Abstract
BACKGROUND AND PURPOSE
Cannabidiol (CBD) has emerged as an interesting compound with therapeutic potential in several CNS disorders. However, whether it can modulate synaptic activity in the CNS remains unclear. Here, we have investigated whether CBD modulates synaptic transmission in rat hippocampal cultures and acute slices.
EXPERIMENTAL APPROACH
The effect of CBD on synaptic transmission was examined in rat hippocampal cultures and acute slices using whole cell patch clamp and standard extracellular recordings respectively.
KEY RESULTS
Cannabidiol decreased synaptic activity in hippocampal cultures in a concentration-dependent and Pertussis toxin-sensitive manner. The effects of CBD in culture were significantly reduced in the presence of the cannabinoid receptor (CB1) inverse agonist, LY320135 but were unaffected by the 5-HT1A receptor antagonist, WAY100135. In hippocampal slices, CBD inhibited basal synaptic transmission, an effect that was abolished by the proposed CB1 receptor antagonist, AM251, in addition to LY320135 and WAY100135.
CONCLUSIONS AND IMPLICATIONS
Cannabidiol reduces synaptic transmission in hippocampal in vitro preparations and we propose a role for both 5-HT1A and CB1 receptors in these CBD-mediated effects. These data offer some mechanistic insights into the effects of CBD and emphasize that further investigations into the actions of CBD in the CNS are required in order to elucidate the full therapeutic potential of CBD.
Keywords: cannabidiol, hippocampus, synaptic transmission, 5-HT1A, CB1
Introduction
The Asian hemp plant, Cannabis sativa, has been used therapeutically for centuries but until recently most research has focused on Δ9-tetrahydrocannabinol (THC), the most abundant phytocannabinoid in cannabis. THC, acting via the cannabinoid CB1 or CB2 receptors (nomenclature follows Alexander et al., 2009), is thought to underlie most of the therapeutic uses of cannabis, which include use as an analgesic, an antiepileptic and an antiemetic (see Ben Amar, 2006; Turcotte et al., 2010). However, there remains controversy over the therapeutic use of cannabis because of the adverse psychotomimetic effects of THC and its potential to cause cognitive impairments (Fletcher and Honey, 2006; D'Souza et al., 2008). Nevertheless, cannabis contains numerous cannabinoids and these different constituents may modulate the effects of THC and have useful effects of their own. This has led to intense investigation into the therapeutic potential of other, non-psychoactive, constituents of cannabis (Russo and Guy, 2006; Pertwee, 2008; Izzo et al., 2009).
In particular cannabidiol (CBD), which lacks negative psychotropic effects when administered to humans even in high doses (Bhattacharyya et al., 2009), has emerged as an interesting compound due to its potential therapeutic application in a number of neurological and neuropsychiatric disorders including addiction, diabetic neuropathy, epilepsy, schizophrenia and stroke and it is currently used at a 1:1 ratio with THC in Sativex® for the treatment of chronic pain in patients suffering from multiple sclerosis and advanced cancer (see Russo and Guy, 2006; Izzo et al., 2009). Despite the fact that CBD is used clinically, the exact mechanism(s) by which it exerts its therapeutic action remains unclear, although several targets have been identified which could underlie its therapeutic actions in CNS disorders (see Russo and Guy, 2006; Pertwee, 2008 and Izzo et al., 2009). In contrast to THC, CBD has a low affinity for CB1 receptors and acts as a CB1 receptor antagonist and a CB2 receptor inverse agonist (Showalter et al., 1996; Bisogno et al., 2001; Thomas et al., 2007). Furthermore, CBD has been proposed as an agonist at 5-HT1A receptors (Russo et al., 2005) and this has been suggested to mediate the anxiolytic and anti-ischaemic properties of CBD (Campos and Guimarães, 2008; Resstel et al., 2009). CBD also acts either as an agonist or antagonist at transient receptor potential cation channels (TRPs; De Petrocellis et al., 2008; Qin et al., 2008) and has also been shown to inhibit the Cav3 subfamily of Ca2+ channels (Ross et al., 2008). In addition, CBD can modulate intracellular Ca2+ levels when tested on cultured hippocampal neurons, an effect proposed to be mediated via Cav1 Ca2+ channels, Ca2+ release from intracellular stores and the modulation of mitochondrial function (Drysdale et al., 2006; Ryan et al., 2009).
Despite increasing evidence to suggest that CBD has multiple targets (Pertwee, 2008; Izzo et al., 2009), the effect of CBD on basal synaptic transmission has yet to be determined although a recent study has demonstrated clear anti-epileptiform and antiseizure activity (Jones et al., 2010). In the present study, we have examined the effect of CBD upon synaptic activity in primary hippocampal cultures and acute hippocampal slices. Our data show for the first time that CBD is able to modulate basal hippocampal synaptic activity and we suggest a role for CB1 and 5-HT1A receptors in these observations. These data provide some mechanistic insights into the effects of CBD and highlight that further investigations into the actions of CBD in the CNS are required in order to fully elucidate the contribution of CBD to the detrimental and therapeutic roles of cannabis.
Methods
Primary hippocampal cultures
All animal care and experimental procedures were in accordance with UK Home Office guidelines. Primary hippocampal cultures were prepared as described previously (Greenwood et al., 2007). Briefly, 1- to 2-day-old Sprague Dawley rats were killed by cervical dislocation and decapitated. Once the hippocampi were removed and triturated, cells were plated at a density of 3 × 105 cells·mL−1 onto poly-L-lysine coated coverslips. Cultures were incubated in a medium consisting of Neurobasal-A Medium (Invitrogen, Paisley, UK) supplemented with 2% (v/v) B-27 (Invitrogen) and 2 mM L-glutamine and maintained in a humidified atmosphere at 37°C/5% CO2 for 13–16 days in vitro (DIV). After 5 DIV, cytosine-D-arabinofuranoside (10 µM) was added to inhibit glial cell proliferation. All experiments were performed on cells taken from at least three separate cultures obtained from different rats.
Hippocampal slice preparation
Sprague-Dawley rats (16–20 days old) were killed by cervical dislocation and decapitated. The brains were rapidly removed and placed immediately in an ice-cold (0–3°C), oxygenated (95% O2/5% CO2) cutting solution containing (in mM): sucrose 26, NaHCO3 2, NaH2PO4 2, MgSO4 3, KCl 2, CaCl2 and d-glucose 10. Parasagittal whole brain slices (400 µm) were cut using a vibratome and placed into oxygenated artificial cerebrospinal fluid (aCSF) with the same composition as the cutting solution, but with NaCl (124 mM) replacing the sucrose. Hippocampal regions were then dissected free and placed in a submerged holding chamber containing aCSF continuously bubbled with 95% O2/5% CO2. Slices were allowed to equilibrate for a minimum of 1 h at room temperature prior to use.
Electrophysiology
Cultured neurones
Cells were perfused at 2 mL·min−1 with a HEPES-buffered saline (HBS) containing (in mM): NaCl 140, KCl 5, MgCl2 2, CaCl2 2, HEPES 10, d-glucose 10, pH was adjusted to 7.4 and osmolarity adjusted to 310 mOsm with sucrose if required. Current clamp recordings were made using whole cell patch clamp in current clamp mode with glass pipettes (4–6 MΩ) filled with a internal solution containing (in mM): KCl 150, MgCl2 1, CaCl2 1, HEPES 10, EGTA 0.5, Mg-ATP 3, GTP 0.3, pH was adjusted to 7.2 and osmolarity was adjusted to 290 mOsm using sucrose if required. Data were acquired with an Axopatch-200B amplifier (Molecular Devices, Sunnyvale, CA, USA) and using WinEDR v 2.7.9 software (J. Dempster, University of Strathclyde, Glasgow, UK). No capacitance and series resistance compensation were applied. All drugs were added via the perfusate. In experiments where CBD alone was applied, it was added for 5 min following a 5 min steady baseline period and then washed out with perfusate. In experiments where antagonists were used, antagonists at the stated concentration were applied for 5 min following a 5 min baseline period. Subsequently, antagonist + CBD were added together for a further 5 min period. Finally, all drugs were washed out with perfusate. Data were analysed offline using WinEDR v 2.7.9.
Acute slice electrophysiology
Slices were transferred to a submerged recording chamber continually perfused with oxygenated aCSF at a flow rate of 1–2 mL·min−1. Extracellular field excitatory postsynaptic potential (fEPSP) recordings were obtained in response to low frequency (0.033 Hz) stimulation of the Schaffer collateral-commissural pathway by a bipolar stimulating electrode and a borosilicate glass recording electrode filled with 4 M NaCl placed in the stratum radiatum of area CA1. Paired-pulse experiments were performed with an inter-stimulus interval of 50 ms. All data were acquired using an Axopatch-2B amplifier (Molecular Devices) and using WinWCP v 2.7.9 software (J. Dempster, University of Strathclyde, Glasgow, UK) with the fEPSP slope analysed online and reanalysed offline using WinWCP v 2.7.9.
Statistical analysis
All data are expressed as mean ± SEM. Data were compared by paired or unpaired Student's t-tests, or one-way analysis of variance with Tukey's comparison as appropriate with P < 0.05 considered significant.
Materials
All chemicals were obtained from Sigma-Aldrich (Poole, UK) or VWR (East Grinstead, UK) except for THC and CBD (Tocris, Bristol, UK or THC Pharma, Frankfurt, Germany), AM251, DL-(2R)-amino-5-phosphonovaleric acid (AP5) and 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide (NBQX) (Ascent Scientific, Bristol, UK) and 8-hydroxy-2(di-N-propylamino)tetralin (8-OH-DPAT), LY320135, WAY100135 and WIN 55,212-2 (Tocris).
Results
Cannabidiol reduces spontaneous action potential frequency in cultured hippocampal neurones
Application of CBD reduced the synaptically driven spontaneous action potential (AP) frequency observed in primary hippocampal cultures in a concentration-dependent manner (0.1 µM, no significant change; 1 µM, 88 ± 8% decrease; 10 µM, 100% decrease; all n= 5, P < 0.001, Figure 1A, B). Confirmation of the synaptically driven nature of the spontaneous APs was shown by their sensitivity to the ionotropic glutamate receptor antagonists, DL-AP5 (100 µM) and NBQX (20 µM) (data not shown). In contrast, CBD was without effect on the resting membrane potential (Vm) at all concentrations tested (0.1 µM, 0.2 ± 2.1 mV from a control Vm of −60.7 ± 2.1 mV; 1 µM, 1.5 ± 1.6 mV from a control Vm of −57.6 ± 1.6 mV; 10 µM, 0.3 ± 0.7 from a control Vm of −62.0 ± 3.7 mV; all n= 5 and P > 0.05). Similar reductions in spontaneous AP frequency were observed following the application of the CB1 agonist, WIN 55,212-2 (0.1 µM Figure 1B) and the CB1 partial agonist, THC (10 µM, Figure 1B), as has been shown previously (Shen and Thayer, 1999; Bajo et al., 2009; Roloff and Thayer, 2009). To determine if the effects of CBD were mediated by a Gαi/o G-protein coupled receptor (GPCR), experiments were performed on cultures treated with Pertussis toxin (PTX, 200 ng·mL−1, 18 h). The CBD-induced reduction in spontaneous AP frequency was abolished following PTX treatment, compared to CBD alone (1 µM, Figure 1B).
Figure 1.
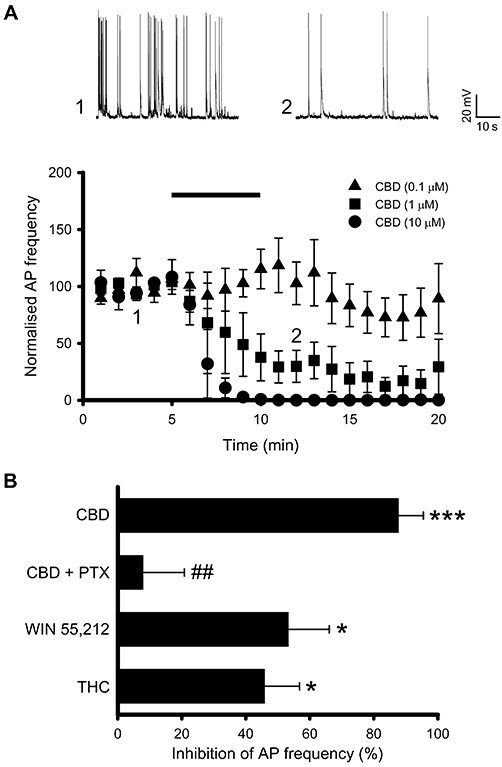
Cannabidiol (CBD) decreases spontaneous action potential (AP) firing frequency in hippocampal cultures, in a concentration-dependent and Pertussis toxin (PTX)-sensitive manner. (A) Time course revealing that AP firing frequency is reduced by CBD (1 & 10 µM). Representative traces illustrating spontaneous AP firing in the absence and presence of CBD (1 µM) are shown for the time points indicated. (B) Summary data showing that AP firing frequency in primary hippocampal cultures was reduced by CBD (1 µM) in a PTX-sensitive manner and by the cannabinoid receptor agonists, WIN 55,212 and Δ9-tetrahydrocannabinol (THC). *P < 0.05, ***P < 0.001, ##P < 0.01 compared with CBD alone.
In order to examine whether the CBD-induced effects involved CB1 receptor activation, we utilized the proposed CB1 receptor antagonist AM251 and the structurally dissimilar CB1 receptor inverse agonist LY320135. Application of AM251 (30 nM) resulted in an initial dramatic increase in AP firing rate, presumably due to the inhibition of the endogenous cannabinoid tone, that in the majority of cases led to a significant depolarization of the membrane potential and, as a consequence, to a loss of AP firing (Figure 2A). Thus the effect of AM251 on CBD-induced effects could not be investigated in our culture system. In contrast, application of LY320135 (1 µM) by itself had no significant effect on the AP firing frequency (14.9 ± 4.2% decrease, n= 7) but did significantly inhibit the decrease in AP firing frequency observed following CBD (1 µM) application, compared to CBD alone (Figure 2C). With CBD proposed as a 5-HT1A receptor agonist, we examined in our culture preparation whether the 5-HT1A receptor was involved in the CBD-mediated effects. Application of the 5-HT1A receptor agonist, 8-hydroxy-2(di-N-propylamino)tetralin (8-OH-DPAT, 10 µM) significantly reduced the spontaneous AP frequency (Figure 2B, C) without affecting the Vm (1.2 ± 1.3 mV decrease from a control Vm of −59.4 ± 1.2, n= 5). The ability of 8-OH-DPAT to reduce spontaneous AP frequency was significantly inhibited in the presence of the 5-HT1A receptor antagonist, WAY100135 (300 nM), compared with 8-OH-DPAT alone (Figure 2C). However, WAY100135 (300 nM) had no effect on CBD-mediated inhibition (1 µM) (Figure 2C), suggesting that in our culture system, the effects of CBD and 8-OH-DPAT were mutually exclusive.
Figure 2.
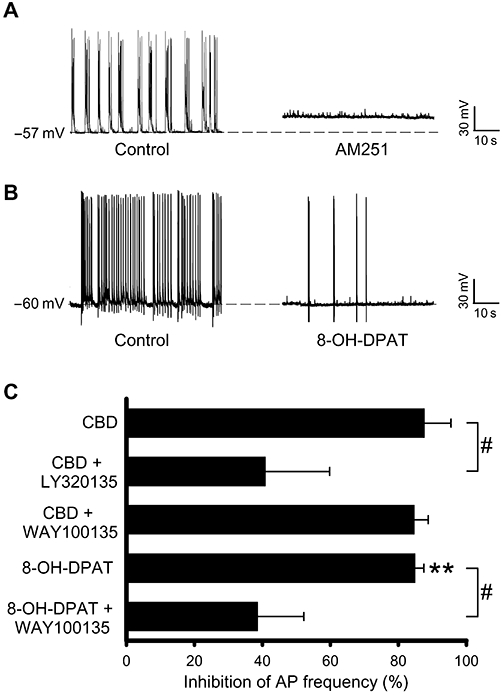
Cannabidiol (CBD)-mediated decrease in action potential (AP) firing frequency in hippocampal cultures is sensitive to the cannabinoid receptor inverse agonist, LY320135. (A) Representative traces illustrating the depolarization and consequent elimination of spontaneous APs observed following the application of AM251 (30 nM). (B) Representative traces showing the reduction in AP firing in the presence of the 5-HT1A agonist, 8-OH-DPAT. (C) Summary data showing the effect of LY320135 and WAY100135 on CBD and 8-OH-DPAT-mediated inhibition of AP firing. **P < 0.01, # P < 0.05 compared with CBD or 8-OH-DPAT alone.
Cannabidiol inhibits synaptic transmission in hippocampal slices
Having established the effect of CBD application in hippocampal cultures, we next investigated whether CBD modulates synaptic transmission in acute hippocampal slices. Application of CBD (10 µM) reversibly inhibited fEPSPs by 39 ± 13% (n= 6, P < 0.05, Figure 3A). The CBD-induced inhibitions were associated with a significant increase in the fEPSP paired-pulse facilitation ratio which increased from the control ratio of 1.65 ± 0.07 to 2.09 ± 0.13 in the presence of CBD (n= 6, P < 0.01, Figure 3B). This finding, aligned with the PTX sensitivity of the CBD effects observed in primary cultures, suggest that a presynaptic GPCR underlies the CBD-mediated inhibition of synaptic transmission. As in experiments in the culture preparation, we utilized the proposed CB1 receptor antagonist, AM251 and the CB1 receptor inverse agonist LY320135 in our slice preparation to investigate the involvement of CB1 receptors. To our surprise, both AM251 (2 µM) and LY320135 (1 µM) abolished the inhibition of synaptic transmission by CBD (Figure 4A, E). Despite the lack of effect of the 5-HT1A receptor antagonist WAY100135 on CBD-mediated effects in cultures, we also investigated the role of 5-HT1A receptors in the CBD-mediated effects on synaptic transmission in the slice preparation. Strikingly, WAY100135 (300 nM) abolished the CBD mediated inhibition of synaptic transmission (Figure 4B, E). In addition and similar to its effect in cultures, 8-OH-DPAT (10 µM), like CBD, significantly inhibited basal synaptic transmission (Figure 4C, E). However, in contrast to CBD, it was without effect on the fEPSP paired-pulse facilitation ratio (control 1.71 ± 0.11, 8-OH-DPAT 1.73 ± 0.13). With evidence existing for AM251 acting via non-CB1 receptor mechanisms (Pertwee, 2005), the specificity of the two putative CB1 receptor inhibitors was investigated against 8-OH-DPAT-mediated inhibitions. Intriguingly, AM251 (2 µM) significantly inhibited the 8-OH-DPAT (10 µM)-induced depression (Figure 4D, E) whereas LY320135 (1 µM) was without effect (Figure 4E).
Figure 3.
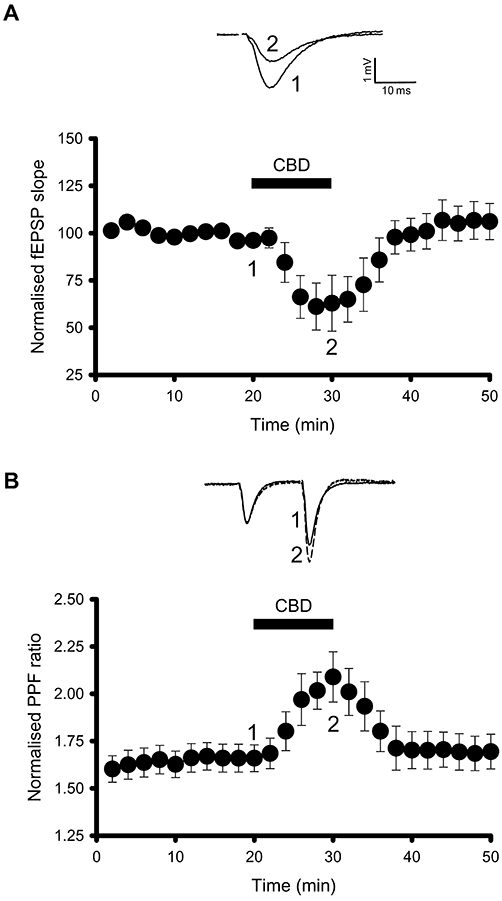
Cannabidiol (CBD) inhibits synaptic transmission in acute slices from the CA1 region of the hippocampus. (A) CBD (10 µM) application reversibly inhibits field excitatory postsynaptic potentials (fEPSP). Representative fEPSP traces are shown for the time points indicated. (B) CBD inhibition of synaptic transmission is associated with an increase in paired-pulse facilitation (PPF) ratio. Scaled fEPSP traces from (A) are shown to demonstrate the increase in paired-pulse facilitation.
Figure 4.
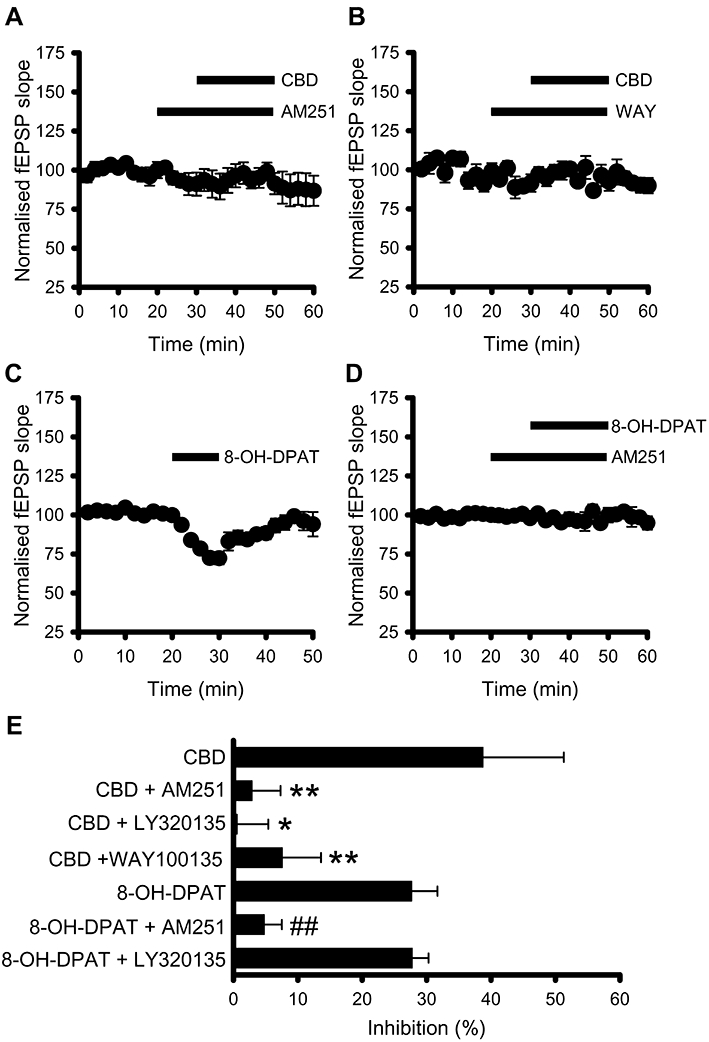
Cannabidiol (CBD)-mediated inhibition of synaptic transmission in acute hippocampal slices is sensitive to 5-HT1A and cannabinoid receptor (CB1) antagonism. (A) CBD (10 µM)-mediated inhibition of synaptic transmission was abolished by the proposed CB1 receptor antagonist AM251 (2 µM). (B) Inhibition of transmission by CBD was significantly reduced in the presence of the 5-HT1A receptor antagonist, WAY100135 (300 nM). (C) 8-OH-DPAT (10 µM) reversibly inhibits field excitatory postsynaptic potentials (fEPSP). (D) AM251 (2 µM) inhibits the 8-OH-DPAT (10 µM)-mediated inhibition of fEPSPs. (E) Summary data showing that CBD-mediated effects were significantly reduced in the presence of 5-HT1A and CB1 receptor antagonists. *P < 0.05, **P < 0.01 compared with CBD alone. ##P < 0.01 compared with 8-OH-DPAT alone.
Discussion
The physiological actions of CBD, a purported non-psychoactive constituent of cannabis, have recently been an area of intense interest especially as it has been proposed to be beneficial in a number of clinical settings. In the present study, we show for the first time that CBD inhibits basal synaptic activity in both hippocampal cultures and acute slices.
Unlike THC, whose physiological effects are thought to be mediated predominantly through CB receptors, CBD has been shown to modulate a multitude of signalling mechanisms (Russo and Guy, 2006; Pertwee, 2008; Izzo et al., 2009). Utilizing in vitro CNS preparations, CBD has been shown to increase intracellular Ca2+ levels when investigated in cultured hippocampal neurons, an effect proposed to be mediated via Cav1 Ca2+ channels, Ca2+ release from intracellular stores and the modulation of mitochondrial function (Drysdale et al., 2006; Ryan et al., 2009). However, these studies were not extended to include the effect of CBD on synaptic activity although CBD did reduce intracellular Ca2+ levels under conditions of high neuronal excitability or epileptiform activity (Ryan et al., 2009), a finding supported by a recent study which shows that CBD has anti-epileptiform activity in hippocampal slice models of epilepsy (Jones et al., 2010). Our findings suggest a mechanism by which CBD inhibits synaptic activity in hippocampal culture preparations. We propose that CBD acts via a Gαi/o coupled GPCR as its effect was abolished following pre-incubation with the Gαi/o G-protein uncoupler, PTX. However, as spontaneous APs observed in our investigation are synaptically driven and with CBD decreasing intracellular Ca2+ levels in the neuronal soma under certain conditions (Ryan et al., 2009), a direct action of CBD on presynaptically located Ca2+ channels or their inhibition through the activation of presynaptic GPCRs cannot be unequivocally ruled out. Indeed, an inhibition of Cav3-type Ca2+ channels by CBD has been shown in heterologous expression systems and sensory neurones (Ross et al., 2008). However, as this Ca2+ channel subtype is not thought to be involved in neurotransmitter release within the hippocampus (Wheeler et al., 1994; Catterall, 2000), the action of CBD on this channel subtype probably does not underlie its effect in the current study. Moreover, modulation of intracellular stores and mitochondria has also been shown to affect synaptic transmission in CNS preparations (Billups and Forsythe, 2002; Fitzjohn and Collingridge, 2002; Le Magueresse and Cherubini, 2007); however, their potential role in the CBD-mediated effects observed here are beyond the focus of the present study.
As THC and WIN 55,212-2 both decreased synaptic activity in agreement with previous studies (Shen and Thayer, 1999; Ohno-Shosaku et al., 2005; Straiker and Mackie, 2005; Bajo et al., 2009), this indicates that the CBD-mediated effects are not an artefact of our culture preparation. This was further verified by observations that CBD inhibited basal synaptic transmission and increased the paired-pulse facilitation ratio in acute hippocampal slices. An increase in paired-pulse facilitation ratio is indicative that CBD is acting via a presynaptic receptor (Baskys and Malenka, 1991) and this finding, aligned to the sensitivity to PTX of the CBD-mediated actions in hippocampal cultures, suggested that the actions of CBD were mediated, at least in part, via a presynaptic Gαi/o coupled GPCR. Hence we investigated the potential role, or lack thereof, of CB1 receptors in the CBD-mediated effects. To our initial surprise, we observed a decrease in the CBD-mediated effects in slices by the proposed CB1 receptor antagonist AM251 and the structurally dissimilar CB1 receptor inverse agonist LY320135. As mentioned earlier, CBD is proposed to have a low affinity for CB1 receptors with no agonist activity and potentially act as a CB1 receptor antagonist (Showalter et al., 1996; Bisogno et al., 2001; Thomas et al., 2007; Jones et al., 2010). It is therefore unlikely that the observed CBD effects are mediated through a direct activation of CB1 receptors. However, an alternative explanation may account for the effects seen with AM251 and LY320135. As CBD has been shown to inhibit the reuptake and metabolism of the endocannabinoid anandamide (Rakhshan et al., 2000; Bisogno et al., 2001), the application of CBD could lead to an increase in the endocannabinoid tone and thus lead to a decrease in synaptic transmission via CB1 receptor activation, as has been shown previously for exogenously applied endocannabinoids in culture (Straiker and Mackie, 2005; Hashimotodani et al., 2007) and in slices under conditions where degradation was inhibited (Bajo et al., 2009). It should be noted, however, that care needs to be taken when interpreting the results obtained using AM251 as this proposed selective CB1 receptor antagonist abolished the effect of 8-OH-DPAT in the present study, indicating non-CB1 receptor effects, as proposed previously (Pertwee, 2005). Despite this, the results obtained with LY320135 do indeed suggest a role for CB1 receptors in the CBD-mediated effects. Taken together, these data imply the direct activation of 5-HT1A receptors and the indirect activation of CB1 receptors in the CBD-mediated effects. However, other signalling mechanisms previously shown to be modulated by CBD may also contribute to the observed effects and cannot be excluded.
An area of current interest regarding the actions of CBD is its proposed action as a putative agonist at the 5-HT receptor subtype, 5-HT1A (Russo et al., 2005). Other studies also support the involvement of 5-HT1A receptors in CBD-mediated effects as 5-HT1A receptor antagonists have been shown to block the anxiolytic effects of CBD observed in animal models of anxiety, depression and stress (Campos and Guimarães, 2008; Resstel et al., 2009; Zanelati et al., 2010). In our experiments, WAY100135 abolished the inhibitory effects of CBD on basal synaptic transmission in acute slices implicating 5-HT1A receptors in the CBD-mediated effects observed here. In agreement with this, activation of 5-HT1A receptors with 8-OH-DPAT inhibited basal hippocampal synaptic transmission, as shown in previous studies (Schmitz et al., 1995; 1998; Pugliese et al., 1998). This raises the possibility that CBD could potentially modify hippocampal-dependent behaviours through the activation of 5-HT1A receptors. However, the effects of 5-HT1A receptor activation on synaptic transmission in acute slices have been proposed to be mediated via postsynaptic activation of G-protein-coupled inwardly rectifying K+ channels (Andrade and Nicoll, 1987; Lüscher et al., 1997) and the lack of effect of 8-OH-DPAT on fEPSP paired-pulse ratio in the current study is consistent with this. It should be noted that 8-OH-DPAT reduced spontaneous AP firing in cultures without affecting the membrane potential. 5-HT receptor-mediated K+ channel activation has been shown to run down in cultures in whole cell mode (Yakel et al., 1988) whilst differences between cultures and slices have been reported previously with regard to the K+ channel type involved following 5-HT receptor activation (Premkumar and Gage, 1994). As our culture experiments are in whole cell mode and are performed only when a stable firing frequency has been obtained, we suggest this is the explanation for the lack of effect on membrane potential seen with 8-OH-DPAT. However, further investigation into the disparity on the membrane potential effects between culture and slice preparations is beyond the scope of this study.
In conclusion, we have shown that CBD reduces synaptic activity in in vitro hippocampal preparations and propose a role for the direct activation of 5-HT1A receptors along with an indirect activation of CB1 receptors in these CBD-mediated effects. These data offer some clues into the mechanisms through which CBD may be reducing regional brain activity (Fusar-Poli et al., 2009; Bhattacharyya et al., 2010) and epileptiform activity (Jones et al., 2010) as reported in recent studies and suggest that further investigations into the actions of CBD in the CNS are required in order to fully elucidate its therapeutic potential.
Acknowledgments
C.J.L. has a John Anderson Scholarship awarded by the University of Strathclyde.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AP5
(2R)-amino-5-phosphonovaleric acid
- CB1
cannabinoid receptor
- CBD
cannabidiol
- DIV
days in vitro
- fEPSP
field excitatory postsynaptic potentials
- GPCR
G-protein coupled receptor
- HBS
HEPES-buffered saline
- NBQX
2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide
- 8-OH-DPAT
8-hydroxy-2(di-N-propylamino)tetralin
- THC
Δ9-tetrahydrocannabinol
Conflicts of interest
There are no conflicts of interest regarding the content of this manuscript.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Roberto M, Schweitzer P. Differential alteration of hippocampal excitatory synaptic transmission by cannabinoid ligands. J Neurosci Res. 2009;87:766–775. doi: 10.1002/jnr.21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskys A, Malenka RC. Agonists at metabotropic glutamate receptors presynaptically inhibit EPSCs in neonatal rat hippocampus. J Physiol. 1991;444:687–701. doi: 10.1113/jphysiol.1991.sp018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O' Carroll CM, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta-9-Tetrahydrocannabinol. Arch Gen Psychiatry. 2009;66:442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology. 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- Drysdale AJ, Ryan D, Pertwee RG, Platt B. Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology. 2006;50:621–631. doi: 10.1016/j.neuropharm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Collingridge GL. Calcium stores and synaptic plasticity. Cell Calcium. 2002;32:405–411. doi: 10.1016/s0143416002001999. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Honey GD. Schizophrenia, ketamine and cannabis: evidence of overlapping memory deficits. Trends Cogn Sci. 2006;10:167–174. doi: 10.1016/j.tics.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by Delta9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2009;24:1–12. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connolly CN. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J Biol Chem. 2007;282:26235–26244. doi: 10.1074/jbc.M704488200. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, et al. Cannabidiol displays anti-epileptiform and anti-seizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332:569–577. doi: 10.1124/jpet.109.159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Cherubini E. Presynaptic calcium stores contribute to nicotine-elicited potentiation of evoked synaptic transmission at CA3-CA1 connections in the neonatal rat hippocampus. Hippocampus. 2007;17:316–325. doi: 10.1002/hipo.20271. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. Neuron. 1997;19:687–695. [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Maejima T, Kano M. Calcium signaling and synaptic modulation: regulation of endocannabinoid-mediated synaptic modulation by calcium. Cell Calcium. 2005;38:369–374. doi: 10.1016/j.ceca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Gage PW. Potassium channels activated by GABAB agonists and serotonin in cultured hippocampal neurons. J Neurophysiol. 1994;71:2570–2575. doi: 10.1152/jn.1994.71.6.2570. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Passani MB, Corradetti R. Effect of the selective 5-HT1A receptor antagonist WAY100135 on the inhibition of e.p.s.ps produced by 5-HT in the CA1 region of rat hippocampal slices. Br J Pharmacol. 1998;124:93–100. doi: 10.1038/sj.bjp.0701807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhshan F, Day TA, Blakely RD, Barker EL. Carrier-mediated uptake of the endogenous cannabinoid anandamide in RBL-2H3 cells. J Pharmacol Exp Ther. 2000;292:960–967. [PubMed] [Google Scholar]
- Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;156:181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff AM, Thayer SA. Modulation of excitatory synaptic transmission by Delta 9-tetrahydrocannabinol switches from agonist to antagonist depending on firing rate. Mol Pharmacol. 2009;75:892–900. doi: 10.1124/mol.108.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J Biol Chem. 2008;283:16124–16134. doi: 10.1074/jbc.M707104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;29:2053–2063. doi: 10.1523/JNEUROSCI.4212-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin reduces inhibition via 5-HT1A receptors in area CA1 of rat hippocampal slices in vitro. J Neurosci. 1995;15:7217–7225. doi: 10.1523/JNEUROSCI.15-11-07217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Gloveli T, Empson RM, Heinemann U. Comparison of the effects of serotonin in the hippocampus and the entorhinal cortex. Mol Neurobiol. 1998;17:59–72. doi: 10.1007/BF02802024. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer SA. Delta9-tetrahydrocannabinol acts as a partial agonist to modulate glutamatergic synaptic transmission between rat hippocampal neurons in culture. Mol Pharmacol. 1999;55:8–13. doi: 10.1124/mol.55.1.8. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte D, Le Dorze JA, Esfahani F, Frost E, Gomori A, Namaka M. Examining the roles of cannabinoids in pain and other therapeutic indications: a review. Expert Opin Pharmacother. 2010;11:17–31. doi: 10.1517/14656560903413534. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Trussell LO, Jackson MB. Three serotonin responses in cultured mouse hippocampal and striatal neurons. J Neurosci. 1988;8:1273–1285. doi: 10.1523/JNEUROSCI.08-04-01273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelati T, Biojone C, Moreira F, Guimarães F, Joca S. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT receptors. Br J Pharmacol. 2010;159:122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


