergic, sensory and nitrergic), the mechanisms involved and the possible effects of rosuvastatin on these changes.
EXPERIMENTAL APPROACH
Male Wistar rats were divided into three groups. (i) rats fed a standard diet (control group); (ii) rats fed a HFD (33.5% fat) for 7 weeks; and (iii) rats fed a HFD and treated with rosuvastatin (15 mg·kg−1·day−1) for 7 weeks. Segments of isolated mesenteric arteries were exposed to electric field stimulation (EFS) with or without tetrodotoxin, phentolamine, 7-nitroindazole (7NI) or Nω nitro-L-arginine methyl ester (L-NAME). Noradrenaline, ATP and NO release, and nNOS expression were also measured.
KEY RESULTS
EFS induced a greater frequency-dependent contraction in obese than in control rats. In HFD rats, phentolamine reduced contractions elicited by EFS, but noradrenaline release was greater and ATP release decreased. L-NAME and 7NI increased contractions to EFS in segments from control rats, but not in those from HFD rats. NO release and nNOS expression were lower in arterial segments from HFD rats than in control rats. All these changes in HFD rats were reversed by treatment with rosuvastatin.
CONCLUSIONS AND IMPLICATIONS
Neural control of mesenteric vasomotor tone was altered in HFD rats. Enhanced adrenergic and diminished nitrergic components both contributed to increased vasoconstrictor responses to EFS. All these changes were reversed by rosuvastatin, indicating novel mechanisms of statins in neural regulation of vascular tone.
Keywords: obesity, statins, adrenergic innervation, nitrergic innervation
Introduction
Dietary factors, especially hypercaloric diets, are a determinant in the etiology of obesity in humans. Numerous studies in rodents have shown that chronic consumption of high-fat diets (HFDs) induces increased gain in weight along with metabolic and cardiovascular alterations such as insulin resistance, dyslipidaemia, hypertension, cardiac haemodynamic disturbances and renal function impairment (Williams et al., 2003; de las Heras et al., 2009).
Accumulating experimental and clinical evidence indicates that obesity is associated with vascular function alterations. Endothelial dysfunction, due to altered NO release and metabolism, has been reported as participating in these vascular alterations (Javeshghani et al., 2009; Belin de Chantemèle et al., 2010; Miâdi-Messaoud et al., 2010). The angiotensin II system also seems to be involved in obesity-associated alterations in both humans and rats (de las Heras et al., 2009; Elmarakby and Imig, 2010; Katragadda and Arora, 2010; Rueda-Clausen et al., 2010). The sympathetic nervous system seems to participate in the pathological cardiovascular and metabolic alterations associated with obesity (Prior et al., 2010). However, certain controversial results have been reported and appear to be related to the specific vascular bed studied. The sympathetic nervous system is activated in human and experimental obesity produced by overfeeding, and has been proposed as one of the main mechanisms leading to the development of hypertension. This is probably attributable to the activation of the sympathetic outflow to the kidneys, and, perhaps, to insulin resistance (Straznicky et al., 2008).
Vascular innervation plays a key role in the regulation of tone in certain vascular beds such as the mesenteric territory. Three components have been described as participating in nervous control of vascular tone of rat mesenteric artery: adrenergic, sensory and nitrergic (Kawasaki et al., 1988; Li and Duckles, 1992; Boeckxstaens and Pelckmans, 1997; Marín and Balfagón, 1998). Alterations of the functional role of these components have been associated with several experimental and pathophysiological circumstances including ageing, hypertension and diabetes (Ferrer et al., 2000b; 2001; 2003; Marín et al., 2000; Minoves et al., 2002; Hatanaka et al., 2006; Iwatani et al., 2008).
Drugs targeting the cardiovascular system such as adrenoceptor antagonists, angiotensin converting enzyme inhibitors (ACEI) and angiotensin AT1 receptor antagonists (ARBs) have shown beneficial effects in metabolic and cardiovascular disorders associated with obesity through several mechanisms involving endothelial factors, inflammation and oxidative stress (de las Heras et al., 2009; Elmarakby and Imig, 2010; Katragadda and Arora, 2010). Statins have also shown anti-inflammatory effects, as well as improvement in NO availability through hypolipidemic and pleiotropic mechanisms (Tan et al., 2002; Gelosa et al., 2007). However, the effects of statins on alterations of vascular innervation associated with obesity, have not been investigated. Thus, in the present study we aimed to investigate: (i) whether HFD-induced obesity in rats was associated with changes in the function of the three components of mesenteric innervation, as well as the possible mechanisms involved; and (ii) the effects of a statin treatment on the possible functional mesenteric alterations observed in HFD-induced obesity.
Methods
Experimental procedure
All animal care and experimental procedures complied with the European Union guidelines on the ethical care of experimental animals, and US National Institutes of Health guidelines. The study was conducted with male Wistar rats (250 g; Harlan Ibérica SL, Barcelona, Spain). Animals were divided into three groups. (i) rats fed a standard diet for 7 weeks as a reference group for normal conditions (control); (ii) rats fed a HFD (33.5% fat) (Harlan Teklad #TD.03307, MN, USA) for 7 weeks (de las Heras et al., 2009); and (iii) rats fed the same HFD (33.5% fat) and treated with rosuvastatin (15 mg·kg−1 day) for 7 weeks. The dose of rosuvastatin was chosen from pilot studies from previous reports (Di Napoli et al., 2005; Otto et al., 2005; Schäfer et al., 2005), where this drug showed a hypolipidemic effect in experimental models. Body weight was measured once a week. Food and water intake were determined throughout the experimental period.
After 7 weeks of treatment, the animals were killed; the first branch of the mesenteric artery was carefully dissected, cleaned of connective tissue and placed in Krebs-Henseleit solution (KHS, in mmol·L−1: NaCl 115, CaCl 2 2.5, KCl 4.6, KH2PO4 1.2, MgSO4. 7H2O 1.2, NaHCO3 25, glucose 11.1, Na2EDTA 0.03) at 4°C. Body weight at the end of the experiment was normalized to tibia length, because of the important body weight differences between HFD rats and controls. Tibia length is used to compare parameters in animals where the main variable in the model is body weight (Khamaisi et al., 2007; de las Heras et al., 2009; Xu et al., 2009).
Lipid profile
Plasma concentrations of total cholesterol, triglycerides (TG) and HDL-cholesterol were determined using specific quantitative sandwich enzyme immunoassay (R&D Systems, Minneapolis, MN, USA). As the calculation of LDL cholesterol could underestimate the lipid alterations in situations where TG are elevated, as in our model of obesity, non-HDL cholesterol was calculated. Non-HDL cholesterol (which includes both LDL and VLDL cholesterol) is considered more appropriate in conditions of high TG.
Vascular reactivity
The method used for isometric tension recording has been described in full elsewhere (Nielsen and Owman, 1971). Two parallel stainless steel pins were introduced through the lumen of the vascular segment: one was fixed to the bath wall, and the other connected to a force transducer (Grass FTO3C; Quincy, MA, USA); this was connected in turn to a model 7D Grass polygraph. For electric field stimulation (EFS) experiments, segments were mounted between two platinum electrodes 0.5 cm apart and connected to a stimulator (Grass, model S44) modified to supply the appropriate current strength. Segments were suspended in an organ bath containing 5 mL of KHS at 37°C continuously bubbled with a 95% O2-5% CO2 mixture (pH of 7.4). Experiments were performed in endothelium-denuded segments in order to eliminate complex interferences of vasoactive substances released from endothelial cells [such as NO, endothelin-1, prostacyclin (PGI2), angiotensin II] as we have previously demonstrated (Ferrer et al., 1992; 2000a; Iwatani et al., 2008). Interrelationships among the many endothelium-derived agents and the components of mesenteric innervation would make the study extremely complicated. In addition, endothelium denudation avoids possible actions by different drugs used on endothelial cells that could lead to misinterpretation of the results. Endothelium was removed by gently rubbing the luminal surface of the segments with a thin wooden stick. The segments were subjected to a tension of 0.5 g, which was readjusted every 15 min during a 90-min equilibration period before drug administration. After this, the vessels were exposed to 75 mmol·L−1 KCl to check their functional integrity. Endothelium removal did not alter the contractions elicited by 75 mmol·L−1 KCl. After a washout period, the absence of vascular endothelium was tested by the inability of 10 µmol·L−1 ACh to relax segments precontracted with 1 µmol·L−1 noradrenaline.
Two consecutive frequency-response curves to EFS (1, 2, 4, 8 and 16 Hz) were performed in mesenteric segments from all experimental groups. The parameters used for EFS were 200 mA, 0.3 ms, 1–16 Hz, for 30 s with a one-minute interval between each stimulus, which is the time required to recover basal tone. These parameters were used in previous studies due to their similarity to physiological conditions (Marín and Balfagón, 1998). A washout period of at least 1 h was necessary to avoid desensitization between consecutive curves.
To evaluate whether EFS-induced contractile response had a neural origin, the blocker for nerve impulse propagation, tetrodotoxin (TTX; 0.1 µmol·L−1) was added to the bath 30 min before EFS.
To determine the contribution of adrenergic innervation to EFS-induced responses in segments from control, HFD and HFD + rosuvastatin rats, 1 µmol·L−1 phentolamine, a non-specific α-adrenoceptor antagonist (Marín and Balfagón, 1998), was added to the bath 30 min before performing the frequency-response curve.
In order to analyse the possible participation of sensory innervation on EFS-induced response, 0.5 µmol·L−1 the fragment of calcitonin-gene related peptide (CGRP 8-37), a CGRP receptor antagonist, was added to the bath 30 min before performing the frequency–response curve in segments from all of the experimental groups.
To determine the participation of NO in EFS-induced response in all groups of rats, the non-selective NO synthase (NOS) inhibitor, Nω nitro-L-arginine methyl ester (L-NAME; 0.1 µmol·L−1), 1 µmol·L−1 1400W, a selective inhibitor of inducible NOS inhibitor, or 0.1 mmol·L−1 7-nitroindazole (7NI), a specific inhibitor of neuronal NOS, was added to the bath 30 min before performing the frequency–response curve.
The vasoconstrictor response to exogenous noradrenaline (1 nmol·L−1–10 µmol·L−1) in presence/absence of L-NAME (0.1 µmol·L−1), and 7NI (0.1 mmol·L−1) were tested in arteries from all experimental groups. In addition, the vasodilator response to NO donor diethylamine NONOate (DEA-NO; 0.1 nmol·L−1–0.1 mmol·L−1) was tested in arteries from all experimental groups. Artery function was checked after the entire experiment by exposing arteries to 75 mmol·L−1 KCl.
Noradrenaline, CGRP, and ATP release
Endothelium-denuded segments from control, HFD and HFD + rosuvastatin rats were preincubated for 30 min in 5 mL of KHS at 37°C and continuously gassed with a 95% O2–5% CO2 mixture (stabilization period). This was followed by two washout periods of 10 min in a bath of 0.4 mL of KHS. Then, the medium was collected to measure basal release. Once the bath was refilled, arteries were subjected to cumulative 30-second EFS periods at 1, 2, 4, 8 and 16 Hz at 1 min intervals. At the end of EFS stimulation, the medium was collected to measure neurotransmitter release. Samples were frozen in liquid nitrogen and stored at –70°C until the assay was performed. To measure the release of noradrenaline, CGRP or ATP we used a noradrenaline research EIA (Labor Diagnostica Nord, Gmbh & Co., KG, Nordhon, Germany), a rat CGRP enzyme immunoassay kit (Spibio, Bertin Group, Montigny le Bretonneux, France), or an ATP Colorimetric/Fluorometric Assay kit (Abcam, Cambridge, UK). Assays were performed following the manufacturer's instructions. Results were expressed as ng noradrenaline·mL−1 per mg of tissue, pg CGRP·mL−1 per mg tissue, or nmol ATP·mg−1 tissue.
Release of NO
NO release was measured as previously described (del Campo et al., 2009). Endothelium-denuded segments from control, HFD and HFD + rosuvastatin rats were subjected to a resting tension of 0.5 g as indicated for reactivity experiments. After an equilibration period of 60 min in HEPES buffer (in mmol·L−1: NaCl 119; HEPES 20; CaCl2 1.2; KCl 4.6; MgSO4 1; KH2PO4 0.4; NaHCO3 5; glucose 5.5; Na2HPO4 0.15; pH 7.4) at 37°C, arteries were incubated with the fluorescent probe 4,5-diaminofluorescein (DAF-2; 2 µmol·L−1) for 45 min. Then, the medium was collected to measure basal NO release. Once the organ bath was refilled, 30 s cumulative EFS periods at 1, 2, 4, 8 and 16 Hz at 1 min intervals were applied. At the end of EFS stimulation, the medium was collected to measure EFS-induced NO release. The fluorescence of the medium was measured at room temperature using a spectrofluorimeter (LS50 Perkin Elmer instruments, Waltham, MA, USA, FL WINLAB Software) with excitation wavelength set at 492 nm and emission wavelength at 515 nm.
The EFS-induced NO release was calculated by subtracting basal NO release from that evoked by EFS. Also, blank samples were collected in the same way as from segment-free medium in order to correct for background emission. To confirm the neural origin of NO, some assays were performed in the presence of 0.1 mmol·L−1 7-NI or 0.1 µmol·L−1 TTX. The amount of NO released was expressed as arbitrary units·mg−1 tissue.
Western blot analysis of neuronal NOS
For Western blot analysis of nNOS expression, endothelium-denuded mesenteric segments from all experimental groups were homogenized in a boiling buffer composed of 1 mM sodium vanadate, 1% sodium dodecyl sulfate (SDS), and 0.01 M pH 7.4 Tris–HCl. Homogenates containing 30 µg protein were electrophoretically separated on a 7.5% SDS–polyacrylamide gel, and then transferred to a polyvinyl difluoride membrane (Bio-Rad Immun-Blot) overnight at 4°C, 230 mA, using a Bio-Rad Mini Protean III system (Bio-Rad Laboratories, Hercules, CA, USA) containing 25 mM Tris, 190 mM glycine, 20% methanol, and 0.05% SDS. The membrane was blocked for 1 h at room temperature in Tris-buffered-saline solution (100 mM, 0.9% w/v NaCl, 0.1% SDS) with 5% powdered fat-free milk before being incubated overnight at 4°C with mouse monoclonal antibody against nNOS (1:2000 dilution, Transduction laboratories). After washing, the membrane was incubated with anti-mouse horseradish peroxidase–conjugated immunoglobulin G antibody (Amersham International Plc, Little Chalfont, UK). The membrane was thoroughly washed and the immunocomplexes were then detected using an enhanced horseradish peroxidase/luminol chemiluminescence system (ECL Plus, Amersham International Plc) and subjected to autoradiography (Hyperfilm ECL, Amersham International Plc). Signals on the immunoblot were quantified using a computer program (NIH Image V1.56, National Institute of Health, Bethesda, MD, USA). The same membrane was used to determine α-actin expression, and the content of the latter was used to correct protein expression in each sample by means of a monoclonal anti α-actin antibody (1:2000 dilution, Sigma, Madrid, Spain). Rat brain homogenates were used as positive control.
Data analysis
The responses elicited by EFS and noradrenaline were expressed as a percentage of the initial contraction elicited by 75 mmol·L−1 KCl for comparison between experimental groups. The relaxation induced by DEA-NO was expressed as a percentage of the initial contraction elicited by noradrenaline. Results are given as mean + SEM. Statistical analysis was done by comparing the curve (1 to 16 Hz) obtained in the presence of the different substances with the control curve by means of repeated measure analysis of variance (anova) followed by Bonferroni's test.
For the experiments on NO, CGRP, ATP and noradrenaline release, the statistical analysis was done using Student's t-test for unpaired experiments. P < 0.05 was considered significant.
Materials
L-noradrenaline hydrochloride, ACh chloride, CGRP, CGRP 8-37, diethylamine NONOate diethylammonium salt, TTX, 1400W, L-NAME hydrochloride, 7-nitroindazole, phentolamine, tempol and DAF-2 were purchased from Sigma-Aldrich. Stock solutions (10 mmol·L−1) of drugs were made in distilled water, except for noradrenaline, which was dissolved in a NaCl (0.9%)-ascorbic acid (0.01% w/v) solution, and 7NI and tempol, which were dissolved in DMSO. The final DMSO concentration did not alter any of the responses in the current studies. These solutions were stored at −20°C and appropriate dilutions were made in KHS on the day of the experiment. Drug and receptor nomenclature follows Alexander et al. (2009).
Results
General characteristics
From the first week onwards, body weight gain was significantly higher in rats fed a HFD compared with rats fed a standard diet (Figure 1).The difference in body weight at the end of experiment was 27% (Table 1). Treatment with rosuvastatin did not affect body weight in HFD rats. Food intake was lower (P < 0.05) in HFD rats compared with controls throughout the experiment. Treatment with rosuvastatin did not modify food intake in HFD rats (Table 1).
Figure 1.
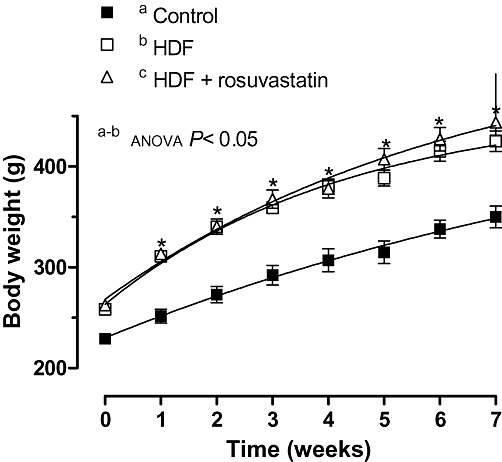
Weekly increases in body weight in control, high-fat diet (HFD) and HFD + rosuvastatin rats. Results are expressed as mean ± SEM n= 6–10 animals per group. *P < 0.05 HFD versus control rats for each week (Bonferroni test).
Table 1.
Final values of body weight, food intake and biochemical parameters
| Control | HFD | HFD + rosuvastatin | |
|---|---|---|---|
| Body weight (g·cm−1) | 321 ± 13 | 423 ± 14* | 437 ± 8.3* |
| Food intake (g) | 6.94 ± 0.36 | 3.41 ± 0.27* | 3.59 ± 0.4* |
| Total cholesterol (mg·L−1) | 458 ± 34 | 470 ± 35 | 418 ± 40 |
| High-density lipoprotein (HDL) cholesterol (mg·L) | 356 ± 16 | 358 ± 22 | 340 ± 24 |
| Non-HDL cholesterol (mg·L−1) | 102 ± 20 | 152 ± 31 | 78 ± 19# |
| Triglycerides (mg·L−1) | 508 ± 35 | 1072 ± 9* | 710 ± 52# |
At the end of the experimental period of 7 weeks, we measured body weight (normalised to tibia length and expressed as g·(cm tibia)−1, plasma total cholesterol: HDL-cholesterol, non-HDL cholesterol and triglycerides, in rats fed a standard diet (Control), high-fat diet (HFD) and rats fed a high-fat diet treated with rosuvastatin at 15 mg·kg−1·day−1 (HFD + rosuvastatin) for 7 weeks. Data shown are means ± SEM.
P < 0.05 versus control;
P < 0.05 versus HFD.
Lipid profile
Plasma total cholesterol and HDL-cholesterol levels were comparable in the three groups. Non-HDL cholesterol levels were comparable in control and HDF rats. However, treatment with rosuvastatin reduced (P < 0.05) non-HDL cholesterol levels in HFD rats.
TG levels were higher (P < 0.05) in rats given a HFD than in controls, and these elevated levels were restored to normal values by treatment with rosuvastatin (Table 1).
Vascular reactivity
Vasoconstrictor response induced by 75 mmol·L−1 KCl was similar in segments from all groups of rats (control, 1028 ± 64 mg; HFD, 912 ± 60; HFD + rosuvastatin, 943 ± 95 mg; P > 0.05; n= 10 per group). No differences were found between KCl vasoconstrictor response at the beginning and at the end of the experiment (control, 1019 ± 85 mg; HFD, 928 ± 64; HFD + rosuvastatin, 979 ± 97 mg; P > 0.05; n= 10 per group).
EFS induced a frequency-dependent contraction, which was greater (P < 0.05) in HFD than in control rats (Figure 2). Treatment with rosuvastatin reduced EFS-induced contractions to a level similar to that in control rats (Figure 2). EFS-induced contractions were practically abolished in segments from all experimental groups by the nerve impulse propagation blocker, TTX (0.1 µmol·L−1; Figure 2).
Figure 2.
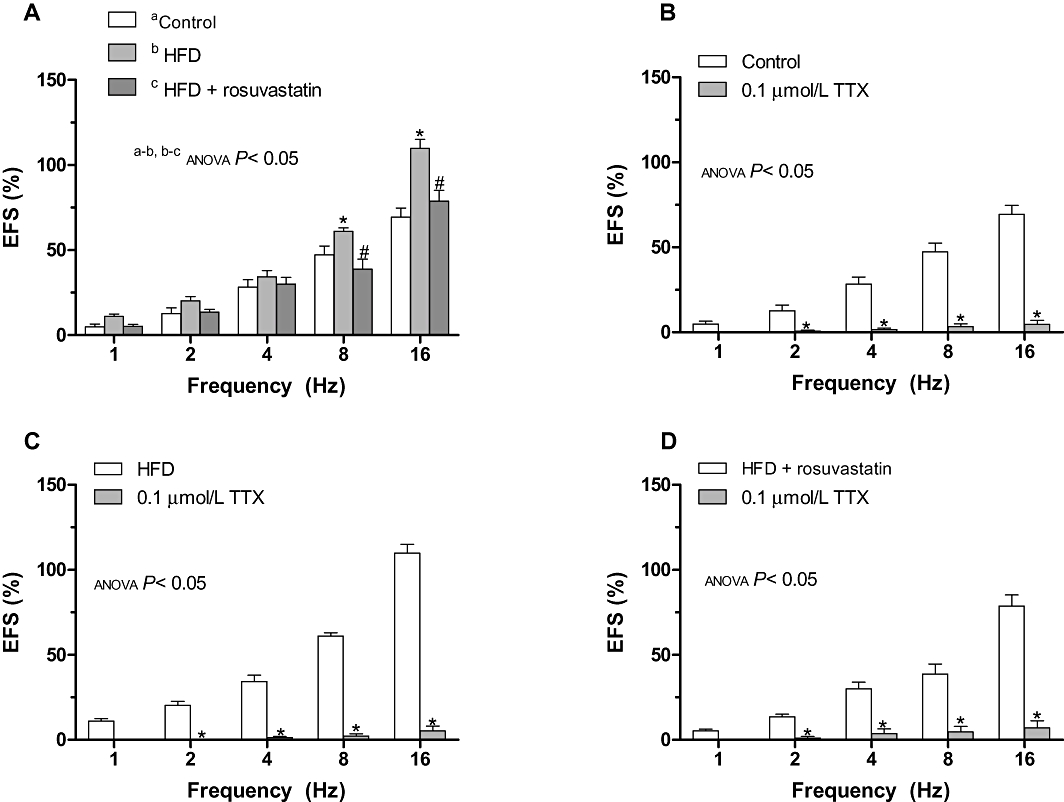
(A) Vasoconstrictor response to electric field stimulation (EFS) in segments from control, high-fat diet (HFD) and HFD + rosuvastatin rats. *P < 0.05 versus control animals for each frequency (Bonferroni test). #P < 0.05 versus HFD animals for each frequency (Bonferroni test). Effect of 0.1 µmol·L−1 TTX on the vasoconstrictor response induced by EFS in segments from control (A), HFD (B) and HFD + rosuvastatin (C) rats. Results (mean ± SEM) are expressed as a percentage of previous contraction elicited by KCl. n= 6–10 animals per group. *P < 0.05 versus conditions without specific inhibitor for each frequency (Bonferroni test).
The contraction elicited by EFS was significantly reduced by the non-selective α-adrenoceptor antagonist, phentolamine (1 µmol·L−1), in segments from all groups of rats, suggesting noradrenaline participation. The decrease was higher in HFD rats than in controls, and was similar to that observed in HFD + rosuvastatin rats (Figure 3).
Figure 3.
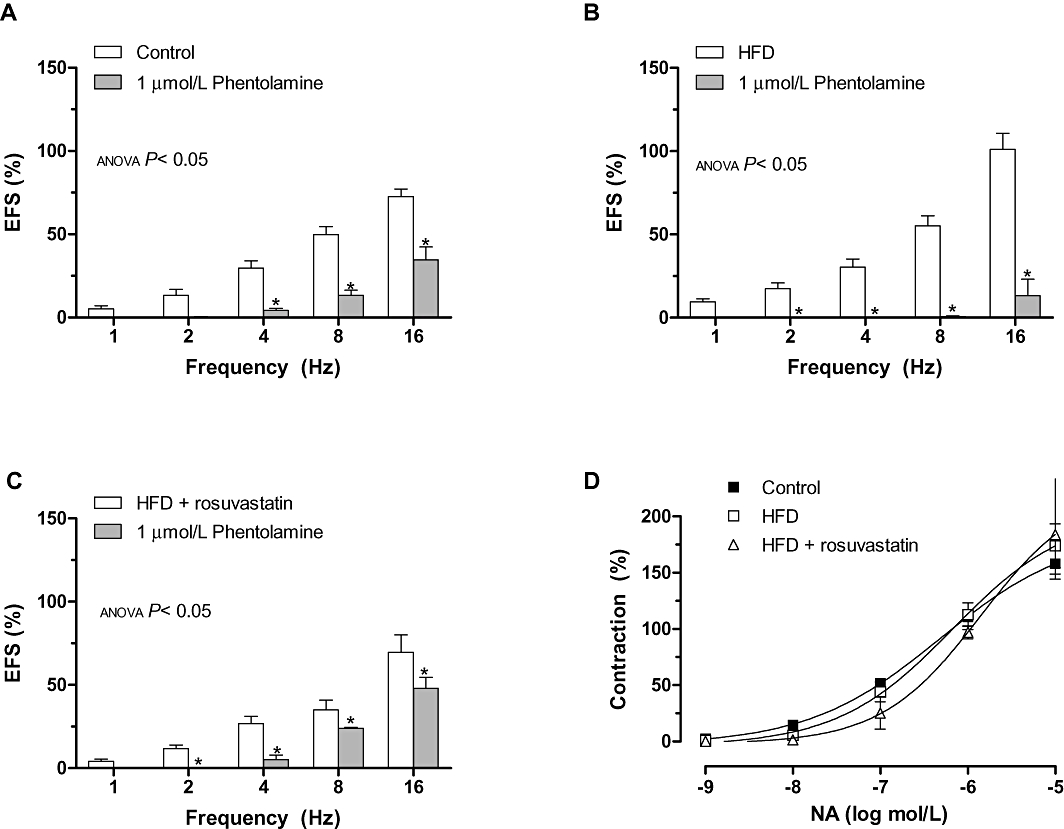
Effect of preincubation with 1 µmol·L−1 phentolamine on the vasoconstrictor response induced by electric field stimulation (EFS) in mesenteric segments from control (A), high-fat diet (HFD) (B) and HFD + rosuvastatin (C) rats. (D) Vasoconstrictor response to exogenous noradrenaline in segments from control, HFD and HFD + rosuvastatin rats. Results (mean ± SEM) are expressed as a percentage of previous contraction elicited by KCl. n= 6–10 animals per group. *P < 0.05 versus conditions without specific inhibitor for each frequency (Bonferroni test).
The contractile response induced by exogenous noradrenaline (0.1 nmol·L−1–10 µmol·L−1) was similar in mesenteric segments from all experimental groups (Figure 3).
The vasodilator response induced by exogenous CGRP was similar in all experimental groups (Figure 4). The CGRP receptor antagonist CGRP 8-37 (0.5 µmol·L−1) did not modify the contractile response induced by EFS in any of the experimental groups, indicating that the sensory nerves did not contribute to the observed effects (Figure 4).
Figure 4.
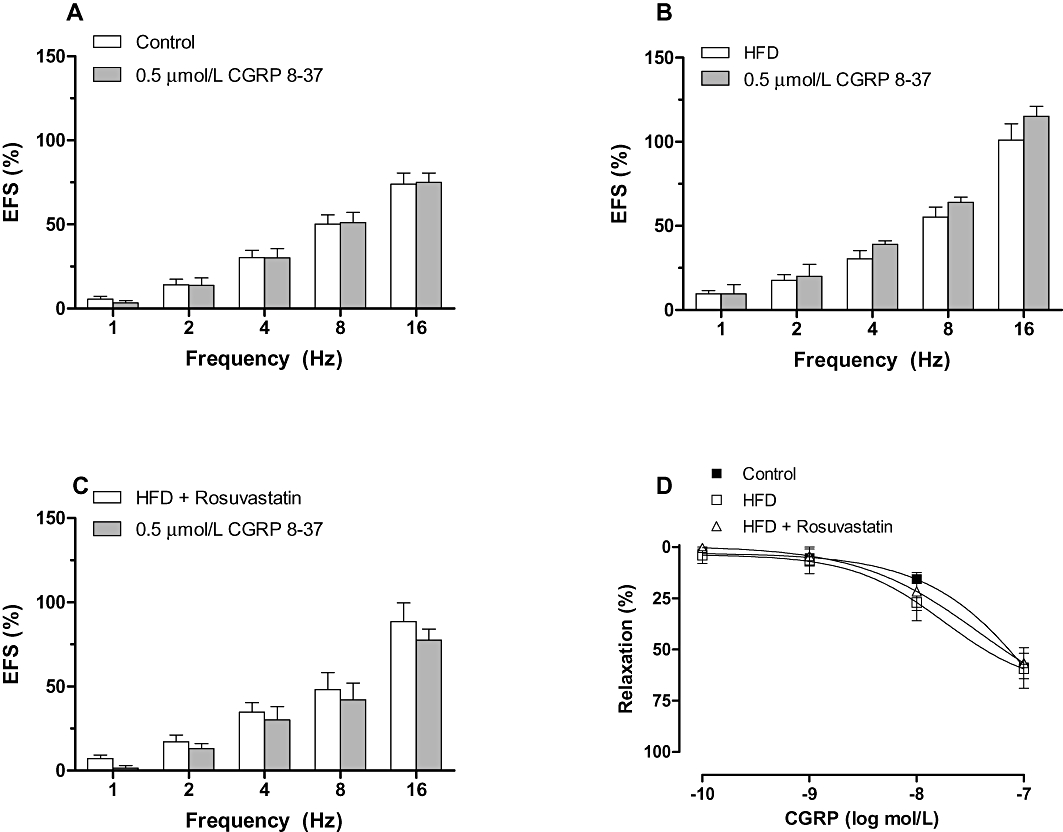
Effect of preincubation with 0.5 µmol·L−1 CGRP 8-37 on the vasoconstrictor response induced by electric field stimulation (EFS) in mesenteric segments from control (A), high-fat diet (HFD) (B) and HFD + rosuvastatin (C) rats. Results (mean ± SEM) are expressed as a percentage of previous contraction elicited by KCl. n= 6–10 animals per group. (D) Vasodilator response to exogenous CGRP in segments from control, HFD and HFD + rosuvastatin rats. Results (mean ± SEM) are expressed as a percentage of previous contraction elicited by noradrenaline. n= 6–10 animals per group.
The contraction induced by EFS was increased by preincubation with L-NAME and 7NI in segments from control and HFD + rosuvastatin rats, but not in HFD rats (Figure 5). The effect in presence of L-NAME was higher than that observed in presence of 7NI. The specific iNOS inhibitor 1400W did not modify EFS-induced contraction in any of the experimental groups (Figure 5). In noradrenaline-precontracted mesenteric segments (995 + 126 mg), DEA-NO (0.1 nmol·L−1–0.1 mmol·L−1) induced a similar concentration-dependent relaxation in segments from all of the experimental groups (Figure 6).
Figure 5.
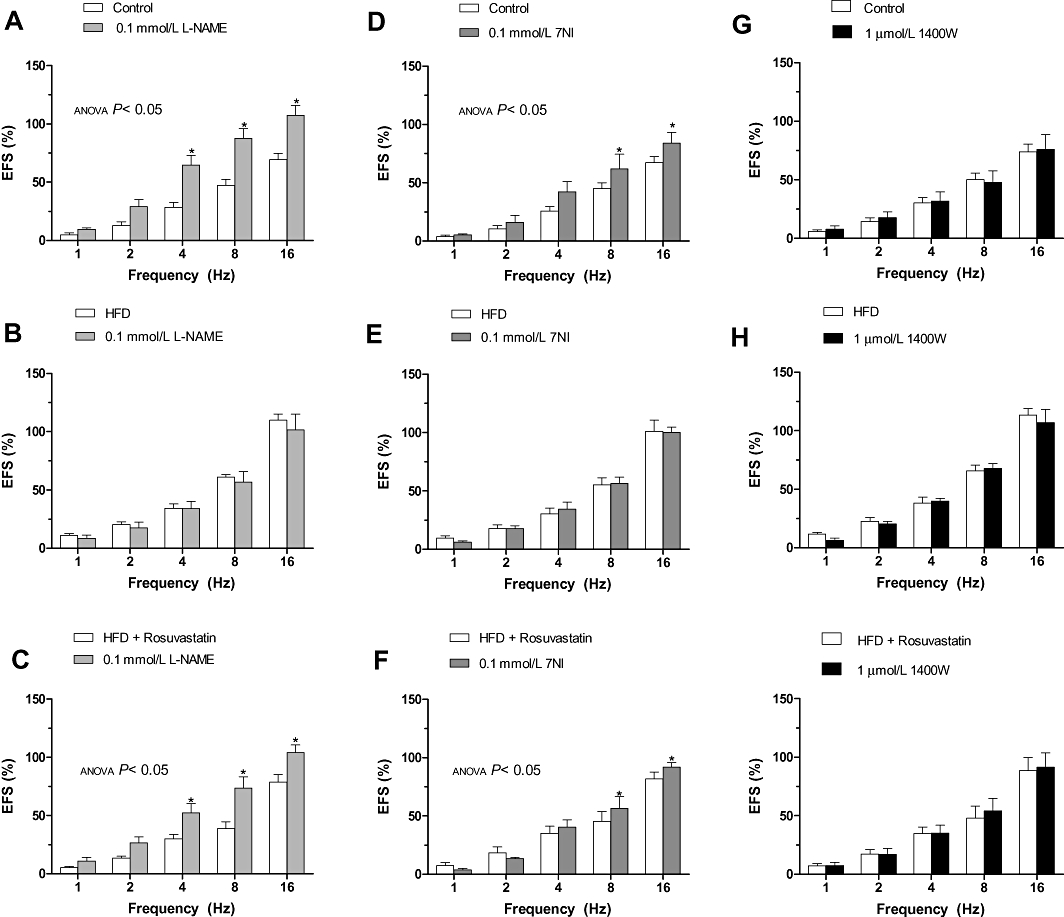
Effect of preincubation with 0.1 mmol·L−1 Nω nitro-L-arginine methyl ester (L-NAME) (A–C), 0.1 mmol·L−1 7-NI (D–F) or 1 µmol·L−1 1400W (G–I) on the vasoconstrictor response induced by electric field stimulation (EFS) in mesenteric segments from control (A,D,G), high-fat diet (HFD) (B,E,H) and HFD + rosuvastatin (C,F,I) rats. Results (mean ± SEM) are expressed as a percentage of previous contraction elicited by KCl. n= 6–10 animals per group. *P < 0.05 versus conditions without specific inhibitor for each frequency (Bonferroni test).
Figure 6.
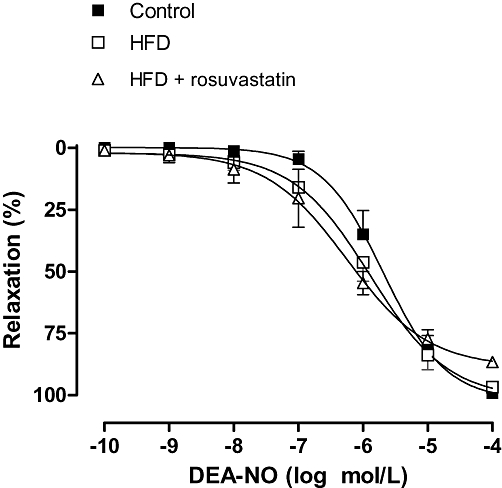
Relaxation to diethylamine NONOate (DEA-NO) in segments from control, high-fat diet (HFD) and HFD + rosuvastatin rats. Results (mean ± SEM) are expressed as a percentage of previous contraction elicited by noradrenaline. n= 6–10 animals per group.
In addition, 7NI decreased noradrenaline-induced contraction in mesenteric segments from all of the experimental groups to a similar extent, whereas it was not modified by preincubation with L-NAME (Figure 7, Table 2).
Figure 7.
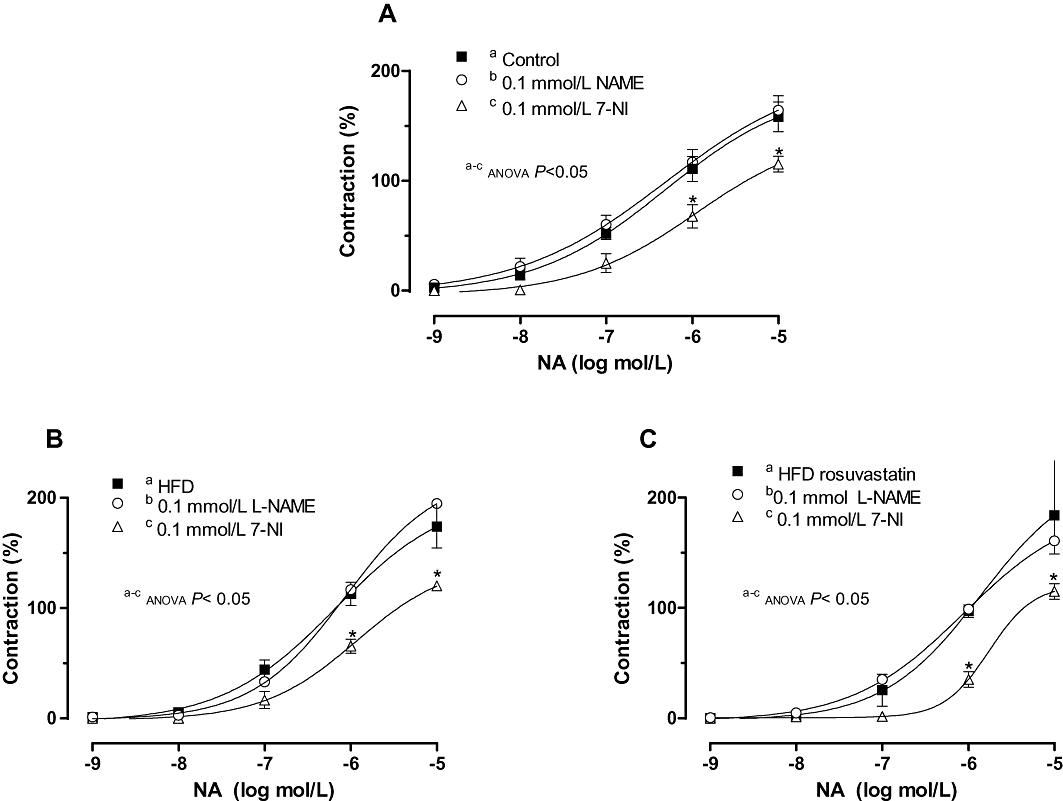
Effect of preincubation with 0.1 mmol·L−1 Nω nitro-L-arginine methyl ester (L-NAME) or 0.1 mmol·L−1 7-NI on the vasoconstrictor response elicited by exogenous noradrenaline in mesenteric segments from control (A), high-fat diet (HFD) (B) and HFD + rosuvastatin (C) rats. Results (mean ± SEM) are expressed as a percentage of previous contraction elicited by KCl. n= 6–10 animals per group. *P < 0.05 versus conditions without specific inhibitor for each frequency (Bonferroni test).
Table 2.
Effect of preincubation with L-NAME (0.1 mmol·L−1) or 7-nitroindazole (7NI; 0.1 mmol·L−1) on the vasoconstrictor responses to noradrenaline (Emax and pEC50) in mesenteric segments from control, HFD and HFD + rosuvastatin rats
| Control | HFD | HFD + rosuvastatin | ||||
|---|---|---|---|---|---|---|
| Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | |
| Untreated | 204.0 ± 38.8 | 6.17 ± 0.31 | 204.0 ± 39.8 | 6.17 ± 0.31 | 225.5 ± 85.2 | 5.85 ± 0.53 |
| L-NAME | 200.6 ± 18.2 | 6.31 ± 0.42 | 222.1 ± 43.5 | 6.06 ± 0.28 | 192.7 ± 32.7 | 6.06 ± 0.26 |
| 7NI | 141.7 ± 12.3* | 5.93 ± 0.11 | 141.7 ± 12.3* | 5.93 ± 0.12 | 121.2 ± 24.2* | 5.76 ± 0.29 |
Results are expressed as mean ± SEM. Six to 10 animals in each group.
P < 0.05 versus untreated rats.
Emax, maximal effect; HFD, high-fat diet; pEC50, −log EC50; L-NAME, ω nitro-L-arginine methyl ester;
Noradrenaline release
Both basal and EFS-induced noradrenaline releases were greater in HFD than in control rats (Figure 8A). Treatment with rosuvastatin reduced noradrenaline release to control levels (Figure 8A).
Figure 8.
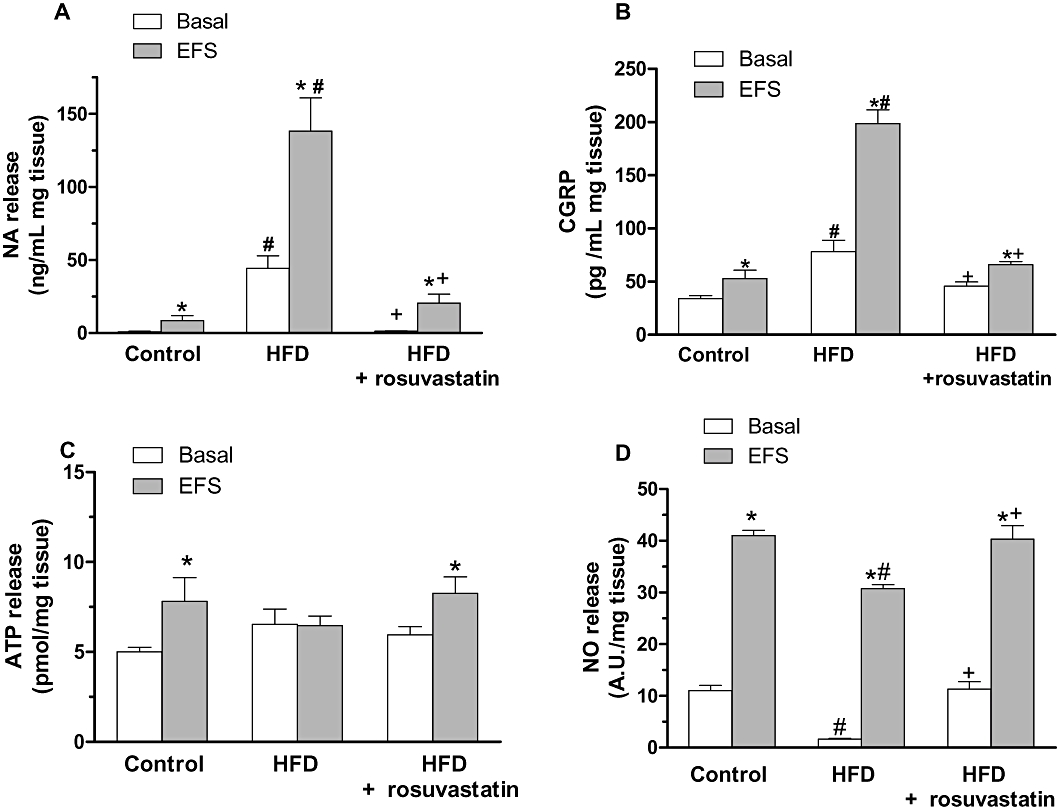
Basal and electric field stimulation (EFS)-induced noradrenaline (A), calcitonin gene-related peptide (CGRP) (B), ATP (C) and nitric oxide (NO) (D) release in segments from control, high-fat diet (HFD) and HFD + rosuvastatin rats. Results (mean ± SEM) are expressed as ng noradrenaline·mL−1 mg tissue, pg CGRP·mL−1 mg tissue, pmol ATP·mg−1 tissue and AU·mg−1 tissue. *P < 0.05 versus basal; #P < 0.05 versus control; +P < 0.05 versus HFD. n= 4–6 animals per group.
CGRP release
Both basal and EFS-induced CGRP releases were greater in HFD than in control rats (Figure 8B). Treatment with rosuvastatin reduced CGRP release to control levels (Figure 8B).
ATP release
Basal ATP release was similar in mesenteric segments from, control, HFD and rosuvastatin-treated HFD rats (Figure 8C). EFS induced ATP release segments in control and rosuvastatin-treated rats, but not in HFD animals (Figure 8C).
NO release
Both basal and EFS-induced release of NO were lower in HFD than in control mesenteric segments (Figure 8D). Treatment with rosuvastatin increased both basal and EFS-induced NO release to control levels (Figure 8D). In addition, 7NI (0.1 mmol·L−1) and TTX (0.1 µmol·L−1) practically abolished EFS-induced NO release in arteries from both groups (Figure 9A–C).
Figure 9.
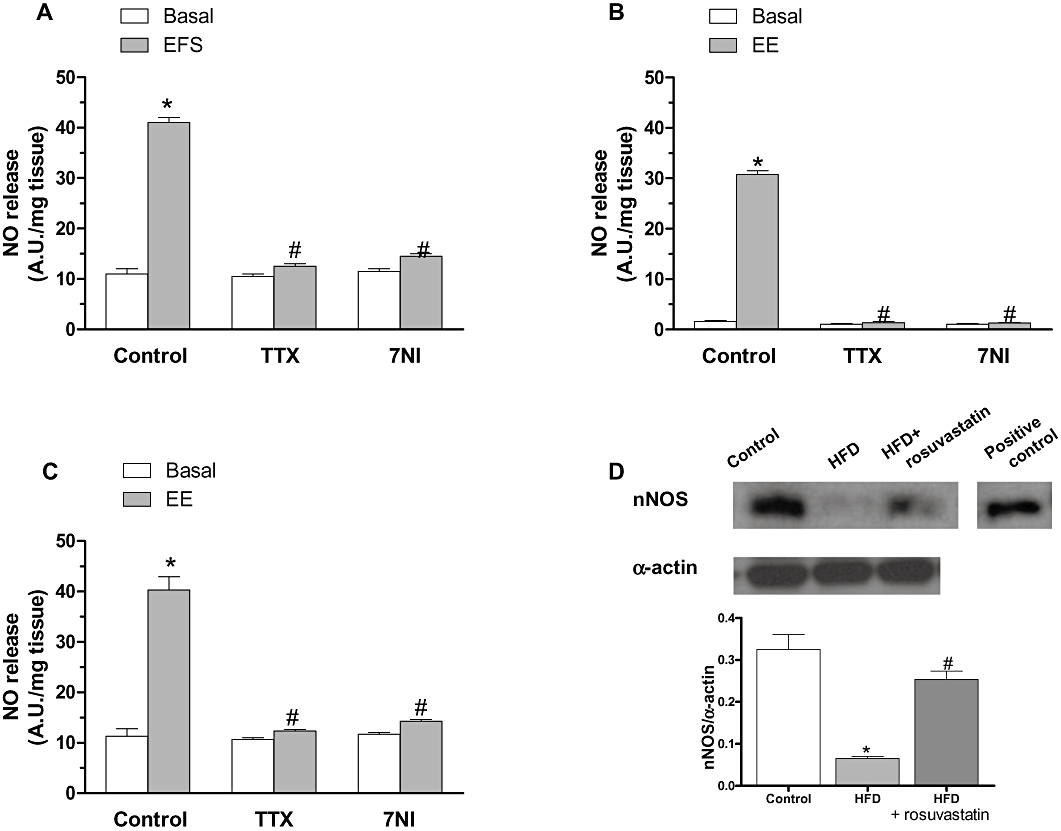
Effect of preincubation with 0.1 µmol·L−1 tetrodotoxin (TTX) or 0.1 mmol·L−1 7-nitroindazole (7NI) on basal and electric field stimulation (EFS)-induced NO release in segments from control (A), high-fat diet (HFD) (B) and HFD + rosuvastatin (C) rats. Results (mean ± SEM) are expressed as AU mg−1 tissue. *P < 0.05 versus basal; #P < 0.05 versus control. n= 4–6 animals per group. (D) Expression of nNOS in segments from control, HFD and HFD + rosuvastatin rats. The blot is representative of four separate segments from each group. Lower panel shows densitometric analysis of nNOS expression. Results (mean ± SEM) are expressed as the ratio of the signal obtained for nNOS and that obtained for α-actin. *P < 0.05 versus basal; #P < 0.05 versus control; +P < 0.05 versus HFD.
nNOS protein expression
HFD-rats presented a reduced (P < 0.05) nNOS protein expression compared with controls. Treatment with rosuvastatin partially prevented (P < 0.05) this reduction (Figure 9D).
Discussion
The main findings of the present work show that HFD-induced obesity produces alterations of the adrenergic and nitrergic innervation of rat mesenteric artery, which are restored by treatment with rosuvastatin.
In the three experimental groups, EFS produced a frequency-dependent contraction, which was abolished in the presence of TTX, indicating that the vasomotor responses are the result of neurotransmitter release from perivascular nerves. HFD produced an increased response to EFS, which was not attributable to changes in the intrinsic contractile machinery as demonstrated by the similar vasconstrictor response to KCl in the three experimental groups. The enhanced response to EFS was normalized by treatment with rosuvastatin. To our knowledge, this is the first study to show this novel effect of statins in the regulation of vascular innervation. The decrease in non-HDL cholesterol levels could be attributed to the observed effects of the statin, although other well-known described effects of statins, such as antioxidant and anti-inflammatory effects, might have contributed to the vascular effects of rosuvastatin (Devaraj et al., 2006; Cachofeiro et al., 2008; Chen et al., 2009).
As mentioned in the Introduction, adrenergic, sensory and nitrergic innervation are involved in the vasomotor response to EFS in rat mesenteric artery (Kawasaki et al., 1988; Li and Duckles, 1992; Boeckxstaens and Pelckmans, 1997; Marín and Balfagón, 1998). The fact that EFS-induced net response is a contraction suggests a predominant role of the adrenergic component. To explore the participation of adrenergic component in the enhanced response to EFS in obese rats, the effects of the non-selective α-adrenergic antagonist, phentolamine, were analysed. The reduction of contractile response in presence of phentolamine was higher in HFD rats than in controls, confirming a higher participation of adrenergic innervation in arteries from obese rats than those from in normal rats. This is in agreement with previous results showing adrenergic hyperactivity in obesity (Scherrer et al., 1994; Straznicky et al., 2008). The observed adrenergic effect in HFD rats could be mediated by either an enhanced response to noradrenaline or an enhanced release of noradrenaline from the nervous terminal. Contractile response to noradrenaline was comparable in the three experimental groups, whereas basal and EFS-induced release of noradrenaline was higher in obese rats. Thus, the observed enhanced adrenergic participation in HFD rats seems to be due to an increased release of noradrenaline. Furthermore, treatment with rosuvastatin restored noradrenaline release and thus, the participation of adrenergic component in vasoconstrictor response to EFS. It should also be mentioned that in the control group and in the HFD + rosuvastatin group, there was a substantial response to activation of the sympathetic nerves that was phentolamine resistant. The most likely transmitter for this response is ATP. In fact, ATP release was higher in normal and HFD + rosuvastatin rats. The results showing noradrenaline and ATP release confirm the coexistence of different mechanisms modulating sympathetic activation. This novel effect of statins in the regulation of vascular adrenergic innervation could have functional relevance, in addition to its well-known effects on lipid metabolism and atherosclerotic mechanisms.
Although the present results confirm the major role of the adrenergic system in the elevation of mesenteric vascular tone in obesity, the participation of other neural components cannot be ruled out. In certain experimental conditions, such as hyperaldosteronism and hypertension, alterations in sensory function play a key role in the regulation of mesenteric vascular tone (Balfagón et al., 2004; del Campo et al., 2009). Exogenous CGRP produced a mild vasodilatory response, which was comparable in the three groups. However, release of this peptide was higher in HFD rats than in normal, and was restored by rosuvastatin treatment. To analyse the functional role of sensory innervation in obese rats, the effect of the CGRP receptor antagonist, CGRP 8-37, was studied in the constrictor response to EFS. No differences were observed between normal and HFD rats in presence or absence of the antagonist. This indicates that, apart from increased CGRP release in obese rats, the sensory innervation did not participate in the response to EFS in HFD rats. This lack of effect may suggest that the amount of CGRP released is not enough to produced any vasomotor response. As expected, rosuvastatin did not show any additional effect on the unaltered response to EFS in the presence of CGRP 8-37, although the statin restored CGRP release from mesenteric artery.
Several studies have already demonstrated the participation of NO from iNOS in pro-inflammatory states (Guzik et al., 2003). As obesity has been considered as a state of low grade inflammation, we aimed to evaluate the potential participation of iNOS in the release of NO induced by EFS. However, preincubation with iNOS inhibitor 1400W did not modify vasoconstrictor response to EFS, indicating that iNOS is not involved in the different observed vascular responses in HFD rats. We previously showed that EFS stimulation produced neural release of NO (Ferrer et al., 2004; Xavier et al., 2004). Consequently, we studied the effects of the non-specific NOS inhibitor, L-NAME, or the specific nNOS inhibitor, 7NI, in the enhanced response to EFS in HFD rats. Normal but not HFD rats showed an enhanced response to EFS in presence of 7NI and L-NAME, where the effect of the latter was more marked than that of 7NI, probably due to the fact that 7NI also decreases the vasoconstrictor response to noradrenaline. This indicates that the participation of neural NO in the control of vasomotor response in the mesenteric artery is abolished in obese rats. This reduced participation of NO in HFD rats could be due to a reduction in NO production or a diminished response to this agent in vascular smooth muscle. Responses to the NO donor DEA-NO were comparable in the three groups, suggesting that a different mechanism was responsible for the observed effects. Basal and stimulated NO release was reduced in obese rats, and almost abolished in presence of TTX, indicating that the diminished participation of nitrergic innervation in obese rats is due to a reduction of NO release. As previously suggested in normal rats (Hatanaka et al., 2006) NO released from perivascular nerves, inhibits neurogenic noradrenaline release, modulating adrenergic neurotransmission. This effect could also be valid in obese rats. Therefore, it could be proposed that the observed enhanced contractile response in mesenteric segments from HFD rats is the result of, at least, two effects: an increased participation of adrenergic innervation, along with a reduction in the nitrergic component. This latter notion was confirmed by the diminished nNOS expression observed in HFD rats. Treatment with rosuvastatin restored nNOS expression, as previously reported for other NOS isoforms (Di Napoli et al., 2005; Landsberger et al., 2005), and NO release, and consequently the participation of nitrergic innervation in the response to EFS in mesenteric arteries from obese rats. Restoring nitrergic function is a novel effect of rosuvastatin, further supporting the importance of statins in the regulation of vascular innervation. Furthermore, the different patterns of noradrenaline, ATP, CGRP and NO release also support the complex interactions modulating mesenteric innervation (Dunn et al., 1999; Brock and Tan, 2004).
Finally, it should be mentioned that the presence of endothelium could affect the observed EFS responses, as previously described (Iwatani et al., 2008). However, interaction between endothelium-derived factors and EFS responses are multiple and very complex. Additionally, it should also be considered that specific inhibitors used in the different experiments could also interfere with endothelium-dependent responses, making results more difficult to interpret. Thus, further studies will be needed to analyse endothelium-EFS interactions under the present experimental conditions.
It should be also emphasized that all observed effects of rosuvastatin were not due to direct effects of the statin on the mesenteric vascular wall. In fact, incubation up to three hours with rosuvastatin did not modify the response to EFS in any experimental group. Thus, other mechanisms, such as reduction of non-HDL cholesterol, as well as numerous pleiotropic effects could have contributed to the observed effects of rosuvastatin on mesenteric innervation.
In summary, the present results indicate that neural control of mesenteric vasomotor tone is altered in HFD rats. Both an enhanced adrenergic component and a diminished nitrergic component contribute to the observed increase in the vasoconstrictor response to EFS. All of these effects were restored by treatment with rosuvastatin, indicating unknown mechanisms of statins in the neural regulation of vascular tone.
Acknowledgments
We thank Mrs Sandra Ballesteros for her technical assistance. This work was supported by grants from Comisión Interministerial de Ciencia y Tecnología de España (SAF2006/0788, SAF2007-61595, SAF2009-10374 and DEP2006-56187-C04-04), Fondo de Investigaciones Sanitarias (FIS PI 060133), and Red Cardiovascular del Fondo de Investigaciones Sanitarias (RD06/0014/0007, RD06/0014/0011). B Martín-Fernández received a fellowship from AstraZeneca Farmacéutica, Spain, S.A.
Glossary
Abbreviations
- 7NI
7 nitroindazole
- CGRP
calcitonin gene-related peptide
- DEA
NO, diethylamine NONOate
- EFS
electric field stimulation
- HDL
high-density lipoprotein
- HFD
high-fat diet
- KHS
Krebs Henseleit solution
- LDL
low-density lipoproteins
- L-NAME
Nω nitro-L-arginine methyl ester
- nNOS
neuronal nitric oxide synthase
- PGI2
prostaglandin I2
- TG
triglyceride
- TTX
tetrodotoxin
- VLDL
very low density lipoprotein
Conflicts of interest
None to declare.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfagón G, Márquez-Rodas I, Alvarez Y, Alonso MJ, Cachofeiro V, Salaices M, et al. Aldosterone modulates neural vasomotor response in hypertension: role of calcitonin gene-related peptide. Regul Pept. 2004;120:253–260. doi: 10.1016/j.regpep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Belin de Chantemèle EJ, Vessières E, Guihot AL, Toutain B, Loufrani L, Henrion D. Cyclooxygenase-2 preserves flow-mediated remodelling in old obese Zucker rat mesenteric arteries. Cardiovasc Res. 2010;86:516–525. doi: 10.1093/cvr/cvp411. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens GE, Pelckmans PA. Nitric oxide and the non-adrenergic non-cholinergic neurotransmission. Comp Biochem Physiol A Physiol. 1997;118:925–937. doi: 10.1016/s0300-9629(97)00022-4. [DOI] [PubMed] [Google Scholar]
- Brock JA, Tan JH. Selective modulation of noradrenaline release by alpha 2-adrenoceptor blockade in the rat-tail artery in vitro. Br J Pharmacol. 2004;142:267–274. doi: 10.1038/sj.bjp.0705779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:4–9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- del Campo L, Ferrer M, Balfagón G. Hypertension alters the function of nitrergic and sensory innervation in mesenteric arteries from female rats. J Hypertens. 2009;27:791–799. doi: 10.1097/HJH.0b013e32832531e6. [DOI] [PubMed] [Google Scholar]
- Chen YX, Wang XQ, Fu Y, Yao YJ, Kong MY, Nie RQ, et al. Pivotal role of inflammation in vascular endothelial dysfunction of hyperlipidemic rabbit and effects by atorvastatin. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.06.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Devaraj S, Chan E, Jialal I. Direct demonstration of an antiinflammatory effect of simvastatin in subjects with the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4489–4496. doi: 10.1210/jc.2006-0299. [DOI] [PubMed] [Google Scholar]
- Di Napoli P, Taccardi AA, Grilli A, De Lutiis MA, Barsotti A, Felaco M, et al. Chronic treatment with rosuvastatin modulates nitric oxide synthase expression and reduces ischemia-reperfusion injury in rat hearts. Cardiovasc Res. 2005;66:462–471. doi: 10.1016/j.cardiores.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Brock JA, Hardy TA. Electrochemical and electrophysiological characterization of neurotransmitter release from sympathetic nerves supplying rat mesenteric arteries. Br J Pharmacol. 1999;128:174–180. doi: 10.1038/sj.bjp.0702760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci (Lond) 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer M, Encabo A, Marín J, Balfagón G. Vasoconstrictive effects of angiotensin I and II in cat femoral arteries. Role of endothelium. Gen Pharmacol. 1992;23:1171–1175. doi: 10.1016/0306-3623(92)90307-6. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Alonso MJ, Salaices M, Marín J, Balfagón G. Increase in neurogenic nitric oxide metabolism by endothelin-1 in mesenteric arteries from hypertensive rats. J Cardiovasc Pharmacol. 2000a;36:541–547. doi: 10.1097/00005344-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Marín J, Balfagon G. Diabetes alters neuronal nitric oxide release from rat mesenteric arteries. Role of protein kinase C. Life Sci. 2000b;66:337–345. doi: 10.1016/s0024-3205(99)00595-0. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Alonso MJ, Salaices M, Marín J, Balfagón G. Angiotensin II increases neurogenic nitric oxide metabolism in mesenteric arteries from hypertensive rats. Life Sci. 2001;68:1169–1179. doi: 10.1016/s0024-3205(00)01029-8. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Sánchez M, Minoves N, Salaices M, Balfagón G. Aging increases neuronal nitric oxide release and superoxide anion generation in mesenteric arteries from spontaneously hypertensive rats. J Vasc Res. 2003;40:509–519. doi: 10.1159/000075183. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Sánchez M, Martín MC, Márquez-Rodas I, Alonso MJ, Salaices M, et al. Protein kinase A increases electrical stimulation-induced neuronal nitric oxide release in rat mesenteric artery. Eur J Pharmacol. 2004;487:167–173. doi: 10.1016/j.ejphar.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Gelosa P, Cimino M, Pignieri A, Tremoli E, Guerrini U, Sironi L. The role of HMG-CoA reductase inhibition in endothelial dysfunction and inflammation. Vasc Health Risk Manag. 2007;3:567–577. [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- Hatanaka Y, Hobara N, Honghua J, Akiyama S, Nawa H, Kobayashi Y, et al. Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J Pharmacol Exp Ther. 2006;316:490–497. doi: 10.1124/jpet.105.094656. [DOI] [PubMed] [Google Scholar]
- de las Heras N, Martín-Fernández B, Miana M, Ballesteros S, Oubiña MP, López-Farré AJ, et al. The protective effect of irbesartan in rats fed a high fat diet is associated with modification of leptin-adiponectin imbalance. J Hypertens. 2009;27:37–41. doi: 10.1097/01.hjh.0000358836.64052.43. [DOI] [PubMed] [Google Scholar]
- Iwatani Y, Kosugi K, Isobe-Oku S, Atagi S, Kitamura Y, Kawasaki H. Endothelium removal augments endothelium-independent vasodilatation in rat mesenteric vascular bed. Br J Pharmacol. 2008;154:32–40. doi: 10.1038/bjp.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javeshghani D, Schiffrin EL, Sairam MR, Touyz RM. Potentiation of vascular oxidative stress and nitric oxide-mediated endothelial dysfunction by high-fat diet in a mouse model of estrogen deficiency and hyperandrogenemia. J Am Soc Hypertens. 2009;3:295–305. doi: 10.1016/j.jash.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Arora RR. Role of angiotensin-converting enzyme inhibitors in vascular modulation: beyond the hypertensive effects. Am J Ther. 2010;17:11–23. doi: 10.1097/MJT.0b013e31815addd9. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Khamaisi M, Søndergaard M, Segev Y, Dagnaes-Hansen F, Jensen TG, Landau D, et al. Differential effects on kidney and liver growth of a non-viral hGH-expression vector in hypophysectomized mice. Growth Horm IGF Res. 2007;17:279–287. doi: 10.1016/j.ghir.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Landsberger M, Jantzen F, Könemann S, Felix SB. Blockade of geranylgeranylation by rosuvastatin upregulates eNOS expression in human venous endothelial cells. Biochem Biophys Res Commun. 2005;336:1005–1009. doi: 10.1016/j.bbrc.2005.08.225. [DOI] [PubMed] [Google Scholar]
- Li YJ, Duckles SP. Effect of endothelium on the actions of sympathetic and sensory nerves in the perfused rat mesentery. Eur J Pharmacol. 1992;210:23–30. doi: 10.1016/0014-2999(92)90647-m. [DOI] [PubMed] [Google Scholar]
- Marín J, Balfagón G. Effect of clenbuterol on non-endothelial nitric oxide release in rat mesenteric arteries and the involvement of β-adrenoceptors. Br J Pharmacol. 1998;124:473–478. doi: 10.1038/sj.bjp.0701856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín J, Ferrer M, Balfagon G. Role of protein kinase C in electrical-stimulation-induced neuronal nitric oxide release in mesenteric arteries from hypertensive rats. Clin Sci (Lond) 2000;99:277–283. [PubMed] [Google Scholar]
- Miâdi-Messaoud H, Chouchane A, Abderrazek E, Debbabi H, Zaouali-Ajina M, Tabka Z, et al. Obesity-induced impairment of endothelium-dependent vasodilation in Tunisian women. Int J Obes (Lond) 2010;34:273–279. doi: 10.1038/ijo.2009.231. [DOI] [PubMed] [Google Scholar]
- Minoves N, Balfagón G, Ferrer M. Role of female sex hormones in neuronal nitric oxide release and metabolism in rat mesenteric arteries. Clin Sci (Lond) 2002;103:239–247. doi: 10.1042/cs1030239. [DOI] [PubMed] [Google Scholar]
- Nielsen KC, Owman C. Contractile response and amine receptor mechanisms in isolated middle cerebral artery of the cat. Brain Res. 1971;27:33–42. doi: 10.1016/0006-8993(71)90370-2. [DOI] [PubMed] [Google Scholar]
- Otto A, Fontaine D, Fontaine J, Berkenboom G. Rosuvastatin treatment protects against nitrate-induced oxidative stress. J Cardiovasc Pharmacol. 2005;46:177–184. doi: 10.1097/01.fjc.0000167010.98177.78. [DOI] [PubMed] [Google Scholar]
- Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, et al. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Lahera V, Calderon J, Bolivar IC, Castillo VR, Gutierrez M, et al. The presence of abdominal obesity is associated with changes in vascular function independently of other cardiovascular risk factors. Int J Cardiol. 2010;139:32–41. doi: 10.1016/j.ijcard.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Fraccarollo D, Eigenthaler M, Tas P, Firnschild A, Frantz S, et al. Rosuvastatin reduces platelet activation in heart failure: role of NO bioavailability. Arterioscler Thromb Vasc Biol. 2005;25:1071–1077. doi: 10.1161/01.ATV.0000161926.43967.df. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Randin D, Tappy L, Vollenweider P, Jéquier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–447. doi: 10.1007/s11906-008-0083-1. [DOI] [PubMed] [Google Scholar]
- Tan KC, Chow WS, Tam SC, Ai VH, Lam CH, Lam KS. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:563–568. doi: 10.1210/jcem.87.2.8249. [DOI] [PubMed] [Google Scholar]
- Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30:769–778. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]
- Xavier FE, Salaices M, Márquez-Rodas I, Alonso MJ, Rossoni LV, Vassallo DV, et al. Neurogenic nitric oxide release increases in mesenteric arteries from ouabain hypertensive rats. J Hypertens. 2004;22:949–957. doi: 10.1097/00004872-200405000-00017. [DOI] [PubMed] [Google Scholar]
- Xu KZ, Zhu C, Kim MS, Yamahara J, Li Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J Ethnopharmacol. 2009;123:280–287. doi: 10.1016/j.jep.2009.03.035. [DOI] [PubMed] [Google Scholar]


